Abstract
Mesenchymal cell motility is characterized by a polarized distribution of actin filaments, with a network of short branched actin filaments at the leading edge, and polymers of actin filaments arranged into distinct classes of actin stress fibers behind the leading edge. Importantly, the distinct actin filaments are characteristically associated with discrete adhesion structures and both the adhesions and the actin filaments are co-ordinately regulated during cell migration. While it has long been known that these macromolecular structures are intimately linked in cells, precisely how they are co-ordinately regulated is presently unknown. Live imaging data now suggests that the focal adhesions may act as sites of actin polymerization resulting in the generation of tension-bearing actin bundles of actin filaments (stress fibers). Moreover, a picture is emerging to suggest that the tropomyosin family of proteins that can determine actin filament dynamics may also play a key role in determining the transition between adhesion states. Molecules such as the tropomyosins are therefore tantalizing candidates to orchestrate the coordination of actin and adhesion dynamics during mesenchymal cell migration.
Key words: actin, focal adhesion, tropomyosin, migration, mesenchymal
Rapidly following the first description of electron dense adhesion plaques to the underlying matrix in migrating fibroblasts in the 1970s,1 it became apparent that there is a close relationship between adhesion sites and filaments of polymerized actin. Subsequently, it emerged that coordinated regulation of the adhesion sites and the actin filaments is integral to the regulation of cell migration. This critically underpins mesenchymal cell motility, characterized by the formation and disassembly of adhesion plaques, as cells grab and release the surrounding extra-cellular matrix.2 A major question in mesenchymal migration has been how the dynamics of the actin filaments are coordinated with the formation and disassembly of adhesion plaques (adhesion dynamics) and recent advances have begun to shed light on the potential cross-talk coordinating these macro-molecular structures.
Groups of liganded, cross-linked transmembrane integrin receptors form the basis of the adhesion plaques/focal adhesions/focal contacts and these structures integrate biophysical and biochemical processes to regulate mesenchymal motility. Since the discovery that the focal adhesions are signalling centres there has been an ever-expanding catalogue of molecules that associate with focal adhesions—aptly labeled the “adhesome” by one of the key labs in the field.3 Included among the adhesome constituents are proteins that can associate with both the integrin cytoplasmic tails and actin filaments and thus bridge the adhesions to actin filaments. While anchoring of bundles of actin filaments, known as stress fibers, at the cytoplasmic end of the focal adhesion site is well established, exactly how these two structures become associated has been an important area of research.
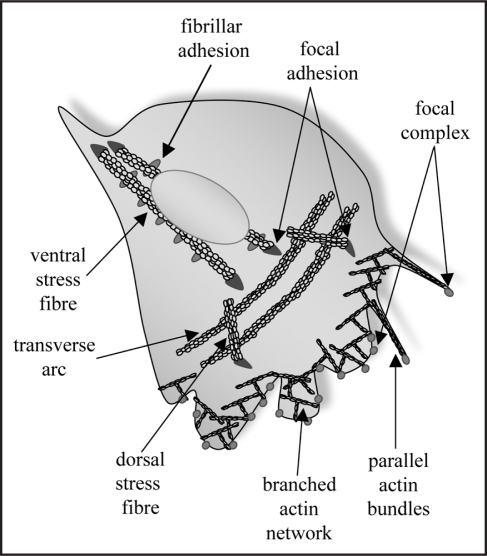
Seminal work from Alan Hall's lab established that the Rho family of small GTPases are critical regulators of discrete actin filament populations associated with distinct adhesion complexes and cellular morphologies4 (Fig. 1). Thus, Rac activation stimulates the formation of small (<1 µm), dot-like focal complexes/pre-cursor adhesions located in a meshwork of short, branched actin filaments at the periphery of the lamellipodia. Similarly, Cdc42 stimulates small focal complexes, but in this case the adhesions are found at the tip of filopodia—short, thin membrane protrusions containing parallel bundles of actin filaments. In contrast, Rho activity causes the formation of longer (2–5 µm), dash-shaped focal adhesions, associated with actin stress fibers. Among other specialized sites of adhesion5 the fibrillar/3-dimensional adhesions form in response to contractility of the actin stress fiber causing translocation of the engaged fibronectin receptors along the ventral surface of the cell. Thus, each of the described adhesion types is characteristically associated with a distinct arrangement of actin polymers.
Figure 1.
Schematic representation of adhesions (fibrillar adhesions, focal adhesions and focal complexes) and associated actin filaments (parallel bundles, branched networks, dorsal and ventral stress fibres and transverse arcs). This schematic emphasizes the polarized nature of the adhesions and filaments: in an actively moving cell focal complexes and the branched actin network are found at the leading edge of the cell, while the dorsal fibres emanate from focal adhesions located just behind the leading edge and the transverse arcs are arrayed parallel to the leading edge. Notably, excessive transition to ventral stress fibres and fibrillar adhesions is inhibitory to cell migration and polarized 2-dimensional migration.11
The co-dependence of actin stress fibers and focal adhesions on RhoA activity again emphasises the intimate connection between these two structures, however does not clarify how they come to be associated in cells. Moreover, some actin filament bundles have an associated focal adhesion at only one end, others have one at either end, while a third class are devoid of associated focal adhesions.6 These filament populations are characteristically observed in mesenchymal cells and are named dorsal fibers, ventral fibers and transverse arcs, respectively (Fig. 1). As implied by their names each filament type is typically found either extending from the leading edge of the cell back towards the dorsal surface, running along the ventral surface, or arrayed in arcs that run parallel to the leading edge. Recent live imaging studies have begun to yield mechanistic insight into the formation of these distinct actin filament populations.7 Imaging of cells expressing fluorescently tagged zyxin to monitor focal adhesions and actin or a-actinin to monitor actin filaments, revealed that actin polymerization can occur at the cytoplasmic face of a focal adhesion. Continued actin polymerization away from the focal adhesion site leads to the formation of dorsal stress fibers that typically intersect with transverse arc filaments towards their distal end. In one intriguing model it has been suggested that the intervening transverse arcs may bridge two dorsal fibers and that myosin-induced tension them pulls on the two dorsal fibers to produce a single ventral stress fiber with focal adhesions at either end6,7 and punctate fibrillar adhesions aligned along the ventral fiber. Importantly, the transition between both actin filament types and adhesion types is a highly regulated process and the balance of these transitions is directly correlated with a cell's migratory behavior. Our present understanding of this process has arisen from the study of cells in 2-dimensional cultures where the actin fiber types and associated focal adhesions are easily discernible and manipulable. An important avenue of future research will be to investigate this series of transitions under 3-dimensional culture conditions that approximate the in vivo environment that confronts migrating cells.
Present models for mesenchymal migration propose an orderly transition between adhesion structures. Thus a sub-set of focal complexes formed at the cell's leading edge are targeted for transition to a focal adhesion. How this subset is singled out is not entirely clear, however Rho GTPase activity is required and almost certainly the transition is signalled by the recruitment of specific proteins such as α5β1 integrin heterodimers, zyxin8 or α-actinin.9 The transition to the focal adhesion then provides traction for the forward movement of the cell via contractility transmitted through associated actin stress fibers. Some of the focal adhesions then slide to the rear of the cell where they are subsequently broken down or detached, to promote tail release and forward movement. Live imaging of focal adhesions in regions of active membrane extension have clearly shown that focal adhesions in this area are also highly dynamic with many seen to turnover during relatively short (minutes) time-frames.10,11 It is significant that there is a bi-phasic migration response to cell adhesion: both too little and too much adhesion can reduce cell migration speeds.12 Hence presumably, molecules that can regulate the rate of focal adhesion disassembly at the leading edge may be important regulators of cell migration. This may be further generalised to any biological process requiring the extension and stabilization of membrane. For example, neurite extensions may depend on the stability of focal adhesions at the tip of the extending process.13,14
A number of signalling proteins have been implicated in the regulation of focal adhesion turnover, with the two of the most studied being FAK and Src kinase.10 To date, less attention has been paid to molecules that affect actin filament dynamics which may reinforce or disrupt focal adhesions. The actin cytoskeleton is involved in most—if not all—cellular functions and so not surprisingly the proteins that determine actin functions are many and varied. Included among these regulators is the tropomyosin family of actin-associating proteins.15 The tropomyosins form continuous head-to-tail polymers that sit along the major groove formed by the twisting together of two strands of actin polymer. The association of tropomyosin with actin filaments alters the association of actin regulators—possibly by changing the filament structure and alternatively exposing or hiding interaction sites. Exogenous expression of the tropomyosin isoform Tm5NM1 causes enhanced myosin II recruitment to actin filaments both in vitro and in vivo16 leading to enhanced acto-myosin contractility. The non-muscle tropomyosins are a large multi-isoform family that are developmentally regulated and show both spatially and temporally restricted expression. Increasing investigations of this family of proteins has added to a growing list of associated biological functions.17 In our recent work, we showed that elevated Tm5NM1 expression results in stabilization of focal adhesions and a switch to an increased fibrillar adhesion/ventral stress fiber phenotype, resulting in loss of cell motility.11 Moreover, our data suggest that this effect is due to reduced actin filament dynamics generated by Tm5NM1 overexpression. We reported distinct focal adhesion effects by two different isoforms—Tm3 and Tm5NM1,11—and indeed this holds true for all of the tropomyosin isoforms we have tested to date (Bach and O'Neill GM, unpublished data). Similarly, a previous study found that exogenous skeletal muscle α-tropomyosin drove increased focal adhesion turnover and correspondingly increased cell migration in nonmuscle cells.18 Thus a picture is emerging suggesting that the tropomyosin expression profile may be a key determinant of adhesion dynamics. Therefore, molecules such as the tropomyosins that determine the actin filament state also play an important role in determining the rates of transition between different adhesion states.
An important question that now needs to be addressed is the mechanism that links tropomyosin expression to adhesion dynamics. This might represent simply a direct mechanical effect, whereby reduced actin stress fiber dynamics stabilizes the associated focal adhesion. However, we found strikingly altered activation of adhesion signalling molecules, dependent on Tm5NM1 expression. Importantly, reduced phosphorylation of adhesion-associated proteins in cells overexpressing Tm5NM1 was paralleled by a correspondingly elevated phosphorylation in mouse embryo fibroblasts derived from a homozygous Tm5NM1 deletion mouse model.11 Thus we favour the idea that the expression of Tm5NM1 may not only change actin filament dynamics, but may also regulate the activity of adhesion-associated proteins. The phosphorylation of adhesion-associated molecules is a critical determinant of adhesion dynamics and the transition between adhesion states10,19,20 however, the mechanisms regulating the spatial and temporal activation of these molecules is not clear. To date, we do not know the sequence of events governing Tm5NM1 association with the actin filaments, nor do we know whether there is any spatial difference in Tm5NM1 association with actin stress fibers; but these are important questions to answer if we are to understand how endogenously expressed Tm5NM1 might take part in regulating the transition between adhesion states during cell migration.
Adhesion molecules such as p130Cas have been suggested to be activated via mechanosensory mechanisms21 and we found altered p130Cas phosphorylation in cells with elevated Tm5NM1 expression11 raising the notion that Tm5NM1 may form part of a mechanosensory circuitry. Alternatively, clusters of specific integrin receptors are linked to discrete down-stream signalling events,22 therefore if Tm5NM1 expression results in a preferential stabilization of α5β1 integrin heterodimers (characteristic of fibrillar-type adhesions) this could cause altered signal transduction. It has also recently been suggested that the regulation of lipid membrane order may have consequent effects on the organization of signalling molecules at focal adhesion sites;23 potentially Tm5NM1 association with actin stress fibers may result in altered membrane lipid order. Finally, the trafficking of Src kinase to the focal adhesion sites is an actin-dependent process24 and conceivably Tm5NM1-dependent stabilization of actin dynamics may interfere with this process. Thus the challenge now is to determine exactly how actin filament-dependent regulation of focal adhesion structures can inform the activation of signalling molecules at these sites.
Acknowledgements
G.O. is supported by grants from the National Health and Medical Research Council of Australia (#512251) and the New South Wales Cancer Council (RG 09-16).
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/9468
References
- 1.Abercrombie M, Heaysman EM, Pegrum SM. The locomotion of fibroblasts in culture IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nobes C, Hall A. Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 5.Geiger B, Bershadsky A, Pankov R, Yamada KM. Extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 6.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fiber assembly. J Microscopy. 2008;231:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 7.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 9.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 11.Bach CTT, Creed S, Zhong J, Mahmassani M, Schevzov G, Stehn J, et al. Tropomyosin isoform expression regulates the transition of adhesions to determine cell speed and direction. Mol Cell Biol. 2009;29:1506–1514. doi: 10.1128/MCB.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varnum-Finney B, Reichardt LF. Vinculin-deficient PC12 cell lines extend unstable lamellipodia and filopodia and have a reduced rate of neurite outgrowth. J Cell Biol. 1994;127:1071–1084. doi: 10.1083/jcb.127.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunning PW, O'Neill GM, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 16.Bryce NS, Schevzov G, Ferguson V, Percival JM, Lin JJ, Matsumura F, et al. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol Biol Cell. 2003;14:1002–1016. doi: 10.1091/mbc.E02-04-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Neill GM, Stehn J, Gunning PW. Tropomyosins as interpreters of the signalling environment to regulate the local cytoskeleton. Sem Cancer Biol. 2008;18:35–44. doi: 10.1016/j.semcancer.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–187. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- 20.Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 21.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–526. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]