SUMMARY
Gastrointestinal motility is ensured by the correct coordination of the enteric nervous system and the visceral smooth muscle cells (SMC), and defective development of SMCs result in gut malformations and intestinal obstructions. In order to identify the molecular mechanisms that control the differentiation of the visceral mesenchyme into SMCs in the vertebrate stomach, we developed microarrays to analyze the gene expression profiles of undifferentiated and differentiated avian stomachs. We identify Scleraxis, a basic-helix-loop-helix transcription factor, as a new marker of stomach mesenchyme and find that expression of Scleraxis defines the presence of two tendons closely associated to the two visceral smooth muscles. Using targeted gene misexpression, we show that FGF signaling is sufficient to induce Scleraxis expression and to establish two tendon domains adjacent to the smooth muscle structures. We also demonstrate that the tendon organization is perturbed by altering Scleraxis expression or function. Moreover, using primary cells derived from stomach mesenchyme, we find that undifferentiated stomach mesenchyme can give rise to both SMCs and tendon cells. These data show that upon FGF activation, selected stomach mesenchymal cells are primed to express Scleraxis and to differentiate into tendon cells. Our findings identify a new anatomical and functional domain in the vertebrate stomach that we characterize as being two intermuscular tendons closely associated with the visceral SMC structures. We also demonstrate that coordinated development of both tendon and smooth muscle domains is essential for the correct morphogenesis of the stomach.
Keywords: Animals; Animals, Genetically Modified; Avian Proteins; antagonists & inhibitors; genetics; metabolism; Base Sequence; Basic Helix-Loop-Helix Transcription Factors; antagonists & inhibitors; genetics; metabolism; Cell Differentiation; Chick Embryo; DNA; genetics; Enteric Nervous System; embryology; physiology; Fibroblast Growth Factors; genetics; metabolism; Gastrointestinal Motility; Gene Expression Profiling; Gene Expression Regulation, Developmental; Gene Targeting; Mesoderm; cytology; embryology; metabolism; Models, Biological; Molecular Sequence Data; Muscle, Smooth; cytology; embryology; metabolism; Myocytes, Smooth Muscle; cytology; metabolism; Oligonucleotide Array Sequence Analysis; Signal Transduction; Stomach; embryology; metabolism; Tendons; embryology; metabolism
Keywords: FGF pathway, Scleraxis, Gut development, Tendon, Visceral smooth muscle, Chick
INTRODUCTION
The gastrointestinal (GI) tract is a remarkably complex, three-dimensional, specialized system derived from a simple tubal structure. The GI tract is composed of three germ layers: mesoderm (which forms the smooth muscle layer), endoderm (which forms the epithelial lining), and ectoderm (which includes the enteric nervous system (ENS)). The primitive gut is initially a straight tube. As they develop, each region of the gut is characterized by a unique morphology discernible by gross and microscopic examination. These tissues show regional specific differentiation along the antero-posterior axis. This regionalization is maintained throughout life and is essential for the normal function of the adult gut. Candidate factors involved in gut development include genes that were first identified in Drosophila. These comprise homeotic (Hox and Nkx) and secreted factors (Bone Morphogenetic Protein (BMP) and Hedgehog) (de Santa Barbara et al., 2002).
The motility of the GI tract is ensured by the correct coordination of the visceral smooth muscle cells (SMC) and the autonomous ENS. ENS originates from neural crest cells that migrate from the dorsal region of the neural tube and colonize the whole gut to establish its innervation (Wallace and Burns, 2005). SMCs derive from the splanchno-pleural mesoderm and will form the undifferentiated visceral mesenchyme (Robert, 2000). Few have investigated the molecular mechanisms involved in the differentiation of the visceral mesenchyme into SMCs. SMCs are present in both vascular and digestive systems, however the digestive tract is the most abundant contributor of SMC in humans (Gabella, 2002). In the chick, the gizzard (muscular stomach or antrum) has a thick layer of smooth muscle that facilitates mechanical digestion, whereas the proventriculus (glandular stomach or fundus), which develops anteriorly to the gizzard, has only a very thin smooth muscle layer (Robert, 2000). The intestines show modest development of smooth muscle layers. In the avian stomach, SMC differentiation is observed from embryonic day (E) 9. Thus, the chick stomach offers the ideal model to elucidate the molecular mechanisms that control visceral SMC differentiation. In the GI tract, Bmp4, a ligand that belong to the transforming growth factor β (TGF-β) superfamily, is expressed in the mesenchyme of the whole chick gut with the exception of the gizzard (Roberts et al., 1998). When over-expressed, Bmp4 causes a reduction in the thickness of the smooth muscle layer of the stomach demonstrating a regulatory role in gut muscle growth (Roberts et al., 1998). Conversely, the homeotic gene Bapx1 is expressed only in the chick gizzard mesenchyme and acts as a represser of Bmp4, therefore modulating gizzard smooth muscle development (Nielsen et al., 2001). In addition, we have investigated the function of the BMP pathway during visceral SMC differentiation and found that aberrant modulation of BMP activity altered this process (de Santa Barbara et al., 2005).
In order to identify factors that trigger and control differentiation of visceral SMC, we carried out a microarray screen to isolate candidate genes. We identified Scleraxis, a member of the basic-helix-loop-helix (bHLH) family of transcription factors, which is expressed in tendon cells of the stomach adjacent to the visceral SMC. We then used the avian retroviral system to specifically misexpress or inactivate Scleraxis in the stomach mesenchyme and showed that Scleraxis expression defines the intermuscular tendon domains that are established in close association with visceral SMC.
MATERIALS AND METHODS
Chick embryonic gastrointestinal tissues
Fertilized White Leghorn eggs were obtained from Haas Farm, France. Tissues were staged according to Hamburger and Hamilton for early embryogenesis Stages and by embryonic day (E) for gastrointestinal tract analysis (Hamburger and Hamilton, 1951).
Retroviral misexpression studies
The Fgf8 (Brent and Tabin, 2004), sFgfR2b (Mandler and Neubuser, 2004) and GFP (Moniot et al., 2004) viral constructs were previously described. ShScleraxis associated with mouse U6 promoter and full-length avian Scleraxis cDNA were cloned into the shuttle vector Slax and then subcloned into the RCAS(A) vector. Full-length avian Scleraxis cDNA was cloned in frame in the Slax-Engrailed vector and then subcloned into the RCAS(A) vector. All vectors were transfected into avian DF-1 cell lines, and viruses harvested and titered using standard techniques. To target the presumptive stomach mesenchyme, misexpression experiments were performed on stage 9–10 embryos as previously described (Moniot et al., 2004).
Primary cell cultures derived from stomach mesenchyme
Gizzards from Stage 25 (referred to as E5 gizzards) were harvested in PBS solution. After collagenase treatment (Sigma) at room temperature for 12 min, we isolated the mesenchymal layer using fine forceps (Simon-Assman and Kédinger, 2000). Individual mesenchymal cells were plated on dishes and kept in culture for 24 (E5+1D) and 72 hr (E5+3D) in DMEM, 10% Fetal Bovine Serum in the absence or presence of the different avian retroviruses.
Expression analyses
In situ hybridization experiments on whole tissues/embryos and paraffin sections were carried out as previously described (Moniot et al., 2004). Different chick templates for antisense riboprobes were obtained by PCR amplification using specific primer sets (Table S2). The following plasmids were used: αSMA, Type I Collagen, Fgf7, Fgf10, FgfR1 (gift from D. Duprez), FgfR2 (gift from C. Tabin), Fjx (gift from P. Francis-West), Scleraxis (gift from D. Duprez), Sox10 (Moniot et al., 2004) and Tenomodulin. Immunohistochemistry was performed on paraffin sections using polyclonal antibodies against αSMA (Sigma) and Phospho-Histone H3 Ser10 (Upstate), and monoclonal antibodies against Type I Collagen, GAG, and Decorin (Developmental Hybridoma Bank (DSHB)).
In cellulo in situ hybridization was performed as previously described (Gregoire et al., 2006). Methyl violet staining was used as described (Bi et al., 2007). Immunofluorescence was performed using monoclonal antibodies directed against Tenascin (DSHB) and Type I Collagen (DSHB), and polyclonal antibodies against Caldesmon (Sigma), Desmin (Sigma) and SOX9 (Moniot et al., 2004). The Alexa 488 anti-mouse and Alexa 555 anti-rabbit secondary antibodies (Invitrogen) were used and nuclei were stained with DAPI (Molecular Probes). Cells were mounted in FluorSave reagent (Calbiochem).
For microarray experiments and quantitative RT-PCR amplification, RNAs were extracted with the RNeasy Kit (Qiagen). Biotinylated complementary RNAs were hybridized to the Affymetrix GeneChip Chicken Genome Arrays using standard manufacturer’s protocols (Affymetrix, IRB, CHU Montpellier, France). Fluorescence intensities were quantified and analyzed using the GCOS software (Affymetrix) (Table S1). Scleraxis expression was quantified by quantitative RT-PCR amplification using LightCycler technology (Roche Diagnostics; 95°C for 10s, 60°C for 5s, 72°C for 10s). PCR primers are listed in Table S2. mRNA values were determined by LightCycler analysis software (version 3.1), according to the standard curves. Data were represented as the relative mean level of Scleraxis expression relative to 18S standard expression.
For electron microscopy, tissues were immersed in a solution of 3.5% glutaraldehyde in phosphate buffer (0.1M, pH 7.4) overnight at 4°C. They were then washed and post-fixed in 1% osmic acid plus 0.8% potassium ferrocianide in the dark and room temperature for 2h. After washes, tissues were dehydrated in graded ethanol solutions and embedded in EmBed 812 DER 736. Thin sections (85nm; Leica-Reichert Ultracut E) were collected. Sections were counterstained with uranyl acetate and lead citrate and observed using a Hitachi 7100 transmission electron microscope at the CRIC facility (C. Cazevieille, Montpellier, France).
Photography
Images of whole-mount tissues and paraffin sections were collected with a Nikon DXM1200 camera connected to a Nikon Multizoom AZ100 microscope.
RESULTS
Intermuscular tendons are present in the vertebrate stomach
In order to identify factors that play a role at the onset of differentiation of the visceral SMC, we used microarrays to compare the gene expression profiles of undifferentiated E6 and differentiated E9 chick gizzards (Fig. 1A; Table S1). We observed a higher expression of well known smooth muscle markers, such as Caldesmon, Desmin, Smooth Muscle Myosin, Smooth Muscle Actin, and of more recently discovered ones, such as Smoothelin and SRF in E9 gizzards (Wallace and Burns, 2005; Niessen et al., 2005; Mericskay et al., 2007). We also identified a new cluster composed of genes (i.e., Scleraxis, Decorin, Tenomodulin and Type XII Collagen) that are associated with the development and differentiation of tendon tissues (Tozer and Duprez, 2005).
Fig. 1. Intermuscular tendons are present in the embryonic stomach.
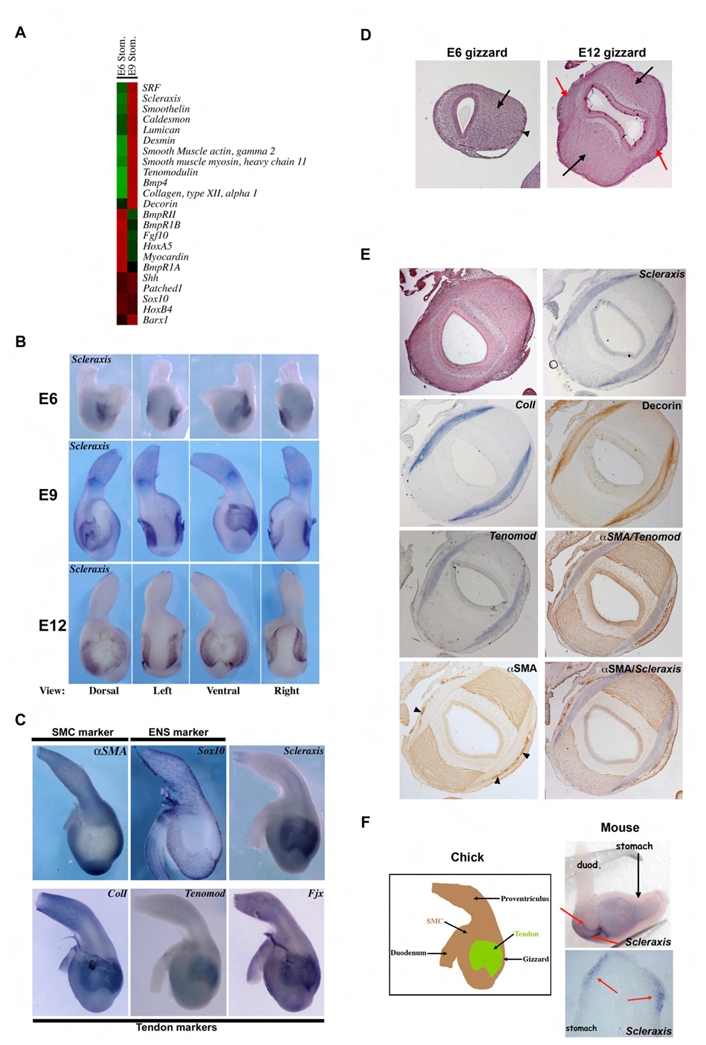
(A) Relative expression level of transcripts in E6 and E9 chick gizzards; the highest signals are in red and lowest in green. At E9, smooth muscle and tendon markers are expressed at higher levels than at E6. (B) Expression pattern of Scleraxis in the stomach by whole-mount in situ hybridization using an antisense Scleraxis riboprobe. Scleraxis expression is restricted to two newly identified domains located on the dorsal and ventral sides of the gizzard. From E6 to E9, Scleraxis expression domain widens. At E12, Scleraxis expression is restricted to the boundaries of these initial domains. (C) Whole-mount in situ hybridization on E9 stomachs. αSMA is expressed in SMC and Sox10 in ENS cells. Scleraxis, Type I Collagen (Coll), Tenomodulin and Four jointed (Fjx) are expressed mainly in the tendon domains. All tendon markers show a pattern of expression that overlaps with that of Scleraxis while they are absent from the smooth muscle domains and ENS cells. (D) Histology of E6 and E12 gizzards. At E6 the visceral mesenchyme is homogenous (arrow) in spite of the presence of migrating enteric nervous cells on the outer layer (arrowhead). At E12, the gizzard is composed of two well differentiated smooth muscle structures (black arrows) adjacent to two domains constituted of connective tissue (red arrows). (E) Serial transversal sections of an E9 gizzard. Scleraxis, Tenomodulin and Type I Collagen are detected by in situ hybridization, and αSMA and Decorin by immunostaining. Two differentiated smooth muscle structures are associated with the two emerging domains of connective tissues characterized by the specific expression of Scleraxis and of the tendon cell markers Type I Collagen, Decorin and Tenomodulin. αSMA labels the two smooth muscle areas as well as the monolayer of smooth muscle tissue surrounding the vasculature (arrowheads). In situ hybridization of Scleraxis or Tenomodulin followed by αSMA immunodetection on the same sections demonstrated the presence of two tendon structures closely associated with the visceral smooth muscle structures of the gizzard. (F) Left panel: Schematic representation of avian E9 stomach indicating the presence of intermuscular tendons. Visceral SMC domain (brown area), and well organized tendon domain (green area). Right panels: In situ hybridization on mouse E13 stomachs using a mouse Scleraxis antisense riboprobe (whole-mount and section). Two Scleraxis expression domains (red arrows) were visible.
In this study, we focused on Scleraxis, a member of the bHLH family of transcription factors previously reported as an early marker of tendons and tenocytes (Edom-Vovard et al., 2001; Schweitzer et al., 2001). We next examined the expression profile of Scleraxis during GI tract development and found it expressed in two specific subdomains of the stomach mesenchyme from E6 to E9 and at the boundaries of these two domains after SMC differentiation (E12) (Fig. 1B). At E9, Scleraxis was expressed also in the caecum, a structure that separates the colon from the small intestine (Supplemental Fig. S1).
The mesenchyme of the avian embryonic stomach is composed of visceral SMC (that express αSMA, a SMC-specific factor) and intercalated ENS cells (positive for Sox10) to allow autonomous contraction. However, Scleraxis and αSMA or Sox10 expressions were mutually exclusive (Fig. 1C) suggesting that Scleraxis is expressed neither in visceral SMC nor in ENS cells. Analyses by light microscopy of the embryonic gizzard showed a homogenous visceral mesenchymal structure with migrating ENS cells in the outer layer at E6 (Fig. 1D). Conversely, at E12, the gizzard consisted of two well differentiated smooth muscle areas both associated with two connective structures (Fig. 1D), which, at E9, appeared to be close to the differentiating SMC (Fig. 1E). These connective tissues were positive for Scleraxis, Tenomodulin, another gene identified by our microarray screen and other tendon markers, such as Type I Collagen (Ros et al., 1995), Four-jointed (Yamaguchi et al., 2006), and Decorin, that showed an expression pattern overlapping with that of Scleraxis (Fig. 1C,E) in these two connective tissues. Conversely, Scleraxis and αSMA were detected in two specific and mutually exclusive domains (Fig. 1E). Furthermore, we found that, in the mouse, Scleraxis expression was limited to two small domains in the antrum that correspond to the avian gizzard (Fig. 1F).
An early study highlighted the intimate relation between muscle bundles and these two connective structures in the adult avian gizzard (Watzka, 1932); two centra tendinea were observed in the adult gizzard and were characterized as rich in collagen fibrils (McLelland, 1979; Gabella, 1985). Attachment of two skeletal muscles through a central structure was also described in the diaphragm. This was considered to be another category of tendon and was named intermuscular tendon (Ackerman and Greer, 2007; Murchison et al., 2007). Taken together, these data indicate that, in the stomach, Scleraxis exhibits a restricted expression pattern that defines two tendons closely associated to the two visceral smooth muscles (Fig. 1F). We define them as intermuscular tendons.
FGF signaling pathway is necessary and sufficient to establish the tendon domains
Previous studies have shown that the Fibroblast Growth Factor (FGF) signaling pathway is required for induction of Scleraxis expression in somitic tendon progenitors and for its maintenance in chick limb tendons (Brent and Tabin, 2004; Edom-Vovard et al., 2002). In order to identify the FGF ligand(s) and receptor(s) that could be responsible for the initiation of Scleraxis expression in E6 stomach, we quantified by RT-PCR their levels in E6 gizzards and detected robust expression of eight FGF ligands and three FGF receptors (Fig. 2A). Whole-mount in situ hybridization revealed that Fgf7, Fgf10, FgfR1 and FgfR2 were expressed in the gizzard mesenchyme from E6 (Fig. 2B and Fig. S2). The other detected FGF ligands were mainly expressed in the epithelial layer (unpublished data). We then investigated whether the FGF signaling pathway could regulate Scleraxis expression. For this aim we perturbed the FGF pathway by misexpressing an inhibitory form of mouse FGFR2 IIb receptor (referred to as sFgfR2b) that is secreted and preferentially sequesters the FGF ligands 1, 3, 7 and 10 (Mandler and Neubuser, 2004), and then monitored Scleraxis expression by in situ hybridization and quantitative RT-PCR (Fig. 2C,D). In the gizzard, infection with RCAS-sFgfR2b retroviruses led to down-regulation of Scleraxis expression and size reduction of the two specific domains (Fig. 2C), while expression of RCAS-GFP, a negative control, had no effect on Scleraxis (compare Figs 2C with 1B). Efficient retroviral infection of the mesenchyme was confirmed by immunohistochemistry using antibodies against the avian retroviral GAG protein (α3C2) or by in situ hybridization using a riboprobe against the avian retroviral envelop gene (env) (data not shown). We monitored Scleraxis mRNA level by quantitative RT-PCR and observed a strong decrease in RCAS-sFgfR2b stomach; this finding was supported also by the reduction of Fgf10 mRNA in this condition (Fig. 2D). Conversely, ectopic activation of the FGF signaling pathway along the GI tract following RCAS-Fgf8 misexpression caused multiple morphological defects mainly in the proventriculus, the gizzard and the caecum (Fig. 2E and Fig. S3). Fgf8-infected stomachs showed ectopic expression of Scleraxis associated with other tendon markers, such as Type I Collagen and Tenomodulin (Fig. 2E and Fig. S4). Moreover, in Fgf8-infected stomachs, we observed reduced expressions of αSMA in the areas of ectopic expression of tendon markers (Fig. 2E and Fig. S3). To analyze the effect of FGF activation on cell proliferation, we used antibodies directed against phosphorylated Histone 3B (PH3), a standard cell cycle marker of the G2/M transition (Fig. 2F). We did not observe significant differences in the number of proliferative cells in the gizzard SMCs following infection of RCAS-Fgf8 or RCAS-GFP, indicating that FGF activation does not induce a global up-regulation of the mitotic potential. These data suggest that FGF signaling pathway controls positively the establishment of tendons in the developing stomach and recruits mesenchymal cells toward a tendon fate.
Fig. 2. The FGF signaling pathway is necessary and sufficient to establish the stomach tendon structures.
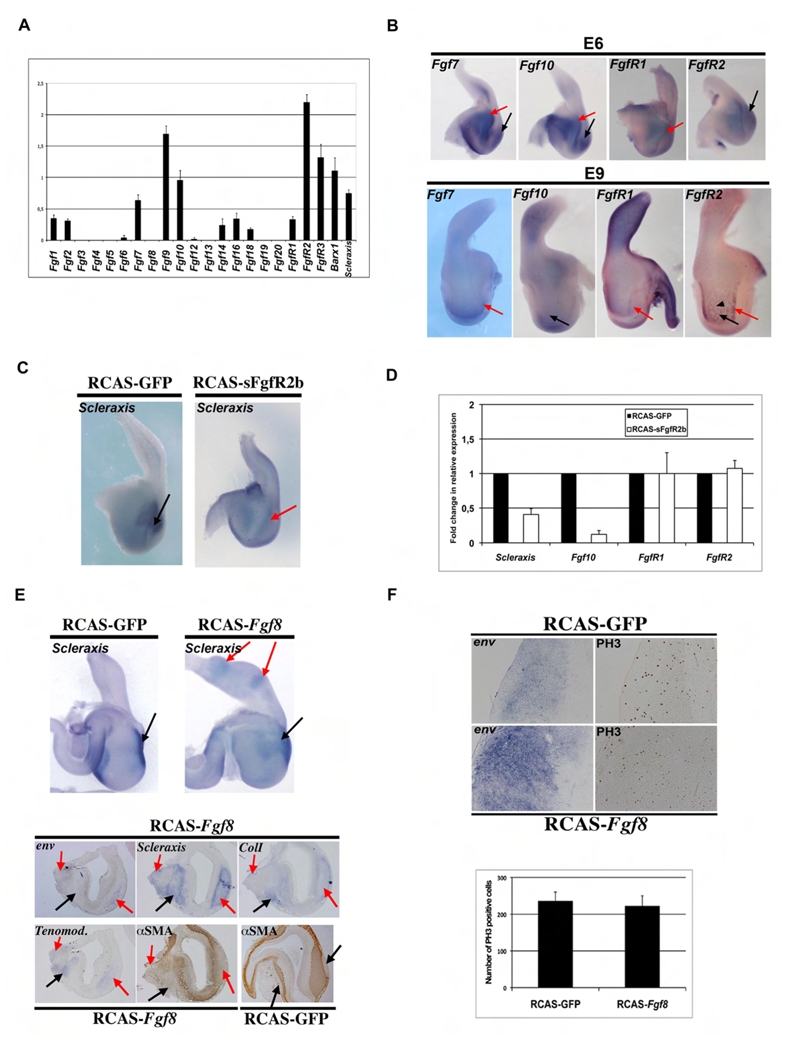
(A) Semi-quantitative RT-PCR analysis of FGF ligands and receptors expression in E6 gizzards. Shown are the mean values +/− SEM relative to the 18S ribosomal RNA control. Each measurement was done on 4 independent cDNA preparations (n= 4). (B) Whole-mount in situ hybridization on E6 and E9 stomachs. At E6, Fgf7, Fgf10, FgfR1, and FgfR2 are widely expressed in the gizzard mesenchyme. At E9, Fgf7 and FgfR1 expression becomes restricted to the mesenchyme close to the edge of the tendon domains, and Fgf10 and FgfR2 are both expressed in ENS cells; in addition, FgfR2 expression defines the tendon borders. Red arrows indicate the tendon area, black arrows the mesenchyme or smooth muscle domain, arrowhead the ENS cells. (C) Inhibition of the FGF signaling pathway in the stomach affects Scleraxis expression. Whole-mount in situ hybridization with an antisense Scleraxis riboprobe on E9 stomachs in which GFP (RCAS-GFP) and sFgfR2b (RCAS-sFgfR2b) are misexpressed. GFP misexpression does not alter stomach morphology, while sFgfR2b misexpression affects slightly the size of the gizzard. Scleraxis expression domain is down-regulated in sFgfR2b-misexpressing stomachs (red arrow), while is not affected in GFP-misexpressing stomachs (black arrow). (D) Inhibition of the FGF signaling pathway in the stomach affects Scleraxis and Fgf10 but not FgfR1 and FgfR2 expression. Quantitative RT-PCR experiments on E7 stomachs in which RCAS-GFP (control) and RCAS-sFgfR2b were misexpressed. sFgfR2b infected stomachs express 60% less Scleraxis and 90% less Fgf10 than controls. Each measurement was done on two independent cDNA preparations (n= 4). (E) Activation of the FGF signaling pathway in the stomach affects the expression of Scleraxis and specific tendon markers. Whole-mount in situ hybridization using an antisense Scleraxis riboprobe on RCAS-GFP and RCAS-Fgf8 E9 stomachs. Ectopic activation of the FGF pathway through Fgf8 misexpression induces ectopic mesenchymal buddings associated with ectopic expression of Scleraxis in the proventriculus (red arrows). Serial adjacent longitudinal sections of a RCAS-Fgf8 E9 stomach analyzed by in situ hybridization with specific antisense riboprobes directed against Env, Scleraxis, Tenomodulin and Type I Collagen and by immunohistochemistry with an anti-αSMA antibody. Longitudinal section of a RCAS-GFP E9 stomach analyzed by immunohistochemistry with an anti-αSMA antibody. Areas with RCAS-Fgf8 expressing cells, detected by Env, are characterized by the presence of Scleraxis and other tendon markers, such as Tenomodulin and Type I Collagen and by reduction of the αSMA positive domain. Black arrows indicate endogenous tendon structures and red arrows ectopic tendons. (F) Serial longitudinal sections of E9 stomach infected with RCAS-Fgf8 and RCAS-GFP retroviruses and analyzed by in situ hybridization with an Env riboprobe and by immunohistochemistry with an anti-PH3 antibody. Positive signals were counted in three comparable sections. Misexpression of Fgf8 in the gizzard had no effect on proliferation.
Tendon structures are essential for the development of the stomach
Scleraxis belongs to the bHLH family of transcription factors and is one of the rare transcription factors expressed at the onset of tendon development (Edom-Vovard et al., 2001; Schweitzer et al., 2001). In order to investigate more directly the role of Scleraxis in stomach development, we used a loss-of-function technique to in vivo deliver ShScleraxis in the chick stomach (Fig. 3A, B) (Harpavat and Cepko, 2006). First, we tested the efficiency of RCAS-ShScleraxis in primary cells derived from stomach mesenchyme and observed a robust reduction of Scleraxis mRNA (Fig. 3C) without any side effect on the identity of the infected cells (data not shown). In embryonic stomachs, RCAS-ShScleraxis led to strong down-regulation of Scleraxis expression (Fig. 3D) and to some minor defects, such as a smaller gizzard and a straight proventriculus (Fig. 3D). Strong retroviral infection was correlated with decreased expression of Scleraxis, Type I Collagen and Tenomodulin (Fig. 3E), suggesting a reduction in size of the tendon domains in the developing stomach. Conversely, we observed an increase of the territory labeled by αSMA, a SMC marker (Fig. 3E).
Fig. 3. Inhibition of Scleraxis expression in the stomach impairs its normal development.
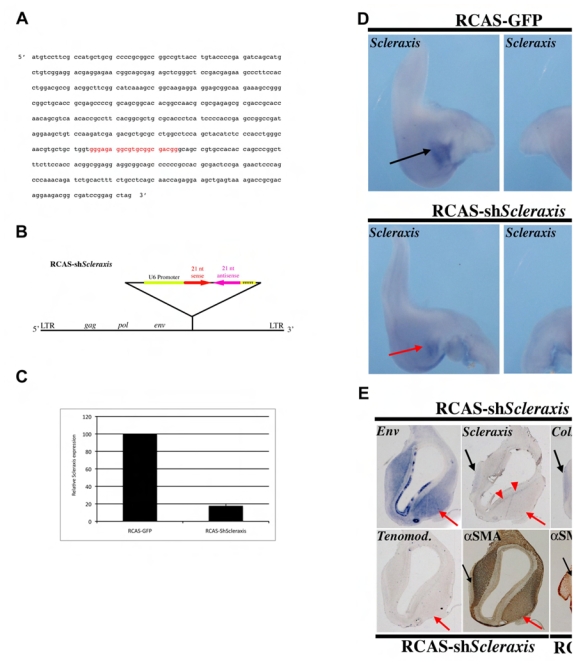
(A) Sequence of the sense oligonucleotide (21 red nucleotides) from the avian Scleraxis coding sequence to make the hairpin and to specifically inhibit its expression. (B) To deliver ShScleraxis into the chick stomach in vivo, the hairpin, whose expression was under the control of the mouse U6 promoter, was cloned into the RCAS viral vector. (C) Real-Time RT-PCR amplification of dissected E5 gizzard mesenchymal-derived cells cultured for 3 days after infection with RCAS-ShScleraxis. Infected cells express 85% less Scleraxis transcripts compared to control cells (RCAS-GFP). Each measurement was done on two independent cDNA preparations (n= 4). (D) Inhibition of Scleraxis expression in RCAS-ShScleraxis stomachs. Whole-mount in situ hybridization using the antisense Scleraxis riboprobe on E6 stomachs in which RCAS-GFP and RCAS-ShScleraxis are misexpressed. RCAS-ShScleraxis stomachs present a slight defect characterized by thinner gizzard and straight proventriculus compared to RCAS-GFP controls. These defects are associated with a diminution of Scleraxis expression. Black arrows indicate normal Scleraxis expression in the tendon structures and red arrows show the decrease of Scleraxis expression in the tendon domains. (E) Serial longitudinal sections of an E9 RCAS-ShScleraxis stomach analyzed by in situ hybridization with specific antisense riboprobes directed against Env, Scleraxis, Type I Collagen and Tenomodulin and by immunohistochemistry with an anti-αSMA antibody. Longitudinal section of a RCAS-GFP E9 stomach analyzed by immunohistochemistry with an anti-αSMA antibody. Areas with RCAS-ShScleraxis expressing cells, detected with the Env probe, are characterized by a strong decrease in the expression of Scleraxis, Type I Collagen and Tenomodulin and an increase of the αSMA positive domain. Note that aberrant localization of few Scleraxis and Type I Collagen expressing cells in the submucosal layer close to the gastric epithelium (red arrowheads). Black arrows indicate endogenous tendon structures and red arrows areas where RCAS-ShScleraxis is strongly expressed.
We next used a gain of function approach and ectopically misexpressed full-length Scleraxis along the GI tract (Fig. 4). Although we misexpressed Scleraxis in different regions of the digestive system, we observed effects only in the stomach (Fig. 4). Scleraxis misexpression induced a gross phenotype characterized by aberrantly dilated gizzard associated with curved proventriculus (Fig. 4A). RCAS-Scleraxis expressing cells were highly concentrated and organized around territories of endogenous expression (Fig. 4A), whereas the muscle domains were strongly reduced as revealed by an αSMA riboprobe (Fig. 4A). Although RCAS-Scleraxis expression was correlated with αSMA inhibition, we did not observe ectopic induction of Type I Collagen as we did in the case of RCAS-Fgf8 misexpression (compare Figs 4B with 2E).
Fig. 4. Misexpression of Scleraxis in the whole stomach inhibits SMC differentiation.
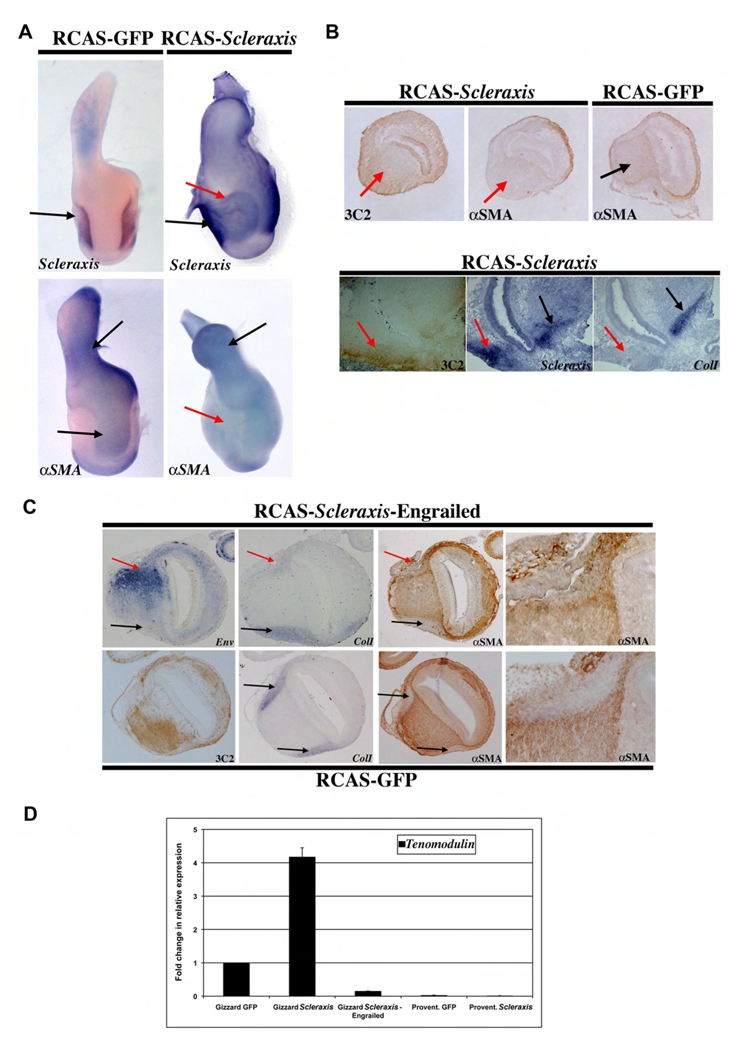
(A) Whole-mount in situ hybridization using the Scleraxis and αSMA riboprobes on E9 stomachs misexpressing GFP or Scleraxis. Ectopic Scleraxis expression (RCAS-Scleraxis) induces a gross phenotype characterized by dilated gizzard associated to a curved proventriculus compared to RCAS-GFP controls. Furthermore, RCAS-Scleraxis inhibits the expression of the SMC marker αSMA. Upper black arrows indicate endogenous tendon structures; lower black arrows indicate normal SMC domain and red arrows perturbation of the gizzard mesoderm. (B) Serial longitudinal sections of E9 RCAS-Scleraxis and RCAS-GFP stomachs analyzed by in situ hybridization with specific Scleraxis, and Type I Collagen ripobrobes and by immunohistochemistry with anti-GAG (3C2) and anti-αSMA antibodies. Areas with many RCAS-Scleraxis expressing cells, detected by the anti-GAG antibody, are associated with inhibition of αSMA expression, but not with induction of Type I Collagen expression. Black arrows indicate endogenous tendon structures and red arrows ectopic Scleraxis expression domains. (C) Serial longitudinal sections of E9 stomach infected with RCAS-Scleraxis-Engrailed or RCAS-GFP retroviruses analyzed by in situ hybridization with Env and Type I Collagen riboprobes and by immunohistochemistry with anti-αSMA and 3C2 antibodies. Misexpression of Scleraxis-Engrailed in tendon domains is associated with repression of Type I Collagen expression in the infected tendon and defective tendon structures. Misexpression of GFP had no effect on tendon development. Black arrows indicate endogenous tendon structures and red arrows Scleraxis-Engrailed expression domains. (D) Scleraxis misexpression in the gizzard increases Tenomodulin expression. Quantitative-RT-PCR experiments on E9 stomachs in which RCAS-GFP (control), RCAS-Scleraxis or RCAS-Scleraxis-Engrailed were misexpressed. RCAS-Scleraxis infected gizzards express 4.2 fold more Tenomodulin than controls, whereas RCAS-Scleraxis-Engrailed infected gizzards express 90% less Tenomodulin transcripts than controls. Ectopic expression of Scleraxis in the proventriculus does not induce Tenomodulin in this tissue. Each measurement was done on three independent cDNA preparations (n= 4).
Scleraxis is considered to be a gene activator and recently Type I Collagen has been identified as a target in tendon fibroblasts (Lejard et al., 2007). Therefore, we thought that by converting Scleraxis into a transcriptional repressor, we could block expression of target genes and counteract its activator function. For this aim, we fused the Engrailed repressor domain in frame to Scleraxis and misexpressed RCAS-Scleraxis-Engrailed in the stomach to repress endogenous Scleraxis function. In embryonic stomachs, RCAS-Scleraxis-Engrailed led to some minor defects, comparable to those observed in RCAS-ShScleraxis stomachs (data not shown). Type I Collagen expression was strongly inhibited in the infected tendon domain and this inhibition was associated with defects in the tendon structure (Fig. 4C). Recently others genes known to be expressed specifically in the tendons, such as Tenomodulin and Type XIV Collagen, were demonstrated to be targeted by Scleraxis in the limb (Shukunami et al., 2006; Murchison et al., 2007). Differently from the limb tendon, we found that Type XIV Collagen was not expressed in the intermuscular tendon but only in gastric ENS cells (Fig. S6). Using quantitative RT-PCR to analyze the expression levels of Tenomodulin, we observed that Scleraxis misexpression in the gizzard up-regulated Tenomodulin, whereas ectopic expression of Scleraxis in the proventriculus did not (Fig. 4D). Moreover, Scleraxis-Engrailed expression in the gizzard strongly repressed Tenomodulin expression (Fig. 4D). These findings demonstrate that, during the development of the intermuscular tendon, Scleraxis is upstream of Type I Collagen and Tenomodulin and strongly suggest that Type I Collagen and Tenomodulin are in vivo targets of Scleraxis in the stomach. However, Scleraxis alone cannot induce ectopic expression of Type I Collagen, suggesting the necessity of additional interacting partner(s).
We then analyzed by electron microscopy the ultrastructure of visceral SMC after infection with different retroviral constructs (Fig. 5). In control gizzards (Fig. 5A), tendon cells showed predominantly rough endoplasmic reticulum and mitochondria and were surrounded by collagen fibrils; visceral SMC, on the other hand, presented mainly intracellular actin formation and thick cytoplasm. In addition, tendon cells were elongated, whereas SMC were rounded. In RCAS-Fgf8 gizzards, SMC were elongated with numerous filopodia and no intracellular actin formation, but numerous organelles and ectopic collagen fibrils in the intercellular region. These ectopic fibrils were not observed in RCAS-Scleraxis SMC. However, ectopic expression of Scleraxis in the gizzard SMC inhibited the formation of intracellular actin and was associated to the presence of numerous organelles (Fig. 5A). Tendon cells, in which sFgfR2b, ShScleraxis and Scleraxis-Engrailed were misexpressed, showed absence or strong reduction of collagen fibrils in the intercellular region (Fig. 5B). Moreover, in the three conditions ectopic cells with thick cytoplasm, which are not observed in normal tendon cells, were present (compare Fig. 5B with 5A).
Fig. 5. Ultrastructural analysis of E9 gizzard sections following retroviral misexpression.
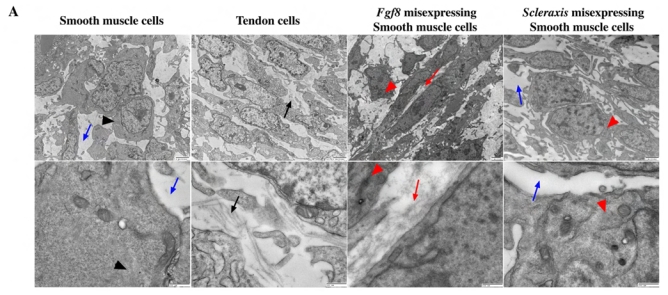
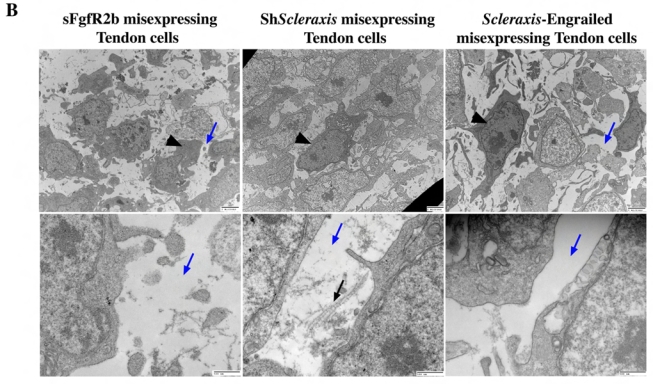
(A) Analysis of E9 gizzard misexpressing GFP, Fgf8 or Scleraxis. Control (RCAS-GFP) SMCs are rounder with few mitochondria and have actin filaments in the cytoplasm. Control tendon cells are aligned and surrounded by collagen fibrils. In addition, they show predominantly rough endoplasmic reticulum and mitochondria. RCAS-Fgf8 SMCs present aberrant filopodia, no intracellular actin formation and numerous organelles. RCAS-Scleraxis SMC also show aberrant filopodia, disorganized intracellular actin and increased number of organelles. Fgf8 misexpression is also associated with ectopic collagen fibrils in the intercellular region that are not observed in RCAS-Scleraxis SMC. Black arrows indicate extracellular collagen fibrils; black arrowheads indicate intracellular actin formation; blue arrows show the absence of extracellular matrix; red arrows show ectopic collagen fibrils and red arrowheads the absence of intracellular actin formation. (B) Analysis of tendon cells from E9 gizzards misexpressing sFgfR2b, ShScleraxis or Scleraxis-Engrailed. RCAS-sFgfR2b tendon cells present aberrant morphology with dark cytoplasm (arrowhead), and without collagen fibrils (blue arrows). RCAS-ShScleraxis tendon cells present a moderate phenotype with a strong decrease of collagen fibril production (blue and black arrows), dark cytoplasm and rounder morphology (arrowhead). RCAS-Scleraxis-Engrailed gizzards also have tendon cells with dark cytoplasm (arrowhead) and strong inhibition of collagen fibril production (blue arrow). For all sections (A and B), the scale bars represent 2 mm (upper panels) and 500 nm (lower panels).
In summary, our data indicate that tendon structures are important for the regulated development of the stomach and their differentiation takes place in close relationship with that of visceral smooth muscles.
Undifferentiated stomach mesenchymal cells give both SMCs and tendon cells
We showed that tendon and smooth muscle domains are closely associated in the stomach and that activation of the FGF pathway in the anterior and posterior parts of the stomach mesenchyme induce ectopic tendon domains (Fig. 2), suggesting that modulation of this pathway in the visceral mesenchyme is sufficient to pattern the tendon. To evaluate the capacity of undifferentiated visceral mesenchyme to give rise to tendon cells, we set up primary cell cultures derived from Stage 25 gizzards before the onset of Scleraxis expression in the stomach. Throughout the study we refer to stage 25 gizzard mesenchyme as E5 gizzard mesenchyme. To this aim, we adapted a reliable technique that allowed us to enzymatically separate the gizzard mesenchyme from its endodermal counterpart (Fig. 6A) (Simon-Assman and Kedinger, 2000). Next, E5 mesenchymal cells were cultured for one (E5+1D) and three days (E5+3D). At E5+1D, we observed that some isolated, scattered cells were positive for Scleraxis expression; at E5+3D, we observed the presence of several colonies that were all positive for Scleraxis expression (Fig. 6B). All Scleraxis positive colonies were also visualized with methyl violet, a specific histological tendon dye (Fig. 6C) (Bi et al., 2007). We then analyzed these colonies and found that they strongly expressed Type I collagen and Tenascin (tendon markers) (Tozer and Duprez, 2005), faintly Desmin (mesenchymal derived cell marker) and no Caldesmon (SMC marker) or SOX9 (chondrocyte marker) (Lefebvre et al., 1997) (Fig. 6D). Conversely, we observed robust expression of Caldesmon and Desmin in the cells adjacent to the colonies, suggesting that visceral SMC organized around these structures (Fig. 6D).
Fig. 6. Characterization of distinct cell populations in primary cell cultures derived from E5 gizzard mesenchyme.
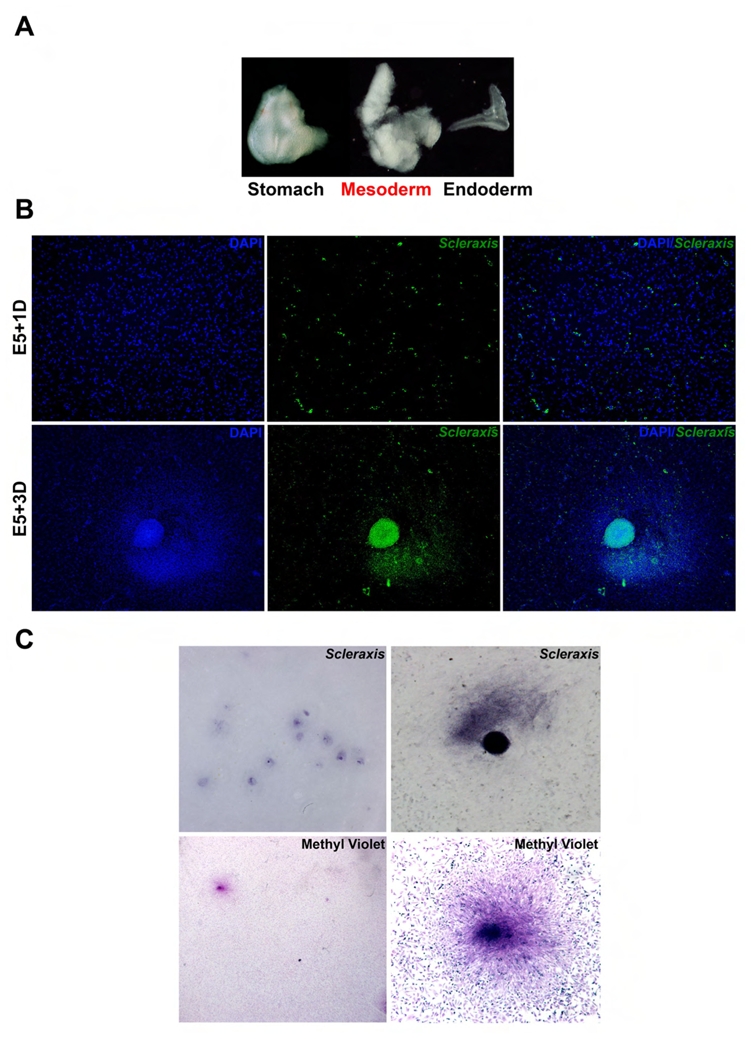
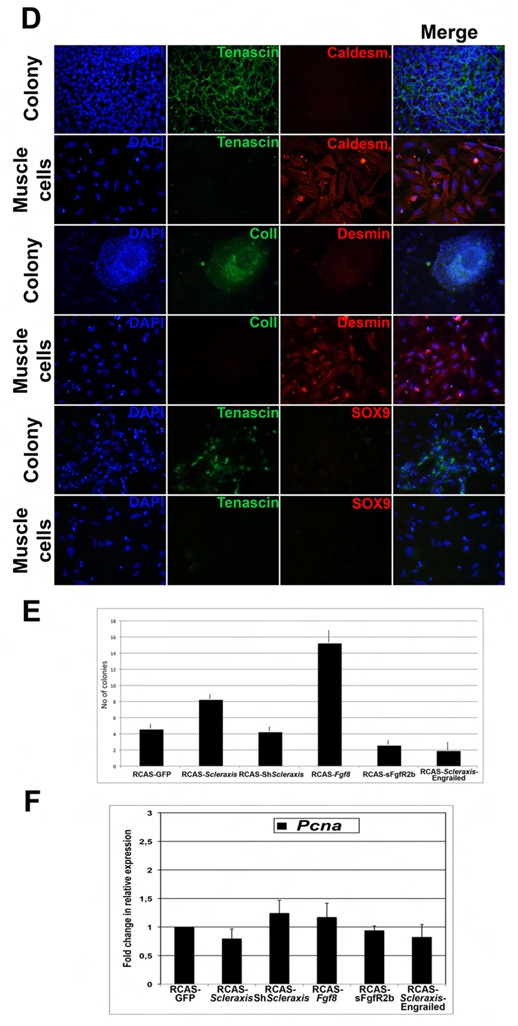
(A) Enzymatic dissociation and microdissection of an E5 gizzard into mesenchyme and endoderm. The undifferentiated mesenchymal cells will be cultured for one (E5+1D) and three days (E5+3D). (B) In cellulo in situ hybridization using the antisense Scleraxis riboprobe on E5+1D and E5+3D mesenchymal-derived cells. Nuclei were visualized with DAPI. At E5+1D, Scleraxis positive cells are isolated (upper panel), whereas at E5+3D they have aggregated into colonies (lower panel). Scleraxis expression is colored using the Photoshop software. (C) Analysis of E5+3D mesenchymal-derived cells by in cellulo in situ hybridization using the antisense Scleraxis riboprobe (upper panel) and methyl violet staining (lower panel). Clusters of cells expressing Scleraxis are also positive for methyl violet, a dye that labels cells containing high-level organelles. (D) Characterization of E5+3D mesenchymal-derived cells by immunofluorescence. Nuclei were visualized with DAPI. The tendon specific markers Tenascin and Type I Collagen were used as well as the SMC marker Caldesmon, the mesenchymal cell marker Desmin, and the chondrocyte marker SOX9. Colonies were all positive for the tendons markers, but not for Caldesmon or SOX9. The scattered cells adjacent to the colonies presented strong expression of muscle markers, but no expression of tendon or chondrocyte markers. These results demonstrate that the mesenchymal-derived cultures contain two types of cells: tendon cells aggregated into colonies, and SMC, represented by the scattered cells. (E) Colony-forming efficiency assessed by methyl violet staining of E5+3D primary cells derived from the mesenchyme of gizzards and infected with RCAS-GFP, RCAS-Scleraxis, RCAS-ShScleraxis, RCAS-Fgf8, RCAS-sFgfR2b, or RCAS-Scleraxis-Engrailed for three days. Fgf8 and Scleraxis misexpression have a strong positive effect on the number of colonies, while ShScleraxis misexpression moderately inhibits their formation. Scleraxis-Engrailed and sFgfR2 are both potent inhibitors of cell clusters’ formation. Each measurement was done on three independent experiments (n= 3). (F) Analysis of Pcna expression by quantitative RT-PCR amplification of E5+3D culture cells following infection with different retroviral constructs. Each measurement was done on two independent cDNA preparations (n= 4). Perturbation of Scleraxis expression by different means has moderated impact on Pcna expression.
We have shown that modulation of the FGF pathway and deregulation of Scleraxis expression are associated with reciprocal changes in visceral SMC and tendon cells of the stomach. We therefore assessed the colony-forming efficiency of E5 gizzard mesenchymal primary cultures in which we deregulated Scleraxis expression or the FGF pathway. The colony-forming efficiency was evaluated by methyl violet staining (Fig. 6E). We observed that RCAS-Scleraxis and even more RCAS-Fgf8 misexpression increased the number of colonies (Fig. 6E). RCAS-ShScleraxis did not affect the number of colonies, whereas RCAS-Scleraxis-Engrailed strongly inhibited their formation. However, RCAS-ShScleraxis colonies were smaller with few cells compared to the control colonies (data not shown). We also analyzed the proliferation rate in these different cultures by immunohistochemistry using anti-PH3 antibodies (data not shown) and by quantitative RT-PCR to evaluate the level of Pcna RNAs (Fig. 6F) and did not observe, like in vivo, strong changes that could be responsible of the differences in colony formation.
These data suggest that E5 gizzard undifferentiated mesenchyme can differentiate at least into two distinct cell types: visceral SMC and tendon cells. These data also indicate that the FGF pathway and Scleraxis are essential for the formation and differentiation of intermuscular tendons.
DISCUSSION
In this work we show that embryonic stomach is characterized by the presence of two tendon domains (intermuscular tendons) close to the gastric visceral smooth muscle structures. Moreover, we propose that coordinated development of tendon and gastric smooth muscle structures is necessary to ensure the correct development of the stomach. This notion is supported by the findings that: 1) inhibition of tendon formation induced stomach malformations, and 2) the increase of tendon territories happened at the expenses of visceral SMCs and impaired their differentiation in vivo. Moreover, we show that undifferentiated stomach mesenchyme can give both SMC and tendon cells, suggesting that the development of this intermuscular tendon cells appear in an autonomous way from the stomach mesenchyme through the FGF pathway and under Scleraxis control.
Presence of intermuscular tendons in the stomach
The majority of smooth muscle forms continuous structures devoid of attachments. However, few smooth muscles are attached to rigid structures (i.e., the anococcygeus, the rectococcygeus, and the costouterus muscles) (Gabella, 1985). The rectococcygeus muscle, for instance, is attached to the anterior surface of the second and third coccygeal vertebrae and is inserted onto the posterior surface of the rectum through anchoring tendons. In this study, we describe two tendons that connect two smooth muscle bundles in the stomach. The connection of two muscle bundles through a connective structure, which is defined as intermuscular tendon, has been previously described, for example, in the diaphragm (Ackerman and Greer, 2007). The localization of two intermuscular tendons in the stomach suggests that their physiological role is to diminish the high tension generated by the periodic contraction of the powerful stomach smooth muscle structures.
In this study, we found that some specific tendon markers (i.e., Scleraxis, Tenomodulin, Four jointed, Type I Collagen and Decorin) are associated with the development and differentiation of these structures. However, other limb tendon specific markers are not expressed. Indeed, a putative target gene of Scleraxis, Type XIV Collagen is not expressed in the intermuscular tendons but in the gastric ENS cells. These molecular differences between intermuscular tendons and force-transmitting or anchoring tendons suggest that generation of tendon structure or the organization of the tissue attachment in these tendon subgroups require molecular modulation.
Determination of the intermuscular tendon domain
At E6, Scleraxis expression defines two domains that give rise to the tendon structures of the stomach. These two domains are induced by the mesenchymal FGF signaling pathway. We also demonstrate that inactivation of Scleraxis expression extinguishes the tendon domains, whereas ectopic expression blocks the differentiation of the visceral mesenchyme into SMC. Tendons are defined as the connective tissues that connect/attach muscle to bone (musculoskeletal system) and muscle to muscle (diaphragm system) (Tozer and Duprez, 2005; Ackerman and Greer, 2007). Tendon cells can have different embryological origins; for instance, body wall tendons derive from one specific somitic compartment, the syndetome (Brent et al., 2003), and craniofacial tendons from cranial neural crest cells (Crane and Trainor, 2006). No embryonic origin was demonstrated for the intermuscular tendons in the diaphragm (Ackerman and Greer, 2007). The digestive tract mesenchyme derives from the splanchnopleural mesoderm and is also colonized by neural crest-derived (Le Douarin and Teillet, 1973; Barlow et al., 2008) and mesothelium-derived vascular SMC cells (Wilm et al., 2005). Chick/quail chimera experiments demonstrated that colonization by neural crest-derived cells of the stomach gives rise to ENS cells (Le Douarin and Teillet, 1973) and not to the tendon domains we identified in this work. An early study of the muscle-tendon structures in the avian gizzard noted the intimate relation between muscle bundles and tendons in this organ and suggested that both derive from the same cell type (Watzka, 1932), Our finding that mesenchymal FGF signaling triggers Scleraxis expression and consequently the determination of the two tendon domains in vivo and in primary cell culture suggests that, upon FGF activation, selected stomach mesenchymal cells are primed to differentiate into tendon cells and to express Scleraxis.
Differentiation of the intermuscular tendon structure
As previously reported (Brent and Tabin, 2004; Edom-Vovard et al., 2002), we found that the mesenchymal FGF signaling pathway positively regulates Scleraxis expression and induces differentiation of the two tendon structures and expression of differentiation markers, such as Type I Collagen, and Tenomodulin, in different parts of the digestive tract. Conversely, Scleraxis is essential for the development of the tendons and Tenomodulin expression. However, Scleraxis misexpression alone cannot induce ectopic expression of Type I Collagen, whereas the transcriptional represser Scleraxis-Engrailed inhibits Type I Collagen expression in vivo. Scleraxis is a bHLH transcription factor that can interacts with the bHLH factor E47 to regulate the expression of Type I Collagen in tendon fibroblasts (Lejard et al., 2007). All these data suggest that Scleraxis requires additive transcription factors or interacting partners to control the differentiation process initiated by activation of the FGF pathway. We can also imagine that Scleraxis proteins present in the muskoskeletal in the intermuscular tendons might interact with different tissue-specific partner(s) to reinforce these tissue specificities. In addition, other signaling pathways activated by FGF can cooperate with Scleraxis to induce tendon differentiation. Different studies showed that the FGF-WNT regulatory network controls mesenchyme development. Recently, mesenchymal FGF signaling has been reported to regulate β-catenin-mediated WNT signaling in lung mesenchyme (Yin et al., 2008). The canonical Frizzled receptor Fz8 is expressed in the stomach (Theodosiou and Tabin, 2003) and its expression pattern overlaps with that of Scleraxis suggesting that Scleraxis and WNT canonical pathways are induced after activation of the FGF pathway. In future studies we will try to identify the WNT ligand that interacts with Fz8 and may allow cooperation with Scleraxis.
In summary, we show that the vertebrate stomach harbors two tendon domains located in the antrum/muscular stomach, which are closely associated with the visceral smooth muscle structures. The possible physiological function of these domains might be to ensure elasticity of the stomach during the contraction of the two massive visceral muscles.
Fig. 7. Model of the molecular pathways and the tissue interactions during stomach development.
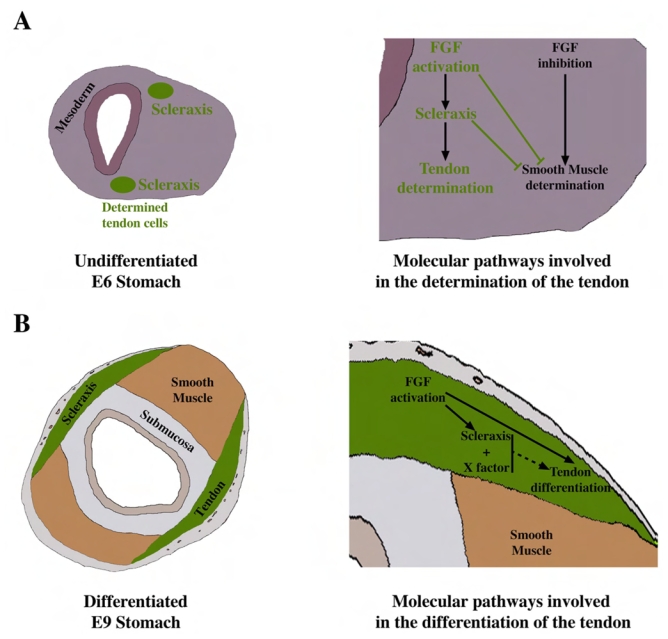
(A) Schematic representation of avian E6 stomach and the molecular pathways involved in the determination of the tendon domains. At E6, the mesenchymal layer is composed of undifferentiated visceral mesenchyme and two emerging committed tendon cell domains (green areas). The cells of these domains, upon mesenchymal FGF activation, express Scleraxis and become committed to the tendon lineage while their differentiation into SMC is inhibited. (B) Schematic representations of avian E9 stomach and the molecular pathways involved in the differentiation of the intermuscular tendons. At E9, the mesenchymal layer is divided in two differentiated visceral SMC domains (brown areas), submucosa (grey area) and two well organized tendon domains (green areas). Mesenchymal FGF activation allows the differentiation of the tendons which express Scleraxis. Scleraxis with additional transcription factors or interacting partners (X factor) might ensure the differentiation of tendon cells.
Acknowledgments
The authors thank Drs. D. Duprez, P. Francis-West, A. Neubuser, and C. Tabin for reagents. We thank Dr. S. Faure and the members of INSERM ERI 25 for discussions. This work was supported by grants from INSERM, Agence Nationale pour la Recherche (ANR-07-JCJC-0112), Association Française contre les Myopathies (N°11877) and Association contre le Cancer (N°3725) to P.d.S.B. L.L.G was supported by the Ministère de I’Education et de la Recherche and C.N. by an AFM postdoctoral fellowship.
References
- Ackerman KG, Greer JJ. Development of the diaphragm and genetic mouse models of diaphragmatic defects. Am J Med Genet C Semin Med Genet. 2007;145C:109–16. doi: 10.1002/ajmg.c.30128. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–91. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–96. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–86. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, van den Brink GR, Roberts DJ. Molecular etiology of gut malformations and diseases. Am J Med Genet. 2002;115:221–30. doi: 10.1002/ajmg.10978. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, Williams J, Goldstein AM, Doyle AM, Nielsen C, Winfield S, Faure S, Roberts DJ. Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development. Dev Dyn. 2005;234:312–22. doi: 10.1002/dvdy.20554. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Bonnin M, Duprez D. Fgf8 transcripts are located in tendons during embryonic chick limb development. Mech Dev. 2001;108:203–6. doi: 10.1016/s0925-4773(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–66. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Gabella G. Chicken gizzard. The muscle, the tendon and their attachment. Anat Embryol. 1985;171:151–62. doi: 10.1007/BF00341409. [DOI] [PubMed] [Google Scholar]
- Gabella G. Development of visceral smooth muscle. Results Probl Cell Differ. 2002;38:1–37. doi: 10.1007/978-3-540-45686-5_1. [DOI] [PubMed] [Google Scholar]
- Gregoire D, Brodolin K, Mechali M. HoxB domain induction silences DMA replication origins in the locus and specifies a single origin at its boundary. EMBO Rep. 2006;7:812–6. doi: 10.1038/sj.embor.7400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:4992. [PubMed] [Google Scholar]
- Harpavat S, Cepko CL. RCAS-RNAi: a loss-of-function method for the developing chick retina. BMC Dev Biol. 2006;6:2. doi: 10.1186/1471-213X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1 (II) collagen gene. Mol Cell Biol. 1997;17:2336–46. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1 (I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–75. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–43. doi: 10.1242/dev.01203. [DOI] [PubMed] [Google Scholar]
- McLelland J. Systema digestorium. In: Baumel JJ, editor. Nomina Anatomica Avium. London: Academic Press; 1979. pp. 267–285. [Google Scholar]
- Mericskay M, Blanc J, Tritsch E, Moriez R, Aubert P, Neunlist M, Feil R, Li Z. Inducible mouse model of chronic intestinal pseudo-obstruction by smooth muscle-specific inactivation of the SRF gene. Gastroenterology. 2007;133:1960–70. doi: 10.1053/j.gastro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Moniot B, Biau S, Faure S, Nielsen CM, Berta P, Roberts DJ, de Santa Barbara P. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. 2004;131:3795–804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nielsen C, Murtaugh LC, Chyung JC, Lassar A, Roberts DJ. Gizzard formation and the role of Bapx1. Dev Biol. 2001;231:164–74. doi: 10.1006/dbio.2000.0151. [DOI] [PubMed] [Google Scholar]
- Niessen P, Rensen S, van Deursen J, De Man J, De Laet A, Vanderwinden JM, Wedel T, Baker D, Doevendans P, Hofker M, et al. Smoothelin-a is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Roberts DJ. Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn. 2000;219:109–20. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1047>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Ros MA, Rivero FB, Hinchliffe JR, Hurle JM. Immunohistological and ultrastructural study of the developing tendons of the avian foot. Anat Embryol (Berl) 1995;192:483–96. doi: 10.1007/BF00187179. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P, Kedinger M. Tissue recombinants to study extracellular matrix targeting to basement membranes. Methods Mol Biol. 2000;139:311–9. doi: 10.1385/1-59259-063-2:311. [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Tabin CJ. Wnt signaling during development of the gastrointestinal tract. Dev Biol. 2003;259:258–71. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–36. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–82. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- Watzka M. Sehnen glatter Muskelfasern. Z mikr-anat Forsch. 1932;30:23–8. [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of SMCs of the gut vasculature. Development. 2005;132:5317–28. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Parish J, Akita K, Francis-West P. Developmental expression of the chick four-jointed homologue. Dev Dyn. 2006;235:3085–91. doi: 10.1002/dvdy.20946. [DOI] [PubMed] [Google Scholar]
- Yin Y, White AA, Huh SU, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–36. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


