Abstract
Fast methods to validate relevant candidate genes associated to fruit ripening are needed specially to associate gene function to the overwhelming amount of leads provided by genomic projects. In tomato, the use of Fruit VIGS in a Del/Ros1 background as described in the Plant Physiology by Orzaez et al. 2009, overcomes the difficulties associated to low efficiency VIGS in tomato and increases the reliability and throughput of this fast reverse genetic assay. The advantages of this transient assay system are discussed here for the case of gene functions associated to fruit ripening and quality traits. The possibility of using other reporters or even the development of transient overexpression assays in the fruit is also discussed.
Key words: virus induced gene silencing, VIGS, transient, reverse genetics, tomato, fruit anthocyanins
Introduction
Fleshy fruits bedeck seeds with attractive wrappings of colors, flavors, aromas, shapes and textures, a chemically encoded message that informs dispersers about palatable, nutritious and healthy compound rich organs. Perhaps to avoid betraying frugivores' evolutionary expectations, fruits are compelled to outperform by offering a complex mixture of nutrients and even health-promoting metabolites that contribute to increase fitness. To facilitate consumption, fruits undergo ripening at their final stage of development, a unique developmental program that transform mature fruits in a vehicle of seeds and compounds that (1) are healthy/energy-rich; (2) edible for the disperser, i.e., free of anti-nutrients and other toxic compounds and, (3) display morphological and chemical cues, often based in secondary metabolites,1 that make them sensory catching and differentiate them from other berries (often cheating about their contents as in protein- based sweeteners).2 Underlying the accomplishment of this set of features operates an intricate genetic network that is only partially understood.
Tomato is the most widely used model species for the study of berry/fleshy fruit biology. A combination of classic and marker-assisted genetics as well as molecular biology approaches has contributed to elucidate the key signaling routes in tomato fruit ripening, which are known to be orchestrated by ethylene. During ripening, the berry fruit becomes ready for animal consumption: ethylene promotes cell wall loosening and the reduction of alkaloid content (easing edibility), triggers the accumulation of sugars and organic acids, and activates carotenoid (lycopene) biosynthesis (an eye-catching, health-promoting compound), among other associated processes whose underlying molecular mechanisms have been at least partially unveiled.3 However most of the ripening associated processes are still poorly understood. For instance, information on enzymes involved in volatile emission, flavonoid biosynthesis, transport and glycosilation; alkaloid biosyntheses, etc., is still very limited and their biotechnological potential remains largely unexploited.4,5
Current Biotechnological Burdens in Fleshy Fruit Biotechnology
Marker-assisted direct genetics has proven success in identifying genetic determinants of fruit quality traits as Brix,6 and other traits associated to domestication, as fruit size,7–9 but discovery pace is slow and often low throughput. In the same line, biotechnological tools for investigating fruit development and metabolism are still rather rudimentary. Thus, reverse genetics is mainly based in stable transformation methods, which are slow and too often dependent on personal skills difficult to transfer from laboratory to laboratory. Alternative transformation methods like floral dip are promising but not widespread and the possibility of resulting in high chimerism is not fully discarded.10 Dedicated fruit expression cassettes are limited, and only recently have started to become available.11 In this scenario, virus-induced gene silencing technology (VIGS) appeared as an attractive alternative for fast reverse genetics in berry fruits.
Virus Induced Gene Silencing in Fruit Genetics
Several methods, which induce transient endogenous gene silencing in fruit-bearing plants have been developed, relying simply in transient RNAi expression12 or assisted by viral replication.13 Among them probably the most popular is the one using the tobacco rattle virus machinery (TRV).14,15 TRV-derived vectors display efficient silencing effects in vegetative tomato tissues with little viral symptoms. Unfortunately, the spread of virus-induced silencing signal through the plant is rather unpredictable in nature and especially low unefficient in mature organs as flowers or fruits. It has been described that the extent of VIGS can be controlled by manipulating external environmental conditions (light, temperature, humidity),16 however no fully satisfactory conditions ensuring that the silencing signal fully reaches all tissues in all fruits have been described. Moreover, some conditions may compromise fruit set or normal fruit development. A substantial improvement in the technique was provided by directly delivering the TRV infective clone into the developing fruit (Fruit VIGS), ensuring that silencing signal reaches all fruits under analysis.17,18 However the level and extension of silencing on the different tissues often escapes control, making it difficult to obtain reliable data. This is especially important when subsequent quantitative analysis (e.g., metabolomics) is required to have an assessment of gene function.
The Lazarillo Strategy
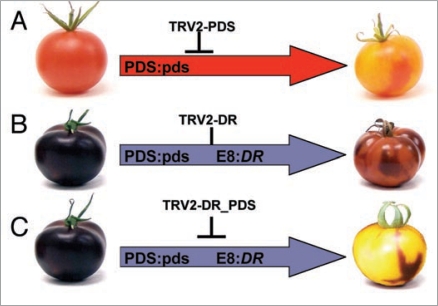
Examples of successful VIGS and fruit VIGS experiments have been reported in the literature but they deal with genes whose depletion produce a visually identifiable phenotype (changes in shape or color). Otherwise, the experimenter goes blind when dissecting silenced and non-silenced tissues. A strategy to overcome this limitation is the use of a sort genetic guide-dog (lazarillo), which labels the extension and intensity of silencing. Petunia endogenous chalcone synthase (CHS) gene has been used as lazarillo for labeling VIGSed areas in the study of genes involved in aroma's biosynthesis.19 VIGSed areas in the petals are highlighted by the removal of purple anthocianins, a result of the absence of CHS activity. Inspired in petunia's example, it was reasoned that using anthocyanin-promoting transgenes as guide-dogs could be of general application for many plants species that do not accumulate anthocyanins. In our recent paper on anthocyanin-guided VIGS, a transgenic genetic module composed by Rosea1 and Delila snapdragon transcription factors (DR) was used as guided-dog for VIGS in the tomato fruit. It was shown that tandem arrangement of DR and a gene of interest (GOI) within TRV vector, efficiently linked GOI silencing to its lazarillo, therefore providing a guide for dissecting silenced tissues in the fruit (Fig. 1). Anthocyanin-guided VIGS was used in combination with chemometric analysis to identify changes in volatile emissions produced by the depletion of tomato phytoene desaturase, lypoxygenase C and odorant-1 genes in the fruit.
Figure 1.
Anthocyianin-guided fruit VIGS. (A) Wild type tomatoes were fruit-VIGSed for the model gene of interest (GOI) phytoene desaturase (PDS) using a TRV construct carrying a PDS fragment (TRV-PDS), and the resulting ripen fruits appeared decorated with red (non-silenced) and yellow (PDS-silenced, lycopene deprived) sectors. (B) E8::Del/E8::ros1 (E8:RD) tomatoes VIGSed for the DR transgenic module using a TRV-DR construct yielded red and purple sectors corresponding to DR-silenced and DR-non-silenced areas. (C) Co-silencing of DR and GOI (PDS) was demonstrated by agroinjecting a TRV construct containing tandemly arranged DR and PDS gene fragments (TRV-DR_PDS). The resulting fruits showed only purple (non-silenced) and yellow (PDS and DR co-silenced) sectors. To facilitate genotype recognition, fruit on the left side of the figure are shown with their final ripen phenotype. It has to be noticed however that VIGS treatments were always performed on green tomatoes, and therefore pigments (either lycopene or anthocyanins) were always absent in the silenced sectors.
Anthocyanin-Guided VIGS Advantages and Limitations
In our experimental setup, a DR module is integrated first into the tomato genome under the control of ripening-specific E8 promoter using established stable transformation procedures. This setup restricts antocyanin accumulation to the ripening fruit, thus reducing any possible deleterious effect during fruit/plant development. Agrobacterium-shuttled TRV infective clones are delivered by injection into the fruit at the mature green stage at the latest. Consequently, anthocyanin accumulation is prevented rather that reverted in silenced fruit sectors, minimizing any influence of transgene expression. Moreover, in this methodology each fruit becomes a biological replica (in opposition to each plant-a replicate of hpRNA methodology), making it well suited for large scale reverse genetics projects.
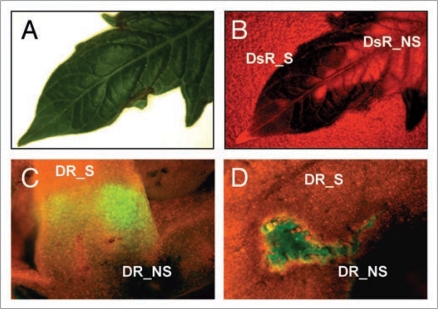
However, not everything affecting fruit composition takes place during ripening. There is compelling evidence that e.g., hormone signaling during early fruit development determines to a great extend its final composition.20 In addition, genetic factors affecting fruit size and shape are known to be operative early in fruit development. In the E8-driven version, anthocyanin-guided VIGS applicability can not be used for investigating genes operating before ripening. Moreover, it is unlikely that DR activity could be extended to the entire fruit development without introducing detrimental effects in fruit growth (results not shown). A possible alternative consists in the use of DsRED, a highly stable red fluorescent reporter protein constitutively expressed in the fruit/plant. In contrast to UV-excited GFP and its derivatives, DsRED excitation length wave resides in the green range of the spectrum and shows little chlorophyll interference, making it specially fitted for plant macroscopic applications. Tomato plants constitutively expressing DsRED show high fluorescent levels in fruits and leaves. Moreover, DsRED accumulation in these plants can be prevented using a TRV-based VIGS strategy (Fig. 2A and B), making DsRED a promising alternative lazarillo for the analysis of genes operating during earlier stages of fruit development.
Figure 2.
Alternative methods for VIGS and transient expression in fruits. (A) A 35S:DsRed tomato leaf VIGSed with a TRV construct carrying a DsRED fragment observed under normal white light. (B) The same leaf observed with adequate DsRED filters show silenced (DsR_S) and non-silenced (DsR_NS) areas. (C) A TRV-DR vector incorporating a GFP cDNA under a duplicated subgenomic promoter brings GFP transient expression to pericarp and central columella when agroinjected in E8:DR fruits. GFP accumulation is observed around purple areas in the fruit. (D) Similarly, the same TRV construct agroinoculated in 35S:DR tobacco plants show GFP expression in and around purple sectors of the leaf, suggesting that transient gene expression in this system is being repressed by the plant silencing machinery.
What about Transient Overexpression?
Together with gene silencing, transient gene overexpression is one of the most needed tools in fruit biotechnology. Efficient transient gene expression in fruits would facilitate experiments like genetic complementation. Perhaps even more challenging, it would allow using (tomato) fruits as an experimental recipient where heterologous enzymes and regulatory genes can be assayed. Taking into account that tomato fruits are semi-autonomous, self-contained biological entities, with highly active routes involved in secondary metabolite biosynthesis (e.g., carotenoids) and with other dormant routes that can be artificially activated (e.g., anthocyanins), the potential of transient gene expression in fruit biotechnology can not be underestimated. Moderate levels of transient gene expression have been achieved using fruit agroinjection, allowing for instance to monitor the assembly of recombinant antibodies17 or the analysis of promoter activity.21 However, agroinjection is only able to deliver Agrobacterium to those tissues surrounding the placenta, the gel and inner pericarp, but only exceptionally reaches deep inside the tightly packaged pericarp tissues, therefore limiting the applicability of the technique. A way to circumvent this problem is to take advantage of viral cell-to-cell and/or systemic movement to deliver transgenes farther into the pericarp tissues. A TRV vector with a duplicated subgenomic promoter followed by GFP gene (pTRV_GFP) was constructed to test the ability of agrodelivered TRV infectous clones to bring trangene expression into the pericarp layers. Using this strategy, pericarp expression was achieved (Fig. 2C), but the levels of recombinant GFP were rather low, and its extension throught the fruit was limited. The most likely reason for this is the activation of the silencing mechanism in the host. The progress of silencing in this experiment was monitored using a DR module in the vector together with anthocyanin accumulating transgenic host plants as described above. As it can be observed in Figure 2C and D, GFP expression was restricted in and around purple areas of fruits and (tobacco) leaves, and was absent from anthocyanin-depleted purples areas. Therefore, silencing in the fruit needs to be suppressed to achieve good levels of TRV-assisted transient gene expression in the fruit. Further work such as using other viral systems less sensitive to silencing is therefore needed to get an efficient transient fruit overexpression system.
Final Remarks
Edible fruit and frugivore nutrition have evolved in parallel, and not surprisingly, fruit consumed either fresh or as fruit-derived products including dietary supplements, show striking beneficial effects in human health. Such effects are likely due to coordinated synergistic interactions of several molecules within a complex mixture rather than to single molecules. Reverse genetics and metabolic engineering in berry fruits, enhanced by recent enabling technologies, will help to unveil such interactions, their relation with the environment and their role in human health.
Acknowledgements
We wish to thank Prof Cathie Martin for providing Del/Ros1 transgenic tobacco and tomato lines. The work described here was supported BIO2008-034034 grant from the Spanish Ministry of Science and Technology and EUSOL project from EU.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9422
References
- 1.Goff SA, Klee HJ. Plant volatile compounds: sensory cues for health and nutritional value? Science. 2006;311:815–819. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- 2.Cipollini ML, Levey DJ. Secondary metabolites of fleshy vertebrate-dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am Nat. 1997;150:346–372. doi: 10.1086/286069. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16:170–180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrari F, Fernie AR. Metabolic regulation underlying tomato fruit development. J Exp Bot. 2006;57:1883–1897. doi: 10.1093/jxb/erj020. [DOI] [PubMed] [Google Scholar]
- 5.Matas AJ, Gapper NE, Chung MY, Giovannoni JJ, Rose JK. Biology and genetic engineering of fruit maturation for enhanced quality and shelf-life. Curr Opin Biotechnol. 2009;20:197–203. doi: 10.1016/j.copbio.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science. 2004;305:1786–1789. doi: 10.1126/science.1101666. [DOI] [PubMed] [Google Scholar]
- 7.Frary A, Nesbitt TC, Grandillo S, Knaap E, Cong B, Liu J, et al. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289:85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- 8.Cong B, Liu J, Tanksley SD. Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proc Natl Acad Sci USA. 2002;99:13606–13611. doi: 10.1073/pnas.172520999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong B, Barrero LS, Tanksley SD. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat Genet. 2008;40:800–804. doi: 10.1038/ng.144. [DOI] [PubMed] [Google Scholar]
- 10.Yasmeen A, Mirza B, Inayatullah S, Safdar N, Jamil M, Ali S, et al. In planta transformation of tomato. Plant Mol Biol Rep. 2009;27:20–28. [Google Scholar]
- 11.Estornell LH, Orzaez D, Lopez-Pena L, Pineda B, Anton MT, Moreno V, et al. A multisite gateway-based toolkit for targeted gene expression and hairpin RNA silencing in tomato fruits. Plant Biotechnol J. 2009;7:298–309. doi: 10.1111/j.1467-7652.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann T, Kalinowski G, Schwab W. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria x ananassa) by agroinfiltration: a rapid assay for gene function analysis. Plant J. 2006;48:818–826. doi: 10.1111/j.1365-313X.2006.02913.x. [DOI] [PubMed] [Google Scholar]
- 13.Cai XZ, Wang CC, Xu YP, Xu QF, Zheng Z, Zhou XP. Efficient gene silencing induction in tomato by a viral satellite DNA vector. Virus Res. 2007;125:169–175. doi: 10.1016/j.virusres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Dinesh-Kumar SP, Burch-Smith T, Liu Y, Schiff M, Dong Y, Zhu X, et al. Virus-induced gene silencing (VIGS) for gene function studies in plants. Phytopathology. 2007;97:145. [Google Scholar]
- 15.Liu YL, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313x.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 16.Fu DQ, Zhu BZ, Zhu HL, Zhang HX, Xie YH, Jiang WB, et al. Enhancement of virus-induced gene silencing in tomato by low temperature and low humidity. Mol Cells. 2006;21:153–160. [PubMed] [Google Scholar]
- 17.Orzaez D, Mirabel S, Wieland WH, Granell A. Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit. Plant Physiol. 2006;140:3–11. doi: 10.1104/pp.105.068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. Virus-induced gene silencing in tomato fruit. Plant J. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer B, Zvi MM, Ovadis M, Marhevka E, Barkai O, Edelbaum O, et al. Reverse genetics of floral scent: application of tobacco rattle virus-based gene silencing in Petunia. Plant Physiol. 2007;145:1241–1250. doi: 10.1104/pp.107.105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, et al. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009 doi: 10.1105/tpc.108.060830. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estornell LH, Orzaez D, Lopez-Pena L, Pineda B, Anton MT, Moreno V, et al. A multisite gateway-based toolkit for targeted gene expression and hairpin RNA silencing in tomato fruits. Plant Biotechnol J. 2009;7:298–309. doi: 10.1111/j.1467-7652.2009.00402.x. [DOI] [PubMed] [Google Scholar]