Abstract
It is estimated that nearly 50% of the world's population is at risk of zinc (Zn) deficiency. The challenge is therefore to increase the Zn content in edible plant parts in order to improve the nutritional value of staple foods. We recently reported the identification and characterization of three barley genes encoding zinc transport proteins belonging to the ZIP protein family. These proteins are believed to be involved in cellular uptake of Zn2+. In this addendum, the Zn2+ transport capacity of ZIP proteins isolated from barley roots was investigated in response to various pH levels. We show that a lowering of pH induces a better growth at low Zn2+ concentrations of yeast cells expressing ZIP proteins. However, no significant change in transport capacity (Vmax) could be observed for HvIRT1, whereas lowering of pH from 5.5 to 4.2 increased the Vmax value with 64% for HvZIP5. These results indicate that proton activity has an important role in regulating the Zn2+ transport capacity of Zn2+ specific ZIP transport proteins. This information will increase the understanding of ZIP proteins and facilitate engineering of genotypes able to grow efficiently on marginal soils.
Key words: ZIP proteins, barley, zinc transport, pH
Introduction
Zinc is an essential nutrient for plants and humans. It has a critical role of numerous metalloenzymes and Zn-dependent transcription factors. Zn deficiency are causing severe health complications, such as impairments in physical development, immune system dysfunction and mental disorders.1,2 At the Copenhagen Consensus 2008 conference, Zn and Vitamin A malnutrition were ranked number one among the biggest global challenges (www.copenhagenconsensus.com). Large resources have also been devoted to improve the Zn content in cereal crops by bio-fortification.3–5 In plants, Zn deficiency results in extensive oxidative damage with negative impact on plant growth and quality.6 Membrane proteins facilitating Zn2+ transport play important roles in supplying and maintaining the sufficient levels of Zn2+ for optimal activity of Zn dependent key enzymes and metabolic processes. ZIP proteins have been shown to be involved in Zn2+ uptake, transport and homeostasis within plants.7 Identification and characterization of the individual proteins and how these proteins are regulated will contribute to an improved understanding of the Zn2+ homeostasis in plants. Our results indicate that the activity of Zn2+ specific HvZIP proteins, except HvIRT1, is significantly influenced by the proton activity on the apoplastic side. Consequently, the mechanism in which ZIP proteins transport Zn2+ is not solely dependent on the Zn2+ concentration in the cell and apoplast but also influenced by the physiological pH of the tissue.
Function of ZIP Proteins
ZIP transporters are identified from a number of plant species, mainly dicots; reviewed by Grotz and Guerinot (2006).7 They are involved in transport of varies metal ions, such as Mn2+, Fe2+/Fe3+, Cd2+, Co2+, Cu2+, Ni2+ and especially Zn2+. The IRT1 homologues, AtIRT1, OsIRT1 and HvIRT1 have been localized to the plasma membrane and they have been proposed to have their main role in Fe2+/Fe3+ and Mn2+ uptake, respectively.8–10 In addition, OsZIP4 has been appointed a role in Zn2+ uptake as this protein is localized to the plasma membrane, whereas GmZIP1 is specifically expressed in the peribacteroid membrane in root nodules of soybean plants.11,12 The cell- and tissue specific localization of the remaining identified ZIP proteins are currently unknown and therefore the specific role, besides being involved in metal homeostasis, awaits further studies. The number of ZIP genes in Arabidopsis (15 putative ZIP proteins)7 and rice (12 putative ZIP proteins)12 is rather remarkable as the high number may indicate some redundancy. Varying substrate specificities and differential expression at different membranes and tissues during plant development, can to some extent explain the abundancy of ZIP genes present in both monocot and dicot plant species. So far there has been identified five putative barley ZIP proteins and these has been named according to nomenclature used for the rice homologous.13 The three ZIP proteins analysed in the reference study seem to be Zn2+ specific and the expression level for HvZIP3 and HvZIP5 are induced by a Zn deficiency treatment whereas HvZIP8 was constitutively expressed.13
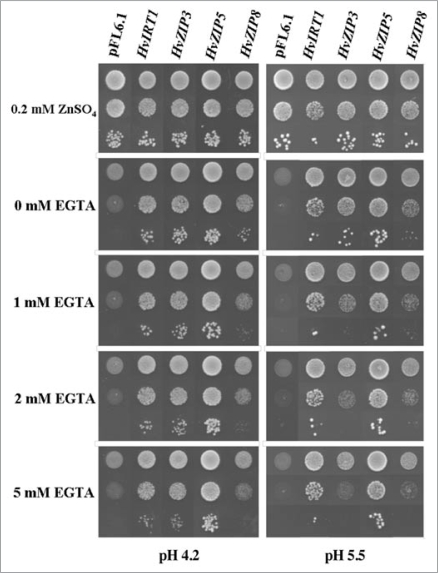
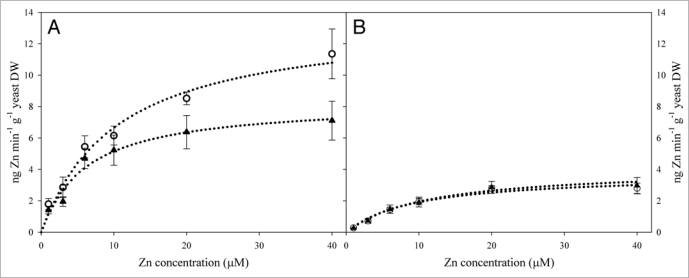
pH Regulation of Zn2+ Transport Capacity
In a previous study characterizing the human hZIP2 Zn2+ transporter, increasing pH levels led to higher Zn2+ uptake rates when expressed in erythroleukemia cells.14 From this study it was also observed that hZIP2 mediated Zn2+ uptake was stimulated by a HCO3− treatment, suggesting a Zn2+-HCO3− co-transport mechanism. In our studies we have tried to confirm this striking pH effect on ZIP mediated Zn2+ transport by analyzing the growth of Δzrt1Δzrt2 yeast cells transformed with barley ZIP genes at different pH levels. In the complementation study there was observed a clear tendency toward an improved growth rate of Δzrt1Δzrt2 yeast cells transformed with HvZIP genes on medium with pH 4.2 compared to pH 5.5 (Fig. 1). In contrast, lowering of pH from 5.5 to 4.2 did not seem to have a positive effect on the growth rate of HvIRT1 transformed Δzrt1Δzrt2 yeast cells. A more conclusive result were obtained using the isotope 67Zn in an experimental setup investigating the Zn2+ concentration-dependent transport kinetics in Δzrt1Δzrt2 yeast cells. The 67Zn2+ uptake kinetics mediated by HvIRT1 were not significantly different when performed in uptake medium with pH 4.2 compared to pH 5.5 (Fig. 2B). In contrast, the Vmax for HvZIP5 mediated uptake was increased with 64% at pH 4.2 relative to pH 5.5 (Fig. 2A). Based on the increased complementation, a similar induction in uptake rate can be hypothesized for HvZIP3 and HvZIP8 mediated uptake (Fig. 1). The study by Gaither and Edie (2000)14 was therefore not confirmed in the present study as we observed a completely reverse effect in response to decreasing the pH on the apoplastic side of plant ZIP transport proteins, in terms of the Zn2+ transport capacity. However, it should be noted that it has previously been observed that Zn2+ uptake mediated by IRT1 from Arabidopsis only was measurable at low pH (pH 4.2).15 The authors speculated that the Zn2+ binding of the IRT1 transporter are influenced by the pH as Zn2+ transport was observed only at pH 4.2 and the Fe2+/Fe3+ transport could be decreased by a Zn2+ addition at pH 6.0. We would therefore propose that the induced Zn2+ uptake mediated by ZIP proteins at low pH observed in the current study could be due to a changed ion binding capacity to the ZIP transporters. The increased Zn2+ binding of the protein would increase the Zn2+ ion density at the site of transport and facilitate an increased Zn2+ flux through the ZIP protein. Furthermore, the conformation of the ZIP proteins could also be influenced by lowering the pH and thereby change the transport capacity, but this awaits further studies before conclusive evidence can be provided.
Figure 1.
Complementation by HvZIP cdnas of the yeast mutant Δzrt1Δzrt2 defective in Zn2+ uptake at pH 4.2 and pH 5.5. Yeast cells transformed with the empty vector pFL6.1 or with a pFL6.1 construct containing either HvZIP3, HvZIP5, HvZIP8 or HvIRTI were spotted on Sd medium as described elsewhere (Pedas et al., 2009) with either 50 mm succinic acid/Tris base pH 4.2 or 5.5 with reduced content of free Zn controlled by EGTA. The Δzrt1Δzrt2 yeast strain showed a strong phenotype and therefore an additional supply of 0.2 mm ZnSO4 was necessary for obtaining control growth. Plates were incubated for three to five days at 30°C.
Figure 2.
Functional expression of HvZiP5 (A) and Hvirt1 (B) in Δzrt1Δzrt2 yeast cells. The concentration-dependent Zn uptake was determined by measuring 67Zn uptake rates over a range of substrate concentrations during a period of 15 min. Yeast media as described elsewhere (Pedas et al. 2009)13 with either 50 mM succinic acid/Tris base pH 4.2 (open symbols) or 5.5 (filled symbols) with varying 67Zn concentrations were added HvZIP5, HvIRT1 or pFL6.1 transformed yeast cells to an OD600 of 0.75. After the uptake period, the yeast cells were washed three times with ice-cold water, freeze dried, weighed, and acid digested in a microwave oven (multiwave 3000, software version 1.24, Anton Paar GmbH, Graz, Austria). The samples were subsequently diluted with ultra-pure water (Milli-Q Element, Millipore, MA) and measured on ICP-MS (Agrilent 7500ce; Agilent Technologies). HvIRT1 and HvZIP5 dependent transport activity were calculated by subtracting empty vector pFL6.1 mediated uptake rates from the corresponding HvIRT1 and HvZIP5 values, respectively. Each point represents the mean of triplicate measurements and error bars represent ± SE.
Areas of Further Exploration
The presented data suggest a possible interaction between proton activity and ZIP mediated Zn2+ uptake. Only a few studies have investigated the activity of ZIP proteins in vivo, mostly in terms of identification of amino acid residues important for transport activity.14,16 An urgent need for investigating the precise mechanism in which the transport of metal ions occur via ZIP proteins are therefore required. Expression in heterologous systems such as Xenopus Oocytes could elucidate more aspects on the potential role of proton activity and membrane potentials. Furthermore, a crystal structure would also reveal the roles of important residues in the trans-membrane domains and the cytoplasmic loop containing the metal binding region,17 in terms of potential metal ion binding sites.
Conclusions
In the referenced study, a successful identification and characterization of three new Zn2+ specific ZIP transporters from barley roots was reported.13 In contrast to a previous study showing a positive correlation between increasing pH levels and Zn2+ transport rate mediated by the hZIP2 protein, we observed a positive effect on the growth of Δzrt1Δzrt2 yeast cells transformed with ZIP cDNAs by lowering the pH from 5.5 to 4.2 in the yeast media. This observation was further substantiated by measurements of Zn2+ concentration-dependent uptake kinetics, as the Vmax for yeast cells transformed with HvZIP5 was increased with 64% at pH 4.2 compared to pH 5.5. In contrast, no effect was observed for HvIRT1 transformed yeast cells, indicating that Zn2+ transport mechanism may be different between ZIP proteins and their closely related IRT1 homologues.
Table 1.
Kinetic parameters for 67Zn influx in the Δzrt1Δzrt2 yeast mutant strain mediated by HvIRT1 or HvZIP5 illustrated in Figure 1.
| pH | HvIRT1 | HvZIP5 | ||
| Vmax | Km | Vmax | Km | |
| ng Mn min−1 g−1 yeast DW | µM | ng Mn min−1 g−1 yeast DW | µM | |
| 4.2 | 3.7 ± 0.8a | 0.3 ± 2.4a | 13.6 ± 2.9a | 10.4 ± 5.4a |
| 5.5 | 4.1 ± 1.2a | 11.0 ± 5.9a | 8.3 ± 1.5b | 6.1 ± 3.3a |
Values with the same superscript letter were not significantly different between pH levels The results are presented as average ± 95% confidence limits of three independent regressions (n = 3).
Acknowledgements
This work was supported by grant from the Ministry of Sciences, Technology and Innovation (grant no. 274-06-0325).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9375
References
- 1.Hotz C, B KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:94–204. [PubMed] [Google Scholar]
- 2.Cakmak I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008;302:1–17. [Google Scholar]
- 3.Palmgren MG, Clemens S, Williams LE, Kraemer U, Borg S, Schjorring JK, et al. Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 2008;13:464–473. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Stomph TJ, Jiang W, Struik PC. Zinc biofortification of cereals: rice differs from wheat and barley. Trends Plant Sci. 2009;14:123–124. doi: 10.1016/j.tplants.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 5.White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- 6.Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochem Biophys Acta. 2006;1763:595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Pedas P, Ytting CK, Fuglsang AT, Jahn TP, Schjoerring JK, Husted S. Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiol. 2008;148:455–466. doi: 10.1104/pp.108.118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat J-F, et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ Plant J. 2006;45:335–446. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- 11.Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udwardi MK, et al. GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem. 2002;277:4738–4746. doi: 10.1074/jbc.M106754200. [DOI] [PubMed] [Google Scholar]
- 12.Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, et al. OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot. 2005;56:3207–3214. doi: 10.1093/jxb/eri317. [DOI] [PubMed] [Google Scholar]
- 13.Pedas P, Schjoerring JK, Husted S. Identification and characterization of zinc-starvation-induced ZIP transporters from barley roots. Plant Physiol Biochem. 2009;47:377–383. doi: 10.1016/j.plaphy.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 15.Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- 16.Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng BH, Guerinot ML, Eide D, Saier JMH. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]