Abstract
Aquaporins (AQPs) are channel proteins that facilitate and regulate the permeation of water across biological membranes. Black mMexican sweet suspension cultured cells are a convenient model for studying the regulation of maize AQP expression and activity. Among other advantages, a single cell system allows the contribution of plasma membrane AQPs (PIPs, plasma membrane intrinsic proteins) to the membrane water permeability coefficient (Pf) to be determined using biophysical measurement methods, such as the cell pressure probe or protoplast swelling assay. We generated a transgenic cell culture line expressing a tagged version of ZmPIP2;6 and used this material to demonstrate that the ZmPIP2;6 and ZmPIP2;1 isoforms physically interact. This kind of interaction could be an additional mechanism for regulating membrane water permeability by acting on the activity and/or trafficking of PIP hetero-oligomers.
Key words: aquaporin, suspension cultured cells, hetero-oligomerization, maize, plasma membrane intrinsic protein, protein interaction, water movement
Black Mexican Sweet (BMS) Suspension Cell Cultures as a Tool for Studying the Water Relation at the Cell Level
Tight regulation of water movement in plant tissues is essential for their growth and development. Water transport through cell membranes depends on the activity of channel proteins called aquaporins (AQPs). Thirteen plasma membrane AQPs or PIPs (plasma membrane intrinsic proteins), clustered in two sequence-related subgroups, PIP1 and PIP2, have been identified in maize.1,2 When expressed alone in mesophyll protoplasts, ZmPIP1s are retained in the endoplasmic reticulum (ER), whereas ZmPIP2s are targeted to the plasma membrane.3 However, when ZmPIP1s and ZmPIP2s are co-expressed, they form hetero-oligomers and are colocalized in the plasma membrane.3 Post-translational modifications, including phosphorylation and protonation, have been shown to regulate PIP trafficking and water channel activity.5–7 These data highlight the importance of AQP regulation in the modulation of cell membrane water permeability.
BMS maize cultured cells and, more generally, plant cultured cells are useful systems for studying AQP expression and regulation.8–10 The whole plant is a complex multicellular entity made up of a wide variety of cell types organized into highly regulated tissues that differ in their gene expression profile and functions. In contrast, a cell culture system consists of a homogenous cell population originating from a single cell type and may, in this sense, be seen as a complementary “low complexity” system to the whole plant. This lower complexity might be an advantage when it comes to dissecting fine molecular tuning mechanisms such as that regulating cell water permeability via AQPs. Moreover, cell cultures possess inherent advantages, such as a fast, reproducible and well characterized growth rate and a high yield of plant material. In the case of a monocot species, such as maize, transgenic cell lines are more easily generated than whole transformed plants, a process that requires experienced personnel and large plant growth infrastructures.
It was recently shown that a cell pressure probe can be used with cultured cells to determine the membrane water permeability coefficient (Pf).11 As protoplasts can also be rapidly prepared from cultured cells, Pf values obtained using the cell pressure probe can be compared to the results of the protoplast swelling assay.9 The combination of these biophysical methods with biochemical approaches allows the contribution of the AQP to the Pf to be determined in a well defined single cell system, thus bypassing the difficulties encountered at the whole plant level in assessing the contribution of the apoplastic path. We have shown that the Pf of BMS cells increases significantly at the end of the logarithmic growth phase and during the steady-state phase compared to the lag phase, as do mRNA and protein levels of several PIPs, demonstrating a positive correlation between AQP abundance in the plasma membrane and the cell Pf.10 However, the Pf values obtained using the cell pressure probe were much higher than those measured by protoplast swelling, suggesting that protoplast preparation somehow affects the membrane water permeability and, thus, AQP activity.
Different Plasma Membrane PIP2 Aquaporins Physically Interact
Using Förster resonance energy transfer and fluorescence lifetime imaging microscopy, we previously showed that ZmPIP1s and ZmPIP2s physically interact in maize mesophyll protoplasts to regulate PIP1 trafficking from the ER to the plasma membrane.3 The interaction between ZmPIP1;2 and ZmPIP2;1 was confirmed by immunoprecipitation experiments using microsomal extracts from maize roots and BMS cells and by nickel affinity chromatography after expression in Xenopus oocytes.3,4 We used BMS cells to determine whether different PIP2 isoforms also interact. As BMS cells can be easily transformed by biolistic bombardment, we decided to generate cell lines expressing ZmPIP2;6 fused to a 6His and a c-myc tag at the N-terminus.
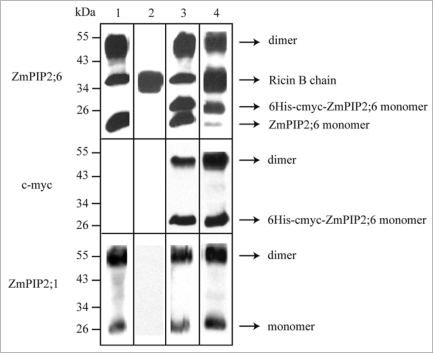
The microsomal fraction from a transgenic line expressing the tagged ZmPIP2;6 was solubilized using octyl-β-D-thioglucopyranoside and subjected to Ni-column chromatography and the bound proteins immunoblotted using anti-ZmPIP2;1, anti-ZmPIP2;6, or anti-c-myc antibodies. Interestingly, ZmPIP2;1 co-purified with the tagged ZmPIP2;6 (Fig. 1), demonstrating a physical interaction between ZmPIP2;1 and ZmPIP2;6. The intermediate band of 35 kD detected by the anti-ZmPIP2;6 antibodies was analyzed by mass spectrometry and shown to correspond to a homolog of the rice ricin B chain and not to a modification of ZmPIP2;6. Ricin B chain shares an epitope of 5 amino acid residues with the 17 amino acid peptide used to prepared the anti-ZmPIP2;6 antibodies.12
Figure 1.
ZmPIP2;1 and ZmPIP2;6 physically interact in BMS suspension cells. A transgenic cell line expressing 6His-cmyc-ZmPIP2;6 under the control of the ubiquitin promoter in the pAHC25 vector was obtained by biolistic bombardment.15 Proteins in the microsomal fraction of wild-type BMS cells or BMS cells expressing 6His-cmyc-ZmPIP2;6 were solubilized using 0.035% (w/v) octyl-β-D-thioglucopyranoside for 2 h at room temperature on a rotating wheel.3,4,10 After centrifugation at 100,000 g for 30 min, the supernatant was subjected to Ni-column purification (Qiagen, Germany) and the proteins separated by SDS-PAGE and analyzed by western blotting using antibodies raised against ZmPIP2;1 or ZmPIP2;6 (raised in our laboratory) or against cmyc (Santa Cruz Biotechnology, CA) as described previously.10,12 Lanes 1 and 3: microsomal fraction of wildtype BMS cells (1) or BMS cells expressing 6His-cmyc-ZmPIP2;6 (3); lanes 2 and 4: nickel column-purified proteins from wild-type BMS cells (2) or BMS cells expressing 6His-cmyc-ZmPIP2;6 (4). The ricin B chain homolog was purified due to the presence of a high number of histidine residues at its N-terminus. The antibodies used for the detection are indicated on the left and the identity of the immunodetected bands is indicated on the right.
These data showed, for the first time, that ZmPIP2s not only interact with ZmPIP1s, but also with other ZmPIP2s. It was demonstrated that the interaction between ZmPIP2s and ZmPIP1s is required for PIP1 trafficking to the plasma membrane in order to modulate the plasma membrane water permeability.4 The interaction between different ZmPIP2s, mainly localized in the plasma membrane under normal conditions, could provide cells with an additional mechanism for regulating their membrane permeability by modifying water channel activity and/or trafficking of PIP2 hetero-oligomers. Post-translational modifications, such as phosphorylation, might also play a role, for example by promoting this interaction or by acting on the activity and/or trafficking of the complexes.13,14 However, these ideas are speculative and further work is required to reveal the mechanism of formation, and the role and regulation, of these hetero-oligomers.
Conclusions
BMS maize cultured cells constitute a useful tool for studying AQP regulation and activity. We used this system to demonstrate that different ZmPIP2 isoforms physically interact. The role of the formation of ZmPIP2 hetero-oligomers remains to be determined. This study emphasizes the usefulness of a low complexity cell system in elucidating the role of an AQP in the cell water relation and is a complementary approach to studies at the whole plant level. Such a system might be of particular interest when studying the trafficking or post-translational modifications of given AQP isoforms and the connection between these regulation mechanisms and changes in membrane water permeability.
Acknowledgements
This work was supported by grants from the Belgian National Fund for Scientific Research (FNRS), the Interuniversity Attraction Poles Programme-Belgian Science Policy, and the “Communauté française de Belgique-Actions de Recherches Concertées”. D.C. is a research fellow at the “Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture”.
Footnotes
Previously published online: w,ww.landesbioscience.com/journals/psb/article/9484
References
- 1.Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000;122:1025–1034. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci USA. 2007;104:12359–12364. doi: 10.1073/pnas.0701180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell. 2003;16:215–228. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaumont F, Moshelion M, Daniels MJ. Regulation of plant aquaporin activity. Biol Cell. 2005;97:749–764. doi: 10.1042/BC20040133. [DOI] [PubMed] [Google Scholar]
- 6.Luu DT, Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 2005;28:85–96. [Google Scholar]
- 7.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 8.Kobae Y, Mizutani M, Segami S, Maeshima M. Immunochemical analysis of aquaporin isoforms in Arabidopsis suspension-cultured cells. Biosci Biotechnol Biochem. 2006;70:980–987. doi: 10.1271/bbb.70.980. [DOI] [PubMed] [Google Scholar]
- 9.Moshelion M, Moran N, Chaumont F. Dynamic changes in the osmotic water permeability of protoplast plasma membrane. 2004;135:2301–2317. doi: 10.1104/pp.104.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moshelion M, Hachez C, Ye Q, Cavez D, Bajji M, Jung R, et al. Membrane water permeability and aquaporin expression increase during growth of maize suspension cultured cells. Plant Cell Environ. 2009 doi: 10.1111/j.1365-3040.2009.02001.x. in press. [DOI] [PubMed] [Google Scholar]
- 11.Gerbeau P, Amodeo G, Henzler T, Santoni V, Ripoche P, Maurel C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002;30:71–81. doi: 10.1046/j.1365-313x.2002.01268.x. [DOI] [PubMed] [Google Scholar]
- 12.Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Molecular Biology. 2006;62:305–323. doi: 10.1007/s11103-006-9022-1. [DOI] [PubMed] [Google Scholar]
- 13.Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, et al. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol Cell Proteomics. 2008;7:1019–1030. doi: 10.1074/mcp.M700566-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Van Wilder V, Miecielica U, Degand H, Derua R, Waelkens E, Chaumont F. Maize plasma membrane aquaporins belonging to the PIP1 and PIP2 subgroups are in vivo phosphorylated. Plant Cell Physiol. 2008;49:1364–1377. doi: 10.1093/pcp/pcn112. [DOI] [PubMed] [Google Scholar]
- 15.Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]