Abstract
Nitric oxide (NO) is involved together with reactive oxygen species (ROS) in the activation of various stress responses in plants. However, the biochemical mechanisms by which ROS and NO participate, and the potential interaction between these molecules are still unclear. Ozone (O3) can be used as a tool to elicit ROS-activated stress responses and to activate cell death in plant leaves. We have recently shown that O3 induced a rapid accumulation of NO in Arabidopsis leaves and at late time points NO production coincided with the formation of hypersensitive response like lesions.1 Experiments using O3 and the NO-donor SNP alone or in combination indicated that both molecules are capable of activating a large set of stress related genes. In combined treatment, NO attenuated O3-induction of salicylic acid (SA) biosynthetic and signaling genes, and reduced SA accumulation. In addition, NO can elevate the levels of ethylene in several mutants. Thus, NO is a modifier of ROS signaling.
Key words: cell death, reactive oxygen species, ozone, nitric oxide, ethylene
Ozone (O3) is an air pollutant which in sensitive plants causes a reduction in photosynthesis and growth, ultimately leading to decreased growth and crop yields. induces the production of ROS by the O3 cells affected.2 These ROS include super-oxide anion (O2.-), hydrogen peroxide (H2O2) and hydroxyl radical (OH.-). The O3-induced quick burst of ROS resembles the oxidative burst in the hypersensitive response (HR) in incompatible plant pathogen interactions.3 The responses that follow the ROS burst include changes in the synthesis and accumulation of the plant stress hormones ethylene, salicylic acid (SA) and jasmonic acid and changes in gene expression.4,5 Eventually, the production of ROS can lead to HR-like cell death.2,5
In addition to ROS and hormones, nitric oxide (NO) has a prominent role in the defense against pathogens.6 Previous studies have shown that NO is quickly produced after pathogen attack and is involved in cell signaling during HR in plants.7,8 NO efficiently induces changes in gene expression9 suggesting that it has an active role in modifying plant stress responses. An important feature of NO is its lipophilic nature which enables its diffusion across plant membranes. NO can alter post-translational signaling by nitrosylation and/or direct binding to active centers of proteins, collectively making NO an effective signaling molecule.6
Recently we have shown that NO can modify cell death responses, signaling and gene expression in O3-exposed Arabidopsis plants.1 O3 induced NO production in a transient manner and production of NO occurred mainly in the area of O3-induced microlesions. Transcript profiling indicated a role for NO in attenuation of certain classes of O3 induced genes, many of which were related to SA biosynthesis or SA signaling. Furthermore, using mutants with altered levels of NO production we demonstrated that functional NO production is required for proper plant O3 responses. One of the earliest plant responses to O3 is ethylene production.2 A few studies indicate that NO and ethylene could interact in regulating stress responses, for example NO treatments induce production of ethylene in Arabidopsis and tobacco,10,11 and NO and ethylene are proposed to act together to regulate some O3 induced genes, exemplified by alternative oxidase AOX.11
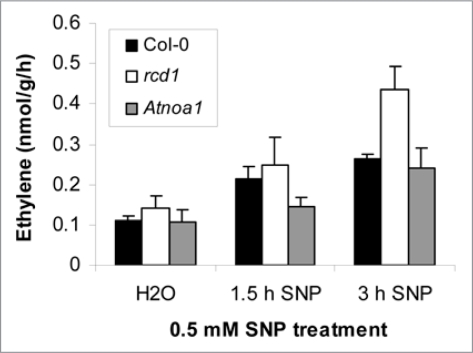
To further dissect the interaction between NO and O3 we observed that both SNP (sodium nitroprusside) and O3 treatment of Col-0 increased expression of the ethylene biosynthesis related genes, ACS6 and ACC oxidase.1 O3 induced ACS6 expression correlates with ethylene formation.12 To confirm that SNP induce ethylene production we measured ethylene production in Col-0 and two mutants, rcd1 (NO overproducer) and Atnoa1/rif1 (reduced NO-production). SNP treatment increased ethylene production, particularly in the rcd1 mutant (Fig. 1). Interestingly, combined SNP + O3 treatment led to attenuation of ACS6 expression1 indicating that one role of NO during O3 could be to modulate ethylene responses.
Figure 1.
NO activates ethylene biosynthesis. 0.5 mM SnP treatment of Col-0, rcd1 and Atnoa1 lead to increase in ethylene formation.
Both SA and ethylene are regulators of ROS induced cell death.2,4 We propose that NO has a role in O3 induced signaling by modifying SA and ethylene levels and gene expression levels. Ethylene is known to be involved in lesion propagation and the elevated ethylene levels in all genotypes tested, especially in the O3 sensitive rcd1 (Fig. 1) suggests that NO indeed has a role in modifying hormone biosynthesis and accumulation.
Abbreviations
- NO
nitric oxide
- ROS
reactive oxygen species
- O3
ozone
- HR
hypersensitive response
- SA
salicylic acid
- SNP
sodium nitroprusside
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9428
References
- 1.Ahlfors R, Brosché M, Kollist H, Kangasjärvi J. Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. Plant J. 2009;58:1–12. doi: 10.1111/j.1365-313X.2008.03756.x. [DOI] [PubMed] [Google Scholar]
- 2.Kangasjärvi J, Jaspers P, Kollist H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005;28:1021–1036. [Google Scholar]
- 3.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 4.Overmyer K, Brosché M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 5.Overmyer K, Brosché M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, et al. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiol. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Czymmek KJ, Shapiro AD. Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the arabidopsis hypersensitive response. Mol Plant Microbe Interact. 2003;16:962–972. doi: 10.1094/MPMI.2003.16.11.962. [DOI] [PubMed] [Google Scholar]
- 9.Grun S, Lindermayr C, Sell S, Durner J. Nitric oxide and gene regulation in plants. J Exp Bot. 2006;57:507–516. doi: 10.1093/jxb/erj053. [DOI] [PubMed] [Google Scholar]
- 10.Magalhaes JR, Monte DC, Durzan D. Nitric oxide and ethylene emission in Arabidopsis thaliana. Physiol Mol Biol Plants. 2000;2:117–127. [Google Scholar]
- 11.Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, et al. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, et al. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]