Abstract
Nitric oxide's (NO) involvement in breaking seed dormancy has been demonstrated in previous research but its action mechanism remains to be clarified. We observed that a rapid accumulation of NO induces an equally rapid decrease of abscisic acid (ABA) that is required for the NO's action in Arabidopsis. In addition, the NO-induced ABA decrease correlates with the regulation of CYP707A2 transcription and the (+)-abscisic acid 8′-hydroxylase (encoded by CYP707A2) protein expression. By analyzing cyp707a1, cyp707a2 and cyp707a3 mutants, we found that CYP707A2 plays a major role in ABA catabolism during the first stage of imbibition. Fluorescent images demonstrate that NO is released rapidly in the early hours at the endosperm layer during imbibition. Evidently such response precedes the enhancement of ABA catabolism which is required for subsequent seed germination.
Key words: seed dormancy, germination, nitric oxide (NO), (+)-abscisic acid 8′-hydroxylase, ABA, CYP707A2
Seed germination is a complex process and incorporates events that commence with the uptake of water by the quiescent dry seed and terminate with the elongation of the embryonic axis.1,2 Seeds of most angiosperms are dormant at maturity and this dormancy must be broken before germination can occur.3 Seed dormancy is a very complicated problem and is described as “one of the least understood phenomena in seed biology” and remains confusing despite much recent progress.3 It has been defined as the incapacity of a viable seed to germinate under favorable external conditions. 4 Seed dormancy can be explained as coat-imposed dormancy or embryo dormancy. In Arabidopsis it is controlled by both seed coat and embryo.3,5–7
ABA plays an important role in a number of physiological processes such as seed maturation, growth and developmental regulations, seed dormancy and adaptation responses to environmental stresses.8–11 In addition, ABA has been shown as an important positive regulator of both the induction of dormancy during seed maturation and the maintenance of the dormant state in imbibed seeds following shedding.12,13 Previous research indicates that seed dormancy is controlled by ABA and correlates with both ABA biosynthesis and catabolism.31 In Arabidopsis, CYP707 As family plays an important role in ABA catabolism during imbibition.30 Our results indicate that CYP707A2 plays a major role in ABA catabolism during imbibition and regulates seeds dormancy.
NO acts as a signaling molecule and plays an important role in plants and animals.14–16 In plants, previous studies have shown that NO can be emitted by plant cells and act as a growth regulator.17,18 NO induces seed germination in replacement of red light,17 affects growth and development,19 increases iron homeostasis and accelerates plant cell senescence.20,21 Furthermore, NO has been suggested to be involved in resistant responses to drought, salinity, heat stress, diseases, programmed cell death and ultraviolet-B radiation.18,22 Some research also indicates that NO participates in seed dormancy and germination control.17–19 Our results give evidences that NO regulates seed dormancy and germination and such action needs CYP707As family's participation. NO can break the dormancy of wild type Arabidopsis seeds, but it can't break cyp707a2 mutant seed dormancy.
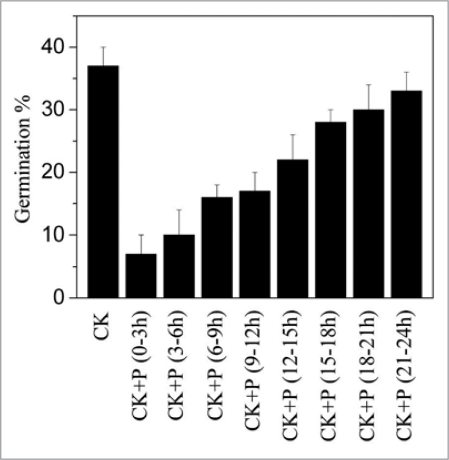
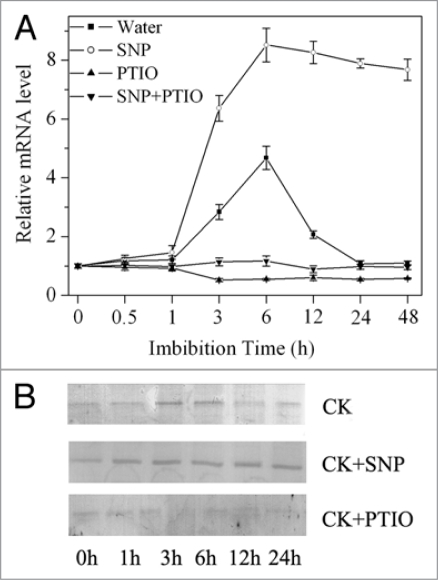
The relationship between NO signaling and ABA response has been demonstrated by some investigators.23–26 For example, ABA-induced guard cell closure needs NO participation.23,26–28 However the mechanism of NO affecting seed dormancy is not clear so far. Our study demonstrates that the rapidly accumulated NO induces an equally rapid ABA decrease that is required for seed dormancy break and germination in Arabidopsis. Our results indicate that seeds rapidly accumulate NO at first 3 h imbibition, and if NO was removed at this time the seed dormancy was enhanced. As shown in Figure 1, when NO was scavenged with PTIO at first 9 h of imbibition for 3 h, seed dormancy was enhanced substantially. NO induces ABA catabolism mainly by regulating the expression of ABA catabolism gene family CYP707A, especially by regulating the expression of CYP707A2 and ABA 8′-hydroxylase encoded by CYP707A2 (Fig. 2A and B).
Figure 1.
effects of nitric oxide (NO) produced at different hours during imbibition on dormancy break. Seeds were imbibed with water and were scavenged for NO with c-PtiO at each 3 h at the first 24 h imbibition. Germination rate was detected after 7 d of imbibition.
Figure 2.
Effect of NO on CYP707A2 gene expression and ABA 8′-hydroxylase expressions encoded by CYP707A2. (A) changes of CYP707A2 transcripts in freshly harvested WT seeds imbibed with water, SNP and c-PTIO. (B) western-blot analysis of ABA 8′-hydroxylase expressions in seeds of Arabidopsis during germination when treated with water (CK), sodium nitroprusside (SNP) or c-2-phenyl-4,4,5,5-tetramethyl imidazoline-1-oxyl-3-oxide (c-PTIO). Freshly harvested seeds were germinated under different treatments. Proteins were extracted and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were immunodetected with antibody (produced in rabbit) against ABA 8′-hydroxylase encoded by CYP707A2. Each treatment was immunodetected with antibody.
We also observed that NO accumulation mostly visible at aleurone layer. Earlier study showed that aleurone layer responds to NO and the response is necessary for seed dormancy.29 Although a lot of studies have indicated that NO is necessary for seed dormancy control, how NO is released during imbibition remains to be clarified. Whether it is by nitrate reductase, NOS or some other ways is not clear. In addition, if NO is not released by a single pathway, very possibly it is so, how these processes are regulated and/or coordinated are interesting questions. All of these need further studies.
In summary, our results demonstrate that CYP707A2 and ABA 8′-hydroxylase encoded by CYP707A2 play central role that is involved in ABA catabolism during imbibition in the seeds of Arabidopsis. NO acts crucially in regulating the transcription of CYP707A2 and ABA 8′-hydroxylase encoded by CYP707A2. Our results also demonstrate that a rapidly accumulated NO at the first stage of imbibition is required for rapid ABA catabolism and seed dormancy break. The remaining question is how NO is rapidly accumulated at this stage is still vague. It needs more experimental evidence to illustrate this mechanism in the future work.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9532
References
- 1.Bewley JD, Black M. Seeds: Physiology of development and germination. New York NY, USA: Plenum Press; 1994. [Google Scholar]
- 2.Holdsworth MJ, Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, afterripening, dormancy and germination. New Phytol. 2009;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 3.Bewley JD. Seed Germination and Dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 1997;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 5.Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa M, Hanada A, Yamauchi Y, Kuwalhara A, Kamiya Y, Yamaguchi S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller K, Tintelnot S, Leubner-Metzger G. Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006;47:864–877. doi: 10.1093/pcp/pcj059. [DOI] [PubMed] [Google Scholar]
- 8.Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1998;39:439–473. [Google Scholar]
- 9.Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwabara A, Ikegami K, Koshiba T, Nagata T. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae) Planta. 2003;217:880–887. doi: 10.1007/s00425-003-1062-z. [DOI] [PubMed] [Google Scholar]
- 11.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signaling. Curr Opin Plant Biol. 2003;6:470–479. doi: 10.1016/s1369-5266(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 14.Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 15.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:1–7. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D. Nitric oxide in plants: The biosynthesis and cell signaling properties of a fascinating molecule. Planta. 2005;221:1–4. doi: 10.1007/s00425-005-1494-8. [DOI] [PubMed] [Google Scholar]
- 17.Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyls elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 18.Beligni MV, Lamattina L. Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 2001;24:267–278. [Google Scholar]
- 19.Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 20.Murgia I, Delledonne M, Soave C. Nitric oxide mediates iron-induced ferritin accumulation in Arabidopsis. Plant J. 2002;30:521–528. doi: 10.1046/j.1365-313x.2002.01312.x. [DOI] [PubMed] [Google Scholar]
- 21.Leshem YY, Wills RBH, Ku VVV. Evidence for the function of the free radical gas-nitric oxide as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem. 1998;36:825–833. [Google Scholar]
- 22.Garcia-Mata C, Lamattina L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001;126:1196–1204. doi: 10.1104/pp.126.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarath G, Hou G, Baird LM, Mitchell RB. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta. 2007;226:697–708. doi: 10.1007/s00425-007-0517-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang AY, Jiang MY, Zhang JH, Tan MP, Hu XL. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175:35–60. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 26.Neill SJ, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 27.Neill SJ, Desikan D, Clarke A, Hancock JT. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002;128:13–16. [PMC free article] [PubMed] [Google Scholar]
- 28.Desikan R, Griffiths R, Hancock J, Neill SJ. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bethke PC, Libourei IG, Aoyama N, Chung YY, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, et al. CYP707A1 and CYP707A2, Which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Ann Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]