Abstract
Unicellular spore cells, designated as monospores (also called archeospores), are well known as migrating plant cells, in which establishment of the anterior-posterior axis directs asymmetrical distribution of F-actin. Since the mechanisms of cell polarity formation are not yet fully elucidated in monospores, we investigated the roles of phosphoinositide signaling systems and Ca2+ mobilization in migration. Although we have already found the critical involvement of phosphatidylinositol 3-kinase in the establishment of cell polarity, we recently demonstrated the important roles of extracellular Ca2+ influx, phospholipase C (PLC) and phospholipase D (PLD). The remarkable characteristics of these factors are that Ca2+ influx depends on photosynthetic activity and that PLC and PLD play roles in the establishment and maintenance of cell polarity, respectively. These findings could provide new insight into the regulation of migration in eukaryotic cells.
Key words: Ca2+ influx, cell polarity, phospholipase C, phospholipase D, photosynthesis, Porphyra yezoensis
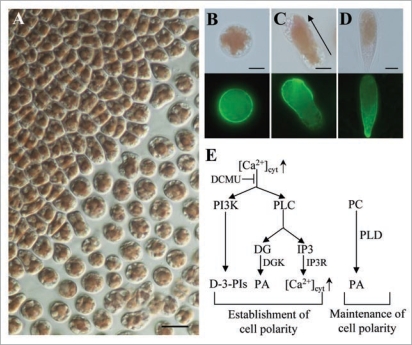
Monospores are responsible for asexual and clonal propagation of the marine multicellular red algae Porphyra and have an exceptional characteristic as migrating plant cells.1–5 Monospores possess a round shape just after release from gametophytic blades (Fig. 1A and B), then undergo morphological change during migration. The establishment of cell polarity leads to the determination of anterior-posterior axis and asymmetrical localization of F-actin (Fig. 1C). After migration, monospores adhere to the substratum in which the apical-basal axis has been established for further development (Fig. 1D). Asymmetrical distribution of F-actin is also found in chemotaxic migration of Dictyostelium cells and leukocytes.6,7 In these cells, reciprocal local accumulation of phosphoinositides, such as phosphatidylinositol-3,4,5-trisphosphate [PtdIns(3,4,5)P3] at the leading edge and phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] at the trailing side, is critical for the establishment of cell polarity. Phosphatidylinositol 3-kinase (PI3K) and PtdIns(3,4,5)P3-specific D-3-phosphatase PTEN have been identified as key modulators in the establishment of cell polarity, bringing asymmetrical distribution of these two phosphoinositides in plasma membranes.6,8 Similarly, we found the involvement of PI3K activity in the establishment of cell polarity in migrating monospores,3 suggesting the evolutional conservation of the function of PI3K in migrating eukaryotic cells. On the other hand, the importance of cell wall synthesis has been found in the maintenance of the cell polarity during monospore migration4 as reported in Fucus zygotes.9,10 Therefore, the establishment and maintenance of cell polarity are thought to be regulated separately in monospores of P. yezoensis. In this addendum, further evidence of differential regulation of cell polarity formation by extracellular Ca2+ influx and phospholipases in migrating monospores of red algae is documented according to our recent report.5
Figure 1.
Establishment and maintenance of cell polarity in monospores from the red alga P. yezoensis. (A) Discharge of unicellular monospores from a multicellular gametophytic blade of P. yezoensis strain TU-1. Scale bar = 20 µm. (B–D) Asymmetrical distribution of F-actin during early development of monospores. F-actin was stained with alex Flour 488 phalloidin. (B) Discharged monospore. (C) Migrating monospore. (D) Adhering monospore. Upper and lower photos in each panel show bright-field and fluorescent images, respectively. Arrow in (C) indicates the direction of migration. Scale bars = 5 µm. (e) Schematic representation of our working hypothesis about the formation of cell polarity required for monospore migration. Photosynthesis-dependent [Ca2+]cyt increase regulates PLC and PI3K for the establishment of cell polarity, while PLD is required for the maintenance of the established cell polarity. DG, diacylgycerol; IP3, inositol-1,4,5-trisphosphate; IP3r, IP3 receptor; PC, phosphatidylcholine.
Photosynthesis-Dependent Extracellular Ca2+ Influx in Cell Polarity Establishment
Since migration and early development of monospores are completely inhibited in the dark, it is clear that monospore migration requires light illumination. There are two possibilities how light promotes mono-spore migration: first is sensing of the light direction by photoreceptors as found in Fucoid zygotes,11 and the other is the energy supply via photosynthesis. Here, since unilateral light did not influence the direction of migration, and DCMU, a photosynthesis inhibitor, completely inhibited migration, the establishment of cell polarity required for migration depends on photosynthetic activity in red algal monospores.
In animal cells, it is well known that Ca2+ influx triggers the establishment of cell polarity.12,13 Moreover, Ca2+ influx is also critical for polarized tip growth of root hairs and pollen tubes in plants.14,15 In P. yezoensis, monospore migration was completely inhibited by treatment with the Ca2+ chelator EGTA and Ca2+ channel blocker LaCl3, whereas Ca2+ ionophore A23187 accelerated monospore migration. Thus, channel-mediated extracellular Ca2+ influx promotes the establishment of cell polarity following monospore migration. It is noteworthy that monospores treated with ionophore A23187 were able to migrate in the dark, indicating that artificial Ca2+ influx can mimic the photosynthesis- dependent monospore migration.
Taken together with the above findings, it was concluded that photosynthetic activity regulates extracellular Ca2+ influx to promote the establishment of cell polarity in monospores. Photosynthesis- mediated Ca2+ influx is a novel system for increasing cytosolic Ca2+ concentration in plants, which has been reported only in blue light-stimulated Ca2+ uptake via the Ca2+ channel in guard cells.16
Functional Diversity between Phospholipases C and D in Monospore Migration
Recently, phosphatidic acid (PA) has been recognized as a second messenger involved in diverse cellular processes such as tip growth, stress response and acclimation, and auxin signal transduction.17,18 Phospholipase C (PLC) catalyzes the generation of two second messengers, namely, inositol-1,4,5-trisphoshphate and diacylglycerol from PtdIns(4,5)P2, the latter of which is immediately converted to PA via phosphorylation by diacylglycerol kinase, although phospholipase D (PLD) directly produces PA from phosphatidylcholine.17,18 Thus, PLC and PLD play different roles in the production of PA.
There is evidence indicating the functional involvement of PLC and PLD in polarity establishment of mammalian cells and Fucus zygotes.19,20 We also found the critical involvement of PLC in the establishment of cell polarity, based on complete inhibition of monospore migration and asymmetrical distribution of F-actin by treatment with a PLC inhibitor, U73122. In contrast, the inhibition of PLD by 1-butanol prevents migration but not asymmetrical distribution of F-actin, although further incubation resulted in the loss of the F-actin asymmetry. These findings demonstrate the functional diversity between PLC and PLD; that is, PLC is required for the establishment of cell polarity as PI3K, whereas PLD maintains cell polarity. Such diversity resembles the differential involvement of PI3K and cell wall synthesis in the establishment and maintenance of cell polarity in monospores.3,4
Perspectives
The involvement of photosynthesis-dependent extracellular Ca2+ influx and phospholipases in cell migration has been shown in the process of cell polarity formation in monospores from P. yezoensis (Fig. 1E). Since little is known how photosynthesis regulates Ca2+ influx, further studies should focus on photosynthesis-dependent activation of the Ca2+ channel and targets for increased Ca2+ influx during monospore migration. For instance, the catalytic activity of PLC depends on Ca2+ concentration, which in turn is mediated by the Ca2+-binding module EF hand motif.21 On the other hand, since PI3K activity also depends on Ca2+,12 PLC and PI3K are thought to be candidates of the Ca2+ target. Moreover, functional diversity of PLC and PLD in the formation of cell polarity was also presented. Such a differential involvement of these two phospholipases has also been observed in PtdIns(4,5)P2-dependent activation of the tobacco ourtward-rectifying K channel and the development of the brown alga Silvetia compressa.20,22 Thus, the interrelationship between PLC and PLD in physiological regulations appears to be conserved in eukaryotic cells. In the future, elucidation of the relationship between photosynthesis-dependent Ca2+ influx and functional diversity among phospholipases and PI3K must be addressed to understand how monospores migrate through a photosynthesis- and phosphoinositide signaling-dependent manner.
Acknowledgements
This study was supported by Grants-in-Aid for Scientific Research (C; no. 21580213) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- PLC
phospholipase C
- PLD
phospholipase D
- PI3K
phosphatidylinositol 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol-3,4,5-trisphosphate
- PtdIns(4,5)P2
phosphatidylinositol-4,5-bisphosphate
- PA
phosphatidic acid
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9534
References
- 1.Ackland JC, West JA, Scott J, Zuccarello GC, Broom J. Biology of Porphyra pulchella sp. nov. from Australia and New Zealand. Algae. 2006;21:193–208. [Google Scholar]
- 2.Ackland JC, West JA, Pickett-Heaps J. Actin and myosin regulate pseudopodia of Porphyra pulchella (Rhodophyta) archeospores. J Phycol. 2007;43:129–138. [Google Scholar]
- 3.Li L, Saga N, Mikami K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J Exp Bot. 2008a;59:3575–3586. doi: 10.1093/jxb/ern207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Saga N, Mikami K. Effects of cell wall synthesis on cell polarity in the red alga Porphyra yezoensis. Plant Signal Behav. 2008b;3:1126–1128. doi: 10.4161/psb.3.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koji Mikami, Lin Li, Megumu Takahashi, Naotsune Saga. Photosynthesis-dependent Ca2+ influx and functional diversity between phospholipases in the formation of cell polarity in migrating cells of red algae. J Experimental Botany. 60:3477–3489. doi: 10.4161/psb.4.9.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin T, Xu X, Fang J, Isik N, Yan J, Brzostowski JA, et al. How human leukocytes track down and destroy pathogens: lessons learned from the model organism Dictyostelium discoideum. Immunol Res. 2009;43:118–127. doi: 10.1007/s12026-008-8056-7. [DOI] [PubMed] [Google Scholar]
- 8.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 9.Kropf DL, Kloareg B, Quatrano RS. Cell wall is required for fixation of the embryonic axis of Fucus zygotes. Science. 1988;239:187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- 10.Kropf DL. Establishment and expression of cellular polarity in Fucoid zygotes. Microbiol Rev. 1992;56:316–339. doi: 10.1128/mr.56.2.316-339.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlee C, Bouget F-Y, Corellou F. Choosing sides: establishment of polarity in zygotes of fucoid algae. Semi Cell Dev Biol. 2001;12:345–351. doi: 10.1006/scdb.2001.0262. [DOI] [PubMed] [Google Scholar]
- 12.Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci USA. 2007;104:16176–16181. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miedema H, Demidchik V, Véry AA, Bothwell JH, Brownlee C, Davies JM. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytol. 2008;179:378–385. doi: 10.1111/j.1469-8137.2008.02465.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Wu X, Chen Y, Li X, Huang M, Zheng M, et al. Combined proteomic and cytological analysis of Ca2+-calmodulin regulation in Picea meyeri pollen tube growth. Plant Physiol. 2009;149:1111–1126. doi: 10.1104/pp.108.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada A, Shimazaki K. Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant Cell Physiol. 2009;50:360–373. doi: 10.1093/pcp/pcn203. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Xue HW, Chen X, Mei Y. Function and regulation of phospholipid signaling in plants. Biochem J. 2009;421:145–156. doi: 10.1042/BJ20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters NT, Pol SU, Kropf DL. Phospholipid signaling during stramenopile development. Plant Signal Behav. 2008;3:398–400. doi: 10.4161/psb.3.6.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J Biol Chem. 2005;280:21015–21021. doi: 10.1074/jbc.M412123200. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Shor O, Diminshtein S, Yu L, Im YJ, Perea I, et al. Phosphatidylinositol(4,5)bisphosphate inhibits K+-efflux channel activity in NT1 tobacco cultured cells. Plant Physiol. 2008;149:1127–1140. doi: 10.1104/pp.108.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]