Abstract
Epiphytic plants in general and bromeliads in particular live in a water and nutrient-stressed environment often limited in nitrogen. Thus, these plants have developed different ways to survive in such an environment. We focused on Aechmea mertensii (Bromeliaceae), which is both a tank-bromeliad and an ant-garden (AG) epiphyte initiated by either the ants Camponotus femoratus or Pachycondyla goeldii. By combining a study of plant morphology and physiology associated with aquatic insect biology, we demonstrate that the ant species influences the leaf structure of the bromeliad, the structure of the aquatic community in its tank, and nutrient assimilation by the leaves. Based on nitrogen and nitrogen stable isotope measurements of the A. mertensii leaves, the leaf litter inside of the tank and the root-embedded carton nest, we discuss the potential sources of available nitrogen for the plant based on the ant partner. We demonstrate the existence of a complex ant-plant interaction that subsequently affects the biodiversity of a broader range of organisms that are themselves likely to influence nutrient assimilation by the A. mertensii leaves in a kind of plant-invertebrate-plant feedback loop.
Key words: Aechmea mertensii, Camponotus femoratus, nitrogen, nitrogen stable isotope, Pachycondyla goeldii, plant-insect interactions, phytotelmata
Epiphytes, which belong to numerous phylogenetically distant families, make up more than one-third of the total vascular plant biodiversity of neotropical rainforests.1 Epiphytism implies physiological consequences and constraints resulting from the lack of access to what are by far the most important sources of water and nutrients for ground-rooting plants.2 Epiphytic plants therefore have adapted in various ways to their aerial environment; adaptations for nutrient acquisition include growth forms such as “trash-baskets” (e.g., Asplenium), tanks (tank-bromeliads), specific leaf features such as absorbant trichomes (e.g., Tillandsia) and the velamen radicum in aerial roots (Orchidaceae).1,3 Potential nitrogen sources for epiphytes may include (1) canopy-derived detritic nitrogen (mineralization of organic material from the canopy) and (2) atmospheric sources (wet and dry deposition, N2 fixation),4 or may involve (3) interactions with animals.5 Indeed, some epiphytic species develop symbioses with ants, either by providing chambers (domatia) where ants nest or by rooting in ant gardens6 (AGs). By measuring differences in stable isotope composition, Treseder et al. (1995)7 estimated that the myrmecophyte Dischidia major (Asclepiadaceae) derives almost 30% of its nitrogen from debris deposited by the ants living in its domatia.
Bromeliads are commonly found among the epiphytic species that root in ant-gardens. Their leaves form compartments acting as phytotelmata that hold rainwater and therefore provide habitats for aquatic macro- and microorganisms.8,9 The detritus (e.g., windborne particulates, faeces, and dead leaves and animals) that enter the tanks constitute a source of nutrients for the aquatic food web, as well as for the bromeliad.10 Nitrogen is made available through the bacterial decomposition of organic matter, and the presence of arthropod predators in the phytotelmata food web most likely accelerates nitrogen cycling and leaf assimilation.2
Recently, we described the complicated interaction that links an epiphytic ant-garden tank-bromeliad, its aquatic micro-ecosystem and the ants with which they are associated in the tropical forest of French Guiana.11 Aechmea mertensii Schult.f. (Bromeliaceae) is an epiphytic tank-bromeliad that roots on AGs initiated either by the ants Camponotus femoratus Fabr. (living in a parabiotic association with Crematogaster levior Forel) or Pachychondyla goeldii Forel.12 The AGs we studied were situated in tree canopies in pioneer growths along forest edges. By combining ecological studies characterizing the available light for the epiphyte, leaf morpho-anatomy, biochemical studies based on 15N isotope analysis and the richness of the aquatic communities inside of the tanks, we demonstrate the influence of the ant species on the foliar and aquatic community structures and, subsequently, on nitrogen acquisition by the plant.
The ant species either directly or indirectly affects the light incidence, leaf structure, phytotelmata contents and nitrogen content of A. mertensii leaves, which is probably correlated to the plant's fitness since nitrogen is a limiting factor for epiphytic plants. Pachycondyla goeldii and C. femoratus colonize exposed and partially-shaded areas, respectively. Exposed bromeliads (P. goeldii AGs) are smaller and limit direct light incidence by adopting an amphora-shape, whereas those growing in the shade (C. femoratus AGs) are larger and forage for light by developing a wider canopy. However, the contrast in the leaf mass area (LMA) response to light intensity based on the ant partner seems to support the hypothesis that leaf structure is not primarily controlled by light,13,14 but rather directly by the ants or by other abiotic factors controlled by the ant partner. The availability of water and leaf debris from the surrounding vegetation may directly influence the diversity of the aquatic organisms in the phytotelmata that are themselves likely to influence nutrient assimilation by A. mertensii leaves in a kind of plant-invertebrate-plant feedback loop.
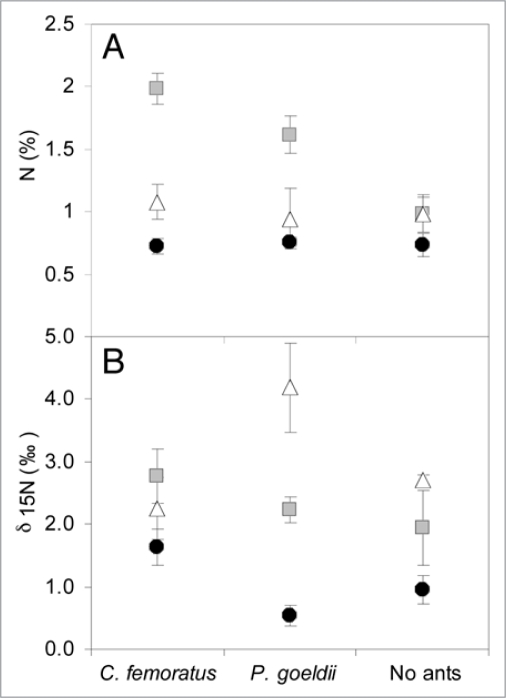
Measurements of the percentage of total nitrogen showed that the carton nest, where the roots are embedded, was characterized by a greater amount of nitrogen compared to the leaf litter inside of the tank for both C. femoratus and P. goeldii AGs (Fig. 1A). While the nitrogen content remained constant for A. mertensii leaves and the leaf litter, the nitrogen content of the carton nest changed with the presence or absence of ants, and according to the ant partner. The carton nests of C. femoratus ants are richer in nitrogen than P. goeldii nests and non-occupied (i.e., abandoned) AGs, which have the lowest nitrogen content. Thus the sources of nitrogen could come from both the carton nest, where the roots are tightly embedded, and the phytotelmata with an average maximum nitrogen content of 2%. Delta 15N values enable us to estimate that A. mertensii may derive nitrogen from both ant species, but was affected differently depending on the ant partner (Fig. 1B). We demonstrate that A. mertensii—C. femoratus/Cr. levior associations can radically increase the amount of nutrients available to the host. Differences in stable N isotopic composition could reflect the diversity and richness of the macro- and microorganisms11 in the phytotelm and/or the diversity of epiphytic species growing in the AGs,12,15 and/or variations in the size of the ant colony living in the AGs. Indeed, C. femoratus/Cr. levior AGs are more densely populated than P. goeldii AGs.12
Figure 1.
(A) Mean (±1 SE) of nitrogen content (%) and (B) δ15N (%) of Aechmea mertensii leaves (filled circles), root-embedded carton-nest (grey square) and phytotelmata leaf litter (empty triangle) for Camponotus femoratus (N = 10), Pachycondyla goeldii (N = 10) and non-occupied (N = 5) AGs.
We have shown that AG ants provide nutrients to their host bromeliad. They either provide nitrogen-rich nutrients by directly or indirectly enhancing the nitrogen uptake of their host plants. Thus ants mediate nutrient uptake as well as phytotelmata contents. Furthermore, the strength of the mutualism appears to be dependent on the ant partner. In contrast to previous studies, our results showed that A. mertensii receives more than twice the amount of nitrogen compared to the myrmecophytic epiphyte Dischidia major7 or the epiphytic fern Antrophyum lanceolatum.16 Such a high level of insect-derived nitrogen could reflect the predominance of a dual nitrogen source derived from animals: one from the phytotelmata-associated organisms and one from the ant-debris (and faeces) in the carton nest. Nevertheless, further research on 15N enrichment is necessary to properly identify which part of the nitrogen input comes from animal remains and which part comes from the roots and phytotelmata. Moreover, it will enable us to identify which of the two AG ant species represents the greatest direct source of nitrogen for the plant.
Acknowledgements
We would like to thank Andrea Dejean for proofreading the manuscript. Support for this study was provided through the Programme Amazonie II of the French Centre National de la Recherche Scientifique (Project 2ID), and the Programme Convergence 2007–2013 (Région Guyane) from the European Community (Project DEGA).
Addendum to: Leroy C, Corbara B, Dejean A, Céréghino R. Ants mediate foliar structure and nitrogen acquisition in a tank-bromeliad. New Phytol. 2009 doi: 10.1111/j.1469-8137.2009.02891.x. In press.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9423
References
- 1.Benzing DH. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 2.Ngai J, Srivastava D. Predators accelerate nutrient cycling in a bromeliad ecosystem. Science. 2006;314:963. doi: 10.1126/science.1132598. [DOI] [PubMed] [Google Scholar]
- 3.Lüttge U. Physiological ecology of tropical plants. Berlin: Springer Verlag; 1997. [Google Scholar]
- 4.Stewart G, Schmidt S, Handley L, Turnbull M, Erskine P, Joly C. 15N natural abundance of vascular rainforest epiphytes: implications for nitrogen source and acquisition. Plant Cell Environ. 1995;18:85–90. [Google Scholar]
- 5.Huxley C. Symbiosis between ants and epiphytes. Biol Rev. 1980;55:321–340. [Google Scholar]
- 6.Davidson DW, Epstein WW. Epiphytic associations with ants. In: Lüttge U, editor. Vascular plant as epiphytes. New York: Springer Verlag; 1989. [Google Scholar]
- 7.Treseder KK, Davidson DW, Ehleringer JR. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature. 1995;375:137–139. [Google Scholar]
- 8.Richardson B. The bromeliad microcosm and assessment of faunal diversity in a neotropical forest. Biotropica. 1999;31:321–336. [Google Scholar]
- 9.Kitching RL. Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 10.Benzing DH. Bromeliaceae: profile of an adaptative radiation. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 11.Leroy C, Corbara B, Dejean A, Céréghino R. Ants mediate foliar structure and nitrogen acquisition in a tank-bromeliad. New Phytol. 2009 doi: 10.1111/j.1469-8137.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- 12.Corbara B, Dejean A, Orivel J. Les “jardins de fourmis”, une association plantes-fourmis originale. L'Année Biologique. 1999;38:73–89. (Fre). [Google Scholar]
- 13.Givnish TJ. Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol. 1988;15:63–92. [Google Scholar]
- 14.Gutschick VP. Biotic and abiotic consequences of differences in leaf structure. New Phytol. 1999;143:3–18. [Google Scholar]
- 15.Orivel J, Dejean A. Selection of epiphyte seed by ant-garden ants. Ecoscience. 1999;6:51–55. [Google Scholar]
- 16.Watkins J, Cardelus C, Mack M. Ants mediate nitrogen relations of an epiphytic fern. New Phytol. 2008;150:5–8. doi: 10.1111/j.1469-8137.2008.02606.x. [DOI] [PubMed] [Google Scholar]