Abstract
Plant hormone abscisic acid (ABA) is found in a wide range of land plants, from mosses to angiosperms. However, our knowledge concerning the function of ABA is limited to some angiosperm plant species. We have shown that the basal land plant Physcomitrella patens and the model plant Arabidopsis thaliana share a conserved abscisic acid (ABA) signaling pathway mediated through ABI1-related type 2C protein phosphatases (PP2Cs). Ectopic expression of Arabidopsis abi1-1, a dominant allele of ABI1 that functions as a negative regulator of ABA signaling, or targeted disruption of Physcomitrella ABI1-related gene (PpABI1A) resulted in altered ABA sensitivity and abiotic stress tolerance of Physcomitrella, as demonstrated by osmostress and freezing stress. Moreover, transgenic Physcomitrella overexpressing abi1-1 showed altered morphogenesis. These trangenic plants had longer stem lengths compared to the wild type, and continuous growth of archegonia (female organ) with few sporophytes under non-stress conditions. Our results suggest that PP2C-mediated ABA signaling is involved in both the abiotic stress responses and developmental regulation of Physcomitrella.
Key words: ABA, ABI1, Physcomitrella patens, PP2C, signaling
PP2C-Mediated ABA Signalingis Conserved Between Physcomitrella and Arabidopsis
Abscisic acid (ABA) is found in a wide variety of land plants, ranging from mosses to angiosperms, and is thought to be involved in plant responses to water stress. Stomatal closure, seed maturation and subsequent acquisition of extreme drought tolerance and dormancy are well-characterized functions of ABA in angiosperms.1 However, ABA function in non-angiosperm plants is unclear. For example, mosses do not have seeds but produce spores, and do not develop stomata in their major tissues protonemata and gametophores. According to the evloutional view, mosses are considered to be the basal land plants. Thus, a comparative approach between mosses and angiosperms will give us new insights into the evolution of ABA function and the signaling pathway in land plants. Marella et al.2 reported that the moss Physcomitrella patens and Arabidopsis have a conserved ABA signaling pathway mediated by ABA INSENSITIVE1 (ABI1)-related protein phosphatases3 and a transcription factor ABA INSENSITIVE3 (ABI3).4 Moreover, Physcomitrella ABI3 orthologous gene (PpABI3A) is able to partially complement the phenotypes of Arabidopsis abi3-6 mutant.5 These findings, with tools for studying gene function including RNA interference, inducible promoters and gene targeting;6 make Physcomitrella a good model system for comparative studies of ABA signaling pathways.
To investigate the function of PP2C-mediated ABA signaling in Physcomitrella, we took a gain-of-function approach in which Arabidopsis abi1-1, a negative regulator of ABA signaling,7–10 was introduced into Physcomitrella under the control of constitutive rice actin promoter (Act::abi1-1).11 The Act::abi1-1 plants showed ABA insensitivity in growth and gene expression, and also exhibited decreased tolerance to osmostress and freezing stress. These results suggest that Physcomitrella and Arabidopsis have conserved PP2C substrates that function as positive regulators in ABA signaling. We also searched the genome database of Physcomitrella for genes encoding PP2Cs, and found 51 putative PP2C genes. Phylogenetic analysis of the amino acid sequences of Physcomitrella PP2Cs and Arabidopsis 76 PP2Cs revealed two Physcomitrella genes (PpABI1A and PpABI1B) belonging to the ABI1-clade.12 It was noteworthy that Arabidopsis ABI1-clade (Group A) contained nine genes, including ABI1, ABI2,3 HAB1,13 HAB213 and PP2CA,14,15 demonstrated to be negative regulators of ABA signaling; however, Physcomitrella had only two genes for Group A. This fact may reflect differences in tissue complexity between Physcomitrella and Arabidopsis, and suggests that land plants developed increased numbers of Group A PP2C genes during their evolution to enable tissue- and organ-specific tuning of ABA signaling.
Taking advantage of Physcomitrella's unique ability for high frequency homologous recombination, we disrupted the PpABI1A gene, which shows a higher expression level compared to PpABI1B.11 ppabi1a plants showed enhanced expression of ABA-inducible genes and enhanced freezing stress tolerance. These and the complementary results from the gain-of-function experiment confirmed that Physcomitrella and Arabidopsis have an evolutionarily conserved regulation mechanisms for ABA signaling, and that PP2C-mediated ABA signaling also regulates abiotic stress responses in the moss Physcomitrella.
PP2C-Mediated ABA Signaling and Developmental Regulation ofPhyscomitrella
ABA in angiosperms is known to function not only in abiotic stress responses but also in developmental regulations, such as lateral root development and seed maturation processes.1,16 It is uncertain if ABA regulates the developmental process in bryophytes. One known effect of exogenous ABA in bryopsida is induction of brood cells in protonemata.17 Brood cells are spherical cells differentiated from rectangle protonemal cells in response to drought or prolonged culture. ABA can efficiently induce brood cell formation. Protonemata elongate their filaments by tip growth of successive perpendicular cell division of apical cells; non-apical cells stop division and growth until the second subapical cells develop new initial cells for lateral growth.18 Interestingly, ABA can induce perpendicular cell division in both apical and non-apical cells that is followed by brood cell formation (data not shown). This suggest that ABA is involved in cell differentiation through cell cycle regulation, an effect not reported by the study of ABA in angiosperms. ppabi1a protonemata showed increased ABA sensitivity for brood cell formation, suggesting that PP2C-mediated ABA signaling is involved in brood cell formation.11
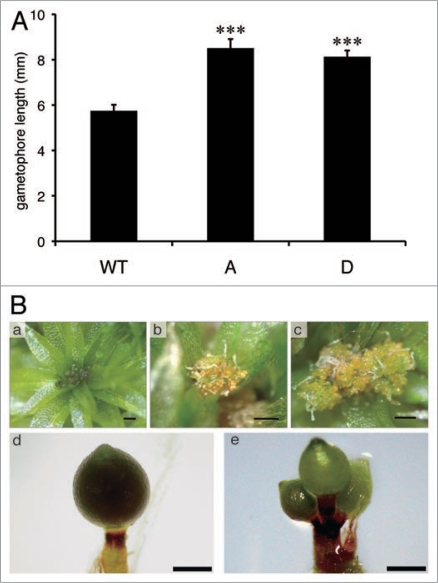
Through the investigation of phenotypes in transgenic plants, we found that Act::abi1-1 plants showed altered morphogenesis even under non-stress conditions. The stem lengths (gametophore heights) of Act::abi1-1 line A and D were 1.4 times longer than that of wild type stems (Fig. 1A). Because mosses do not possess the vascular bundle system, and the gametophore leaves consist of single layer cells without cuticle, maintenance of a low gametophore height is likely one mechanism to ensure adequate water supply and moisture from the ground surface. It is possible that ABA regulates gametophore height to avoid water stress.
Figure 1.
Ectopic expression of Arabidopsis abi1-1 caused developmental alteration of Physcomitrella. (A) Gametophore heights of wild type (WT) and Act::abi1-1 plant lines A and D were measured. Gametophores from each plant grown on Jiffy-7 for one month were randomly selected and the heights were measured under a dissection microscope. Values shown are mean ± SE (n = 30). Asterisks indicate significant changes between Act::abi1-1 plants and WT (***p < 0.001). (B) representative photographs of archegonia development of WT (a) and Act::abi1-1 plant lines a (b) and D (c). Massive growth of yellowish archegonia is observed only in Act::abi1-1 plants but not in the wild type. Representative photographs of sporophytes developed on apexes of WT gametophore (d) and Act::abi1-1 plant line A (e) are shown. notice that WT produces one sporophyte per apex, but Act::abi1-1 plants produce multiple sporophytes per apex. Scale bars, 200 µm.
In Physcomitrella, leafy gametophoes produce the sex organ, gametangia, on its apex. Archegonia (female) and antheridia (male) are produced in the same apex. Water is needed to allow sperm travel to the the archegonia egg cell.19 Although a number of archegonia are produced per apex, normally only one archegonium develops into the sporophyte after fertilization. During sex organ induction at low temperature (15°C), aberrant proliferation of gametangia was observed in Act::abi1-1 plants (Fig. 1B). Although the wild type plant produces a number of archegonia per apex before fertilization, the size as a whole is small (approximately 200 µm in size) and can be observed only microscopically. Many of Act::abi1-1 gametophores, however, produced little to no sporophytes and showed continuous growth of archegonia, resulted in a mass with numerous number of yellowish archegonia (1–2 mm in diameter as a whole) visible with the naked eye. Since Act::abi1-1 plants were able to develop normal spores when gametophores were exposed to enough water (data not shown), the sex organs of the Act::abi1-1 plant itself were likely fertile. Even Act::abi1-1 plants occasionally developed sporophytes, but also frequently made multiple sporophytes per apex, in contrast to the wild type, which usually make one sporophyte per apex (Fig. 1B). Because the sperm need a water film to swim from antheridia to archegonium,19 sperm of the Act::abi1-1 plants might be less tolerant to dryness than that of wild type plants under insufficient water condition. Alternatively, cell surface properties of gametophores in Act::abi1-1 plants might be altered, resulting in defective formation of the water film under low water conditions. We should also consider the possibility that ectopic expression of abi1-1 protein disturbed the developmental process independently of ABA signaling. Evaluation of the double disruption of PpABI1A and PpABI1B is warranted. However, it is possible that PP2C-mediated ABA signaling is involved in the regulation of development of Physcomitrella.
Conclusions
In the referenced study and the addendum, we have shown that the basal land plant moss Physcomitrella and Arabidopsis evolutionarily conserve a PP2C-mediated ABA signaling pathway, suggesting that this pathway participates in regulation not only of abiotic stress responses but also in developmental regulation of Physcomitrella. Recently, ABI1 and related PP2Cs have been show to function with novel ABA receptors, Regulatory Component of ABA Receptor (RCAR)/PYRABACTIN RESISTANCE 1 (PYR1).20,21 Genes encoding these receptors are also found in the Physcomitrella genome, indicating that this model plant is a useful system for comparative analysis of PP2C-mediated ABA signaling in plants.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9464
References
- 1.Finkelstein RR, Gampala SS, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marella HH, Sakata Y, Quatrano RS. Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J. 2006;46:1032–1044. doi: 10.1111/j.1365-313X.2006.02764.x. [DOI] [PubMed] [Google Scholar]
- 3.Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM. Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nambara E, Keith K, McCourt P, Naito S. Isolation of an internal deletion mutant of the Arabidopsis thaliana ABI3 gene. Plant Cell Physiol. 1994;35:509–513. [PubMed] [Google Scholar]
- 6.Quatrano RS, McDaniel SF, Khandelwal A, Perroud P-F, Cove DJ. Physcomitrella patens: mosses enter the genomic age. Curr Opin Plant Biol. 2007;10:1–8. doi: 10.1016/j.pbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein RR, Somerville CR. Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 1990;94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein RR. Abscisic acid-insensitive mutations provide evidence for stage-specific signal pathways regulating expression of an Arabidopsis late embryogenesis-abundant (LEA) gene. Mol Gen Genet. 1993;238:401–408. doi: 10.1007/BF00291999. [DOI] [PubMed] [Google Scholar]
- 9.Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- 10.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu K, Nishikawa Y, Ohtsuka T, Taji T, Quatrano RS, Tanaka S, et al. Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens. Plant Mol Biol. 2009;70:327–340. doi: 10.1007/s11103-009-9476-z. [DOI] [PubMed] [Google Scholar]
- 12.Schweighofer A, Hirt H, Meskiene I. Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 2004;9:236–243. doi: 10.1016/j.tplants.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicolas C, Lorenzo O, et al. Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J. 2004;37:354–369. doi: 10.1046/j.1365-313x.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 14.Sheen J. Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA. 1998;95:975–980. doi: 10.1073/pnas.95.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, et al. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006;140:115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Smet I, Zhang H, Inze D, Beeckman T. A novel role for abscisic acid emerges from underground. Trends Plant Sci. 2006;11:434–439. doi: 10.1016/j.tplants.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Goode JA, Stead AD, Duckett JG. Redifferentiation of moss protonemata: an experimental and immunofluorescence study of brood cell formation. Can J Bot. 1993;71:1510–1519. [Google Scholar]
- 18.Schumaker KS, Dietrich MA. Programmed changes in form during moss development. Plant Cell. 1997;9:1099–2007. doi: 10.1105/tpc.9.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reski R. Development, genetics and molecular biology of mosses. Bot Acta. 1998;111:1–15. [Google Scholar]
- 20.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]