Abstract
The gaseous hormone ethylene has multiple roles in plant development and responses to external cues. Among these is the regulation of ‘Rhizobium’-induced nodulation in legumes. Extensive descriptive literature exists, but has been expanded to allow more mechanistic analysis through the application of genetics. Both mutants and transgenics displaying ethylene insensitivity have now been described, suggesting an intimate interplay of ethylene response, plant development and nodulation.
Key words: ethylene, legume, mutant, nodulation, receptor, symbiosis, transgenics
Nodulation of legumes is significant as the nitrogen fixing symbiosis adds critical available nitrogen to the biosphere. Often major crop plants are either legumes (c.f., soybean, peanut, bean, medics, clovers, chickpea), or they rely on rotational practices involving legumes. Nodule formation occurs predominantly on roots after mitogenic stimulation of cortical and pericycle cells by the Rhizobium-secreted lipo-oligosaccharide Nod-Factor. To date, the timing, the signal, its receptor (a LysM receptor kinase complex), major parts of the down-stream response cascade (involving ion channels, a calcium calmodulin dependent protein kinase, a cytokinin receptor and numerous transcription factors) and the target cell types are known1,2 (Fig. 1A). As a result, nodule ontogeny is an elegant and mature experimental system to analyse plant development, associated signaling and behavior.3 Genetic, genomic, physiological and biochemical tools are currently being combined to optimise the role of legumes in diverse agricultural and silvicultural systems to facilitate more sustainable production of food, feed and biofuels.
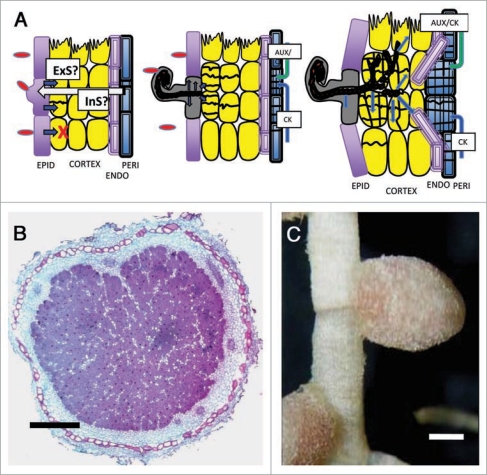
Figure 1.
Critical stages of nodule initiation in determinate nodulating legumes. (A) Rhizobium cells in the soil recognize plant-secreted flavones and attach to the epidermal (EPID) cells. Nod-factor, a lipo-oligosaccharide decorated with various host-range determining moieties, is perceived by the legume epidermal cells which respond in two ways, namely re-arrangement of the cytoskeleton resulting in root hair curling and bacterial entrapment, and induction of ectopic cytokinin signalling (possibly synthesis). These events allow (1) bacterial invasion through an infection thread (as inverted root hair cell wall) and (2) generation of an unknown external signal (ExS) that activates cell division in subepidermal cortical cells [in harmony with an unknown internal signal (InS)]. Blue arrows signify continued Nod Factor stimulation. Note that in indeterminate nodulators such as clover and Medicago, these cell divisions occur in the deep cortex adjacent to the endodermis. These cell divisions occur predominantly (90–96%) opposite xylem pole cells, which is altered in ethylene insensitive mutants/transgenics. The vascular system of the root extends into the nodule to provide hormonal and nutritional transport. An early nodule primordium is deemed non-autonomous, while the more advanced cell cluster is able to generate most of its own hormones for cell proliferation. EPID, epidermis; PERI, pericycle; ENDO, endodermis. The final nodule is a chimeric organ having arisen from both cortical and pericycle origins. (B) cross-section of a fully nitrogen-fixing nodule of soybean (photo: Lisette Pregelj, CILR), and (C) pea (Pisum sativum; photo: Dr. Alex Borisov, St. Petersburg). Bar = 1 mm.
Mutant and Transgenic Analysis using Model Legumes
Ethylene gas exposure, or growth in the presence of ethylene precursors such as ACC, limits nodule numbers per plant through reduced infection by ‘rhizobia’4–6 and decreased ability to initiate cell divisions in target plant zones behind the growing root tip (the ‘zone of nodulation’ characterized by emerging root hairs). The laboratory of Doug Cook and Varma Penmetsa was first to describe legume mutants with increased insensitivity for the classical ‘triple response’ during dark germination.7 Specifically, a Medicago truncatula mutant, called ‘sickle’, because of the characteristic root deformation caused by excessive nodule initiation, was described. Of symbiotic significance, this recessive mutant displayed increased nodule initiation and rhizobial infection. Its symbiotic phenotype was controlled by the root as shown by reciprocal grafts. Recently, the affected gene was cloned and shown to be EIN2,8 a known member of the signal response chain to ethylene in Arabidopsis thaliana. EIN2 is presumed to encode a nuclear membrane component, possibly transmitting a cytoplasmic response in CTR1 to nuclear transcription and response factors.
Recently, Lohar et al.9 reported a similar increased nodulation phenotype in the model legume Lotus japonicus. In contrast to Cook's work, Lotus nodulates in essentially the same steps but the induced nodule primordium has a short life span leading to determinate nodule formation (Fig. 1B); in contrast, Medicago, like pea and clovers, forms indeterminate nodules characterized by a more persistent (but still terminal) meristem (Fig. 1C). Hence, one finds the distinctive difference of spherical (determinate) and cylindrical nodule shapes (indeterminate).
The Lotus ethylene insensitive lines were constructed by transgenic modification using the dominant Arabidopsis ETR1-1 mutant gene.10 ETR1 encodes a two-component histidine kinase receptor, which if mutated prevents ethylene action.11 It most likely functions in the ER membrane. AtETR1-1 was introduced as a single copy under the control of the constitutive 35S CaMV promoter.12 Results were confirmed over several generations with independent lines. Strong ethylene insensitivity was detected in germination tests conducted in the dark. Classical ethylene insensitivity phenotypes included extensive hypocotyl length in triple response assays, reduced number of lateral roots, flower petal retention and delayed flowering and fruit ripening (Fig. 2A and E). Additionally, increased nodule numbers (both in the presence and absence of ACC) (Fig. 2H), disrupted root hair infection and epidermal cell divisions (Fig. 2C and D), altered radial distribution of nodule primordium induction (normally almost exclusively off the xylem pole, but now with an eight-fold increase off the phloem pole; Fig. 2F and G), twisted hypocotyl (Fig. 2B), and increased number of bacteroids per symbiosome (two- to three-fold) were observed.
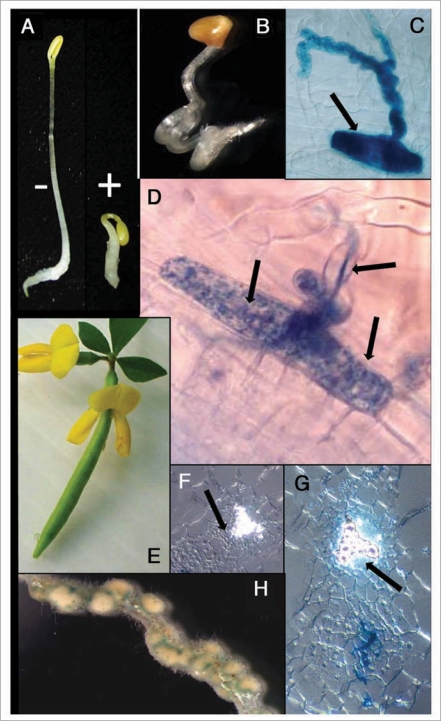
Figure 2.
Ethylene-associated phenotypes. (A) classical triple response of dark grown Lotus japonicus wild-type seedlings grown with (+) and without (−) added ethylene or its precursor ACC; (B) ethylene super-insensitive seedling of line LjETR1-1 with a twisted hypocotyl; (C) Infected root hair but the infection was blocked in transgenic line LjETR1-1 (X-gal staining; Mesorhizobium loti strain NSP2235::lacZ). (D) same as (C), but illustrating epidermal cell divisions (note nuclei). (E) Flower with retained petals and developing seed pod. (F) Root section of wild type showing nodule initiation (lower left) off the xylem pole (see arrow); (G) as (F) but LjETR1-1, but cell division cluster off the phloem pole (see arrow); (H) nodulated Lotus LjETR1-1 root portion showing high density nodulation.
The Lotus ETR1 transgenics nodulated in the presence of inhibitory levels of ACC, but interestingly still were inhibited in primary root growth. This may suggest that the 35S CaMV promoter is not active in the appropriate cell types, or that another receptor (e.g., ERS1) may be critical for root meristem activity. It also suggests that root growth responses and nodulation, although both involving the same target tissues (i.e., the pericycle close to xylem poles) respond in opposing ways (see Fig. 1).
Of interest is a previously unrecognized root hair invasion and cell division response. At a high frequency, root hairs in LjETR1-1 seedlings were filled with bacterial infection threads that did not penetrate into the cortex (Fig. 2C and D). At times these epidermal cells divide repeatedly, but without cell enlargement. One speculates that increased Nod Factor production, concomitant ectopic cytokinin synthesis, and altered plant ‘immune response’, leading to a pseudo-hypersensitive response, cause this abnormal cellular response.
Lotus transgenics, like sickle in Medicago, had wild-type sensitivity to nitrate inhibition of nodulation, suggesting that although nitrate treatment and ethylene responses in legumes are well-documented negative regulators, they do not act in the same pathway.
Similar results were obtained by Nukui and associates in Japan, who transformed Lotus with the less-characterized ethylene ERS1 receptor gene from melon.13 Although these results suggested a broader commonality in ethylene regulation of nodulation, some recent results suggest further complexity. Again EMS-induced ethylene insensitive mutants of L. japonicus were isolated using a Triple Response seedling assay.14 Mutant ENIGMA-1 was isolated together with a second independent, but allelic mutant (ENIGMA-2). Both exhibited recessive, root-controlled phenotypes. Both mutants had reduced nodulation (about 70–75% of wild type Miyakojima), yet both are mutated in LjEIN2! Yet ethylene receptor transgenics of Lotus (i.e., LjETR1-1) showed increased nodulation. All mutants and transgenics possessed strong ethylene insensitivity phenotypes in other developmental processes, supporting the physiological functionality of the genetic alteration. Thus, a paradox exists as the EIN2 mutant of Medicago has increased nodule number whilst an EIN2 mutation in Lotus does not.
Ethylene and Autoregulation of Nodulation
Legumes also control nodule numbers through a systemic process labeled “Autoregulation of Nodulation (AON)”.15 Mutants in this circuit have a supernodulating or hypernodulating phenotype.16 Such mutants were isolated in several legumes, though most advances have so far been made with soybean and Lotus. All AON-deficient mutants display decreased nitrate sensitivity to nodulation. One causative gene is the CLAVATA1-related LRR receptor kinase NARK (nodule autoregulation receptor kinase17). The equivalent gene in Lotus is called HAR1 (hypernodulation and aberrant root18,19), and in Medicago truncatula is called SUNN (supernummary nodulation20). Other loci such as KLAVIER, ASTRAY and TOO MUCH LOVE exist in Lotus and have been partially characterized. Significantly, GmNARK was shown to control nodulation through its expression in the shoot vasculature21,22 and this was confirmed in other legumes.18,19,23 NARK interacts with a protein phosphatase (KAPP24), reminiscent of the interaction seen by CLAVATA1 in the control of the apical meristem.
One presumes that multicellular plants utilise a complex set of LRR receptor kinases to control illegitimate cell divisions; i.e., NARK (and its cousins SUNN and HAR1) are de facto ‘tumor suppressors’.25 Short and long distance signaling molecules are presumed26 and have been isolated as CLE-like peptide-encoding genes (no binding studies yet) in both L. japonicus,27 Medicago (Holsters M, Ghent, personal communication) and soybean (Reid D and Ferguson B, unpublished data; see Fig. 3). It is presumed that the LRR domain is the major binding site for a CLE peptide. Genome-wide expression analysis suggests that root inoculation with Bradyrhizobium via NARK negatively controls the biosynthesis of jasmonic acid in the leaf vasculature.28 A common phenotype in all AON-defective mutants is a partial insensitivity to otherwise inhibitory levels of nitrate, and reduced root systems (with varying degrees of penetrance as Lotus har1 mutants tend to be severely affected, while soybean and Medicago show the response only when extensively supernodulated).
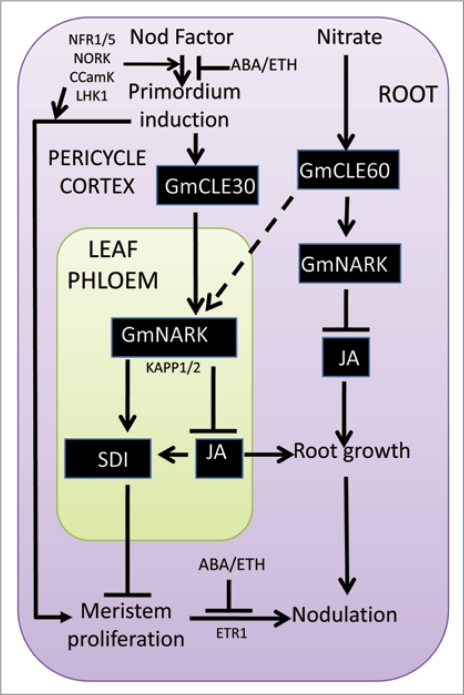
Figure 3.
Model for nodule initiation, local nitrate inhibition and systemic AON control circuits. We propose that Nod factor induces cell proliferation that also leads to production of a CLE-type peptide which either in a complexed form or as a prepeptide travels to the leaf where it interacts with the CLAVATA1-like LRR receptor kinase NARK (or HAR1/SUNN). It is unclear whether jasmonic acid synthesis and response transcript changes are directly or indirectly controlling the release and transport of the SDI (shoot-derived inhibitor), thought to be a small molecular weight substance (possibly a metabolite or its analogue), which activates nodule meristem arrest in the root. The same compound is proposed to be a positive regulator of root meristem. ABA and ethylene inhibit nodulation possibly prior to calcium spiking during Nod Factor perception.6 A secondary role (at least for ethylene) is proposed via radial potentiation for cell divisions of cortical cells. In contrast nitrate activates a related peptide that also is proposed to interact with NARK but only in the root, leading to localised inhibition of nodulation.
Interactions and Cross-Talk
A key question is how does AON interact with ethylene and cytokinin signaling? Does cell division control involve the cytokinin receptor (CRE1/LHK1) known to engage in cross-talk with the related ethylene receptor cascade? Several results permit the conclusion that the AON and ethylene pathways are independent. First, ethylene mutants do autoregulate as shown by their suppressed nodulation pattern below the heavily nodulated region (the ‘sickle’). Second, both soybean and Lotus AON mutants are still ethylene sensitive.29 Third, double mutants of Mtsunn/Mtsickle and Ljhar1/LjETR1-1 do not show an additive phenotype.29,30
However, the jury is still out. Ljhar1-1 mutants possess a strong root phenotype.31,32 Seedling primary root growth and lateral initiation are affected. When mutant and wild-type seedlings are treated with inhibitory levels of the cytokinin BAP, root growth is severely inhibited. Similar findings have been reported using Pisum sativum.33,34 In Lotus, this inhibition is reversed by treatment with AVG or silver ions (both ethylene action inhibitors).31 However, recovery to near wild-type levels of root length occurred preferentially in the har1-1 mutant. We tested this phenomenon, not with phytohormone inhibitors, but by mutant interactions. Confirmed double mutants of Ljhar1-1 and LjETR1 (HE11 and HE19,35) were tested for their root and nodulation phenotypes. Of great interest was the fact that the presence of the ethylene insensitivity receptor suppressed the primary root growth inhibition caused by the absence of the LjHAR1 gene product. Skilled reciprocal grafting of both HE11 and HE19 (har1; ETR1-1) to the Ljhar1-1 mutant created plants that harbored a homozygous har1-1/har1-1 condition in the entire plant, but with the ETR1-1 controlled et hylene insensitivity being active in either shoot or root.30 We conclude that the suppression of the Har1 deficiency induced root dwarfing required ethylene insensitivity in the shoot. We propose more complex signal exchanges exist between the root and the shoot in both the control of nodulation and root growth36 (Fig. 4).
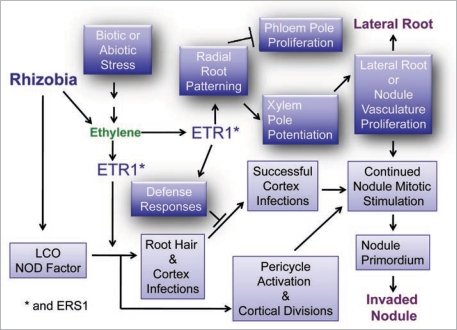
Figure 4.
Conceptual diagram of inter-relations of ethylene regulation during nodulation and root growth. Ethylene works through balanced signalling on root and nodule meristems. Additionally the ‘plant immune response’ is activated leading to concomitant biochemical changes such as alterations of the phenylpropanoid pathway. These in turn affect ‘rhizobia’ as well as endogenous plant processes such as hormone translocation. Mutations in the ETR1 receptor gene are complex to analyse as it is unclear what tissue specificity and distribution this receptor has relative to related receptors like ERS1.
Conclusions
Using transgenics and induced mutants it was possible to dissect mechanisms of ethylene action as it relates to nodule establishment and systemic nodulation control. Paradoxes were revealed, possibly hinting at yet unknown complexities arising from our present ignorance pertaining to hormonal action differentiating determinate (spherical) and indeterminate (cylindrical) nodules (Fig. 1B and C). Clearly regulation of nodule meristem activity is ‘in play’. Further analysis utilizing new functional genomic tools, coupled with attention to cell-specificity and metabolites (as compared to transcript profiling) will be needed. One can be certain that the ethylene story in legume nodulation will reveal many new facets of plant biology, especially concerning cross-talks and interactions.
Acknowledgements
We thank colleagues, students, ARC, NSF and our universities for research support. Yu-Hsiang Lin is thanked for SDI facts. P.M.G., P.K.C. and Q.J. thank the Australian Research Council for provision of a Centre of Excellence grant (CEO348212). G.S. also thanks the US National Science Foundation for research support (grant #DBI-0421620).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9395
References
- 1.Stacey G, Libault M, Brechenmacher L, Wan J, May GD. Genetics and functional genomics of legume nodulation. Curr Opin Plant Biol. 2006;9:1–12. doi: 10.1016/j.pbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Oldroyd GE, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 3.Gresshoff PM. Molecular genetic analysis of nodulation genes in soybean. Plant Breeding Reviews. 1993;11:275–318. [Google Scholar]
- 4.Chan PK, Gresshoff PM. Roles of plant hormones in legume nodulation. Biotechnology 2008. In: Doelle HW, DaSilva EJ, editors. Encyclopedia of Life Support Systems (EOLSS) Oxford UK: EOLSS Publishers; 2009. developed under the auspices of the UNESCO http://www.eolss.net. [Google Scholar]
- 5.Ferguson BJ, Mathesius U. Signaling interactions during nodule development. J Plant Growth Regulators. 2003;22:47–72. [Google Scholar]
- 6.Oldroyd GED, Engstrom EM, Long SL. Ethylene inhibits the nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 8.Penmetsa RV, Uribe P, Anderson J, Lichtenzveig J, Gish J-C, Nam Y-W, et al. The Medicago truncatula of the Arabidopsis EIN2 gene, sickle, is a negative regulator of symbiotic and pathogenic microbial interactions. Plant J. 2008;55:580–595. doi: 10.1111/j.1365-313X.2008.03531.x. [DOI] [PubMed] [Google Scholar]
- 9.Lohar D, Stiller J, Kam J, Stacey G, Gresshoff PM. Ethylene insensitivity conferred by the Arabidopsis Etr1-1 receptor gene alters the nodulation response of transgenic Lotus japonicus. Annals of Botany. 2009;104:277–285. doi: 10.1093/aob/mcp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science. 1993;262:539–545. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, et al. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotech. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- 12.Stiller J, Martirani L, Tuppale S, Chian R-J, Chiurazzi M, Gresshoff PM. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. J Exp Bot. 1997;48:1357–1365. [Google Scholar]
- 13.Nukui N, Ezura H, Minamisawa K. Transgenic Lotus japonicus with an ethylene receptor gene Cm-ERS1/H70A enhances formation of infection threads and nodule primordia. Plant Cell Physiol. 2004;45:427–435. doi: 10.1093/pcp/pch046. [DOI] [PubMed] [Google Scholar]
- 14.Chan PK, Biswas B, Gresshoff PM. ENIGMA, an ethylene insensitive (EIN2) mutant of Lotus japonicus with reduction in infection thread and nodule number (in preparation)
- 15.Gresshoff PM. Post-genomic insights into nodulation. Genome Biol. 2003;4:201. doi: 10.1186/gb-2003-4-1-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean [Glycine max (L.) Merr] mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA. 1985;82:4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searle IR, Men AM, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, et al. Long distance signalling for nodulation control in legumes requires a CLAVATA1-like receptor kinase. Science. 2003;299:108–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- 18.Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, et al. Shoot control of root development is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 20.Schnabel E, Journet E-P, Carvalho-Niebel Fd, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- 21.Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM. Regulation of the soybean-Rhizobium symbiosis by shoot and root factors. Plant Physiol. 1986;82:588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nontachaiyapoom S, Kinkema M, Scott PT, Schenk PM, Men AE, Gresshoff PM. Promoters of orthologous soybean and Lotus japonicus nodulation autoregulation genes interchangeably drive phloem-specific expression in transgenic plants. Mol Plant Microbe Interact. 2007;20:769–790. doi: 10.1094/MPMI-20-7-0769. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Q, Gresshoff PM. Shoot-control and genetic mapping of the har1-1 (hypernodulation and aberrant root formation) mutant of Lotus japonicus. Funct Plant Biol. 2002;29:1371–1376. doi: 10.1071/PP01097. [DOI] [PubMed] [Google Scholar]
- 24.Miyahara A, Hirani TA, Oakes M, Kereszt A, Kobe B, Djordjevic MA, et al. Soybean nodule autoregulation receptor kinase phosphorylates two kinase-associated protein phosphatases in vitro. J Biol Chem. 2008;283:25381–25391. doi: 10.1074/jbc.M800400200. [DOI] [PubMed] [Google Scholar]
- 25.Beveridge CA, Mathesius U, Rose R, Gresshoff PM. Common threads of meristem development and homeostasis: branching, nodules and lateral roots. Curr Opin Plant Biol. 2007;10:44–51. doi: 10.1016/j.pbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Oelkers K, Goffard N, Weiller GF, Gresshoff PM, Mathesius U, Frickey T. Bioinformatic analysis of the CLE signaling peptide family. BMC Plant Biology. 2008;8:1. doi: 10.1186/1471-2229-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, et al. Nod factor/nitrate-induced CLE genes that drive Har1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009;77:67–77. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- 28.Kinkema M, Gresshoff PM. Identification of downstream signals for the soybean autoregulation of nodulation receptor kinase GmNARK. Mol Plant Microbe Interact. 2008;10:1337–1348. doi: 10.1094/MPMI-21-10-1337. [DOI] [PubMed] [Google Scholar]
- 29.Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR. A dual mechanism of nodule control in Medicago truncatula. Plant Physiol. 2003;131:1–11. doi: 10.1104/pp.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Q. Autoregulation of Nodulation and Root Development in the Model Legume Lotus japonicus. Brisbane: University of Queensland; 2009. PhD Dissertation. [Google Scholar]
- 31.Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, de Bruijn FJ, et al. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 2000;23:97–114. doi: 10.1046/j.1365-313x.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 32.Buzas DM, Gresshoff PM. Short and long distance control of root development by LjHAR1 during the juvenile stage of Lotus japonicus. J Plant Physiol. 2007;164:452–459. doi: 10.1016/j.jplph.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Lorteau MA, Ferguson BJ, Guinel FC. Effects of cytokinin on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiol Plant. 2001;112:421–428. doi: 10.1034/j.1399-3054.2001.1120316.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson BJ, Wiebe EM, Emery RJN, Guinel FC. Cytokinin accumulation and an altered ethylene response mediate the pleiotropic phenotype of the pea nodulation mutant R50 (sym16) Can J Bot. 2005;83:989–1000. [Google Scholar]
- 35.Jiang Q, Gresshoff PM. Detection of single nucleotide polymorphisms (SNPs) and transgenes in DNA from small individual seeds and half-cotyledons in Lotus japonicus. Symbiosis. 2005;40:49–53. [Google Scholar]
- 36.Oka-Kira E, Kawaguchi M. Long-distance signaling to control root nodule number. Curr Opin in Plant Biol. 2006;9:496–502. doi: 10.1016/j.pbi.2006.07.012. [DOI] [PubMed] [Google Scholar]