Abstract
The c-Fes protein-tyrosine kinase is associated with growth and differentiation of hematopoietic, neuronal, vascular endothelial and epithelial cell types. In this study, we investigated whether small interfering RNA (siRNA)-mediated knockdown of c-Fes expression affected proliferation of the human renal carcinoma cell lines, ACHN and VMRC-RCW. Immunofluorescence microscopy showed that c-Fes was expressed in both the cytosol and nuclei of these cells, and siRNA treatment preferentially downregulated c-Fes expression in the cytosol. Knock-down of c-Fes inhibited cellular proliferation in a dose-dependent manner with minimal increase in cell death. c-Fes siRNA treatment also downregulated the phosphorylation of Akt1 on S473 and IKKα on T23, and cyclin D1 expression, enhanced the expression of IκBα, and prevented the nuclear localization of NFκB. Treatment with an NFκB inhibitory peptide (SN50) also blocked the proliferation and nuclear localization of NFκB in these cells. The effect of SN50 treatment was not enhanced by c-Fes siRNA, suggesting that downregulation of c-Fes expression inhibited cell cycle progression through the Akt1/NFκB pathway. In contrast to siRNA-mediated knockdown, ectopic expression of either wild-type or kinase-inactive c-Fes in renal carcinoma cells failed to alter their proliferation in vitro and in vivo. Thus, suppression of proliferation resulting from siRNA-mediated knockdown may depend upon an expression of c-Fes protein rather than its kinase activity. Taken together, our results indicate that downregulation of c-Fes expression may be a potential therapeutic strategy for advanced human renal cell carcinoma and inhibition of its kinase activity as an antiangiogenic therapy does not seem to induce the growth of human renal carcinoma cells.
Keywords: human renal carcinoma cells, c-Fes, siRNA, proliferation, Akt1, NFκB
Introduction
The proto-oncogene, c-fes/fps, encodes a non-receptor protein tyrosine kinase that belongs to a family distinct from Src, Abl, and other non-receptor protein tyrosine kinases (1,2). c-Fes, together with the structurally related kinase, c-Fer, are characterized by a long unique N-terminal region featuring a tubulin-binding FCH domain and coiled-coil oligomerization motifs, followed by SH2 and kinase domains. c-Fes expression was originally described in hematopoietic and vascular endothelial cells (3), as well as some neuronal cell types. More recently, c-Fes expression has been reported in epithelial cells as well, including normal colonic epithelial tissue sections (4,5). c-Fes kinase activity appears to be regulated by coiled-coil dependent oligomerization and subsequent autophosphorylation (6). Expression of kinase-inactive c-Fes in cultured vascular endothelial cells exerts a dominant-negative effect on endogenous c-Fes kinase-activity, consistent with this hypothesis (7,8). Using stable cell lines expressing kinase-inactive c-Fes, we demonstrated previously the involvement of c-Fes in a variety of angiogenic cellular responses promoted by fibroblast growth factor-2, angiopoietins, sonic hedgehog, and stromal cell-derived factor-1α (9). Furthermore, downregulation of c-Fes by siRNA inhibited vascular endothelial growth factor-A-induced migration and capillary morphogenesis by endothelial cells (10). In vivo, expression of a membrane-targeted form of c-Fes induced angiogenesis in transgenic mice, leading to multifocal hemangiomas (11). These results suggest that c-Fes could be a potential target for antiangiogenic therapy designated to shutdown intracellular signals mediated by multiple proangiogenic factors concurrently (9).
The c-Fes kinase was originally identified in the context of avian (v-fps) and feline (v-fes) retroviral oncogenes, which cause potent transformation of rodent fibroblasts in vitro. Similarly, mutations in the negative regulatory c-Fes coiled-coil domains lead to upregulation of kinase activity and fibroblast transformation, suggesting a pro-oncogenic role for c-Fes in cancer (12,13). More recently, somatic mutations of c-fes were found in human colorectal cancers (14). However, subsequent studies revealed that these mutations reduced or abolished Fes kinase activity, supporting a tumor suppressor role for c-Fes in the context of colonic epithelial cells (4,5). Indeed, loss of c-Fes expression is a common finding in primary human colorectal cancer specimens, whereas c-Fes expression can be readily detected in normal colonic epithelial cells from the same individuals (5). Along similar lines, introduction of kinase-inactivating c-Fes mutations in a mouse model of breast cancer reduced the latency for tumor formation, an effect that was rescued by a wild-type fes transgene (4). Restoring wild-type Fes expression to Fes-negative colorectal cancer cell lines inhibited anchorage-independent growth in soft agar (5). These observations suggest that c-Fes might have tumor suppressor activity in some epithelial cell types and that inhibition of Fes activity as an antiangiogenic therapy may contribute to the growth of some tumor types in vivo.
Renal cell carcimona is resistant to ordinary chemotherapy or radiotherapy (15). Advanced renal carcinoma is highly vascularized, and represents an excellent candidate for an antiangiogenic therapy (16,17). A recent study has shown that Fes is expressed in renal cell carcinoma tissues (18). However, the role of c-Fes in the progression of renal cell carcinoma is totally unknown. In order to consider an antiangiogenic strategy directed against Fes for the treatment of advanced renal cell carcinoma, it must first be determined how inhibition of c-Fes affects proliferation of renal carcinoma cells. Since c-Fes-selective kinase inhibitors are not currently available, we examined the effect of the downregulation of endogenous c-Fes expression using an siRNA approach or by ectopic expression of dominant-negative Fes on the proliferation of human renal carcinoma cells.
Materials and methods
Materials
Minimal essential medium (MEM), non-essential amino acids (NEAA), anti-vinculin, anti-β-actin, anti-cyclin D1, and anti-FLAG (M2) monoclonal antibodies were purchased from Sigma Chemical Company, St. Louis, MO. Rabbit antiserum that recognizes both c-Fes and c-Fer, denoted Fps-QE, was kindly provided by Dr Peter A. Greer (Queen's Cancer Research Institute, Ontario, Canada). Anti-c-Fes antibodies (N-19, H-65), anti-Akt1 monoclonal antibody, anti-extracellular signal-regulated kinase 1 (ERK1) antibody, anti-IKKα monoclonal antibody, anti-phosphoThr23 IKKα/β polyclonal antibody, anti-NFκB p65 monoclonal antibody, and anti-phosphotyrosine antibody (PY99) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antiphospho-mitogen-activated protein kinase (MAPK) polyclonal antibody, anti-phospho Akt 1 (pS473) rabbit monoclonal antibody, and anti-IκBα mouse monoclonal antibody were obtained from Cell Signaling Technology, Inc., (Beverly, MA). The NFκB SN50 cell-permeable inhibitor peptide was purchased from Calbiochem, EMD Biosciences, Inc., La Jolla, CA. HiPerFect® transfect reagent, negative control siRNA, and human c-Fes siRNA (Hs_FES_6_HP Validated siRNA; sequence not available) were purchased from Qiagen (Tokyo, Japan). ON-TARGETplus SMARTpool human c-Fes siRNA (a mixture of 4 duplex siRNA; CGAGGAUCCUGAAGCAGUAUU and 5'-PUACUGCUUCAGGAUCCUCGUU, GGUGUUGGGUGAGCAGAUUUU and 5'-PAAUCUGCUCACCCAACACCUU, GAAGAGUGGUGUUGUCCUGUU and 5'-PCAGGACAACACCACUCUUCUU, and GAAAGUGGAUGGCCCAGCGUU and 5'-PCGCUGGGCCAUCCACUUUCUU, respectively) was purchased from GE Healthcare Bio-science KK (Dharmacon RNA Technologies), Tokyo, Japan.
Cell culture
The human renal carcinoma cell lines, ACHN and VMRC-RCW (19), were cultured in MEM supplemented with NEAA and 10% heat-inactivated fetal bovine serum (FBS).
Transfection of siRNA into ACHN cells and VMRC-RCW cells
ACHN and VMRC-RCW cells were transfected with siRNAs using HiPerFect reagent as described previously (20). In brief, c-Fes and negative control siRNAs were transfected into each cell line, and cells were harvested 2–3 days later for proliferation assay and biochemical analysis.
Cell proliferation assay
Cells were suspended in MEM with NEAA containing 10% FBS and seeded into 24-well plates at a density of 1 × 104 cells/well. One day later, cells were treated with either control or c-Fes siRNA at the indicated concentrations. After 3 days, the cells were detached with trypsin and counted with the use of a hemocytometer. Untreated cells were seeded into 24-well plates at 2 × 104 cells/well and cell number was counted 3 days later.
Labeling index
Labeling indices were examined to determine numbers of cells in S-phase using the Bromodeoxyuridine (BrdU) In Situ Detection Kit® (BD Biosciences Pharmingen, San Diego, CA) as described before (21). In brief, cells treated with either control or c-Fes siRNA were seeded into wells of 48-well culture plates at a density of 2 × 104 cells/cm2. After 2 days, cells were pulse-labeled for 4 h with 10 µM of BrdU. Cells were then fixed with the fixation buffer provided in the kit, treated with 70% ethanol for 20 min, and incubated with 4 M HCl for 20 min. Cells were washed and incubated with biotinylated anti-BrdU antibody, followed by sequential incubation with streptoavidin-horseradish peroxidase and diaminobenzidine. Finally, cells were counter-stained with hematoxylin. At least 500 cells were counted for each well, and labeling indices were determined as labeled nuclei/total nuclei ratios and expressed as percentages.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously (22). In brief, cells were incubated in serum-free medium overnight. Cells were treated in the presence or absence of 10% FBS for 10 min followed by lysis in Triton X-100 lysis buffer. c-Fes and Akt1 were immunoprecipitated, separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene difluoride membranes for immunoblot analysis as indicated. To determine the expression of cyclin D1 and IκBα as well as phosphorylation of MAPK/ERK and IKKα after stimulation with 10% FBS, cells were lysed directly in SDS-sample buffer followed by SDS-PAGE, and immunoblotting. In some cases, blots were stripped and reprobed with control antibodies as described previously (7). In the indicated blots, relative phosphorylation or relative amount was calculated by band intensities determined from scanned images of the blots using NIH Image (22).
Indirect immunofluorescent staining
Indirect immunofluorescent staining was performed as described previously (23). Cells were seeded onto coverslips and cultured overnight. Cells were treated with either control or c-Fes siRNA and the culture was continued for additional 66 h. Cells were washed with PBS, fixed with 3.7% paraformaldehyde, and permeabilized with 0.05% Triton X-100. Cells were incubated with either non-immune (control) rabbit IgG or with anti-c-Fes or anti-c-Fer antibodies, followed by incubation with ant-rabbit IgG labeled with Alexa Fluor 488.
Immunocytochemical staining
Cells expressing NFκB were detected by immunocytochemical staining. Cells were seeded into 24-well culture plates (1 × 104 cells/well) in medium containing 10% FBS. The following day, cells were treated with either control or c-Fes siRNA and the culture was continued for 3 days. Cells were fixed with 3.7% glutaraldehyde solution, permeabilized with 0.05% Triton X-100 in PBS, and incubated with the anti-NFκB antibody. One hour later, cells were washed with PBS and incubated with peroxidase-conjugated anti-rabbit IgG, and NFκB p65 was visualized by using the DAB (black) Substrate Kit (Zymed Laboratories, South San Francisco, CA). At least 500 cells were counted for each well, and the percentage of NFκB-positive cells was determined as the stained cell number/total cell number × 100.
Transfection of wild-type or kinase-inactive c-Fes into ACHN cells
ACHN cells were transfected with the pcDNA3.1/Hygro vector containing FLAG-tagged wild-type or kinase-inactive (K590E) c-Fes (6) using the FuGENE 6 reagent. After 48 h, stably transfected cells were selected with hygromycin. After 2 weeks, expression of exogenous Fes was confirmed in the hygromycin-resistant cell populations by immunoprecipitation followed by immunoblotting.
Tumor growth in nude mice
Animal work was done at the Nagasaki University Graduate School of Biomedical Sciences with Institutional Animal Care and Use Committee approval in accordance with institutional guidelines. Stable ACHN cell lines expressing wild-type or kinase-inactive c-Fes as well as mock-transfeted control cells were inoculated subcutaneously into male Balb/c nu/nu mice (5 × 106 cells/injection). After 21 days, mice were sacrificed and tumor volume was calculated. In some tumors, cystic lesions were observed and the volume of the cystic fluid was deduced from the total volume of the tumor. In each mouse, one control cell line, one wild-type c-Fes cell line, and two kinase-inactive c-Fes cell lines were inoculated at distinct sites to minimize the inter-host variability.
Statistical analysis
Statistical analysis was performed using Mann-Whitney's U test. Differences were considered significant when the P-value was <0.05. Data are presented as mean ± SD.
Results and Discussion
Downregulation of c-Fes reduces proliferation of human renal carcinoma cells in culture
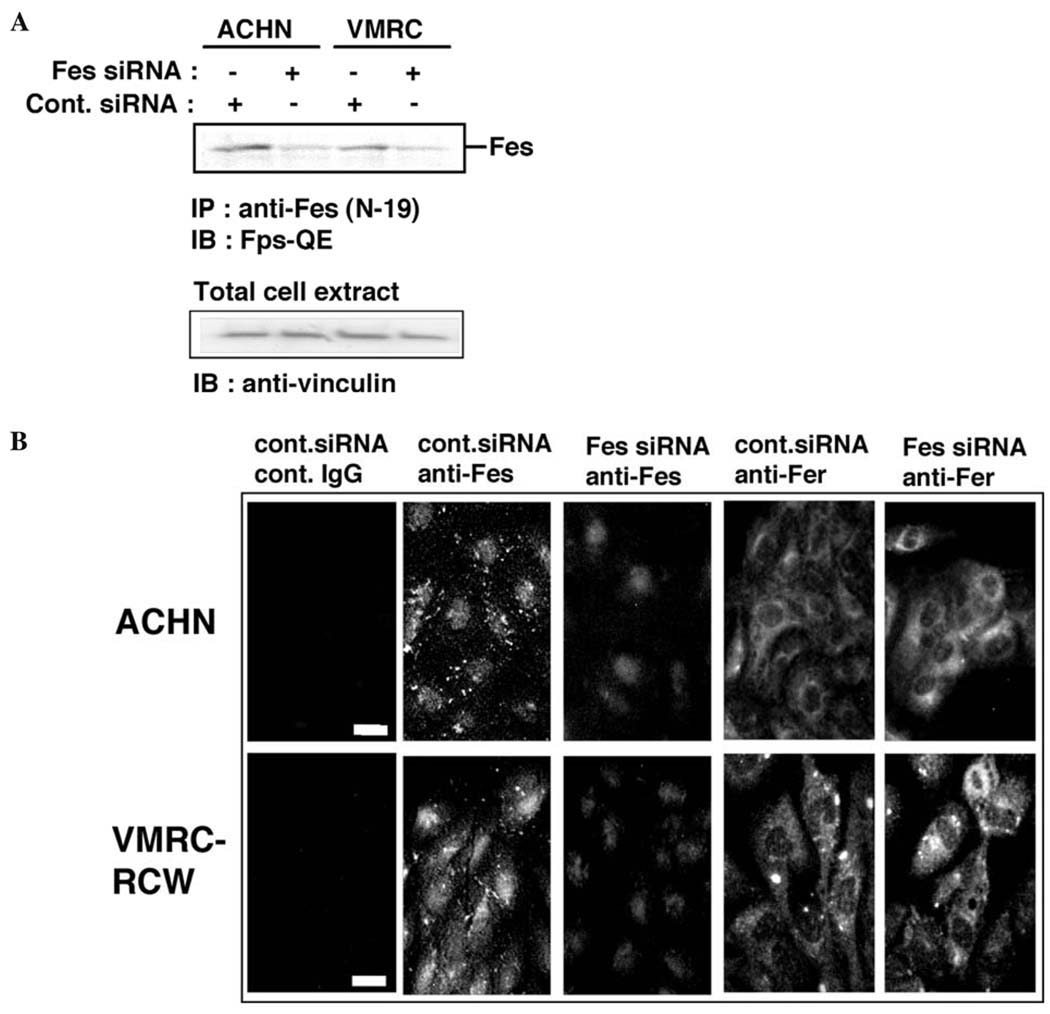
We first examined the effect of c-Fes siRNA on the expression of endogenous Fes in the human renal carcinoma cells, ACHN and VMRC-RCW. As shown in Fig. 1A, c-Fes siRNA at 20 nM efficiently downregulated overall c-Fes expression as determined by immunoblot analysis. Previous studies have established that c-Fes localizes to the cytosol and the nucleus, and localization is dependent upon the cell type and stage of the cell cycle (24–26). To examine whether c-Fes siRNA-treatment preferentially downregulates cytosolic vs. nuclear c-Fes in renal carcinoma cells, we stained the cells with an anti-c-Fes antibody following siRNA-treatment. As shown in Fig. 1B, c-Fes was localized to both the cytoplasm and nucleus of control cells, and siRNA-treatment efficiently downregulated cytoplasmic but not nuclear c-Fes. We also examined the expression pattern of c-Fer, a closely related protein tyrosine kinase. As shown in Fig. 1B, c-Fer localized mainly to the cytoplasm. Treatment with c-Fes siRNA did not alter the expression or localization of c-Fer, providing an important negative control for the c-Fes siRNA-treatment.
Figure 1.
Treatment of human renal carcinoma cells with c-Fes siRNA downregulates the expression of c-Fes protein. (A) Two human renal carcinoma cell lines, ACHN and VMRC-RCW, were treated with either negative control (Cont.) or c-Fes siRNA at 20 nM. Three days later, c-Fes was immunoprecipitated from 95% of total cell lysate and separated by SDS-PAGE. The remaining 5% of the lysate was also separated by SDS-PAGE. Proteins were visualized by immunoblotting with the indicated antibodies. Anti-vinculin signals indicate the relative protein content in each lane. Results are representative of two independent experiments with Hs_FES_6_HP Validated siRNA (Qiagen). Similar results were obtained by using ON-TARGETplus SMARTpool c-Fes siRNA (Dharmacon; not shown). (B) Cells cultured on the coverslips were either treated with negative control (Cont.) or c-Fes siRNA. Three days later, cells were fixed and stained with non-immune rabbit IgG (Cont. IgG) or indicated antibodies. Proteins were visualized by indirect immunofluorescent staining. Bar, 10 µM. Representative results are shown from three independent experiments with ON-TARGETplus SMARTpool c-Fes siRNA. Comparable results were obtained by using Hs_FES_6_HP Validated siRNA (not shown).
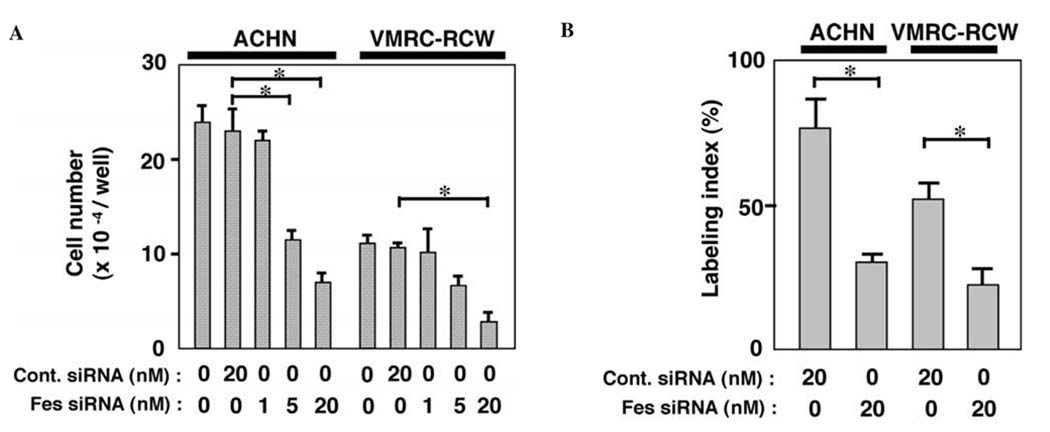
To examine whether the expression of c-Fes is involved in the proliferation of renal carcinoma cells, we treated the cells with either control or c-Fes siRNA and counted viable cells three days later. As shown in Fig. 2A, c-Fes siRNA-treatment significantly inhibited cell proliferation in a dose-dependent manner. c-Fes siRNA-treatment also decreased the number of cells in S-phase (Fig. 2B). Note that treatment with control or c-Fes siRNA did not produce a significant difference in the number of caspase-3-expressing cells (unpublished data), suggesting that c-Fes regulates the proliferation but not the survival of renal carcinoma cells. In these experiments, we observed that c-Fes siRNA obtained from Qiagen (Hs_FES_6_HP Validated siRNA) and ON-TARGETplus SMARTpool human Fes siRNA produced very similar results. Therefore, we used siRNA from Qiagen for subsequent experiments described below.
Figure 2.
(A) Treatment of human renal carcinoma cells with c-Fes siRNA inhibits the proliferation. ACHN or VMRC-RCW cells were seeded in 24-well plates and cultured in the presence of either negative control (Cont.) or c-Fes siRNA at the indicated concentrations for three days. Cells were then detached and counted. Data are expressed as means ± SD of triplicate samples. Reproducible results were obtained from four independent experiments. *P<0.05. (B) ACHN or VMRC-RCW cells were cultured for 2 days. Cells were pulse-labeled with BrdU for 4 h, and nuclei which had incorporated BrdU were visualized by immunocytochemical staining. The labeling index was determined as the labeled nuclei/total nuclei ratio × 100. Values shown are means ± SD for triplicate wells. **P<0.01. Similar results were obtained in two independent experiments.
Downregulation of Fes reduces Akt 1 phosphorylation in human renal carcinoma cells
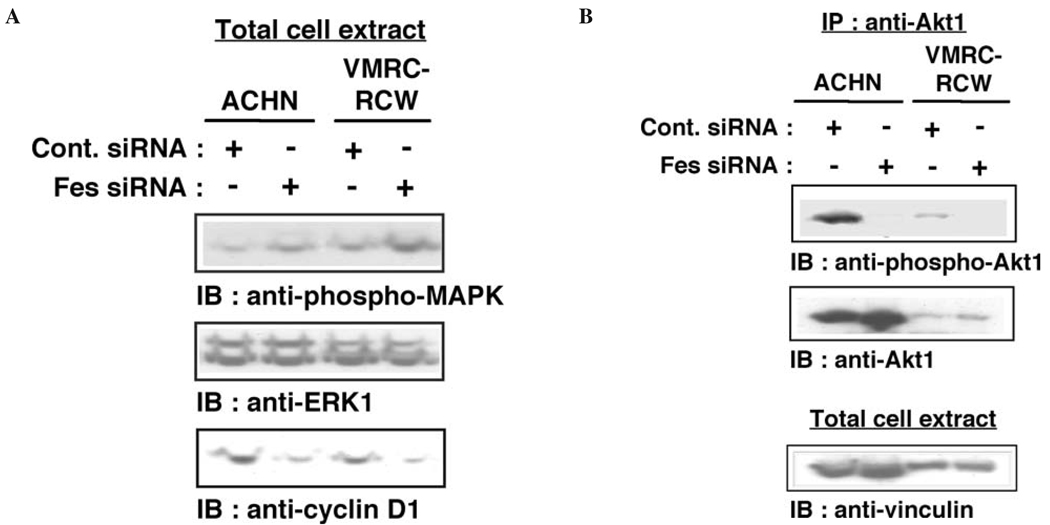
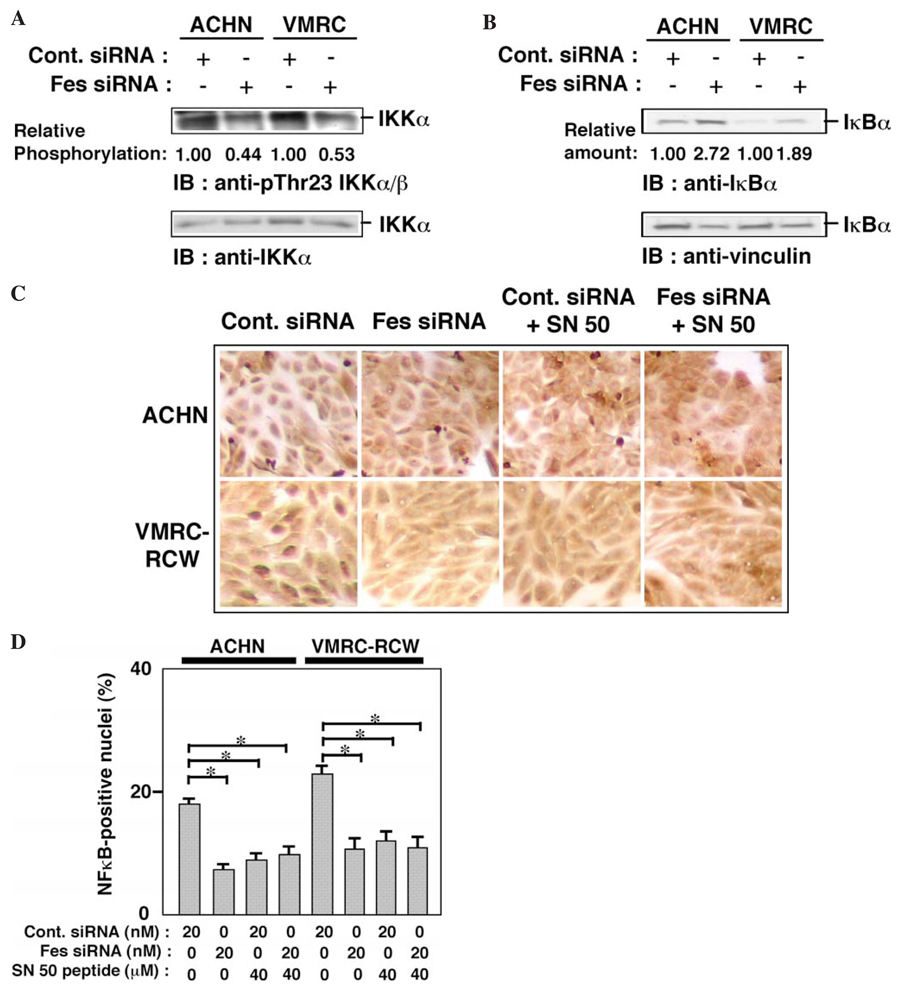
A number of proliferative signaling networks are regulated by c-Fes (1,2), including the Ras/ERK and phosphoinositide 3-kinase (PI3-kinase)/Akt pathways. We examined the effect of siRNA-mediated Fes downregulation on the status of ERK and Akt1 activation in renal carcinoma cells. As shown in Fig. 3A, activated ERK (phospho-MAPK) was not downregulated in cells treated with Fes siRNA. Expression of cyclin D1 was, however, decreased in these cells. These results suggest that treatment with Fes siRNA may inhibit cyclin D1 expression independently of ERK activation. Phosphorylation of Akt1 was downregulated by the treatment with Fes siRNA in both ACHN and VMRC-RCW cells (Fig. 3B). It has previously shown that activated Akt phosphorylates and activates IKKα (23), which induces the proteasomal degradation of IκB and permits nuclear translocation of p65 NFκB (27). Nuclear NFκB activates the transcription of cyclin D1 (28). Downregulation of Fes by siRNA may therefore decrease the expression of cyclin D1 through an NFκB-dependent pathway, thus inhibiting the proliferation of renal carcinoma cells. To test this idea, we examined the phosphorylation of IKKα and found that Thr-23 phosphorylation was reduced in both ACHN and VMRC-RCW cells treated with Fes siRNA (Fig. 4A). We also examined the amount of IκB in siRNA-treated cells. As shown in Fig. 4B, Fes siRNA-treatment upregulated the level of IκB, consistent with this hypothesis.
Figure 3.
Treatment of renal carcinoma cells with c-Fes siRNA reduces cyclin D1 expression and Akt1 phosphorylation. (A) Cells were treated with either negative control (Cont.) or Fes siRNA for three days. Cells were lysed in SDS-sample buffer and proteins were separated by SDS-PAGE. Proteins were visualized by immunoblotting with the indicated antibodies. Representative results are shown from three independent experiments. (B) Akt1 was immunoprecipitated from cells treated with either negative control (Cont.) or c-Fes siRNA for three days. Five percent of the total cellular lysate was used for a loading control (vinculin). Proteins were visualized by immunoblotting with the indicated antibodies. Representative results are shown from two independent experiments.
Figure 4.
Treatment of renal carcinoma cells with c-Fes siRNA reduces IKKα phosphorylation, IκBα expression, and nuclear localization of NFκB. (A) IKKα was immunoprecipitated from cells treated with either negative control (Cont.) or c-Fes siRNA for three days. Proteins were visualized by immunoblotting with the indicated antibodies. Relative phosphorylation was expressed as a ratio of band intensities of phospho-Thr23 IKKα/β/IKKα. Representative results are shown from two independent experiments. (B) Cells were treated with either negative control (Cont.) or c-Fes siRNA for three days. Cells were lysed in SDS-sample buffer and proteins were separated by SDSPAGE. Proteins were visualized by immunoblotting with the indicated antibodies. Relative amount was expressed as a ratio of band intensities of IκBα/vinculin. Representative results are shown from two independent experiments. (C). Cells were seeded onto coverslips and cultured in the presence of either negative control (Cont.) or c-Fes siRNA at indicated concentrations for three days. SN50 peptide was added to the indicated cells on the first and the second day of the culture. (D) Cells positive for nuclear NFκB are expressed as percentages of total cell counts. P<0.05. Representative results are shown from two independent experiments.
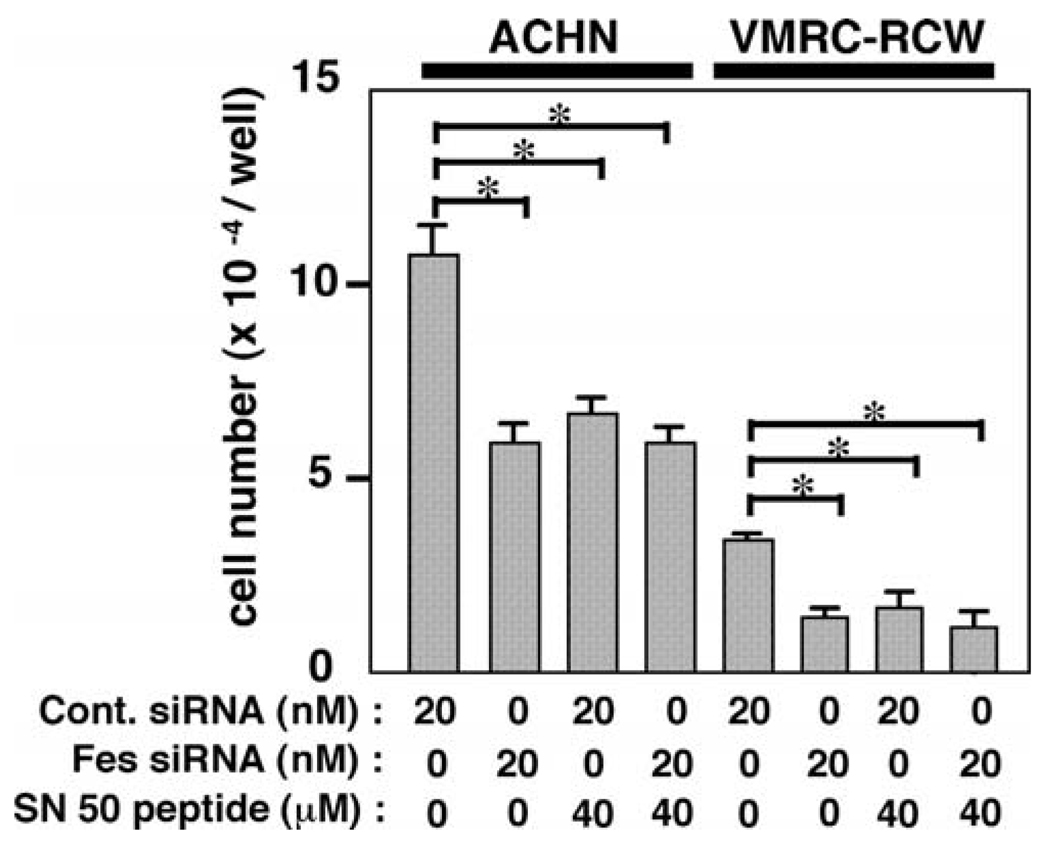
We next examined the effect of SN50, a cell-penetrating peptide, known to inhibit the nuclear translocation of NFκB (29). As shown in Fig. 4C and D, treatment with the SN50 peptide inhibited NFκB nuclear localization as effectively as the Fes siRNA. However, when cells were treated with both Fes siRNA and SN50 peptide, no additive or synergistic effect on the inhibition of NFκB nuclear localization was observed, suggesting that both SN50 and Fes siRNA work via the same pathway. We next examined the effect of SN50 peptide-treatment on cell proliferation of ACHN and VMRC-RCW cells. As shown in Fig. 5, treatment with the SN50 peptide also inhibited cell proliferation, which was not further inhibited by Fes siRNA-treatment. Thus, it seems likely that the treatment with Fes siRNA inhibits renal carcinoma cellular proliferation through inhibition of nuclear localization of NFκB.
Figure 5.
SN50 peptide does not further inhibit proliferation of renal carcinoma cells treated with Fes siRNA. Cells were seeded onto coverslips and cultured in the presence of either negative control (Cont.) or c-Fes siRNA at indicated concentrations for three days. SN50 peptide was added to the cells as indicated on the first and the second day of culture. Cells were then detached and were counted. Data are expressed as means ± SD of triplicate samples. Reproducible results were obtained from four independent experiments. *P<0.05.
Expresssion of either wild-type or kinase-inactive Fes in renal carcinoma cells does not significantly affect their proliferation in vitro or ability to form tumors in nude mice
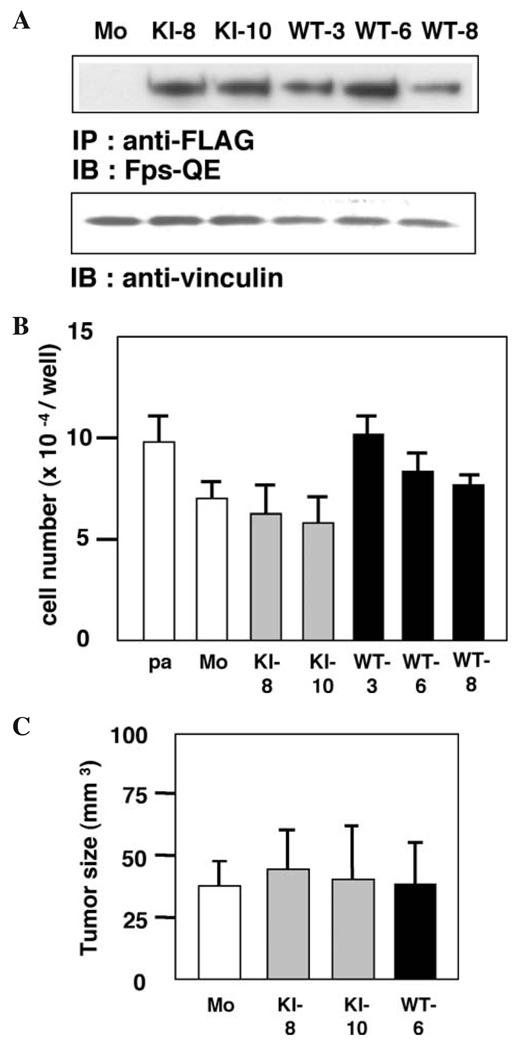
We next established stable ACHN cell lines expressing either wild-type or kinase-inactive c-Fes. As shown in Fig. 6A, two cell populations were derived that express kinase-inactive c-Fes (denoted KE-8 and KE-10 cells, respectively) and three cell populations were derived that express wild-type c-Fes (denoted WT-3, WT-6, and WT-8 cells, respectively). We examined the proliferation of these cells in culture. As shown in Fig. 6B, no significant difference in proliferation was observed between cells expressing wild-type or kinase-inactive c-Fes vs. control cells. We also examined the growth of these cells following xenografting into nude mice. As shown in Fig. 6C, control, WT-6, KE-8, and KE-10 tumors grew to a similar size, suggesting that expression of kinase-inactive c-Fes does not influence the proliferation of renal carcinoma cells in vivo via the dominant-negative effect on kinase activity observed in other cell types (5).
Figure 6.
Expression of wild-type or kinase-inactive c-Fes does not affect ACHN cell growth in culture or tumor formation in nude mice. (A) Confirmation of exogenous c-Fes expression in stably transfected ACHN cells. FLAG-tagged wild-type (WT) or kinase-inactive (KI; K590E substitution) c-Fes was immunoprecipitated from the 95% of the indicated cells lysates. The remaining 5% of the lysate was used for a loading control. Proteins were visualized by immunoblotting with the indicated antibodies. Representative results are shown from three independent experiments. (B) Cells were seeded in 24-well plates and cultured for three days. Cells were detached and were counted. Data are expressed as means ± SD of triplicate samples. Reproducible results were obtained from two independent experiments. (C) Cells were inoculated subcutaneously in the back of Balb/c nu/nu mice (n=4). After 21 days, tumors were excised and measured. Data are expressed as means ± SD of four tumors.
In this study, we provide evidence that siRNA-mediated downregulation of the c-Fes protein results in anti-proliferative effects in renal carcinoma cell lines. These data provide some of the first evidence that c-Fes may have a pro-tumorigenic role in a renal cell carcinoma context. Consistent with this finding is the very recent observation that c-Fes may mediate the proliferation of leukemia cells driven by the KIT D816V mutant (30). In contrast, other data have suggested that Fes may contribute to terminal differentiation, as in the case of K562 chronic myelogenous leukemia cells (31). c-Fes may also serve a tumor suppressor function in the context of other epithelial cancers, including a polyoma middle-T antigen-driven model of breast carcinogenesis (4) and the anchorage-independent growth of colorectal carcinoma cell lines (5).
Interestingly, expression of kinase-inactive c-Fes neither stimulated nor inhibited proliferation of renal carcinoma cells. These results are in contrast to the siRNA knockdown results, and suggest that c-Fes may serve a kinase-independent scaffold function essential for the proliferation of renal carcinoma cells. Intriguingly, a kinase-independent function for the C. elegans ortholog of the c-Fes relative c-Fer has also been described in the context of embryonic morphogenesis (32). Thus, clinical translation of these findings may require a strategy that downregulates c-Fes protein expression rather than simply inhibiting its kinase function. Our findings also suggest that inhibition of c-Fes kinase activity with small molecular weight kinase inhibitors may still represent a viable antiangiogenic strategy for advanced renal cell carcinoma, since blocking kinase activity with the dominant negative c-Fes mutant does not appear to impact renal tumor growth directly. As such inhibitors are developed, data presented here indicate that it will be essential to determine the relationship between c-Fes expression and activity in a wide variety of human tumor types. Suppression of kinase activity may provide benefit in advanced renal cell carcinoma and other tumor contexts but may interfere with c-Fes tumor suppressor function in others, including CML as well as breast and colon cancers. Development of c-Fes-selective inhibitors will be of tremendous value in teasing out the diverse roles of c-Fes in different tissue types.
Acknowledgments
We are grateful to Dr Peter A. Greer (Queen's University, Kingston, Ontario, Canada) for the kind gift of Fps-QE antibody. We thank Mr. T. Shimogama for his excellent and outstanding help. This work was partly supported by Grants-in-Aid from Japan Society for the promotion of Science, 15591698 and 17591686 (S.K.), 17791080 (Y.M.) and NIH grant CA123756 (T.E.S.).
References
- 1.Smithgall TE, Rogers JA, Peters KL, Li J, Briggs SD, Lionberger JM, Cheng H, Shibata A, Scholtz B, Schreiner S, Dunham N. The c-Fes family of protein-tyrosine kinases. Crit Rev Oncog. 1998;9:43–62. doi: 10.1615/critrevoncog.v9.i1.40. [DOI] [PubMed] [Google Scholar]
- 2.Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol. 2002;3:278–289. doi: 10.1038/nrm783. [DOI] [PubMed] [Google Scholar]
- 3.Haigh J, McVeigh J, Greer P. The fps/fes tyrosine kinase is expressed in myeloid, vascular endothelial, epithelial, and neuronal cells and is localized in the trans-Golgi network. Cell Growth Differ. 1996;7:931–944. [PubMed] [Google Scholar]
- 4.Sangrar W, Zirgnibl RA, Gao Y, Muller WJ, Jia Z, Greer PA. An identity crisis for fps/fes: oncogene or tumor suppressor? Cancer Res. 2005;65:3518–3522. doi: 10.1158/0008-5472.CAN-04-3468. [DOI] [PubMed] [Google Scholar]
- 5.Delfino FJ, Stevenson H, Smithgall TE. A growth-suppressive function for the c-fes protein-tyrosine kinase in colorectal cancer. J Biol Chem. 2006;281:8829–8835. doi: 10.1074/jbc.M507331200. [DOI] [PubMed] [Google Scholar]
- 6.Rogers JA, Read RD, Li J, Peters KL, Smithgall TE. Autophosphorylation of the Fes tyrosine kinase. Evidence for an intermolecular mechanism involving two kinase domain tyrosine residues. J Biol Chem. 1996;271:17519–17525. doi: 10.1074/jbc.271.29.17519. [DOI] [PubMed] [Google Scholar]
- 7.Kanda S, Lerner EC, Tsuda S, Shono T, Kanetake H, Smithgall TE. The non-receptor protein-tyrosine kinase c-Fes is involved in fibroblast growth factor-2-induced chemotaxis of murine brain capillary endothelial cells. J Biol Chem. 2000;275:10105–10111. doi: 10.1074/jbc.275.14.10105. [DOI] [PubMed] [Google Scholar]
- 8.Kanda S, Mochizuki Y, Miyata Y, Kanetake H. The role of c-Fes in vascular endothelial growth factor-A-mediated signaling by endothelial cells. Biochem Biophys Res Commun. 2003;306:1056–1063. doi: 10.1016/s0006-291x(03)01106-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanda S, Miyata Y, Kanetake H, Smithgall TE. Non-receptor protein-tyrosine kinases as molecular targets for antiangiogenic therapy. Int J Mol Med. 2007;20:113–121. [PubMed] [Google Scholar]
- 10.Kanda S, Kanetake H, Miyata Y. Downregulation of Fes inhibits VEGF-A-induced chemotaxis and capillary-like morphogenesis by cultured endothelial cells. J Cell Mol Med. 2007;11:495–501. doi: 10.1111/j.1582-4934.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greer P, Haigh J, Mbamalu G, Khoo W, Bernstein A, Pawson T. The Fps/Fes protein-tyrosine kinase promotes angiogenesis in transgenic mice. Mol Cell Biol. 1994;14:6755–6763. doi: 10.1128/mcb.14.10.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng H, Rogers JA, Dunham NA, Smithgall TE. Regulation of c-Fes tyrosine kinase and biological activities by N-terminal coiled-coil oligomerization domains. Mol Cell Biol. 1999;19:8335–8343. doi: 10.1128/mcb.19.12.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HY, Schiavone AP, Smithgall TE. A point mutation in the N-terminal coiled-coil domain releases c-Fes tyrosine kinase activity and survival signaling in myeloid leukemia cells. Mol Cell Biol. 2001;21:6170–6180. doi: 10.1128/MCB.21.18.6170-6180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, Markowitz S, Willson JK, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. Mutational analysis of the tyrosine kinase in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 15.Hutson TE, Figlin RA. Renal cell cancer. Cancer J. 2007;13:282–286. doi: 10.1097/PPO.0b013e318156fe69. [DOI] [PubMed] [Google Scholar]
- 16.Kanda S, Miyata Y, Kanetake H. Current status and perspective of antiangiogenic therapy for cancer: urinary cancer. Int J Clin Oncol. 2006;11:90–107. doi: 10.1007/s10147-006-0565-6. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 18.Karthaus HF, Schalken JA, Feitz WF, Debruyne FM, De Haan PT, Bloemers HP, van de Ven WJ. Expression of the human fes cellular oncogene in renal cell tumors. Urol Res. 1986;14:123–127. doi: 10.1007/BF00255829. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Kanda S, Yamamoto K, Kohno T, Maeda K, Matsuyama T, Kanetake H. Increase in hepatocyte growth factor receptor tyrosine kinase activity in renal carcinoma cells is associated with increased motility through partly phosphoinositide 3-kinase. Oncogene. 2001;20:7610–7623. doi: 10.1038/sj.onc.1204975. [DOI] [PubMed] [Google Scholar]
- 20.Kanda S, Kanetake H, Miyata Y. Elevated expression of ERK 2 in human tumor cells chronically treated with PD98059. Biochem Biophys Res Commun. 2006;345:1481–1486. doi: 10.1016/j.bbrc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 21.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65:6820–6827. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 22.Kanda S, Miyata Y, Kanetake H, Smithgall TE. Fibroblast growth factor-2 induces the activation of Src through Fes, which regulates focal adhesion disassembly. Exp Cell Res. 2006;312:3015–3022. doi: 10.1016/j.yexcr.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–88. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 24.Tagliafico E, Siena M, Zanocco-Marani T, Manfredini R, Tenedini E, Montanari M, Grande A, Ferrari S. Requirement of the coiled-coil domains of p92c-Fes for nuclear localization in myeloid cells upon induction of differentiation. Oncogene. 2003;22:1712–1723. doi: 10.1038/sj.onc.1206279. [DOI] [PubMed] [Google Scholar]
- 25.Zirngibl R, Schulze D, Mirski SE, Cole SP, Greer PA. Subcellular localization analysis of the closely related Fps/Fes and Fer protein-tyrosine kinases suggests a distinct role for Fps/Fes in vesicular trafficking. Exp Cell Res. 2003;266:87–94. doi: 10.1006/excr.2001.5217. [DOI] [PubMed] [Google Scholar]
- 26.Carlson A, Yates KE, Slamon DJ, Gasson JC. Spatial and temporal changes in the subcellular localization of the nuclear protein tyrosine linase, c-Fes. DNA Cell Biol. 2005;24:225–234. doi: 10.1089/dna.2005.24.225. [DOI] [PubMed] [Google Scholar]
- 27.Mercurio F, Manning AM. Multiple signals converging on NF-κB. Curr Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 28.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NK-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 30.Voisset E, Lopez S, Dubreuil P, De Sepulveda P. The tyrosine kinase FES is an essential effecter of KITD816V proliferation signal. Blood. 2007;110:2593–2499. doi: 10.1182/blood-2007-02-076471. [DOI] [PubMed] [Google Scholar]
- 31.Lionberger JM, Smithgall TE. The c-Fes protein-tyrosine kinase suppresses cytokine-independent outgrowth of myeloid leukemia cells induced by Bcr-Abl. Cancer Res. 2000;60:1097–1103. [PubMed] [Google Scholar]
- 32.Putzke AP, Hikita ST, Clegg DO, Rothman JH. Esential kinase-independent role of a Fer-like non-receptor tyrosine kinase in Caenorhabditis elegans morphogenesis. Development. 2005;132:3185–3195. doi: 10.1242/dev.01900. [DOI] [PubMed] [Google Scholar]