Abstract
We examined seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus (family Flaviviridae, genus Flavivirus, WNV) in relation to human WNV disease cases in a five-county area of northeastern Colorado during 2006–2007. Studies along habitat/elevation gradients in 2006 showed that the seasonal activity period is shortened and peak numbers occur later in the summer for Culex tarsalis Coquillett females in foothills-montane areas >1,600 m compared with plains areas <1,600 m in Colorado’s Front Range. Studies in the plains of northeastern Colorado in 2007 showed that seasonal patterns of abundance for Cx. tarsalis and Culex pipiens L. females differed in that Cx. tarsalis reached peak abundance in early July (mean of 328.9 females per trap night for 18 plains sites), whereas the peak for Cx. pipiens did not occur until late August (mean of 16.4 females per trap night). During June–September in 2007, which was a year of intense WNV activity in Colorado with 578 reported WNV disease cases, we recorded WNV-infected Cx. tarsalis females from 16 of 18 sites in the plains. WNV infection rates in Cx. tarsalis females increased gradually from late June to peak in mid-August (overall maximum likelihood estimate for WNV infection rate of 8.29 per 1,000 females for the plains sites in mid-August). No WNV-infected Culex mosquitoes were recorded from sites >1,600 m. The vector index for abundance of WNV-infected Cx. tarsalis females for the plains sites combined exceeded 0.50 from mid-July to mid-August, with at least one site exceeding 1.00 from early July to late August. Finally, we found that abundance of Cx. tarsalis females and the vector index for infected females were strongly associated with weekly numbers of WNV disease cases with onset 4–7 wk later (female abundance) or 1–2 wk later (vector index).
Keywords: Culex pipiens, Culex tarsalis, Colorado, seasonal risk, West Nile virus
Colorado experienced a dramatic West Nile virus (family Flaviviridae, genus Flavivirus, WNV) disease outbreak in 2003 with 2,947 reported cases of human disease and a smaller outbreak in 2007 with 578 reported cases (http://www.cdphe.state.co.us/dc/zoonosis/wnv/). This included two major WNV disease foci: 1) the northern Front Range and northeastern plains and 2) the Grand Junction area in the western part of the state. Knowledge of seasonal patterns of activity for mosquito vectors and WNV is critical for both effective implementation of mosquito control measures and to advise the public regarding critical time periods when use of personal protection measures, such as repellents, should be emphasized. Previous studies on seasonal patterns of risk for exposure to Culex vectors in eastern Colorado were focused narrowly on the Fort Collins–Loveland area and showed that abundance of Culex vectors peaked during July–August (Smith et al. 1993, Bolling et al. 2007). The study by Bolling et al. (2007) also reported on WNV infection rates in various mosquito species but did not specifically present data for seasonal patterns of WNV infection rates. Recently, Kent et al. (2009) reported an increase in WNV infection rates in Culex tarsalis Coquillett from June to August in 2007 in Weld County in the northeastern Colorado plains. A similar pattern with increasing WNV infection rates over the summer has been recorded for this species also in other western states (Bell et al. 2005; DiMenna et al. 2006; Nielsen et al. 2008; Reisen et al. 2008a, 2009). However, studies combining mosquito abundance and WNV infection rate to generate a more comprehensive measure of entomological risk of exposure to WNV, such as the vector index for abundance of WNV-infected mosquitoes, have been scarce (Bell et al. 2005, Gujral et al. 2007).
This study focused on a five-county area in northeastern Colorado and aimed to determine seasonal patterns for 1) abundance of the primary WNV vectors, Culex pipiens L. and Cx. tarsalis, and the nuisance-biter and potential secondary WNV vector Aedes vexans (Meigen); 2) WNV infection rates in Cx. pipiens and Cx. tarsalis females; and 3) the vector index for abundance of WNV-infected Cx. tarsalis females. In addition, we determined whether these entomological risk measures for Cx. tarsalis were associated with the seasonal occurrence of human WNV disease cases.
Materials and Methods
Study Area
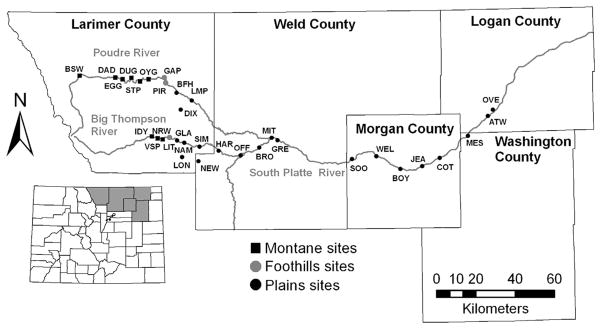
The study area in northeastern Colorado includes the western edge of the Great Plains and the eastern edge of the Rocky Mountains. The climate in this area is characterized by cold winters and hot summers with low humidity. The average annual rainfall in Fort Collins in Larimer County from 1971 to 2000 was 393 mm (Mountain States Weather Services, Fort Collins, CO). Mosquito sampling was conducted along two rivers that emerge from the Rocky Mountains in western Larimer County (Poudre River and Big Thompson River) and then flow into the prairie landscapes characteristic of eastern Colorado (Fig. 1). Both rivers merge into the South Platte River in Weld County (Fig. 1). In the plains, these rivers typically are bordered by a narrow band of forested riparian wetland, dominated by cottonwood (Populus spp.) and willow (Salix spp.) that, in turn, is commonly surrounded by irrigated agricultural land. In the foothills and low montane habitats, the rivers flow through a canyon landscape dominated by grass, shrub, conifers (primarily Ponderosa pine, Pinus ponderosa Dougl. ex Laws.), and aspen (Populus tremuloides Michx.). Mosquito sampling sites were selected within the relatively uniform riparian corridor at sites which could be accessed by automobile. Locations were mapped with a GPS receiver (Trimble Geo XT, Trimble Corp., Sunnyvale, CA) and visualized using ArcGIS 9.3 (ESRI, Redlands, CA). Selected environmental site characteristics are provided in Table 1. Latitude and longitude for sampling sites are included to facilitate future studies to determine if seasonal patterns were affected by climate change.
Fig. 1.
Location of mosquito sampling sites for 2006 (along the Poudre River) and 2007 (along the Big Thompson River and South Platte River). The location of the targeted five-county area in Colorado is shown in the inset map.
Table 1.
Characteristics of mosquito collection sites
| Site coordinatesa |
Mean June–Aug. temp. (°C)b | |||||
|---|---|---|---|---|---|---|
| Site | Habitat | Latitude (N) | Longitude (W) | Elevation (m) | Elevation category (m) | |
| Poudre River sampling transect (2006) | ||||||
| LMP | Plains | 40.59456333 | −105.07842459 | 1,510 | 1,501–1,600 | 20.5 |
| BFH | Plains | 40.63046744 | −105.16906742 | 1,560 | 1,501–1,600 | 19.6 |
| DIXc | Plains | 40.55414868 | −105.14104913 | 1,585 | 1,501–1,600 | 19.8 |
| PIR | Foothills | 40.67531706 | −105.23651783 | 1,610 | 1,601–1,750 | 18.1 |
| GAP | Foothills | 40.70004260 | −105.24424789 | 1,640 | 1,601–1,750 | 18.2 |
| OYG | Montane | 40.69214711 | −105.33747484 | 1,750 | 1,601–1,750 | 16.6 |
| STP | Montane | 40.68193007 | −105.38909379 | 1,860 | >1,750 | 15.9 |
| DUG | Montane | 40.69854287 | −105.44131068 | 2,000 | >1,750 | 15.5 |
| EGG | Montane | 40.69110602 | −105.49463206 | 2,110 | >1,750 | 15.0 |
| DAD | Montane | 40.69959335 | −105.53861231 | 2,130 | >1,750 | 14.5 |
| BSW | Montane | 40.70755489 | −105.75274554 | 2,360 | >1,750 | 11.5 |
| Big Thompson River-South Platte River sampling transect (2007) | ||||||
| OVE | Plains | 40.53981478 | −103.26729278 | 1,215 | 1,201–1,300 | 22.1 |
| ATW | Plains | 40.51132441 | −103.29860284 | 1,219 | 1,201–1,300 | 22.1 |
| MES | Plains | 40.42114686 | −103.42058665 | 1,242 | 1,201–1,300 | 22.0 |
| COT | Plains | 40.32261249 | −103.59151936 | 1,272 | 1,201–1,300 | 22.2 |
| JEA | Plains | 40.28480442 | −103.69612552 | 1,286 | 1,201–1,300 | 22.3 |
| BOY | Plains | 40.27350806 | −103.82703510 | 1,303 | 1,301–1,400 | 22.2 |
| WEL | Plains | 40.33373196 | −103.97041874 | 1,321 | 1,301–1,400 | 21.9 |
| SOO | Plains | 40.32223738 | −104.11763996 | 1,344 | 1,301–1,400 | 21.8 |
| GRE | Plains | 40.41074220 | −104.56347789 | 1,397 | 1,301–1,400 | 21.8 |
| MIT | Plains | 40.42255709 | −104.59813692 | 1,402 | 1,401–1,500 | 21.8 |
| BRO | Plains | 40.37795104 | −104.67287258 | 1,418 | 1,401–1,500 | 21.8 |
| OFF | Plains | 40.34138892 | −104.78297469 | 1,434 | 1,401–1,500 | 21.5 |
| HAR | Plains | 40.36346382 | −104.91709610 | 1,459 | 1,401–1,500 | 21.3 |
| SIM | Plains | 40.38336774 | −105.03109402 | 1,487 | 1,401–1,500 | 20.9 |
| NEWd | Plains | 40.31557104 | −105.03709367 | 1,511 | 1,501–1,600 | 20.9 |
| NAM | Plains | 40.40073803 | −105.12296546 | 1,524 | 1,501–1,600 | 20.6 |
| GLA | Plains | 40.41089097 | −105.16606378 | 1,544 | 1,501–1,600 | 20.4 |
| LONe | Plains | 40.33409016 | −105.13580524 | 1,566 | 1,501–1,600 | 20.6 |
| LIT | Foothills | 40.42509226 | −105.21171030 | 1,598 | 1,501–1,600 | 19.9 |
| NRW | Montane | 40.41515686 | −105.25146509 | 1,688 | 1,601–1,750 | 19.2 |
| VSP | Montane | 40.42004747 | −105.28132071 | 1,737 | 1,601–1,750 | 18.6 |
| IDY | Montane | 40.42918494 | −105.31700388 | 1,840 | >1,750 | 17.3 |
Site locations were determined with a GPS receiver.
Mean values for 1961–1990 were based on GIS-derived data from Climate Source LLC, Corvallis, OR (2- by 2-km spatial resolution).
Located by Dixon Reservoir, ≈7 km south of the Poudre River.
Located by Newell Lake, ≈8 km south of the Big Thompson River.
Located by Lonetree Reservoir ≈7 km south of the Big Thompson River.
Mosquito Collection and Identification
Mosquitoes were collected using CO2-baited Centers for Disease Control and Prevention (CDC) miniature light traps (John W. Hock Company, Gainesville, FL) that were suspended ≈1.5 m above the ground and operated from afternoon (1500 –1700 hours) until morning (0800 –1000 hours). Sampling sites contained two traps baited with ≈1 kg of dry ice and were located directly along the aforementioned rivers. Sampling in 2006 included 10 sites located along the Poudre River and one site by the Dixon Reservoir in Fort Collins. This spanned an elevation gradient from <1,600 m in Fort Collins up to 2,360 m in the Poudre Canyon (Fig. 1). The sites were sampled every 2 wk from mid-April to late October 2006. Sampling in 2007 included 20 sites along the Big Thompson and South Platte rivers and two additional sites located south of the Big Thompson River in the Loveland area. This included an elevation gradient ranging from 1,215 m in the prairie landscape of eastern Colorado to 1,840 m in the montane habitat of the Big Thompson Canyon (Fig. 1). These sites were sampled every 2 wk from mid-June to mid-September 2007. Collected mosquitoes were examined with a dissecting microscope and identified to species by using published keys (Harmston and Lawson 1967, Darsie and Ward 2005). Taxonomic nomenclature for Aedini genera follows Reinert et al. (2004).
Detection of West Nile Virus in Culex Mosquitoes
Culex mosquitoes were examined for presence of WNV RNA following Bolling et al. (2007), with the modifications outlined below. Mosquitoes were identified on a chill table and placed in pools of 1–50 by species, sex, site, trap, and date. Mosquito pools were then stored at −70°C until processed for viral RNA detection.
Each pool was triturated for 45 s with a vortex mixer in a 5-ml round-bottomed polypropylene tube (Becton Dickinson, Franklin Lakes, NJ) by using 1.5 ml of diluent (1× minimal essential medium containing 2% fetal bovine serum, 100 μg/ml penicillin/streptomycin, supplemented with L-glutamine and nonessential amino acids) and four copper-coated steel shot (4.5 mm in diameter; 0.177 caliber). Suspensions were then centrifuged at 3,000 rpm for 10 min at 4°C. Total RNA was extracted from 140 μl of the supernatant by using the QIAamp viral RNA Mini kit (QIAGEN, Valencia, CA). RNA was then eluted in 60 μl of nuclease-free water (Ambion, Austin, TX). Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect viral RNA in the samples. Mosquito pools were first tested using universal flavivirus primers targeting a portion of the NS5 gene (forward MAMD, 5′-AACATGATGGGRAARAGRGARAA-3′; and reverse cFD2, 5′-GTGTCCCAGCCGGCGGTGTCATCAGC-3′) (Scaramozzino et al. 2001). Pools testing positive for flavivirus RNA were then tested for WNV by using primers developed and recommended by the CDC for use in WNV surveillance (forward WN212, 5′-TTGTGTTGGCTCTCTTGGCGTTCTT-3′; and reverse WN619c, 5′-CAGCCGACAGCACTGGACATTCATA-3′) (Gubler et al. 2000, Lanciotti et al. 2000). PCR products were visualized after electrophoresis on a 1% agarose gel stained with ethidium bromide. Negative (no template) and positive controls were included in each RT-PCR run.
Infection rates per 1,000 individuals were calculated as bias-corrected maximum likelihood estimates (MLEs) by using the Excel Add-In PooledInfRate, version 3.0 (Biggerstaff 2006).
Epidemiological Data
Data for 219 WNV disease cases reported in 2007 from the targeted five-county area (Larimer, Weld, Morgan, Washington, and Logan) were provided by the Colorado Department of Public Health and Environment. This included date of onset for each case, located by county, zip code, and census tract of residence but did not include any personal identifiers.
Presentation and Analysis of Data
Presented data for mosquito abundance in 2006 are restricted to the WNV vector Cx. tarsalis and the nuisance biter Ae. vexans because the other locally important WNV vector, Cx. pipiens, was not collected in sufficient numbers for meaningful inclusion in the presentation. To simplify the presentation, seasonal patterns for mosquito abundance and temperature were aggregated into three elevation classes: 1,501–1,600, 1,601–1,750, and >1,750 m (sites falling into each of these categories are shown in Table 1). Mosquito abundance data from 2006 are shown together with mean weekly temperatures determined using HOBO H8 Pro series loggers (Onset Computer Corporation, Pocasset, MA). Seasonal data for mosquito abundance in 2007, which did not span the entire active season, are presented in the context of comparison with WNV infection rates in mosquitoes and human WNV disease cases, and therefore are restricted to the primary WNV vectors Cx. tarsalis and Cx. pipiens.
Presented data for WNV infection in mosquitoes for 2006–2007 are restricted to Cx. tarsalis and Cx. pipiens females. We also present a series of seasonal data for June–September 2007 (aggregated data for the 18 sites in the plains and excluding the foothills-montane sites [LIT, NRW, VSP, and IDY], which yielded very few Cx. tarsalis), including abundance of Cx. tarsalis females (weekly means per trap night), infection rate with WNV per 1,000 Cx. tarsalis females (weekly maximum likelihood estimate for infection rate), and the vector index (Gujral et al. 2007) for abundance of WNV-infected Cx. tarsalis females (weekly mean per trap night × weekly proportion of WNV-infected females).
Statistical tests used are indicated in the text. All statistical analyses were carried out using the JMP 7.0.1 statistical package (SAS Institute, Cary, NC), and results were considered significant when P < 0.05.
Results
Seasonal Patterns of Mosquito Abundance Along Elevation Gradients in the Colorado Front Range Area: 2006
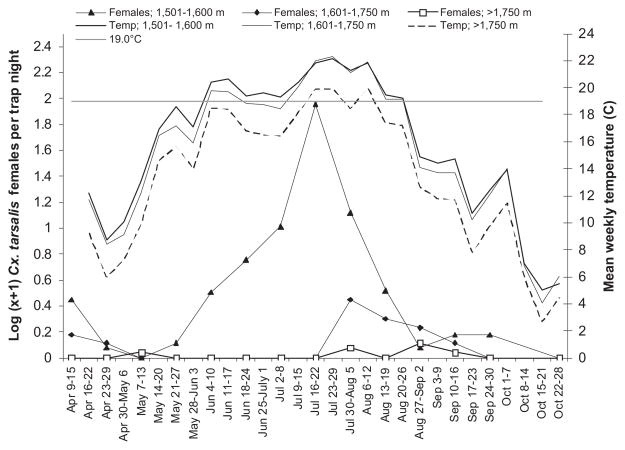
Examination of seasonal abundance patterns for the WNV vector Cx. tarsalis along an elevation gradient extending from plains to montane habitats in the Colorado Front Range area from April to October 2006 showed that 1) peak abundance of females was greater <1,600 m compared with 1,601–1,750 m or >1,750 m; 2) females were collected over a longer time period <1,600 m; and 3) peak abundance occurred earlier <1,600 m (Fig. 2). Cx. tarsalis females were first recorded during mid-April (on the first sampling occasion of the year) for sites located <1,600 m and between 1,601 and 1,750 m; these mosquitoes probably represented overwintered females that emerged and sought blood meals during warm spring weather (Fig. 2). The earliest collection of Cx. tarsalis females from sites >1,750 m occurred in early May.
Fig. 2.
Seasonal patterns of abundance of Cx. tarsalis females in relation to mean weekly temperature by elevation category (1,501–1,600; 1,601–1,750; and >1,750 m) along the Poudre River, April–October 2006.
Below 1,600 m, Cx. tarsalis females were collected on each sampling occasion from late May to late September, with a distinct peak in mid-July. In contrast, not a single Cx. tarsalis female was collected from late May to mid-July between 1,601 and 1,750 m or >1,750 m. At these higher elevations, peak abundances occurred in early August (1,601–1,750 m) or late August (>1,750 m). The period with consecutive collections of Cx. tarsalis females extended from late July to mid-September for the sites between 1,601 and 1,750 m. As illustrated in Fig. 2, Cx. tarsalis abundance increased rapidly when weekly mean air temperatures consistently exceeded 18.5–19.5°C, occurring in late May at elevations <1,600 m and in mid-July at higher elevations.
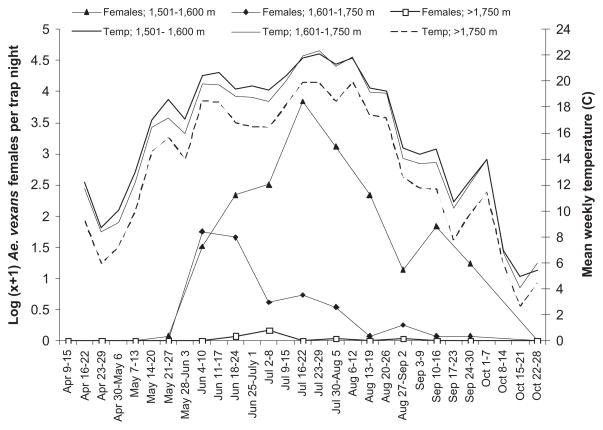
The seasonal abundance patterns for Ae. vexans were similar to Cx. tarsalis <1,600 m, with consistent collections of females from late May to late September and peak abundance occurring in mid-July (Fig. 3). However, at higher elevations the patterns differed between the two species. Ae. vexans females peaked sharply in late May between 1,601 and 1,750 m and declined gradually in abundance thereafter, compared with Cx. tarsalis, which peaked later in the season at 1,601–1,750 m than <1,600 m.
Fig. 3.
Seasonal patterns of abundance of Ae. vexans females in relation to mean weekly temperature by elevation category (1,501–1,600; 1,601–1,750; and >1,750 m) along the Poudre River, April–October 2006.
Seasonal Patterns of Cx. tarsalis and Cx. pipiens in the Northeastern Colorado Plains: 2007
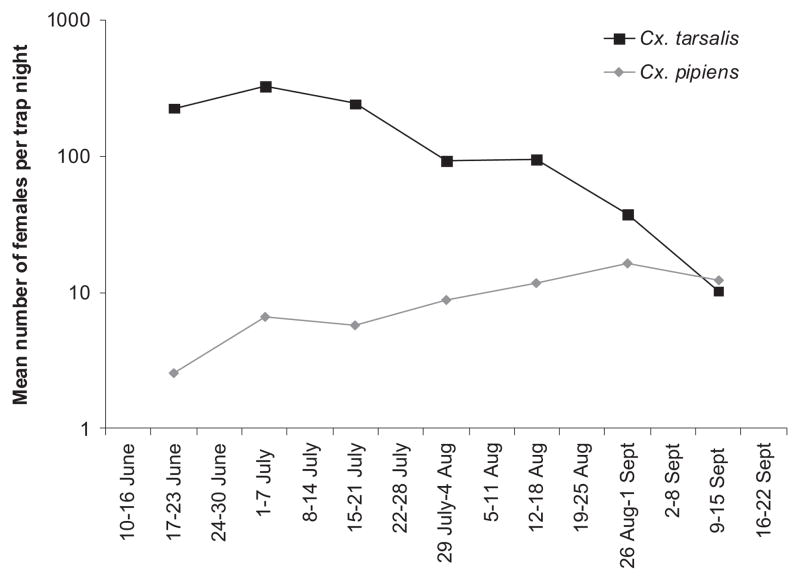
Data from 2007 provided an opportunity to compare seasonal patterns for Cx. tarsalis and Cx. pipiens from late June to mid-September in the northeastern Colorado plains (Fig. 4). This showed that abundance of Cx. tarsalis females peaked in early July and declined gradually thereafter. In contrast, Cx. pipiens females gradually increased in abundance over the sampling period to reach peak numbers in late August.
Fig. 4.
Seasonal patterns of abundance of Cx. tarsalis and Cx. pipiens females for 18 sites in the northeastern Colorado plains, June–September 2007.
Seasonal Patterns of WNV Infection in Culex Mosquitoes: 2006 and 2007
In both 2006 and 2007, WNV-infected Cx. tarsalis females were collected in the plains but not in foothills-montane areas >1,600 m (Tables 2 and 3). WNV activity was limited in the plains sites examined in 2006, with WNV infection in Cx. tarsalis females recorded only in mid-July (Table 2). Sampling in the plains in 2007 included a different set of collection sites and yielded far greater numbers of Cx. tarsalis females and more intense WNV activity compared with 2006. Only two of the 18 sites examined in the plains in 2007 failed to produce infected Cx. tarsalis females. The proportion of plains sites producing infected Cx. tarsalis females ranged from 22% in late June to ≥67% in mid-July and mid-August.
Table 2.
Seasonal pattern of infection with West Nile virus in Cx. tarsalis females in Larimer County, April–September 2006
| Elevation category; habitat; sites | MLE for WNV infection rate per 1,000 femalesa (total no. of females examined) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 Apr. | 28 Apr. | 9 May | 25 May | 7 June | 21 June | 8 July | 19 July | 2 Aug. | 16 Aug. | 30 Aug. | 13 Sept. | 27 Sept. | |
| <1,600 m; Plains; DIX | 0 (11) | 0 (1) | 0 (2) | 0 (9) | 0 (15) | 0 (40) | 15.00 (355) | 0 (12) | 0 (6) | 0 (1) | 0 (3) | 0 (3) | |
| <1,600 m; Plains; LMP | 0 (1) | 0 (12) | 0 (1) | 0 (31) | 0 (16) | 0 (1) | |||||||
| <1,600 m; Plains; BFH | 0 (1) | 0 (1) | 0 (15) | 6.99 (149) | 0 (45) | 0 (7) | 0 (1) | ||||||
| <1,600 m; Plains sites combined; DIX, LMP, BFH | 0 (11) | 0 (1) | 0 (2) | 0 (11) | 0 (28) | 0 (56) | 11.91 (535) | 0 (73) | 0 (14) | 0 (2) | 0 (3) | 0 (3) | |
| 1,601–1,750 m; Foothills-Montane; PIR, GAP, OYG | 0 (3) | 0 (2) | 0 (11) | 0 (6) | 0 (4) | 0 (2) | |||||||
| >1,750 m; Montane; STP, DUG, EGG, DAD, BSW | 0 (1) | 0 (2) | 0 (3) | 0 (1) | |||||||||
Females were tested in pools of 1–50. Bias-corrected maximum likelihood estimates (MLEs) were calculated with the Excel Add-In PooledInfRate, version 3.0 (Biggerstaff 2006).
Table 3.
Seasonal patterns of infection with West Nile virus and Vector Index for Cx. tarsalis females along the South Platte River-Big Thompson River corridor, June–September 2007
| WNV infection rate (IR) per 1,000 femalesa (total no. females examined) and vector index (VI)b |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | 20–22 June |
4–6 July |
17–19 July |
1–3 Aug. |
15–17 Aug. |
29–31 Aug. |
12–14 Sept. |
|||||||
| IR | VI | IR | VI | IR | VI | IR | VI | IR | VI | IR | VI | IR | VI | |
| Plains | ||||||||||||||
| OVE | 0 (129) | 0 | 0 (155) | 0 | 6.62 (507) | 1.68 | 19.5 (208) | 2.03 | 12.4 (257) | 1.60 | 67.1 (44) | 1.48 | 0 (51) | 0 |
| ATW | 1.29 (769) | 0.50 | 0 (1078) | 0 | 13.9 (461) | 3.20 | 0 (285) | 0 | 50.0 (20) | 1.90 | 18.8 (62) | 0.58 | 0 (3) | 0 |
| MES | 0 (812) | 0 | 0.65 (1545) | 0.50 | 4.48 (474) | 1.06 | 0 (28) | 0 | 28.6 (107) | 1.53 | 0 (37) | 0 | 38.9 (31) | 0.60 |
| COT | 0 (105) | 0 | 1.13 (887) | 0.50 | 4.45 (200) | 0.45 | 4.84 (181) | 0.44 | 0 (113) | 0 | 0 (34) | 0 | 0 (13) | 0 |
| JEA | 0 (453) | 0 | 2.41 (1735) | 2.09 | 7.23 (470) | 1.70 | 22.0 (176) | 1.94 | 4.87 (422) | 1.03 | 2.60 (386) | 0.50 | 0 (43) | 0 |
| BOY | 0 (49) | 0 | 0 (74) | 0 | 0 (113) | 0 | 0 (55) | 0 | 0 (22) | 0 | 0 (7) | 0 | 0 (4) | 0 |
| WEL | 0.83 (1175) | 0.49 | 2.31 (891) | 1.03 | 2.56 (1224) | 1.57 | 8.47 (554) | 2.35 | 3.50 (287) | 0.50 | 0 (325) | 0 | 0 (15) | 0 |
| SOO | 1.72 (582) | 0.50 | 0.78 (1275) | 0.50 | 5.20 (1066) | 2.77 | 10.3 (465) | 2.40 | 3.65 (277) | 0.51 | 0 (44) | 0 | 0 (27) | 0 |
| GRE | 0 (412) | 0 | 0 (375) | 0 | 1.05 (951) | 0.50 | 0 (72) | 0 | 0 (62) | 0 | 83.3 (12) | 0.50 | 0 (6) | 0 |
| MIT | 0 (1304) | 0 | 1.39 (1467) | 1.02 | 1.52 (660) | 0.50 | 0 (90) | 0 | 5.33 (376) | 1.00 | 0 (267) | 0 | 0 (79) | 0 |
| BRO | 0 (611) | 0 | 0 (579) | 0 | 1.15 (867) | 0.25 | 2.28 (439) | 0.50 | 8.36 (119) | 0.50 | 0 (32) | 0 | 0 (27) | 0 |
| OFF | 0 (570) | 0 | 0 (980) | 0 | 2.78 (1132) | 1.57 | 4.01 (511) | 1.02 | 8.90 (809) | 3.60 | 0 (37) | 0 | 0 (53) | 0 |
| HAR | 0 (127) | 0 | 5.94 (171) | 0.51 | 5.45 (395) | 1.08 | 8.16 (132) | 0.54 | 0 (87) | 0 | 0 (6) | 0 | 0 (3) | 0 |
| SIM | 0 (43) | 0 | 8.76 (86) | 0.60 | 0 (139) | 0 | 0 (42) | 0 | 29.2 (33) | 0.32 | 0 (10) | 0 | 0 (5) | 0 |
| NEW | 4.07 (249) | 0.51 | 0 (217) | 0 | 2.39 (419) | 0.50 | 0 (45) | 0 | 14.1 (369) | 2.60 | 0 (38) | 0 | 200.0 (5) | 0.50 |
| NAM | 0 (38) | 0 | 0 (51) | 0 | 0 (68) | 0 | 166.7 (6) | 1.00 | 0 (15) | 0 | 0 | 0 (1) | 0 | |
| GLA | 0 (31) | 0 | 0 (88) | 0 | 0 (66) | 0 | 0 (62) | 0 | 0 (4) | 0 | 0 (3) | 0 | 0 (2) | 0 |
| LON | 0 (65) | 0 | 0 (135) | 0 | 0 (154) | 0 | 0 (74) | 0 | 8.85 (94) | 0.42 | 0 (17) | 0 | 0 (3) | 0 |
| Total | 0.53 | 0.12 | 1.13 | 0.37 | 3.59 | 0.88 | 6.59 | 0.61 | 8.29 | 0.80 | 4.49 | 0.17 | 5.41 | 0.06 |
| Foothills-Montane | ||||||||||||||
| LIT | 0 (1) | 0 | 0 (4) | 0 | 0 (18) | 0 | 0 (18) | 0 | 0 (4) | 0 | 0 (3) | 0 | 0 (2) | 0 |
| NRW | 0 | 0 (3) | 0 | 0 (1) | 0 | 0 (1) | 0 | 0 | 0 (1) | 0 | 0 | |||
| VSP | 0 | 0 (20) | 0 | 0 (14) | 0 | 0 (4) | 0 | 0 (2) | 0 | 0 (1) | 0 | 0 | ||
| IDY | 0 (1) | 0 | 0 (2) | 0 | 0 (1) | 0 | 0 | 0 | 0 | 0 | ||||
| Total | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Females were tested in pools of 1–50. Bias-corrected maximum likelihood estimates for WNV infection rates were calculated with the Excel Add-In PooledInfRate, version 3.0 (Biggerstaff 2006).
Vector index was calculated as the mean no. of females per trap night times the proportion of WNV-infected females.
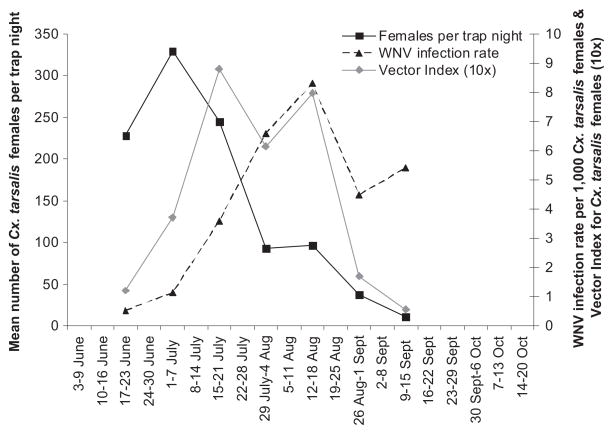
Overall WNV infection rates for Cx. tarsalis females in the 18 plains sites in 2007 increased gradually from late June (MLE of 0.53 per 1,000 females) to reach a peak in mid-August (8.29 per 1,000 females) and then remained high through mid-September (4.49–5.41 per 1,000 females) (Table 3; Fig. 5). Infection rates for individual collections by site and date, where ≥500 Cx. tarsalis females were examined, ranged from 0 to 8.90 per 1,000 females, with most collections that produced infected females falling in the infection rate range of one to three per 1,000 females (Table 3). Very high rates of WNV infection, >10 per 1,000 females, were associated with smaller sample sizes.
Fig. 5.
Seasonal patterns for mean number of Cx. tarsalis females per trap night, WNV infection rate in the females, and vector index for abundance of WNV-infected Cx. tarsalis females (10×) in the northeastern Colorado plains, June–September 2007.
Fewer Cx. pipiens females were collected at most sites, and only five pools were infected with WNV. Infected pools were recorded 20–22 June, 17–19 July, 15–17 August, 29 –31 August, and 12–14 September. The overall infection rate (MLE per 1,000 females) for the plains sites during June–September was 2.10.
Seasonal Patterns of Entomological Risk Measures in Relation to Occurrence of WNV Disease Cases: 2007
For the 2007 data, we explored potential relationships between the seasonal pattern of WNV disease cases in the targeted five-county area (Larimer, Weld, Morgan, Washington, and Logan) and the seasonal patterns for three entomological risk measures combined for the 18 plains sites located within these counties: 1) mean number of Cx. tarsalis females per trap night (Fig. 5); 2) WNV infection rate per 1,000 Cx. tarsalis females (Table 3; Fig. 5); and 3) vector index for abundance of infected Cx. tarsalis females (mean per trap night × proportion of WNV-infected females) (Table 3; Fig. 5). The seasonal patterns for the three different entomological risk measures are shown together in Fig. 5. Abundance of Cx. tarsalis females peaked in early July and declined gradually thereafter, whereas the WNV infection rate in the females increased gradually to reach a peak in mid-August. This resulted in the vector index peaking in mid-July and mid-August. The vector index for all plains sites combined exceeded 0.50 from mid-July to mid-August, and at least one site recorded a vector index ≥0.50 from late June to mid-September and ≥1.00 from early July to late August.
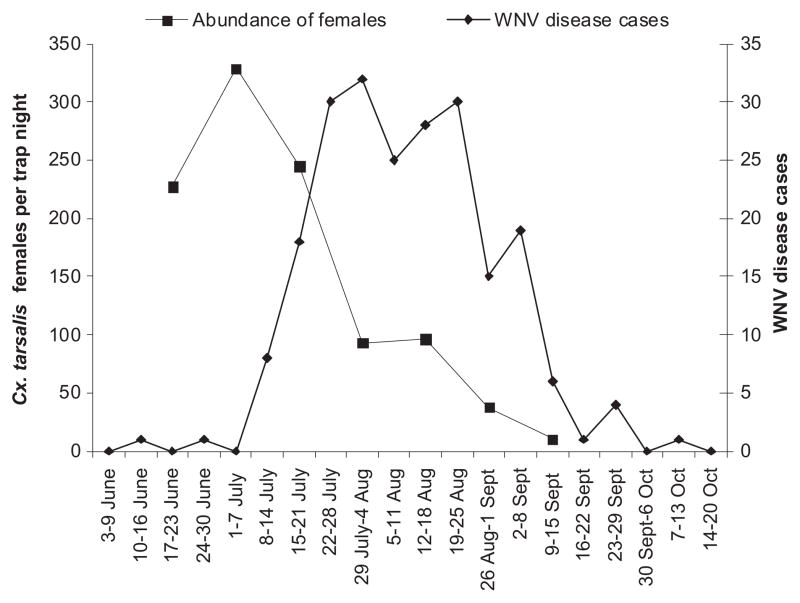
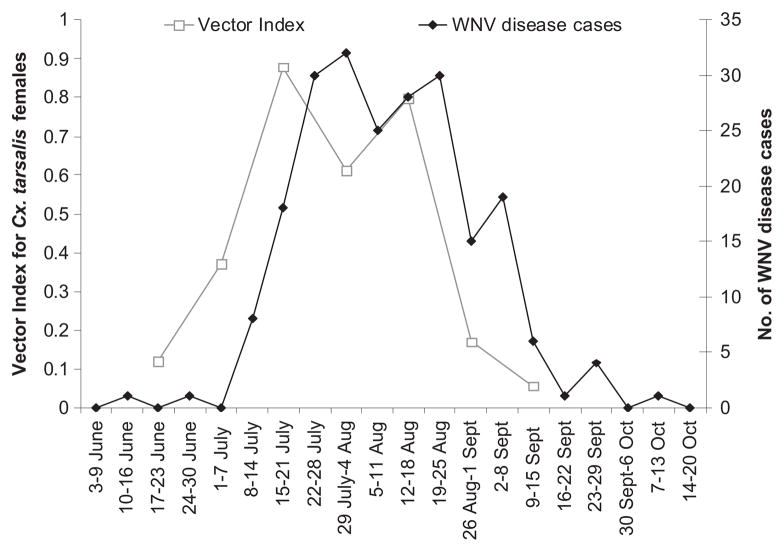
Linear regression models where entomological risk measures were used to predict WNV disease in subsequent weeks, with time-lags ranging from 0 to 8 wk, showed that abundance of Cx. tarsalis females was strongly associated with weekly numbers of WNV disease cases 4–7 wk later and that the vector index was strongly associated with weekly numbers of WNV disease cases 1–2 wk later (Table 4). Weekly patterns for WNV disease cases in relation to abundance of Cx. tarsalis females or vector index are shown in Figs. 6 and 7.
Table 4.
Results for linear regression models to predict numbers of WNV disease cases in subsequent weeks from entomological risk measures, northeastern Colorado, 2007
| Lag time for comparison with weekly no. of WNV disease cases | Abundance of Cx. tarsalis femalesa |
WNV infection rate in Cx. tarsalis femalesb |
Vector index for Cx. tarsalis femalesc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model fit |
ANOVA |
Model fit |
ANOVA |
Model fit |
ANOVA |
|||||||
| Typed | r2 | F1,5 | P | Typed | r2 | F1,5 | P | Typed | r2 | F1,5 | P | |
| + 0 wk | NA | 0.187 | 1.15 | 0.33 | Pos | 0.712 | 12.39 | 0.02 | NA | 0.488 | 4.76 | 0.08 |
| + 1 wk | NA | 0.007 | 0.04 | 0.86 | NA | 0.373 | 2.97 | 0.14 | Pos | 0.786 | 18.37 | 0.008 |
| + 2 wk | NA | 0.102 | 0.57 | 0.48 | NA | 0.061 | 0.33 | 0.59 | Pos | 0.725 | 13.18 | 0.02 |
| + 3 wk | NA | 0.371 | 2.95 | 0.14 | NA | 0.001 | 0.01 | 0.98 | NA | 0.534 | 5.72 | 0.06 |
| + 4 wk | Pos | 0.907 | 49.01 | <0.001 | NA | 0.401 | 3.35 | 0.12 | NA | 0.130 | 0.75 | 0.43 |
| + 5 wk | Pos | 0.729 | 13.47 | 0.01 | NA | 0.477 | 4.55 | 0.08 | NA | 0.054 | 0.28 | 0.61 |
| + 6 wk | Pos | 0.801 | 20.18 | 0.006 | Neg | 0.738 | 14.05 | 0.01 | NA | 0.009 | 0.04 | 0.84 |
| + 7 wk | Pos | 0.911 | 51.30 | <0.001 | Neg | 0.781 | 17.80 | 0.008 | NA | 0.001 | 0.01 | 0.95 |
| + 8 wk | NA | 0.498 | 4.95 | 0.07 | Neg | 0.678 | 10.51 | 0.02 | NA | 0.075 | 0.40 | 0.55 |
All comparisons were based on seven data points for entomological risk measures (taken every 2 wk from late June to mid-September); these data points combined data for 18 plains sites located within the five-county area in northeastern Colorado that was used to determine weekly WNV disease cases.
Mean number of Cx. tarsalis females per trap night.
Maximum likelihood estimate for WNV infection rate per 1,000 Cx. tarsalis females.
Vector index for abundance of WNV-infected Cx. tarsalis females (weekly mean per trap night × weekly proportion of WNV-infected females).
Pos, positive linear relationship; Neg, negative linear relationship; NA, no significant relationship.
Fig. 6.
Seasonal pattern of WNV disease cases in a five-county area (Larimer, Weld, Morgan, Washington, and Logan) in northeastern Colorado, June–September 2007, in relation to abundance of Cx. tarsalis females.
Fig. 7.
Seasonal pattern of WNV disease cases in a five-county area (Larimer, Weld, Morgan, Washington, and Logan) in northeastern Colorado, June–September 2007, in relation to the vector index for abundance of WNV-infected Cx. tarsalis females.
Discussion
This study provided detailed descriptions of seasonal risk patterns for exposure to mosquitoes and WNV in a wide range of habitat types in Colorado, including prairie landscapes in the Great Plains and foothills and montane areas in the Rocky Mountains. Key findings included that 1) the seasonal activity period is shortened and peak numbers occur later in the summer for Cx. tarsalis females in foothills-montane areas >1,600 m compared with plains areas <1,600 m along Colorado’s Front Range; 2) seasonal patterns of abundance for Cx. tarsalis and Cx. pipiens females in the northeastern Colorado plains in 2007 differed in that Cx. tarsalis reached peak abundance in early July, whereas the peak for Cx. pipiens did not occur until late August; 3) WNV-infected Cx. tarsalis females were recorded from nearly all sites sampled in the plains in 2007 with infection rates commonly exceeding one infected female per 1,000 examined; 4) the vector index for abundance of WNV-infected Cx. tarsalis females exceeded 0.50 for the plains sites combined from mid-July to mid-August, with values for at least one individual site exceeding 1.00 from early July to late August; and 5) abundance of Cx. tarsalis females and the vector index for abundance of infected females were strongly associated with weekly numbers of WNV disease cases with onset 4–7 wk later (female abundance) or 1–2 wk later (vector index).
An important limitation of this study is that we were not able to sample all sites during both years. Some of the key findings outlined above, especially the associations between entomological risk measures and human disease cases, need to be corroborated not only in other parts of the western United States but also in future studies in Colorado that span multiple years and can account for between-year variability in weather conditions, mosquito population dynamics, and WNV transmission intensity. Another issue that needs to be addressed in future studies is to clarify the relative roles of Cx. tarsalis versus Cx. pipiens as bridge vectors of WNV to humans in eastern Colorado. Our findings suggest that Cx. tarsalis should be considered a primary vector of WNV to humans in eastern Colorado, but further work is needed to define the local circumstances under which Cx. pipiens also may play an important role in this respect.
Seasonal Patterns of Mosquito Abundance Along Elevation Gradients in the Colorado Front Range
To our knowledge, this is the first study from North America exploiting a natural elevation/climate gradient to determine how seasonal patterns of abundance of adult nuisance-biting or vector mosquitoes change with elevation at the cool edge of the range of the mosquitoes. For the WNV vector Cx. tarsalis, we found dramatic changes in seasonal abundance patterns >1,600 m. Above this elevation threshold, the seasonal activity period for Cx. tarsalis females was shortened (Fig. 2), peak numbers were lower and occurred later in the summer (Fig. 2), and WNV was not detected from the females (Tables 2 and 3). We speculate that these differences were due to 1) temperature conditions at elevations >1,600 m in the Front Range that limited population growth of Cx. tarsalis by slowing larval developmental rates and gonotrophic cycles, and 2) a reduction in the number, size, and persistence of larval habitats due to land use changes such as a lack of irrigation and other human-managed water inputs. This also may keep the overall abundance of Culex vectors below a critical threshold for enzootic WNV transmission to occur. These speculations provoke interesting questions regarding how climate warming in coming decades, should it occur, may impact risk of exposure to Culex vectors and WNV in mountainous areas of the western United States where current climate conditions are marginally suitable for Culex vectors and viral replication, but where nearby lower elevation areas have active WNV transmission foci.
Variability in the seasonal abundance pattern for Ae. vexans along the same elevation gradient was less dramatic. The seasonal pattern observed <1,600 m, with increased abundance from late June to early August, was expected from previous studies conducted in Great Plains landscapes in eastern Colorado and Nebraska (Janousek and Kramer 1999, Bolling et al. 2007). In foothills-montane areas >1,600 m, Ae. vexans exhibited a similar rate of increase as seen in the lower elevation sites from late May to early June. Abundance then stabilized briefly before starting to decline in late June, which differed from the lower elevation sites where abundance continued to increase sharply until mid-July. This resulted in a distinct seasonal pattern >1,600 m characterized by a short period of increasing abundance in late May, a brief peak during the first 2 wk of June, and a slow decline thereafter. We speculate that the seasonal pattern >1,600 m results from a combination of 1) limited access to larval development sites in dry foothills-montane canyon landscapes beyond the initial spring river flooding event and 2) cooler temperatures negatively impacting larval development rates and female gonotrophic cycles.
Seasonal Patterns of Culex Abundance in the Northeastern Colorado Plains
Seasonal patterns of abundance of Cx. tarsalis and Cx. pipiens have been described previously from many parts of the western United States. The single peak seasonal pattern for abundance of Cx. tarsalis females observed in this study in 2006–2007, with elevated abundances occurring from late June to mid-August, agrees with previous studies from Colorado (Tsai et al. 1988, Smith et al. 1993, Bolling et al. 2007), Nebraska (Janousek and Kramer 1999), North Dakota (Bell et al. 2005), Utah (Beadle 1959), Washington (Pecoraro et al. 2007), and northern California (Reisen et al. 1995a). A different seasonal pattern with an earlier spring peak and a second distinct peak in the fall can occur in warmer areas such as southeastern California (Reisen et al. 1995b, 2008a, 2009). Furthermore, an intermediate pattern, with a peak for Cx. tarsalis females in July and a smaller but distinct second peak in September, was reported from the Davis area in central California (Nielsen et al. 2008).
The seasonal pattern observed by us for Cx. pipiens females in 2007, with gradually increasing abundance reaching a peak in late August, agrees with a previous study using light traps in western Colorado (Tsai et al. 1988). Other studies have reported earlier peaks for Cx. pipiens females in late June and July in Washington (Pecoraro et al. 2007) and the Central Valley of California (Nielsen et al. 2008). We also recognize that the data for Cx. pipiens in our study should be interpreted with care because use of CDC light traps can underestimate the abundance of this species compared with efforts that also include gravid traps (Tsai et al. 1988).
With the exception of California (Kliewer et al. 1969; Olson et al. 1979; Reisen et al. 1992, 2008b; Wegbreit and Reisen 2000), there is a lack of long-term studies from the western United States to determine the extent of between-year variability in seasonal abundance patterns for Culex vectors, especially in relation to weather patterns. This is unfortunate because such studies are critical for developing models to forecast Culex vector abundance based on weather patterns. For example, Reisen and colleagues used long-term (1950–2000) data for Cx. tarsalis from mosquito control programs in California to determine impacts of climate variation on mosquito abundance and found strong correlations between spring abundance of Cx. tarsalis and winter–spring precipitation, winter snow pack and winter–spring temperature (Reisen et al. 2008b). Similar studies are needed from the Great Plains WNV disease focus.
Seasonal Patterns for WNV Infection Rates and Vector Index for Cx. tarsalis
The overall seasonal pattern for WNV infection rates in Cx. tarsalis females in the northeastern Colorado plains in 2007 was characterized by a gradual increase in infection rates from late June to late July, peak values occurring during the first half of August, and infection rates remaining high until the study was concluded in mid-September (Table 3). A similar monthly pattern was recorded from June to August for WNV infection rates in Cx. tarsalis females in other parts of Weld County in 2007 (Kent et al. 2009). Other studies have produced similar seasonal patterns for infection of Cx. tarsalis with WNV in California, New Mexico, and North Dakota (Bell et al. 2005; DiMenna et al. 2006; Nielsen et al. 2008; Reisen et al. 2008a, 2009) and with western equine or St. Louis encephalitis viruses in Colorado (Hess and Hayes 1967, Tsai et al. 1988, Smith et al. 1993). We also found considerable variation among trap sites in seasonal patterns for WNV infection rates (Table 3), which underscores the importance of operating multiple trap stations for mosquito-based WNV surveillance. Additional studies are needed to determine optimal combinations of trap densities and trap locations for mosquito-based WNV surveillance in the Great Plains landscape to minimize operational cost without compromising data quality.
The general pattern for WNV infection rates in Cx. tarsalis observed in our study probably reflects the seasonal pattern of intensity of enzootic transmission of WNV, which can be expected to increase over the summer as Culex vectors become more abundant and new generations of WNV-susceptible birds emerge. Furthermore, a temporal shift in feeding behavior of Cx. tarsalis toward increased feeding on mammals from spring to summer has been observed in California and northeastern Colorado (Tempelis et al. 1965, 1967). This phenomenon has been hypothesized to impact seasonal risk of human exposure to Cx. tarsalis and other Culex species (Edman and Taylor 1968, Kilpatrick et al. 2006). However, a recent study from Weld County showed that the percentage of Cx. tarsalis that fed on humans increased from June to July–August but remained <7% for all months examined (Kent et al. 2009). During each month, >75% of Cx. tarsalis bloodmeals came from birds. The epidemiological importance of temporal shifts in feeding behavior remains unclear. We caution against attempting to adjust risk indices for human exposure to WNV-infected Cx. tarsalis based on perceived seasonal changes in feeding behaviors until we have gained a better understanding of the underlying mechanisms. For example, increased feeding on humans in summer may simply reflect changes in human behavior rather than changes in mosquito feeding habits.
The vector index for abundance of WNV-infected vectors, which was developed by the CDC, is in operational use in mosquito control programs in some parts of the western United States, including Colorado but has not received much attention in the published literature. In fact, we are only aware of two previously published studies presenting data on the vector index, or variations of the vector index, for Cx. tarsalis (Bell et al. 2005, Gujral et al. 2007). Our study, which uses a vector index for Cx. tarsalis females to assess risk of exposure to WNV-infected females, clearly demonstrates the value of combining information for vector abundance and WNV infection rates to generate a more meaningful risk index. In Fig. 5, we illustrate how the seasonal pattern for the vector index differs from that of mosquito abundance alone or WNV infection rate alone. In fact, we find it surprising that this type of risk index has taken so long to permeate the WNV literature. Similar risk indices that combine vector abundance and vector infection rate are used extensively to assess risk for exposure to tick-borne pathogens such as Borrelia burgdorferi (Mather et al. 1996, Stafford et al. 1998, Eisen et al. 2004) and also were used previously as measures of risk for exposure to Cx. tarsalis infected with western equine or St. Louis encephalitis viruses (Reeves et al. 1962, Hess and Hayes 1967, Tsai et al. 1988).
Predicting Seasonal Patterns for WNV Disease Cases from Entomological Risk Measures
Our study demonstrates that the number of weekly human cases of WNV disease within the targeted five-county area in 2007 could be predicted by the abundance of Cx. tarsalis females (4–7 wk previously) and by the vector index for WNV-infected Cx. tarsalis females (1–2 wk previously) (Table 4; Figs. 6–7). Interestingly, a previous study on western equine encephalitis virus (family Togaviridae, genus Alphavirus, WEEV) in eastern Colorado in 1965 showed a similar pattern where the weekly numbers of human WEE cases were predicted by abundance of Cx. tarsalis females with a 4-wk time-lag and by abundance of WEEV-infected Cx. tarsalis per trap night with a 2-wk time lag (Hess and Hayes 1967). A recent large-scale study of WEEV transmission to sentinel chickens in central and southeastern California also identified 4–6 wk as the critical time-lag between Cx. tarsalis abundance and sentinel chicken seroconversions (Barker 2008).
Our data for time-lags between entomological risk measures and human WNV disease cases provide critical information for operational surveillance programs to determine time-lags for which these entomological risk measures are meaningful and how they should be used to guide emergency vector control activities. One distinct drawback of using the vector index is the short lead time of 1–2 wk for reliable prediction of human case loads. This underscores the critical need for rapid turnaround of WNV testing of mosquito pools in order for the vector index to be operationally useful. The longer lead time for abundance of Cx. tarsalis females (4 wk) to predict human case loads for WNV disease argues for use of this entomological risk measure. However, the robustness of the predictive capability of this risk measure needs to be evaluated prospectively and corroborated in other areas and over multiple years because the longer time-lag, relative to the vector index, may result in greater sensitivity to weather events such as cold spells that can affect vector population growth and intensity of enzootic WNV transmission. Advances in statistical models for early detection or warning systems (e.g., Chaves and Pascual 2007) also provide new opportunities to explore how entomological data can be used to predict human cases.
Acknowledgments
We gratefully acknowledge the U.S. Forest Service and the cities of Fort Collins and Loveland for permission to collect mosquitoes. Field and laboratory assistance was provided by S. L. Anderson, M. Heersink, J. Holmes, L. Ibarra-Juarez, L. Mahaffey, A. Meyer, and J. Montgomery. The research project was supported, in part, by contract N01-(AI)-25489 from the National Institutes of Health National Institutes of Allergy and Infectious Diseases.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References Cited
- Barker CM. Ph.D. Dissertation. University of California; Davis: 2008. Spatial and temporal patterns in mosquito abundance and virus transmission in California. [Google Scholar]
- Beadle LD. Field observations on the biting habits of Culex tarsalis at Mitchell, Nebraska, and Logan. Utah Am J Trop Med Hyg. 1959;8:134–140. doi: 10.4269/ajtmh.1959.8.134. [DOI] [PubMed] [Google Scholar]
- Bell JA, Mickelson NJ, Vaughan JA. West Nile virus in host-seeking mosquitoes within a residential neighborhood in Grand Forks. North Dakota Vector-Borne Zoonotic Dis. 2005;5:373–382. doi: 10.1089/vbz.2005.5.373. [DOI] [PubMed] [Google Scholar]
- Biggerstaff BJ. PooledInfRate, version 3.0: a Microsoft®Excel®Add-In to compute prevalence estimates from pooled samples. Centers for Disease Control and Prevention; Fort Collins, CO: 2006. [Google Scholar]
- Bolling BG, Moore CG, Anderson SL, Blair CD, Beaty BJ. Entomological studies along the Colorado Front Range during a period of intense West Nile virus activity. J Am Mosq Control Assoc. 2007;23:37–46. doi: 10.2987/8756-971X(2007)23[37:ESATCF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Pascual M. Comparing models for early warning systems of neglected tropical diseases. PLoS Negl Trop Dis. 2007;1:e33. doi: 10.1371/journal.pntd.0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. University Press of Florida; Gainesville, FL: 2005. [Google Scholar]
- DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, LaBeau EM, Hatton ES, Glass GE. Emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Grande Valley. J Med Entomol. 2006;43:594–599. doi: 10.1603/0022-2585(2006)43[594:eownvi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science (Wash, DC) 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Chang CC, Mun J, Lane RS. Acarological risk of exposure to Borrelia burgdorferi spirochaetes: long-term evaluation in northwestern California, with implications for Lyme borreliosis risk-assessment models. Med Vet Entomol. 2004;18:38–49. doi: 10.1111/j.1365-2915.2004.0476.x. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Campbell GL, Nasci RS, Komar N, Petersen L, Roehrig JT. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol. 2000;13:469–475. doi: 10.1089/vim.2000.13.469. [DOI] [PubMed] [Google Scholar]
- Gujral IB, Zielinski-Gutierrez EC, LeBailly A, Nasci R. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg Infect Dis. 2007;13:419–425. doi: 10.3201/eid1303.060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmston FC, Lawson FA. Mosquitoes of Colorado. U.S. Department of Health, Education and Welfare, Public Health Service; Atlanta, GA: 1967. [Google Scholar]
- Hess AD, Hayes RO. Seasonal dynamics of western encephalitis virus. Am J Med Sci. 1967;253:333–348. doi: 10.1097/00000441-196703000-00011. [DOI] [PubMed] [Google Scholar]
- Janousek TE, Kramer WL. Seasonal incidence and geographical variation of Nebraska mosquitoes, 1994–95. J Am Mosq Control Assoc. 1999;15:253–262. [PubMed] [Google Scholar]
- Kent R, Juliusson L, Weissman M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer JW, Miura T, Chapman HC. Seasonal occurrence and physiology of Culex tarsalis in foothills of Fresno County, California. Ann Entomol Soc Am. 1969;62:13–18. doi: 10.1093/aesa/62.1.13. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- Nielsen CF, Armijos MV, Wheeler S, Carpenter TE, Boyce WM, Kelley K, Brown D, Scott TW, Reisen WK. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in northern California. Am J Trop Med Hyg. 2008;78:53–62. [PMC free article] [PubMed] [Google Scholar]
- Olson JG, Reeves WC, Emmons RW, Milby MM. Correlation of Culex tarsalis population indices with the incidence of St. Louis encephalitis and western equine encephalomyelitis in California. Am J Trop Med Hyg. 1979;28:335–343. doi: 10.4269/ajtmh.1979.28.335. [DOI] [PubMed] [Google Scholar]
- Pecoraro HL, Day HL, Reineke R, Stevens N, Withey JC, Marzluff JM, Meschke JS. Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. J Vector Ecol. 2007;32:22–28. doi: 10.3376/1081-1710(2007)32[22:calcfp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Hammon WM, Longshore WA, Jr, McClure H, Geib AF. Epidemiology of the arthropod-borne virus encephalitides in Kern County, California, 1943–1952. Vol. 4. University of California Publications in Public Health; 1962. pp. 1–257. [PubMed] [Google Scholar]
- Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool J Linn Soc. 2004;142:289–368. [Google Scholar]
- Reisen WK, Hardy JL, Presser SB, Milby MM, Meyer RP, Durso SL, Wargo MJ, Gordon E. Mosquito and arbovirus ecology in southeastern California, 1986–1990. J Med Entomol. 1992;29:512–524. doi: 10.1093/jmedent/29.3.512. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Boyce K, Yoshimura G, Lemenager D, Emmons RW. Enzootic transmission of western equine encephalomyelitis virus in the Sacramento Valley of California during 1993 and 1994. J Vector Ecol. 1995a;20:153–163. [Google Scholar]
- Reisen WK, Lothrop HD, Hardy JL. Bionomics of Culex tarsalis (Diptera: Culicidae) in relation to arbovirus transmission in southeastern California. J Med Entomol. 1995b;32:316–327. doi: 10.1093/jmedent/32.3.316. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. Persistent West Nile virus transmission and the apparent displacement of St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008a;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Cayan D, Tyree M, Barker CM, Eldridge B, Dettinger M. Impact of climate variation on mosquito abundance in California. J Vector Ecol. 2008b;33:89–98. doi: 10.3376/1081-1710(2008)33[89:iocvom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring R. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J Med Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramozzino N, Crance JM, Jouan A, DeBriel DA, Stoll F, Garin D. Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39:1922–1927. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Moore CG, Davis T, Savage HM, Thapa AB, Shrestha SL, Karabatsos N. Arbovirus surveillance in northern Colorado, 1987 and 1991. J Med Entomol. 1993;30:257–261. doi: 10.1093/jmedent/30.1.257. [DOI] [PubMed] [Google Scholar]
- Stafford KC, III, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A three-year study of the feeding habits of Culex tarsalis in Kern County, California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Francy DB, Hayes RO, Lofy MF. Variations in feeding patterns of seven culicine mosquitoes on vertebrate hosts in Weld and Larimer Counties, Colorado. Am J Trop Med Hyg. 1967;16:111–119. doi: 10.4269/ajtmh.1967.16.111. [DOI] [PubMed] [Google Scholar]
- Tsai T, Smith G, Ndukwu M, Jakob W, Happ C, Kirk L, Francy D, Lampert K. Entomologic studies after a St. Louis encephalitis epidemic in Grand Junction, Colorado. Am J Epidemiol. 1988;128:285–297. doi: 10.1093/oxfordjournals.aje.a114969. [DOI] [PubMed] [Google Scholar]
- Wegbreit J, Reisen WK. Relationships among weather, mosquito abundance, and encephalitis virus activity in California: Kern County 1990–98. J Am Mosq Control Assoc. 2000;16:22–27. [PubMed] [Google Scholar]