Abstract
Objective
To evaluate whether comprehensive post-discharge care management for stroke survivors is superior to organized acute stroke unit care with enhanced discharge planning in improving a profile of health and well-being.
Methods
This was a randomized trial of a comprehensive post-discharge care management intervention for ischemic stroke patients with NIH Stroke Scale scores ≥1 discharged from an acute stroke unit. An Advanced Practice Nurse (APN) performed an in-home assessment for the intervention group from which an Interdisciplinary Team developed patient-specific care plans. The APN worked with the primary care physician (PCP) and patient to implement the plan over the next 6 months.
Main outcome measures
The intervention and usual care groups were compared using a global and closed hypothesis testing strategy. Outcomes fell into 5 domains: 1) Neuromotor Function, 2) Institution Time or Death, 3) Quality of Life, 4) Management of Risk, and 5) Stroke Knowledge and Lifestyle.
Results
Treatment effect was near zero standard deviations for all but the stroke knowledge and lifestyle domain which showed a significant effect of the intervention (p=0.0003).
Conclusions
Post discharge care management was not more effective than organized stroke unit care with enhanced discharge planning in most domains in this population. The intervention did, however, fill a post-discharge knowledge gap.
Introduction
Stroke is the leading cause of disability, the third leading cause of death, and one of the most expensive medical problems in the United States1. The emergence of specialized inpatient stroke and rehabilitation units that utilize a comprehensive interdisciplinary team approach have demonstrated improvements in post-stroke outcomes2-8. It is unclear whether improved post-discharge care can further optimize post-stroke outcomes.
Numerous studies show that implementation of Wagner’s model for chronic illness care 9, through the use of interdisciplinary teams, can be effective in ensuring comprehensive care of patients with chronic diseases 10-13. Several studies have attempted to incorporate the chronic disease management model into post-hospitalization stroke care, with limited success 14-20. The post-stroke care management model tested here uses a Wagner-like chronic illness model for post-stroke care by including 1) care with an equal emphasis on physical and psychosocial health, 2) an Advanced Practice Nurse care manager (APN-CM) to assess patients’ problems and coordinate care, 3) a team of stroke experts to devise individual care plans, 4) standardized assessments and interventions to ensure consistency in care, and 5) providing primary care physicians (PCPs) with “academic detailing” to support the team’s evidence-based recommendations. We tested this comprehensive care management intervention against organized stroke unit care that included enhanced discharge planning. What is unique from prior attempts to evaluate post-stroke care management is the strategy employed to test its superiority over acute stroke unit care across many outcomes in order to be sensitive to the multi-component effects of items 1-5 above.
Methods
Patients were recruited from the acute stroke unit (SU) at Summa Health System, a 963-bed community teaching hospital in Akron, Ohio. On average, the stroke unit treats 560 stroke patients per year and the unit includes a separate neurological intensive care unit. Subjects were enrolled in the study upon confirmation of ischemic stroke from August 2002-January 2006. The following were the inclusion criteria:
Diagnosis of ischemic stroke.
NIH Stroke Scale score ≥1.
Discharged to home from the acute care hospital, or discharged to home within 8 weeks from a short-term skilled nursing facility (SNF) or acute rehabilitation facility.
Live within 25 miles.
Have no other illness that would dominate post-stroke care
Speak English.
Do not have an endarterectomy planned.
Informed consent was obtained from each participant or a caregiver, for those patients who failed a mental status screening examination 21. Randomization occurred after enrollment and baseline assessments at discharge to home. If patients were discharged to a skilled rehabilitation facility for less than 8 weeks, randomization and baseline measures were obtained at the time of discharge from the facility. The randomization sequence was by permuted blocks of fixed size (10) generated by study biostatisticians. Group assignment was made by a research assistant using the sealed envelope method. Approval for this study was obtained by the IRB at Summa Health System.
Organized Stroke Unit care common to both groups
The SU provides patient-centered care through an interdisciplinary team approach 22. Team members evaluate each patient’s physical and psychosocial needs using standardized assessment tools. The team then develops an individualized evidence-based care plan. Thus, by discharge, all patients should have had all recommended tests performed, an optimized medication regimen in place, and a thorough discharge plan.
Enhanced discharge planning common to both groups
For both the intervention and control groups, the patient’s PCP received a written patient summary generated by the research nurse that summarized all inpatient findings, the patient’s risk factor profile, discharge plans, discharge medications, and all of the baseline assessment data obtained by the research nurse. This was added to usual care to ensure that everything that could be done in the acute care setting was done, making our study a conservative test of the additional benefit of the post-discharge care management.
Baseline data
Information abstracted from patient charts at discharge included discharge instructions, medications, plan of care, and lab/diagnostic testing results. Baseline assessments by a research nurse included a depression screen (using the CES-D 23), the NIH Stroke Scale 24, blood pressure, and an investigator-generated Stroke Knowledge Test.
Treatment of the Intervention Group
This study incorporated recommendations from the National Stroke Association 25, the American Heart Association 26, and the National Clinical Guidelines for Stroke from the Royal College of Physicians 27 into its interventions. By incorporating the best evidence-based recommendations available, it was reasonable to expect a positive impact of the intervention across the chosen important outcomes.
For the intervention group an Advanced Practice Nurse provided care management to patients. The APN-CM performed an in-home assessment within 1 week of discharge. Standard education and intervention protocols for stroke and common post-stroke complications were implemented during the home visit (see reference 28 for a full description of the care management intervention). Results of the home assessment were reviewed by an interdisciplinary post-stroke consultation team (PSC-Team). The core PSC-Team included a geriatrician, community-based general internist, stroke Clinical Nurse Specialist, APN-CM, and physical therapist. Extended team members who were available as-needed included a neurologist, psychologist, pharmacist, physiatrist, social worker, physical therapist, speech therapist, occupational therapist, and dietitian.
The PSC-Team developed patient care plans specific to each problem identified by the APN-CM. A copy of the care plans, evidence-based guidelines, pertinent references, and a short paragraph providing “academic detailing” specific to the patient’s problems were communicated to the patient’s PCP. The APN-CM worked collaboratively with the PCP to implement the recommendations and provide ongoing monitoring over the next 6 months. The intervention included home visits by a physical therapist, as needed, to maximize function, education regarding lifestyle modification, medication reconciliation and pill organizers to optimize stroke risk factor control, collaboration with the local Area Agency on Aging to ensure that needed social services (e.g., meals on wheels, pre-packaged medication systems, home health aides) were in place to maximize quality of life, and frequent assessment and intervention to reduce common post-stroke complications (e.g., depression, incontinence, falls). The patient also received a personalized health record to help them self-manage their risk factors, as well as education regarding stroke warning signs to minimize the effects of a recurrent stroke by seeking treatment at the first sign of stroke symptoms.
Periodic phone calls were used to assess patient changes that warranted further intervention. Additional home visits were made on an as-needed basis. The APN-CM also attended doctor visits with patients as needed. At a minimum, patients were contacted by phone once/week for the first month post-discharge, then once/month until the end of the study (6 months post-discharge).
Treatment of the Control Group
After discharge from the acute stroke unit or short-term rehabilitation, control subjects received usual post-discharge care from their primary care physician. There were no assessments by the research team until after 6-month outcomes were measured. However, as mentioned above, PCPs from both groups were sent a problem list, risk factor profile, discharge plan of care, and discharge medication list at the time of their patients’ discharge from the acute care hospital to home. Control patients also received mailings every 2 months reminding them of their involvement in the study and providing stroke-related patient educational materials.
Outcomes
It is important that the outcomes measured in trials of this nature be meaningful to the patient and society, sensitive to variability in patients’ deficits, reliable and valid, and modifiable by the intervention 29. A major difficulty encountered in stroke outcome studies is that each stroke survivor faces different deficits and different degrees of recovery. Traditional analyses that focus on a single outcome (e.g., readmission rate or functional status) to determine whether the intervention group fared better than the control group may miss small and consistent positive impacts on a wide range of variables 30-33.
Comprehensive assessment of the impact of care management should be sensitive to impairments (motor deficits, depression), disabilities (deficits in activities of daily living), and handicaps (quality of life) 34. Hospital readmissions, other institutionalizations, and death should be measured as indicators of severe complications that can be avoided by a comprehensive care management intervention 29. Furthermore, since there are numerous problems that are prognostic for recurrent stroke (e.g., elevated blood pressure, noncompliance with prescribed medications) or other common post-stroke complications (e.g., falls, incontinence) which a comprehensive care management intervention should improve, any assessment of such an intervention must include measures of the successful management of all of these risk factors 35. Finally, since patients’ knowledge of the symptoms of stroke and appropriate urgent response to them is critical to ensure early presentation for urgent medical treatment should stroke symptoms recur, an assessment of a care management model must also include an assessment of stroke knowledge 36-38.
Many of the issues addressed by the intervention are interdependent and can interact to improve outcomes. For example, effective treatment of depression improves not only depression but it may also result in better function if the patient is more energetic and has greater motivation to comply with medical management recommendations. We hypothesized that, combined, the components of care management listed above would result in fewer hospitalizations, improved quality of life, and superior functional recovery.
The outcomes we selected go beyond the traditional narrow focus on neuromotor function or healthcare utilization by including variables that reflect the process of care management that contribute to the patient’s present and future health and quality of life. We classified these into 5 domains. Objective, clinically relevant performance-based measures were used whenever appropriate:
Neuromotor Function, measured using National Institutes of Health (NIH) Stroke Scale 24, the Timed Up and Go test 39, and the Physical Performance Test 40;
Institution Time and Death, time was measured as days spent hospitalized or in a nursing home during the 6-month follow up period and death (if a patient died, then institution time was measured as the percentage of days in an institution while alive × 180);
Quality of Life (QOL), measured using the SSQOL 41;
Management of Risk for common post-stroke complications and recurrent stroke, measured as successful evidence-based management of systolic blood pressure (< 140mmHg), diastolic blood pressure (< 90mmHg), depression (measured using the CES-D 23), medication appropriateness (measured using an investigator-generated tool), hemoglobin A1c (<6.5%), total cholesterol (<180), and self-reported falls and incontinence;
Stroke Knowledge and Lifestyle Modification, measured using an investigator-generated questionnaire that assesses knowledge of stroke risk factors, appropriate behaviors for stroke risk reduction, appropriate response to stroke symptoms, objective health indicators, and alcohol use and smoking.
Timing of outcome measurement
Outcome measurements were performed at a home visit (when possible) by a research nurse blinded to group assignment at 6 months post-discharge. Duncan et al 42 argue that 6 months is the optimum follow up time for stroke trials. Some measurements were confirmed by review of hospital and PCP records.
Hypothesis Testing
Using a profile of both functional and management outcomes to reflect impacts on the differing dimensions of a care management intervention required a hypothesis testing strategy that would strongly control Type I error while ensuring high power for an alternative of consistent beneficial effects across the outcomes. Comparison of the intervention and control groups was accomplished using global hypothesis testing separately for the functional and management domains 32, 33, 43-44. We spent a double sided alpha=0.05 by using 0.04 for a global test for the first three functional domains, and 0.01 for the last two management domains, where we expected a larger, more direct impact.
Global testing for the functional domains involved testing an average of estimated differences between the groups across all the functional measures after standardization to a standard deviation (SD) of 1 for each measure. The same was done separately for the variables in the management domains. The domains were equally weighted when calculating the global test. For standardization, we used a maximum-likelihood rescaling method by iterating over modeled variances 45,46. For individual domain testing, we used the closure principle to set up a gate keeping strategy 47-50. Only when a global test was significant could we proceed to the next step of testing smaller subsets or individual domains.
Power
A range of global effect sizes consistent with the results of our pilot study of the same intervention 51 indicated that a total sample size of 380 (190 per group) would provide power of 90% or more for each of the global tests. In our pilot trial for the subset of patients similar to those in this trial, the average effect sizes for the three function domains were above 0.35 standard deviations, while for the management domains they were above 0.7 standard deviations 51.
Statistical Models
To increase precision, covariates were included in a multivariate model with overall treatment group effects, group x measure interactions, baseline covariates, and other covariates. Estimates of domain effects were tested as described above, but individual measure treatment effects are also described to fully explain results. The many covariates and outcomes available provided confidence in the missing at random assumptions (conditional on available observations) used in SAS Proc Mixed 46, 53.
Exploratory/sensitivity analyses
We provide information about subgroup interaction effects to further clarify results. As this was an exploratory exercise, no adjustment for multiple testing was provided. Alternative measures of effectiveness including change from baseline assessments and scoring simplifications were used to confirm the primary analysis.
Results
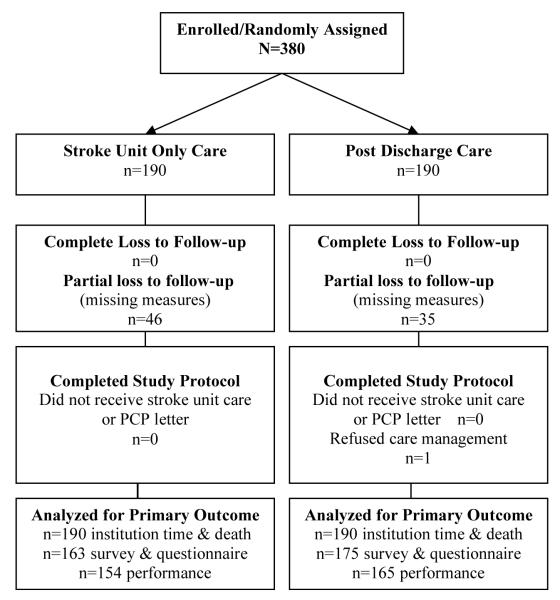
190 patients were randomized into the intervention group and 190 were randomized into the control group (figure 1). For the Institution Time and Death Domain (2), we obtained information on every patient at 6-months post randomization (0% missing). For questionnaire and interview measures in Domains 3, 4, and 5 we obtained 6-month results on 165 (13% missing) and 175 (8% missing) in the usual care and intervention groups, respectively. For performance measures in Domain 1 we obtained 6 month results on 154 (19% missing) and 165 (13% missing) in the usual care and intervention groups, respectively. We had greater missing data for the performance measures, because these required home visits. We found that many in our target population recovered without dysfunction, and were too busy for the lengthy home visit assessment. More patients were available for the phone interview questionnaires. Only one patient refused care management. All analyses followed the intention to treat principle. There were no study related adverse events.
Figure 1.
Flow of participants in STEPS CARE Trial.
Table 1 provides means or percentages for each of the two randomized groups on variables available at baseline (before randomization) and shows that the two groups were highly similar. The table also shows the severity of patient problems and the potential for improvement on these variables. As expected almost all confidence intervals on differences between the two randomized groups included zero.
Table 1.
Group Comparisons at Baseline (Before Randomization) Baseline Variables and Covariates
| Domain/Baseline Variables | Post Discharge Care N=190 mean or %,(SE) |
Stroke Unit Only N=190 mean or %,(SE) |
95% Confidence Interval on difference |
|---|---|---|---|
| Demographic Variable | |||
| Gender (% Men) | 48%, (4.) | 52%, (4.) | (−14., 6.) |
| Age (Average) | 68, (1.) | 69, (1.) | (−4., 2.) |
| Race (% African-American) | 17%, (3.) | 15%, (3.) | (−5., 10.) |
| Marital (% married) | 47%, (4.) | 46%, (4.) | (−9., 11.) |
| Living Arrangement (% home) | 73%, (3.) | 74%,(.3) | (−11., 7.) |
| Covariates | |||
| % Diabetic | 42%, (4.) | 29%, (3.) | (−3., −22.) |
| % discharged to rehab | 38%, (4.) | 41%, (4.) | (−12., 8.) |
| Number Comorbidities (Average) | 0.6, (0.1) | 0. 7, (0.1) | (0.3, 0.0) |
| % prior Myocardial Infarction | 17%, (3.) | 23%, (3.) | (−14., 2.) |
| % prior CVA, TIA, or A Fib | 38%, (4.) | 44%, (4.) | (−16., 4.) |
| Neuromotor Function | |||
| NIH Stroke Scale * (Average) | 2.0, (0.1) | 1.7, (0.1) | (−0.1, 0.7) |
| Ambulation† (Average) | 2.2, (0.1) | 2.2, (0.1) | (−0.2, 0.2) |
| Transfer‡ (Average) | 1.9, (0.1) | 1.8, (0.1) | (−0.1, 0.3) |
| Institution Time or Death | |||
| Hospital Days in Prior Year (Average) | 0.6, (0.3) | 2.1, (0.3) | (−2.3, −0.6) |
| Management of Risk | |||
| % with Systolic | 44%, (4.) | 41%, (4.) | (−7., 13.) |
| Blood Pressure >140mm§ | |||
| % with Diastolic | 6%, (2.) | 5%, (2.) | (−4., 5.) |
| Blood Pressure >90mm§ | |||
| % with total serum cholesterol | 48%, (4.) | 46%, (4.) | (−8., 12.) |
| >180mg/dl§ | |||
| CES-D (Average)¶ | 2.8, (0.2) | 3.1, (0.2) | (−0.7, 0.2) |
| % who fell in prior 2 months | 16%, (3.) | 15%, (3.) | (−6., 8.) |
| % with incontinence | 8.8%, (0.2) | 9.0%, (0.2) | (−0.7, 0.2) |
| Stroke Knowledge and Lifestyle Modification | |||
| % correct answers for Stroke | 76%., (3.) | 75%, (3.) | (−2., 3.) |
| % correct answer for Physical | 52%, (4.) | 50%, (4.) | (−2., 6.) |
| % who smoke | 20%, (3.) | 17%, (3.) | (−6., 10.) |
| % who drink >2 drinks/day | 4%, (2.) | 3%, (1.) | (−3., 5.) |
| % exercising | 73%, (3.) | 80%, (3.) | (−15., 2.) |
Note: statistics are means unless indicated by a % sign
For NIH SS, sum of 11 items, a range of 0-12, lower is better
For ambulation, 1 is independent and 6 is dependent
For transfers, 1 is independent and 6 is dependent
For systolic blood pressure, diastolic blood pressure, and total cholesterol the statistical test used the average number in each group who exceeded the upper limits of normal to be sensitive to people who were out of control
For CESD, sum of 10 items, range from 0-10, lower is better
Table 2 provides individual measure comparisons at 6 months. The means for the two groups are from the full multivariate model so they are adjusted for all covariates and missing data (46,52). The raw change scores from baseline were also compared, when baseline was measured (results not shown), and agree closely with these results. It is important to note that the differences between the two groups in SD units are consistently small for all measures in the first four domains. For example, the post discharge management group’s mean (adjusted for baseline) on the NIH Stroke scale is 1.1 and the control group’s mean was 1.2. The difference of −0.1 is clearly small and this is even more obvious in the column labeled differences in SD units. In SD units the difference is −0.06, and its confidence interval is narrow. A small difference was found consistently across all of the measures except for the Stroke Knowledge and Lifestyle Management Domain. For Domain 5 the differences are largest for the knowledge tests. Thus, these individual measures provide a convincing impression that only Domain 5 was affected by the intervention.
Table 2.
Group Comparisons of Means on Individual Measurement at 6 Months Post Randomization (adjusted for covariates and missing data)
| Domain/Individual Measurements (scoring and Direction) |
6 Mo Score Post Discharge Care Management |
6 Mo Score Stroke Unit Care Only |
Difference Treatment minus Control (diff in SD units) |
95% Confidence Interval on difference |
|---|---|---|---|---|
| Neuromotor Function | ||||
| NIH Stroke Scale (Average)* | 1.1 | 1.2 | −0.11 (−0.06) | (−0.4, 0.2) |
| Physical Performance Test†(Average) | 17.3 | 17.7 | −0.34 (−0.06) | (−1.3, 0.7) |
| Timed Up and Go test‡ (Average) | 20.8 | 18.6 | 2.2 (0.01) | (−0.9, 5.3) |
| Institution Time or Death | ||||
| % died | 4.5% | 3.5% | 2.2 (0.01) | (− 3.1, 5.3) |
| Hospital Days (Average) | 1.6 | 1.4 | 0.2 (0.04) | (−0.7, 1.2) |
| Quality of Life | ||||
| Quality of Life § (Average) | 196 | 199 | −2 (−0.07) | (−9, 5) |
| Management of Risk | ||||
| % with systolic blood pressure | 31.5% | 30.0% | 1.5 (0.03) | (−1.4, 4.0) |
| >140 mmHg * | ||||
| % with diastolic blood pressure | 5.6% | 5.2% | 0.4 (0.02) | (−0.9, 1.7) |
| >90mmHg¶ | ||||
| % with total cholesterol | 35.4% | 30.8% | 4.6 (0.1) | (−1.1, 8.2) |
| >180 mg/dl¶ | ||||
| CES-D (Average)✸ | 2.3 | 2.1 | 0.2 (0.12) | (−0.2, 0.8) |
| % with A1c>6.5%¶ | 28.3% | 22.8% | 5.5 (0.13) | (−0.8, 11.9) |
| % who fell | 38% | 39 | −1.(−0.01) | (−11, 10) |
| % incontinent | 24% | 25% | −1. (−0.03) | (−10, 7) |
| Medication Appropriateness(Average) • | 1.28 | 1.3 | −0.02 (−0.05) | (−0.1, 0.1) |
| % on anticoagulant | 90% | 89% | 1. (0.03) | (−6, 8) |
| Stroke Knowledge and Lifestyle Modification | ||||
| % correct stroke symptoms | 79.% | 76.% | 3. (0.25) | (0.4, 5) |
| % correct physical risk knowledge | 53.% | 48.% | 5. (0.28) | (2., 9.) |
| % who smoke | 14.5% | 12.6% | 1.9 (0.06) | (−2.5, 6.1) |
| % who drink>2 drinks/day | 3.7% | 1.6% | 2.1 (0.13) | (−0.7, 4.9) |
| % exercising | 81% | 71% | 10. (0.24) | (−0.1, 20) |
| % using method for | 79% | 74% | 5. (0.12) | (−4., 15.) |
| for med compliance | ||||
Note: Statistics are means unless indicated by a % sign.
Sum of 11 items, a range of 0-12 lower is better. 14% > NIH=2
Sum of 6 items, a range of 0-24, higher is better
Seconds, lower is better
Sum of 49 items, range from 49-245, higher is better
For systolic blood pressure, diastolic blood pressure, total cholesterol and hemoglobin A1c the statistical test used the average number in each group who exceeded the upper limits of normal to be sensitive to people who were out of control
For CESD, sum of 10 items, range from 0-10, lower is better
For medication appropriateness, possible scores ranged from 0-2
Table 3 provides the primary results of the hypothesis testing. The top of the table provides the estimates of the treatment effect for each of the five domains in standard deviation units. Scoring is such that higher is better and a positive sign means the treatment proved superior to control. It is clear that the effect of the treatment was near zero standard deviations for all but domain 5. Note also that the standard errors are small enough (all <0.11 standard deviations) to suggest that these small care management effects cannot be explained by insufficiently accurate estimates. The formal global and closed testing are provided in the lower section of Table 3. The global test for the 3 function domains proved non-significant at the alpha=0.04 level (p=0.53). As planned, no further testing of the three function domains followed. In contrast, the global test of the management domains was significant at alpha=0.01 (p=0.002). The closed tests for the management of risk domain proved non-significant (p=0.62), while the one for stroke knowledge and lifestyle modification proved significant (p=0.0003).
Table 3.
Domain Effect Sizes and Primary Hypothesis Tests for 6 Month Outcomes
| Domain | Difference between groups in standard deviation units with positive values indicating superior results for intervention group. (Standard Error) |
|
|---|---|---|
| Domain 1 Neuromotor Function | −0.028 | (0.087) |
| Domain 2 Institution Time or Death | −0.042 | (0.084) |
| Domain 3 Quality of Life | −0.049 | (0.11) |
| Domain 4 Management of Risk | 0.024 | (0.048) |
| Domain 5 Stroke Knowledge and Lifestyle | 0.26 | (0.070) |
| Hypothesis Test | |
|---|---|
| P−Value | |
| Global Test for Domain 1, 2, and 3 (Function) | 0.53 |
| Global Test for Domain 4 and 5 (Management) | 0.002 |
| Closed Test for Domain 4 (Management) | 0.62 |
| Closed Test for Domain 5 (Knowledge) | 0.0003 |
Exploratory Subgroup effects
We studied subgroup interaction effects for several variables including the NIH Stroke Scale, ambulation, transfer, hospital days in year prior to study, hypertension, cholesterol, CES-D, incontinence, correct answers for stroke symptoms, stroke risk knowledge, smoking, alcohol use, exercise, gender, race, age, diabetes, discharge destination, number of comorbidities, previous myocardial infarction, prior stroke/transient ischemic attack/atrial fibrillation. Only prior stroke/transient ischemic attack/atrial fibrillation identified a substantial subgroup interaction effect indicating that those patients who had any of these prior events (25% had a prior stroke, 13% had a prior transient ischemic attack, and 12% had atrial fibrillation in this group) benefited more from the care management intervention on Domain 1 specifically. Its effect size was 0.54 standard deviations in favor of prior stroke patients and its p-value was 0.02, but this was not a formal test (it was not an expected result) and we did not adjust for multiple testing when generating p values.
Frequency of and time spent in care management activities
Table 4 shows the average time spent by the care manager on specific intervention activities. Activities are divided by the time spent on each activity according to which domain the specific intervention was expected to affect most. It can be seen that the majority of the care manager’s time was spent on self management and medical management issues with major activities involving patient visits, patient education, and addressing medical issues. Very little time was devoted to addressing Allied Health or psychosocial issues.
Table 4.
Care manager activities by domain Average Time Spent (in minutes) per Patient on Activity and Domain Impacted
| ACTIVITY | Domain 1 Neuromotor Function |
Domain 2 Institution Time or Death |
Domain 3 Quality of Life |
Domain 4 Management of Risk |
Domain 5 Stroke Knowledge and Lifestyle |
Total Time per Activity |
|---|---|---|---|---|---|---|
| Contacts/Education/Meetings. | ||||||
| Median min | 90 | 11 | 6.2 | 33 | 136 | 197 |
| (Mean, SD) | (3.9,5.6) | (0.11, 0.08) | (13.4, 21.3) | (49, 48) | (145, 90) | (213, 104) |
| Number of cases | 37 | 2 | 79 | 181 | 185 | 190 |
| (%) | 20 | (1.1) | (42) | (95) | (97) | (100) |
| Medical follow up | ||||||
| Median min. | 6.3 | 0.16 | 11 | 85 | 13 | 135 |
| (Mean, SD) | (18, 27) | (0.39, 0.55) | (28, 50) | (153, 196) | (28, 39) | (221, 280) |
| Number of cases | 60 | 3 | 73 | 166 | 124 | 190 |
| (%) | (31) | (1.6) | (38.4) | (87.4) | (65.3) | (100) |
| Clerical | ||||||
| Median min | 0.40 | 0 | 1.3 | 53 | 7.5 | 70 |
| (Mean, SD) | (0.42, 0.25) | (1.5, 1.3) | (58, 35) | (12, 11) | (73, 55) | |
| Number of cases | 6 | 0 | 17 | 156 | 71 | 190 |
| (%) | (3.2) | (8.9) | (82.1) | (37.4) | (100) | |
| Allied Health | ||||||
| Median min | 2.79 | 0.79 | 1.3 | 1.8 | 0.16 | 0 |
| (Mean, SD) | (5, 8.6) | (0.12, 0.07) | (2.8, 4.3) | (2.6, 2.1) | (0.14, 0.08) | (11, 28) |
| Number of cases | 53 | 3 | 25 | 34 | 4 | 190 |
| (%) | (27.9) | (1.6) | (13.2) | (17.9) | (2.1) | (100) |
| Psychosocial | ||||||
| Median min | 0.16 | 0 | 12 | 1.37 | 0.37 | 9.0 |
| (Mean SD) | (0.24, 0.20) | (27, 43) | (2, 5.3) | (0.43, 0.31) | (31, 68) | |
| Number of cases | 4 | 0 | 102 | 26 | 7 | 190 |
| (%) | (2.1) | (53.7) | (13.7) | (3.7) | (100) | |
| Total Time per Patient | ||||||
| Per Domain | ||||||
| Median min | 0.63 | 5.5 | 30 | 198 | 165 | 424 |
| (Mean, SD) | (1.7,2.7) | (10.3,13) | (70,107) | (275,260) | (185,125) | (512,301) |
Discussion
The specific aim of this study was to test the superiority of comprehensive interdisciplinary post-discharge stroke care management in improving outcomes for stroke survivors as compared to organized acute stroke unit care with enhanced discharge planning. The results showed no significant superiority of the post-discharge care management intervention on functional outcomes at 6 months. The negative results cannot be explained by inadequate power, poor study design, or lack of comprehensive assessment. As reported by Redfern et al 53, the pilot trial upon which this study was modeled used a high quality intervention design as defined by the UK Medical Research Council Framework. In the pilot trial 22, the Institution Time or Death, Management of Risk, Stroke Knowledge and Lifestyle Modification, and Quality of Life domains all showed differences between groups greater than 0.33 standard deviations so we powered this trial to detect small effect sizes of 0.3 standard deviations. Much smaller effect sizes were seen in the current trial, with the largest being for Stroke Knowledge and Lifestyle Modification (.25) which proved significant, but all others were near zero. Therefore, this trial was sufficiently powered. Similarly, this study was theoretically grounded. Thus, the results of this study indicate that there is no additional benefit of post discharge stroke care management beyond treatment on an organized stroke unit and communication with the PCP of in-patient findings at discharge.
In the pilot trial, the Neuromotor Function domain showed a smaller effect size (.2) than the other domains. This was due to the small percentage of patients in the pilot with substantial neuromotor deficits at baseline (patients with transient ischemic attacks were included in the pilot). For this study we changed the inclusion criteria to patients with ≥1 on the NIH Stroke Scale. Still, the average NIH Stroke Scale score in this study was 1.7 for the intervention group and 2.0 for the control group. Like the pilot, this left little room for improvement. Similarly, both groups appear to have relatively good quality of life at 6 months, which may be due to the fact that the SSQOL heavily reflected function. Thus, organized stroke unit care with enhanced discharge planning may be sufficient for optimizing functional and quality of life outcomes 6 months post-discharge. Comprehensive post-discharge care management may be more appropriate for a population with greater impairment.
Like the Neuromotor Function domain, variables in the Management of Risk domain offered little room for improvement. Average systolic blood pressures were less than 8 mmHg above the target of 140. Total cholesterol levels were only 18 points, on average, higher than the target of 180. Depression, falls, and incontinence were all also relatively good at baseline. We expected all of these variables to be substantially out of control at baseline and post-discharge based on the literature, however we found these variables to be well addressed by the time the patient was discharged from the acute stroke unit and throughout follow up. Again, this indicates that organized interdisciplinary stroke unit care, effective discharge planning, review of medications, and having social services arranged while in the hospital is sufficiently effective in meeting post-discharge needs. This is consistent with previous studies that showed the effectiveness of organized stroke units 2-4, 54.
For the performance measures in Domain 1 we had substantial missing data (19% and 13%, in usual care and post-discharge care respectively), but the differences here were so small between the groups that it is hard to imagine that the missing data would have provided a significant result in favor of post-discharge care. Recall also that we used the missing at random assumption when estimating the treatment effect (46). Most importantly, we have very many baseline covariates and many other 6 month assessments that were not missing on these subjects. All of this concomitant information provides us with confidence in our adjustment for missing data bias (52).
The Stroke Knowledge and Lifestyle Modification domain was the only one to show a significant effect in favor of the intervention indicating that the intervention filled a knowledge and self-management gap despite extensive teaching on the acute stroke unit. These results indicate that the addition of post-discharge education would be a further beneficial enhancement of comprehensive stroke unit care.
Summary
The purpose of this study was to test the superiority of comprehensive interdisciplinary post-discharge care management over organized acute stroke unit care with enhanced discharge planning. We found that the treatment effect was near zero standard deviations for all but the Stroke Knowledge and Lifestyle Modification domain which showed a significant effect of the intervention (p=0.0003). Thus, comprehensive acute stroke unit care with communication to PCPs regarding inpatient findings and discharge care plans appears to be sufficient to optimize important stroke outcomes 6 months post-discharge. Our results indicate, however, that post-discharge stroke education could enhance stroke outcomes even more. Future studies must determine whether care management is more beneficial in populations without acute stroke units and/or with greater baseline need.
Acknowledgements
The authors would like to thank Michele Gareri her diligence in data collection and protocol implementation and Dr Joseph Broderick for lending his expertise in stroke trials. We would also like to thank Dr Robert Lada as well as the entire stroke unit staff for their assistance with this study. We would also like to thank Kim Peterson for her assistance with manuscript preparation.
Grant Support: Supported by the National Institute of Neurological Disorders and Stroke, grant number 5 R01 NS041333-02 and the Summa Hospitals Foundation. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Heart Association . Heart Disease and Stroke Statistics-2007 Update. American Heart Association; Dallas, Texas: 2007. [Google Scholar]
- 2.Stroke Unit Trialists’ Collaboration Organized inpatient (stroke unit) care for stroke. Cochrane Database System Review. 2002;(1):CD000197. doi: 10.1002/14651858.CD000197. [DOI] [PubMed] [Google Scholar]
- 3.Norrving B, Adams R. Organized stroke care. Stroke. 2006;37:326. doi: 10.1161/01.STR.0000200554.95094.09. [DOI] [PubMed] [Google Scholar]
- 4.Klijn C, Hankey G, American Stroke Association. European Stroke Initiative Management of acute ischaemic stroke: new guidelines from the American Stroke Association and European Stroke Initiative. Lancet Neurol. 2003;2(11):698–701. doi: 10.1016/s1474-4422(03)00558-1. [DOI] [PubMed] [Google Scholar]
- 5.Teasell R. Stroke recovery and rehabilitation. Stroke. 2003;34:365. doi: 10.1161/01.str.0000054630.33395.e2. [DOI] [PubMed] [Google Scholar]
- 6.Sulch D, Melbourn A, Perez I, et al. Integrated care pathways and quality of life on a stroke rehabilitation unit. Stroke. 2002;33(6):1600–4. doi: 10.1161/01.str.0000017144.04043.87. [DOI] [PubMed] [Google Scholar]
- 7.Nir Z, Zolotogorsky N, Sugarman H. Structured nursing intervention versus routine rehabilitation after stroke. Am J Phys Med Rehabil. 2004;83:522–529. doi: 10.1097/01.phm.0000130026.12790.20. [DOI] [PubMed] [Google Scholar]
- 8.Bisset A, MacDuff C, Chesson R, et al. Stroke services in general practice - are they satisfactory? Br J Gen Pract. 1997;47:787–793. [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 10.Stuck AE, Siu AL, Wieland GD, et al. Comprehensive Geriatric Assessment: A Meta-Analysis of Controlled Trials. Lancet. 1993;342:1032–36. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 11.Wagner E, Austin B, Von Korff M. Improving Outcomes in Chronic Illness. Manag Care. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 12.Stewart S, Vandenbroeck A, Pearson S, et al. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med. 1999;159:257–261. doi: 10.1001/archinte.159.3.257. [DOI] [PubMed] [Google Scholar]
- 13.Hansen F, Poulsen H, Sorensen K. A model of regular geriatric follow-up by home visits to selected patients discharged from a geriatric ward: A randomized controlled trial. Age Clin Exp Res. 1995;7:202–6. doi: 10.1007/BF03324316. [DOI] [PubMed] [Google Scholar]
- 14.Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ. 1996;312:1642–46. doi: 10.1136/bmj.312.7047.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen H, Schultz-Larsen K, Kreiner S, et al. Can Readmission After Stroke Be Prevented? Stroke. 2000;31:1038–1040. doi: 10.1161/01.str.31.5.1038. [DOI] [PubMed] [Google Scholar]
- 16.Joseph L, Babikian V, Allen N, et al. Risk Factor Modification in Stroke Prevention The Experience of a Stroke Clinic. Stroke. 1999;30:16–20. doi: 10.1161/01.str.30.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg G, Segal M, Berk S, et al. Stroke Transition after Inpatient Rehabilitation. Top Stroke Rehabil. 1997;4(1):64–79. doi: 10.1310/L9B9-6TB8-N4NQ-YRVH. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson P, Wolfe C, Warburton F, et al. A Long-term Follow-up of Stroke Patients. Stroke. 1997;28:507–512. doi: 10.1161/01.str.28.3.507. [DOI] [PubMed] [Google Scholar]
- 19.Sacco R, Benjamin E, Broderick J, et al. Risk Factors. Stroke. 1997;28:1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 20.Mant J, Carter J, Wade D, et al. Family support for stroke: A randomized controlled trial. Lancet. 2000;356:808–13. doi: 10.1016/S0140-6736(00)02655-6. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. JAGS. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Allen K, Hazelett S, Jarjoura D, et al. Effectiveness of a care management model for post discharge stroke/TIA patients: A randomized trial. J Stroke Cerebrovasc Dis. 2002;11:88–98. doi: 10.1053/jscd.2002.127106. [DOI] [PubMed] [Google Scholar]
- 23.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;3:385–401. [Google Scholar]
- 24.Goldstein L, Samsa G. Reliability of the National Institutes of Health Stroke Scale Extension to Non-Neurologists in the Context of a Clinical Trial. Stroke. 1997;28:307–310. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- 25.National Stroke Association National Stroke Association Stroke Prevention Guidelines. J Stroke Cerebrovasc Dis. 1998;7:162–164. [Google Scholar]
- 26.American Heart Association American Heart Association 23rd International Joint Conference on Stroke and Cerebral Circulation, Orlando, Florida. Geriatrics. 1998;53(3):84–87. [Google Scholar]
- 27. http://rcplondon.ac.uk/pubs/journal/journ_34_mar_ed5.htm.
- 28.Allen K, Hazelett S, Jarjoura D, et al. Improving stroke outcomes: implementation of a postdischarge care management model. JCOM. 2004;11(11):707–714. [Google Scholar]
- 29.Duncan D, Williams L, Brass L, et al. Quality Assessment of Stroke Care. Stroke Newsl. 1999:12–15. [Google Scholar]
- 30.Kane R, Ouslander J, Abrass I. Essentials of Clinical Geriatrics. 4 ed McGraw-Hill; New York: 1999. [Google Scholar]
- 31.O’Brien P. Procedures for comparing sample with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 32.Pocock S, Geller N, Tisatis A. The analysis of multiple endpoints in clinical trials. Biometrics. 1987;43:487–98. [PubMed] [Google Scholar]
- 33.Tilley B, Marler J, Geller N, et al. Use of a Global Test for Multiple Outcomes in Stroke Trials with Application to the National Institute of Neurological Disorders and Stroke t-PA Stroke Trial. Stroke. 1996;27(11):2136–2142. doi: 10.1161/01.str.27.11.2136. [DOI] [PubMed] [Google Scholar]
- 34.Duncan P. Evaluating the Outcomes of Stroke. Med Outcomes Trust. 1998;3(3) [Google Scholar]
- 35.Institute of Medicine. Committee on Quality of Care in America . Public Briefing: Crossing the Quality Chasm: A New Health System for the 21st Century. Natl Acad Press; 2001. [Google Scholar]
- 36.Rodgers H, Atkinson C, S B, Suddes M, et al. Randomized Controlled Trial of a Comprehensive Stroke Education Program for Patients and Caregivers. Stroke. 1999;30:2585–2591. doi: 10.1161/01.str.30.12.2585. [DOI] [PubMed] [Google Scholar]
- 37.NSA’s Stroke Prevention Advisory Board National Stroke Association Stroke Prevention Guidelines. J Stroke Cerebrovasc Dis. 1998;7(2):162–164. [Google Scholar]
- 38.Gallagher P. Comprehensive education plan for a discrete stroke population: Needs, considerations, and gaps. Axone. 1999;20(4):88–92. [PubMed] [Google Scholar]
- 39.Podsiadlo D, Richardson S. The timed “Up and Go”: A test of basic functional mobility for frail elderly persons. JAGS. 1991;39:142–48. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 40.Reuben D, Siu A. An Objective Measure of Physical Function of Elderly Outpatients: Physical Performance Test. JAGS. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 41.Williams L, Weinberger M, Harris L, et al. Development of a Stroke-Specific Quality of Life Scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 42.Duncan P, Jorgensen H, Wade D. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31(6):1429–38. doi: 10.1161/01.str.31.6.1429. [DOI] [PubMed] [Google Scholar]
- 43.Pocock S. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis, and interpretation. Control Clin Trials. 1997;18:530–545. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- 44.D’Agostino R, Russell H. Multiple endpoints, multivariable global tests. In: Colton A, editor. Encyclopedia of Biostatistics. John Wiley; New York: 1998. [Google Scholar]
- 45.Roy J. Scaled marginal models for multiple continuous outcomes. Biostatistics. 2003;4:371–383. doi: 10.1093/biostatistics/4.3.371. [DOI] [PubMed] [Google Scholar]
- 46.SAS Proc Mixed. Version 9.0 SAS Institute; Cary, North Carolina: 2004. [Google Scholar]
- 47.Lechmacher W, Wassmer G, Reitmeir P. Procedures for two-sample comparisons with multiple endpoints controlling the experiment-wise error rate. Biometrics. 1991;47:511–512. [PubMed] [Google Scholar]
- 48.Reitmeir P, Wassmer G. One-sided multiple endpoint testing in two-sample comparisons. Commun Stat-Simula. 1996;25:99–117. [Google Scholar]
- 49.Marcus R, Peritz E, Gabriel K. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 50.Jarjoura D, Hua K, Hazelett S, et al. Approaches to inference with multiple endpoints in care management trials. Paper presented at: International Conference on Health Policy Research; Chicago. Illinois; 2003. [Google Scholar]
- 51.Allen K, Jarjoura D, Hazelett S, et al. Effectiveness of Care Management For Secondary Prevention With TIA/NON-Disabled Stroke Survivors. Washington, DC: AGS 2002 Annual Scientific Mtg. [Google Scholar]
- 52.Little R. Modeling the Dropout Mechanism in Longitudinal Studies. Journal of the American Statistical Association. 1995;90:1112–1121. [Google Scholar]
- 53.Judith Redfern, Christopher McKevitt, Charles Wolfe. Development of Complex Interventions in Stroke Care: A Systematic Review. Stroke. 2006;37:2410–2419. doi: 10.1161/01.STR.0000237097.00342.a9. [DOI] [PubMed] [Google Scholar]
- 54.Ovbiagele B, Saver J, Fredieu A, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004;35:1–6. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]