Abstract
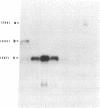
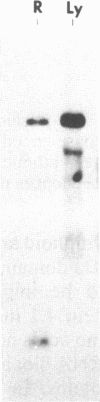
We have conducted comparative analysis of nucleotide sequences encoding erythroid and lymphoid protein 4.1 isoforms. The lymphoid protein 4.1 isoforms exhibit several nucleotide sequence motifs that appear to be either inserted into or deleted from the mRNA sequence by alternative splicing of a common mRNA precursor. One of these motifs, located within the spectrin-actin binding domain, is found only in erythroid cells and is specifically produced during erythroid cell maturation. The selective expression of the alternatively spliced mRNA during erythroid maturation implies the existence of a lineage-specific splicing mechanism whose activity is triggered by terminal maturation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. A., Weissman S. M. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T-cell differentiation. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1997–2001. doi: 10.1073/pnas.84.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Aster J. C., Welsh M. J., Brewer G. J., Maisel H. Identification of spectrin and protein 4.1-like proteins in mammalian lens. Biochem Biophys Res Commun. 1984 Mar 15;119(2):726–734. doi: 10.1016/s0006-291x(84)80311-3. [DOI] [PubMed] [Google Scholar]
- Baines A. J., Bennett V. Synapsin I is a spectrin-binding protein immunologically related to erythrocyte protein 4.1. 1985 May 30-Jun 5Nature. 315(6018):410–413. doi: 10.1038/315410a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia. J Clin Invest. 1971 Dec;50(12):2755–2760. doi: 10.1172/JCI106778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
- Correas I., Speicher D. W., Marchesi V. T. Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem. 1986 Oct 5;261(28):13362–13366. [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Cohen C. M. Platelets contain proteins immunologically related to red cell spectrin and protein 4.1. Blood. 1985 Jan;65(1):52–59. [PubMed] [Google Scholar]
- Goodman S. R., Casoria L. A., Coleman D. B., Zagon I. S. Identification and location of brain protein 4.1. Science. 1984 Jun 29;224(4656):1433–1436. doi: 10.1126/science.6374897. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Appearance of new variants of membrane skeletal protein 4.1 during terminal differentiation of avian erythroid and lenticular cells. Nature. 1985 Jan 17;313(5999):238–241. doi: 10.1038/313238a0. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Weil S. C., Tsiftsoglou A. S., Volloch V., Neumann J. R., Keys C., Housman D. E. Hemin does not cause commitment of murine erythroleukemia (MEL) cells to terminal differentiation. Blood. 1980 Sep;56(3):481–487. [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Leto T. L., Pratt B. M., Madri J. A. Mechanisms of cytoskeletal regulation: modulation of aortic endothelial cell protein band 4.1 by the extracellular matrix. J Cell Physiol. 1986 Jun;127(3):423–431. doi: 10.1002/jcp.1041270311. [DOI] [PubMed] [Google Scholar]
- Linnenbach A. J., Speicher D. W., Marchesi V. T., Forget B. G. Cloning of a portion of the chromosomal gene for human erythrocyte alpha-spectrin by using a synthetic gene fragment. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2397–2401. doi: 10.1073/pnas.83.8.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Ngai J., Stack J. H., Moon R. T., Lazarides E. Regulated expression of multiple chicken erythroid membrane skeletal protein 4.1 variants is governed by differential RNA processing and translational control. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4432–4436. doi: 10.1073/pnas.84.13.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel J. E., Beardsley D. S., Southwick F. S., Lux S. E. An analogue of the erythroid membrane skeletal protein 4.1 in nonerythroid cells. J Cell Biol. 1984 Sep;99(3):886–893. doi: 10.1083/jcb.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]