Native and selenomethionine-labelled FAD synthetase from C. ammoniagenes have been crystallized by the hanging-drop vapour-diffusion method. A MAD data set for SeMet-labelled FAD synthetase was collected to 2.42 Å resolution, while data sets were collected to 1.95 Å resolution for the native crystals.
Keywords: FAD synthetase, Corynebacterium ammoniagenes
Abstract
FAD synthetase from Corynebacterium ammoniagenes (CaFADS), a prokaryotic bifunctional enzyme that catalyses the phosphorylation of riboflavin as well as the adenylylation of FMN, has been crystallized using the hanging-drop vapour-diffusion method at 277 K. Diffraction-quality cubic crystals of native and selenomethionine-labelled (SeMet-CaFADS) protein belonged to the cubic space group P213, with unit-cell parameters a = b = c = 133.47 Å and a = b = c = 133.40 Å, respectively. Data sets for native and SeMet-containing crystals were collected to 1.95 and 2.42 Å resolution, respectively.
1. Introduction
Flavoproteins in general, and flavoenzymes in particular, participate in a large number of metabolic processes in all types of living organisms (DNA repair, cellular respiration, fatty-acid metabolism, photosynthesis, programmed cell death etc.) and are therefore critical for cell survival (Massey, 2000 ▶). The functions of these proteins rely on the properties of their cofactors, FMN or FAD, which are therefore essential for cell function. Both cofactors are synthesized in vivo from riboflavin (RF, vitamin B2) in a two-step process. In the first reaction RF is phosphorylated to FMN by riboflavin kinase (RFK). The produced FMN can then be adenylylated to generate FAD by FMN-adenylyltransferase (FMNAT; Efimov et al., 1998 ▶; Barile et al., 2000 ▶). RFK and FMNAT activities are present in all kingdoms of life from bacteria to mammals. However, whereas in mammals and yeast monofunctional enzymes, RFK and FMNAT, are involved in each of these activities (Santos et al., 2000 ▶), in prokaryotic organisms a single bifunctional enzyme, FAD synthetase (FADS), is in charge of both activities. In prokaryotic FADS the C-terminal domain is homologous to monofunctional RFKs, while the N-terminal domain presents a remote similarity to some nucleotidyltransferases (Manstein & Pai, 1986 ▶; Krupa et al., 2003 ▶). In plants, monofunctional enzymes as well as bifunctional enzymes have been reported. However, these bifunctional enzymes combine RFK and FMNAT activities with other activities (Sandoval & Roje, 2005 ▶; Sandoval et al., 2008 ▶; Giancaspero et al., 2009 ▶). Despite the fact that three-dimensional structures have been reported for several RFKs, even in the presence of ligands (Bauer et al., 2003 ▶; Karthikeyan et al., 2003 ▶), the first structure of a monofunctional FMNAT has only just been reported (Huerta et al., 2009 ▶). Finally, only one structure is available of a bifunctional enzyme, that from Thermotoga maritima (TmFADS; Wang et al., 2003 ▶, 2005 ▶), but no functional studies related to this enzyme are available.
The bifunctional enzyme from Corynebacterium ammoniagenes, CaFADS, has been widely used to prepare FMN and FAD analogues (Murthy & Massey, 1997 ▶). Its initial functional characterization suggested a two-step mechanism, with a single flavin-binding site in which substrates and products bind and are released sequentially (Efimov et al., 1998 ▶). A recent in silico structural model based on the structures of TmFADS and the RFKs of Homo sapiens (HsRFK) and Saccharomyces pombe (SpRFK), as well as a thermodynamic analysis of the interaction of substrates and products (Frago et al., 2008 ▶, 2009 ▶), suggest that CaFADS presents two almost independent domains with two ATP-binding and two flavin-binding sites.
In this context, knowledge of the crystal structure of CaFADS will be essential in order to understand the catalytic mechanism of the bifunctional FADS family. In spite of the fact that TmFADS, HsRFK and SpRFK present sequence similarities to CaFADS of 40.2, 41.4 and 38.2%, respectively (Frago et al., 2008 ▶), structural determination of CaFADS by the molecular-replacement technique using these X-ray structures as initial models was unsuccessful. Therefore, we expressed and crystallized selenomethionine-labelled CaFADS (SeMet-CaFADS) in order to solve this structure by the MAD technique. The preliminary results are presented here.
2. Experimental procedures
2.1. Production and purification of native CaFADS and SeMet-CaFADS
The PET28-CaFADS plasmid coding for native CaFADS was expressed in LB cultures of Escherichia coli BL21 (DE3) by IPTG induction as described previously (Frago et al., 2009 ▶). SeMet-CaFADS was generated by modification of published protocols (Guerrero et al., 2001 ▶; Stols et al., 2004 ▶). For expression of SeMet-CaFADS, non-auxotrophic E. coli BL21 (DE3) cells containing the PET28-CaFADS plasmid were grown at 310 K in M9 minimal salt medium (Sambrook & Russell, 2001 ▶) containing 0.4%(v/v) glucose as a carbon source, 1.5 µM thiamine, 0.15%(v/v) ferrous sulfate chelate solution (Sigma–Aldrich) and 30 µg ml−1 kanamycin. When the culture reached an OD of 0.6–0.8, overexpression was induced with 1 mM IPTG. Simultaneously, an l-amino-acid cocktail including l-Val, l-Leu, l-Ile, l-Lys, l-Phe and l-Thr and freshly prepared SeMet solution were added. The final concentrations of these amino acids in the culture were 50 mg l−1 for Ile, Leu, Val and SeMet, and 100 mg l−1 for Lys, Phe and Thr. Expression was induced overnight (12–14 h) at 310 K and 180 rev min−1.
Both native CaFADS and SeMet-CaFADS were purified following a previously described protocol (Frago et al., 2008 ▶) consisting of 45% ammonium sulfate fractionation followed by sequential Phenyl-Sepharose and DEAE-Cellulose chromatography. Quantification of the protein was performed spectrophotometrically using the experimentally determined extinction coefficient of 28 100 M −1 cm−1 at 279 nm (Frago et al., 2009 ▶).
A molecular weight of 37 123.1 was obtained by electrospray mass spectrometry for the produced SeMet-CaFADS; the theoretical value for the native protein is 36 843.5. This confirmed the incorporation of six Se atoms. Circular dichroism and enzyme-activity assays were carried out in order to confirm that the SeMet-CaFADS was correctly folded and active (data not shown).
2.2. Crystallization
Native CaFADS and SeMet-CaFADS were dialyzed in 20 mM Tris pH 8.0 with 1 mM DTT and concentrated to 10 mg ml−1. Crystallization conditions for the native protein were initially screened at 295 and 277 K using the commercial kits JBScreen 1–4 (Jena Bioscience) and Crystal Screens 1 and 2 (Hampton Research) with the hanging-drop vapour-diffusion method. The drops consisted of 1 µl reservoir solution and 2 µl protein solution (10 mg ml−1). Native crystals were initially obtained at 277 K in a condition containing 1.5 M Li2SO4 and 0.1 M HEPES–NaOH pH 7.5. Crystals grew to maximum dimensions of 0.5 × 0.5 × 0.5 mm after 10 d.
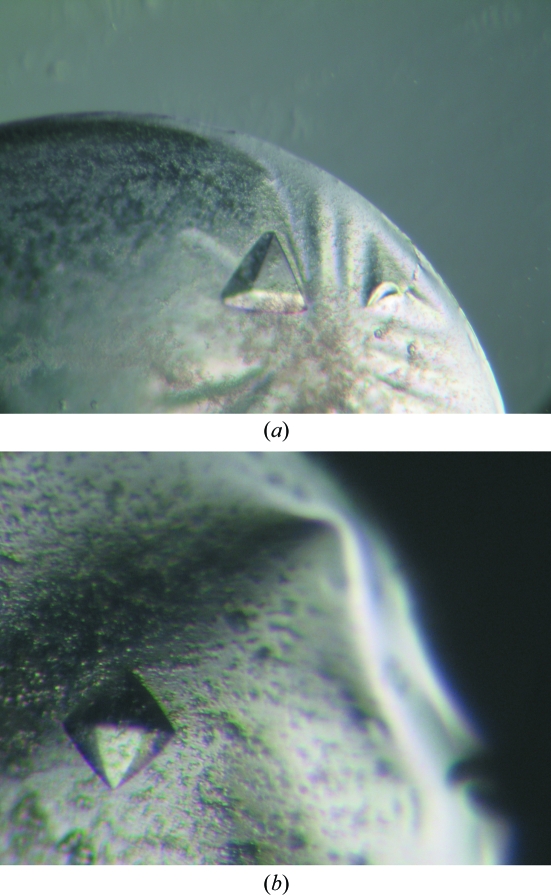
The crystallization conditions for SeMet-CaFADS differed with respect to those for native FADS. High-throughput techniques using a NanoDrop robot (Innovadyne Technologies Inc.) at 295 K were used to assay alternative crystallization conditions for SeMet-CaFADS using a 10 mg ml−1 enzyme sample and the following commercial kits: Crystal Screens I, II and Lite, Index Screen and SaltRx (Hampton Research), PACT Suite and JCSG+ Suite (Qiagen), and Precipitant Synergy (Jena Bioscience). Despite the large number of conditions assayed, no crystal formation was observed. SeMet-CaFADS was then dialyzed in 40 mM potassium phosphate buffer pH 6.8. Using this sample, small crystals were observed in drops containing the crystallization conditions for the native enzyme (1.5 M Li2SO4 and 0.1 M HEPES–NaOH pH 7.5) at 277 K after 10 d. Variation of the protein:precipitant ratio influenced the number of nucleation events as well as the size of the crystals, but changes in the salt concentration or buffer type prevented crystal formation. The best crystals were obtained in drops containing 2 µl reservoir solution and 3 µl protein solution (10 mg ml−1). The streak-seeding technique with native small crystals greatly increased both the reproducibility of crystallization and the size of the crystals. Crystals grew to maximum dimensions of 0.3 × 0.3 × 0.3 mm. Crystals grew from a heavy amorphous precipitate and small salt crystals were often observed in the same drops. SeMet-CaFADS crystals grown by streak-seeding exhibited the same pyramidal habit as the native crystals, while crystals grown without seeding appeared as bipyramids (Fig. 1 ▶).
Figure 1.
Crystals of CaFADS obtained after crystallization trials at 277 K in conditions containing 0.1 M HEPES–NaOH pH 7.5 and 1.5 M lithium sulfate. (a) Native CaFADS crystals and (b) SeMet-CaFADS crystals grown without seeding.
2.3. X-ray diffraction experiments:
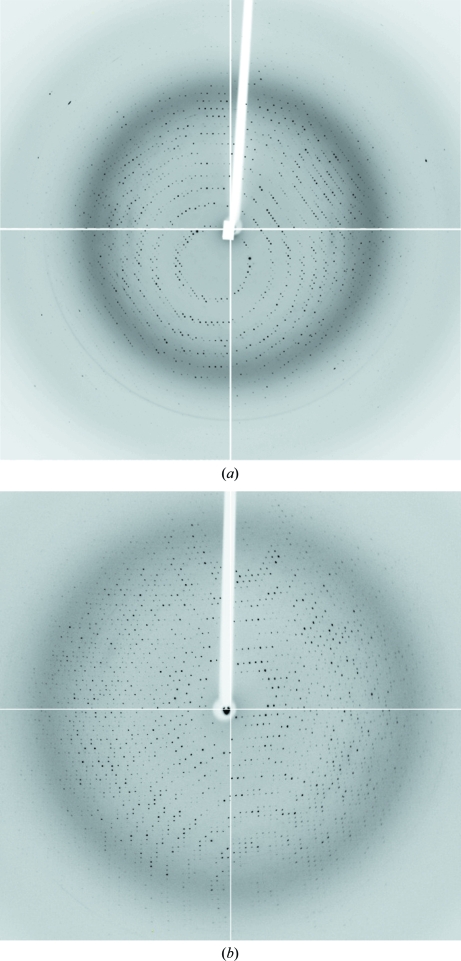
Crystals were flash-cooled using cryoprotectant solutions containing 50% reservoir solution and 50% saturated Li2SO4 solution. Diffraction data sets from native crystals were collected on the ID14-2 beamline at the European Synchrotron Radiation Facility (ESRF, Grenoble; Fig. 2 ▶ a). Data sets were collected at 100 K using a wavelength of 0.93300 Å. The data were processed, scaled and reduced with MOSFLM (Leslie, 2006 ▶) and SCALA (Evans, 2006 ▶) from the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶).
Figure 2.
X-ray diffraction patterns from (a) native CaFADS crystals (oscillation range 1°) and (b) SeMet CaFADS crystals (oscillation range 1°). Reflections were observed to 1.56 and 1.90 Å resolution, respectively.
Diffraction data sets for SeMet-CaFADS crystals were collected on the BM16 beamline at ESRF. The X-ray fluorescence spectrum of selenium was used to determine the optimal wavelengths for data collection. Data sets were collected at three wavelengths (0.97942, 0.97919 and 0.90752 Å; peak, inflection and remote, respectively) at 100 K (Fig. 2 ▶ b). As the crystals did not suffer significant radiation damage, the number of images was increased in order to obtain a greater redundancy of the data. Data sets were processed and scaled using XDS (Kabsch, 1988 ▶) and SCALA.
Data-collection statistics for native CaFADS and SeMet-CaFADS crystals are summarized in Table 1 ▶.
Table 1. Data-collection statistics for native CaFADS and SeMet-CaFADS.
Values in parentheses are for the highest resolution shell.
| SeMet-CaFADS | ||||
|---|---|---|---|---|
| Native CaFADS | Peak | Inflection | Remote | |
| Crystal data | ||||
| Space group | P213 | P213 | ||
| Unit-cell parameter (Å) | a = 133.47 | a = 133.40 | ||
| Data collection | ||||
| Temperature (K) | 100 | 100 | ||
| Wavelength (Å) | 0.93300 | 0.97919 | 0.97942 | 0.90752 |
| Resolution (Å) | 54.23–1.95 (2.06–1.95) | 24.77–2.42 (2.55–2.42) | 24.77–2.41 (2.54–2.41) | 24.77–2.40 (2.52–2.40) |
| Total reflections | 661144 | 631373 | 631754 | 692411 |
| Unique reflections | 57834 | 30479 | 30505 | 31179 |
| Average I/σ(I) | 19.9 (2.7) | 23.6 (4.7) | 23.6 (4.5) | 23.3 (6.1) |
| Completeness (%) | 100 (100) | 99.5 (97.3) | 99.4 (96.2) | 99.9 (100) |
| Redundancy | 11.4 (8.7) | 20.7 (12.6) | 20.7 (12.5) | 22.2 (19.8) |
| Rmerge† | 0.09 (0.46) | 0.11 (0.54) | 0.11 (0.57) | 0.12 (0.65) |
R
merge = 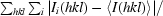
 .
.
3. Results and discussion
Both the native CaFADS crystals and the SeMet-CaFADS crystals grown using seeding belonged to the same cubic space group P213, as suggested by POINTLESS (Evans, 2006 ▶). Their unit-cell parameters were a = b = c = 133.47 and a = b = c = 133.40 Å and they diffracted to 1.95 and 2.42 Å resolution, respectively. SeMet-CaFADS crystals grown without seeding diffracted to 3.5 Å resolution, but determination of the unit cell and space group was ambiguous. Considering the molecular weight of CaFADS and the unit-cell volume, a Matthews coefficient of 2.68 Å3 Da−1 (Matthews, 1968 ▶) with two monomers in the asymmetric unit and a solvent content of 54.2% were obtained.
Structural determination is currently in progress.
Acknowledgments
The authors thank Dr J. M. Mancheño for help during crystal mounting and data collection. We also thank G. Fox from the BM16 beamline at ESRF for support during synchrotron data collection. This work was supported by the Spanish Ministry of Education and Science (BIO2007-65890-C02-01 to MM and BFU2008-01711/BMC to JAH). BH holds a fellowship from the Spanish Ministry of Science and Innovation (FPU program).
References
- Barile, M., Brizio, C., Valenti, D., De Virgilio, C. & Passarella, S. (2000). Eur. J. Biochem.267, 4888–4900. [DOI] [PubMed]
- Bauer, S., Kemter, K., Bacher, A., Huber, R., Fischer, M. & Steinbacher, S. (2003). J. Mol. Biol.326, 1463–1473. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Efimov, I., Kuusk, V., Zhang, X. & McIntire, W. S. (1998). Biochemistry, 37, 9716–9723. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Frago, S., Martinez-Julvez, M., Serrano, A. & Medina, M. (2008). BMC Microbiol.8, 160. [DOI] [PMC free article] [PubMed]
- Frago, S., Velazquez-Campoy, A. & Medina, M. (2009). J. Biol. Chem.284, 6610–6619. [DOI] [PMC free article] [PubMed]
- Giancaspero, T. A., Locato, V., de Pinto, M. C., De Gara, L. & Barile, M. (2009). FEBS J.276, 219–231. [DOI] [PubMed]
- Guerrero, S. A., Hecht, H.-J., Hofmann, B., Biebl, H. & Singh, M. (2001). Appl. Microbiol. Biotechnol.56, 718–723. [DOI] [PubMed]
- Huerta, C., Borek, D., Machius, M., Grishin, N. V. & Zhang, H. (2009). J. Mol. Biol.389, 388–400. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (1988). J. Appl. Cryst.21, 916–924.
- Karthikeyan, S., Zhou, Q., Mseeh, F., Grishin, N. V., Osterman, A. L. & Zhang, H. (2003). Structure, 11, 265–273. [DOI] [PubMed]
- Krupa, A., Sandhya, K., Srinivasan, N. & Jonnalagadda, S. (2003). Trends Biochem. Sci.28, 9–12. [DOI] [PubMed]
- Leslie, A. G. W. (2006). Acta Cryst. D62, 48–57. [DOI] [PubMed]
- Manstein, D. J. & Pai, E. F. (1986). J. Biol. Chem.261, 16169–16173. [PubMed]
- Massey, V. (2000). Biochem. Soc. Trans.28, 283–296. [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Murthy, Y. V. & Massey, V. (1997). Methods Enzymol.280, 436–460. [DOI] [PubMed]
- Sambrook, J. & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press.
- Sandoval, F. J. & Roje, S. (2005). J. Biol. Chem.280, 38337–38345. [DOI] [PubMed]
- Sandoval, F. J., Zhang, Y. & Roje, S. (2008). J. Biol. Chem.283, 30890–30900. [DOI] [PMC free article] [PubMed]
- Santos, M. A., Jimenez, A. & Revuelta, J. L. (2000). J. Biol. Chem.275, 28618–28624. [DOI] [PubMed]
- Stols, L., Millard, C. S., Dementieva, I. & Donnelly, M. I. (2004). J. Struct. Funct. Genomics, 5, 95–102. [DOI] [PubMed]
- Wang, W., Kim, R., Jancarik, J., Yokota, H. & Kim, S.-H. (2003). Proteins, 52, 633–635. [DOI] [PubMed]
- Wang, W., Kim, R., Yokota, H. & Kim, S.-H. (2005). Proteins, 58, 246–248. [DOI] [PubMed]