Neoagarobiose hydrolase from the marine bacterium Saccharophagus degradans 2-40 was overexpressed in Escherichia coli and crystallized in the monoclinic space group C2, with unit-cell parameters a = 129.83, b = 76.81, c = 90.11 Å, β = 101.86°.
Keywords: bioenergy, neoagarobiose, hydrolases
Abstract
Many agarolytic bacteria degrade agar polysaccharide into the disaccharide unit neoagarobiose [O-3,6-anhydro-α-l-galactopyranosyl-(1→3)-d-galactose] using various β-agarases. Neoagarobiose hydrolase is an enzyme that acts on the α-1,3 linkage in neoagarobiose to yield d-galactose and 3,6-anhydro-l-galactose. This activity is essential in both the metabolism of agar by agarolytic bacteria and the production of fermentable sugars from agar biomass for bioenergy production. Neoagarobiose hydrolase from the marine bacterium Saccharophagus degradans 2-40 was overexpressed in Escherichia coli and crystallized in the monoclinic space group C2, with unit-cell parameters a = 129.83, b = 76.81, c = 90.11 Å, β = 101.86°. The crystals diffracted to 1.98 Å resolution and possibly contains two molecules in the asymmetric unit.
1. Introduction
Recently, marine biomass has been highlighted as a raw material for bioenergy production (Horn, 2009 ▶). Specifically, red algal biomass such as Rhodophyta is known to have several advantages over land-plant biomass (cellulosic biomass; Renn, 1997 ▶; Park et al., 2008 ▶). For instance, algal biomass does not compete with food crops, contains a negligible amount of lignin that is resistant to degradation and can be harvested several times a year (Park et al., 2008 ▶). Agar polysaccharide (AP) is a major constituent of red algal biomass and is a copolymer of β-d-galactose and 3,6-anhydro-α-l-galactose, which are polymerized with alternating α-(1,3) or β-(1,4) linkages (Araki, 1956 ▶). Although the metabolic fate of agar polysaccharide is not yet known, many agarolytic bacteria can hydrolyze AP in multiple steps using endo- or exo-β-agarases, yielding neoagarobiose [O-3,6-anhydro-α-l-galactopyranosyl-(1→3)-d-galactose] as the final end product (Ekborg et al., 2006 ▶). Because neoagarobiose is not a fermentable sugar in common bacteria, converting neoagarobiose into monomeric fermentable sugars (e.g. d-galactose) is a crucial step in both metabolizing AP and producing raw materials from red algal biomass in bioenergy production. Neoagarobiose hydrolase (NABH) is known to hydrolyze the α-(1,3)-linkage in neoagarobiose, yielding the monomeric sugars β-d-galactose and 3,6-anhydro-α-l-galactose, and is regarded as a essential enzyme in bacteria that use agar as a sole carbon source (Ekborg et al., 2006 ▶). There have been several studies of the biochemical activity of NABH, but no information at the molecular level such as the sequence, structure or molecular mechanism of the protein is available (Day & Yaphe, 1975 ▶; Sugano et al., 1994 ▶; Suzuki et al., 2002 ▶). Here, we report preliminary crystallization studies of NABH, which will provide information on the saccharification of AP for bioenergy production and help in understanding the metabolic fate of AP in agarolytic bacteria.
2. Protein purification and crystallization
The full-length neoagarobiose hydrolase (NABH) gene (EMBL ID ABD81917), consisting of 1104 nucleotides, was amplified from Saccharophagus degradans 2-40 genomic DNA by PCR (primer 1, 5′-ATGAGCGATTCAAAAGTAAATAAAAAATTG-3′; primer 2, 5′-TACTGCTCCGGAATCGCCTG-3′) and cloned into a pJL vector designed for ligation-independent cloning with a His tag at the carboxy-terminus (Lee & Kim, 2009 ▶). NABH was recombinantly expressed in Escherichia coli B834 (DE3) cells grown in M9 minimal medium supplemented with l-selenomethionine (SeMet) by induction with 0.1 mM isopropyl β-d-1-thiogalactopyranoside. The expression-cell paste was lysed using a sonicator in 20 mM Tris–HCl pH 7.0 and insoluble particles were removed by centrifugation at 100 000g. NABH was purified from the soluble extract by immobilized nickel ion-affinity chromatography (IMAC) with a HisTrap HP 5 ml column (GE Healthcare) followed by anion-exchange chromatography (AIEX) using a HiTrap Q FF 5 ml column (GE Healthcare). Purified α-NABH was desalted to 20 mM Tris pH 7.4 buffer with a HiPrep 26/10 desalting column (GE Healthcare). The desalted sample was concentrated to a final concentration of 9 mg ml−1 using a 10 kDa molecular-weight cutoff spin concentrator. Dithiothreitol was added to the final sample to a concentration of 1 mM. Crystallization trials were performed using the sparse-matrix method (Jancarik & Kim, 1991 ▶) with a Phoenix robot (Art Robbins Instruments) using Index Screen (Hampton Research). Drops were set up in a 96-well crystal plate by mixing equal volumes of protein solution and precipitant solution (0.5 µl each) and equilibrated against a 50 µl reservoir at room temperature. Crystals appeared in four conditions, but the best shaped and best diffracting crystals (Fig. 1 ▶) were obtained using 2.4 M sodium malonate pH 7.0; they appeared overnight and grew to final dimensions of 120 × 120 µm within a week.
Figure 1.
Crystals of neoagarobiose hydrolase crystallized using 2.4 M sodium malonate pH 7.0. These crystals diffracted to 1.98 Å resolution. This picture was taken under polarized light and the crystals are colourless. The dimensions of the crystals in the figure are 120 × 120 µm.
3. X-ray data collection
Crystals were removed from the crystallization plate using a nylon loop and quickly soaked in cryoprotectant solution (3.1 M sodium malonate pH 7.0) before flash-cooling in liquid nitrogen. Diffraction data were collected on the Macromolecular Crystallography Facility beamline 5.0.2 of the Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory (LBNL). All diffraction images were recorded with an ADSC Q315 detector. The best data set was collected using a 90° oscillation range with Δϕ = 1° at 0.9784 Å. All data were processed using HKL-2000 (Otwinowski & Minor, 1997 ▶). The Matthews coefficient (Matthews, 1968 ▶) was calculated using CCP4 (Collaborative Computational Project, Number 4, 1994 ▶). The crystallographic data statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics for the neoagarobiose hydrolase crystal.
Values in parentheses are for the highest resolution shell.
| Space group | C2 |
| Unit-cell parameters (Å) | a = 129.83, b = 76.81, c = 90.11, β = 101.86 |
| Resolution (Å) | 50–1.98 (2.05–1.98) |
| Total No. of observations | 226401 (22764) |
| No. of unique observations | 58856 (5837) |
| Multiplicity | 3.8 (3.9) |
| Data completeness (%) | 98.4 (98.1) |
| 〈I/σ(I)〉 | 25.3 (4.1) |
| Rmerge (%)† | 0.086 (0.46) |
R
merge = 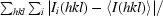
 , where
, where  denotes the sum over all reflections and
denotes the sum over all reflections and 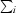 is the sum over all equivalent and symmetry-related reflections.
is the sum over all equivalent and symmetry-related reflections.
4. Results and discussion
NABH, a key enzyme in the agarolytic pathway, contains 368 amino-acid residues and has a molecular weight of 41.6 kDa. Recombinant NABH was expressed in E. coli with a His tag at the C-terminus and was purified by IMAC and AIEX to produce protein of sufficient quality for crystallization trials. The crystals were originally obtained in 0.1 M Tris pH 8.1, 20% PEG 3350, 0.2 M MgCl2 in hanging-drop plates. These crystals were too fragile to be handled for X-ray diffraction testing. Knowing that the protein crystallized and that there were no known crystal structures of homologues, we directly proceeded to crystallization trials of SeMet-derivative protein. The initial crystallization trial was performed by setting up 96 conditions using Index Screen from Hampton Research, which yielded several new crystallization conditions.
The best diffraction-quality crystals of SeMet-derivative protein were obtained using 2.4 M sodium malonate pH 7.0. The crystals diffracted to 1.98 Å resolution using synchrotron radiation at ALS (Fig. 2 ▶). The crystal belonged to the C-centred monoclinic space group C2, with unit-cell parameters a = 129.83, b = 76.81, c = 90.11 Å, α = γ = 90, β = 101.86°. The estimated Matthews coefficient (V M) was 2.65 Å3 Da−1, with a solvent content of 53%, assuming two molecules to be present in the asymmetric unit. The crystals fluoresced weakly at the Se edge (data not shown) and were prone to radiation damage, possibly owing to their small size. Ongoing experiments to improve the SeMet crystals and for cocrystallization with ligands are under way. The crystal structure of NABH will help to elucidate the reaction mechanism of the hydrolysis of neoagarobiose into d-galactose and 3,6-anhydro-l-galactose, which will in turn provide information on the saccharification process of marine biomass for the production of bioenergy.
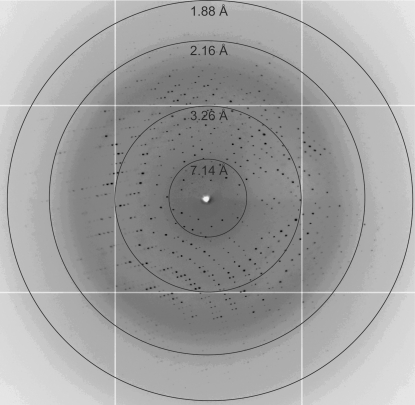
Figure 2.
Diffraction image of neoagarobiose hydrolase crystals obtained using synchrotron radiation at the Advanced Light Source, Lawrence Berkeley National Laboratory (beamline 5.0.2).
Acknowledgments
We are grateful to Professor Sung-Hou Kim for letting us use his facility for protein purification and crystallization, to Dr Jose Henrique Pereira for his help and advice in preparing this manuscript and to the staff of the Berkeley Center for Structural Biology for their help and advice. The Advance Light Source at Lawrence Berkeley National Laboratory is supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Sciences Division of the US Department of Energy under Contract No. DE-AC03-76SF00098.
References
- Araki, C. (1956). Bull. Chem. Soc. Jpn, 29, 543–544.
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Day, D. F. & Yaphe, W. (1975). Can. J. Microbiol.21, 1512–1518. [DOI] [PubMed]
- Ekborg, N. A., Taylor, L. E., Longmire, A. G., Henrissat, B., Weiner, R. M. & Hutcheson, S. W. (2006). Appl. Environ. Microbiol.72, 3396–3405. [DOI] [PMC free article] [PubMed]
- Horn, S. J. (2009). Seaweed Biofuels: Production of Biogas and Bioethanol from Brown Macroalgae. Saarbrücken: VDM Verlag.
- Jancarik, J. & Kim, S.-H. (1991). J. Appl. Cryst.24, 409–411.
- Lee, J. & Kim, S.-H. (2009). Protein Expr. Purif.63, 58–61. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Park, J.-I., Woo, H.-C. & Lee, J.-H. (2008). Korean Chem. Eng. Res.46, 833–844.
- Renn, D. (1997). Trends Biotechnol.15, 9–14.
- Sugano, Y., Kodama, H., Terada, I., Yamazaki, Y. & Noma, M. (1994). J. Bacteriol.176, 6812–6818. [DOI] [PMC free article] [PubMed]
- Suzuki, H., Sawai, Y., Suzuki, T. & Kawai, K. (2002). J. Biosci. Bioeng.93, 456–463. [DOI] [PubMed]