Abstract
Vascular endothelial growth factor (VEGF) and endometrial angiogenesis play a critical role in successful embryonic implantation. Despite many studies of the effects of estrogen and progesterone on VEGF expression, its focal regulation at the site of implantation is unknown. Retinoic acid (RA) has been reported to regulate VEGF in a variety of cell types. Because localized RA synthesis occurs within the periimplantation endometrium, we tested the possibility that RA regulates VEGF production in endometrial stromal cells. Using primary and telomerase-immortalized human endometrial stromal cells, we determined that RA alone did not alter constitutive levels of VEGF production, but markedly amplified secretion when the cells were cotreated with activators of VEGF gene transcription (12-O-tetradecanoyl phorbol-13-acetate, TPA; TGF-β; and IL-1β). Whereas TPA or TGF-β alone stimulated VEGF promoter activity and up-regulated mRNA levels, significant protein secretion was detected only after RA was added to the culture systems. Analysis of retinoids in secretory phase endometrial biopsies indicated that endogenous RA accumulated at concentrations sufficient to induce VEGF secretion. Polyribosome profile analysis showed that the addition of RA to transcriptional activators of VEGF shifted the translational suppressed VEGF mRNA transcripts into larger polyribosome complexes engaged in active translation. Although the precise mechanism(s) of the RA effect remains to be defined, it appears to be mediated by reactive oxygen species; the antioxidant N-acetylcysteine inhibited RA+TPA-stimulated secretion of VEGF by more than 80%. Together, our results demonstrate that in human endometrial stromal cells, RA can combine with transcriptional activators of VEGF to augment VEGF secretion through a translational mechanism of action mediated by reactive oxygen species. These findings suggest a link between the spatiotemporal changes of retinoid synthesis in the periimplantation stroma and the capacity to quickly up-regulate focal VEGF secretion needed to induce early angiogenic events of pregnancy.
In endometrial stromal cells, RA combines with transcriptional activators of VEGF to enhance translational efficiency. This mechanism will quickly upregulate VEGF secretion during early pregnancy.
The critical role of endometrial angiogenesis related to decidualization, early placentation, and embryo survival is widely accepted (1). Coordinated vascular development needs to occur on both maternal and fetal sides of the unique implantation interface. The remarkable finding of Ferrara et al. (2) that haplodeficiency for vascular endothelial growth factor (VEGF) led to embryonic lethality in mice supports the fetal requirement for this angiogenic factor, but it also appears that normal maternal production of VEGF is likewise necessary for decidual vascularization (3). In this regard, two key vascular changes have long been noted to occur during the decidualization process in women during the mid-late secretory phase of the menstrual cycle (days LH+9 to LH+11) (4). The first is a highly localized remodeling of the perivascular stroma surrounding the spiral arterioles, followed by a secondary wave of generalized stromal edema, sometimes referred to as the “pseudodecidual” or “predecidual” reaction. It is very likely that these phenomena are mediated directly via the vascular permeability action of VEGF (5). Dysregulation of these processes can result in defective implantation and early miscarriage. However, even if miscarriage is averted, failure of normal angiogenic growth factor production in early pregnancy is associated with subsequent pregnancy complications including preeclampsia and fetal growth restriction (6,7). The exact mechanism(s) underlying regulation of VEGF and other angiogenic factor production during this crucial period of development is unknown.
Stromal cell decidualization is controlled by a combination of maternal hormones and signals from the implanting embryo that are collectively necessary for successful implantation. Investigations from a number of laboratories have demonstrated the presence of retinoid-binding proteins at the site of decidualization and have established that the action of retinoic acid (RA) is essential for proper decidualization to occur (8). Studies have also shown that the majority of RA is derived from local biosynthesis, and the ability of endometrial cells to convert retinol to RA appears to be under the control of progesterone and correlates with the degree of stromal cell decidualization (9). Although the role of RA in stromal cell decidualization and implantation is unknown, a number of known avenues suggest how this retinoid could impact these processes. These include regulation by RA of certain enzymes [e.g. transglutaminase type II, metalloproteinases (10,11)] and cytokines [e.g. TGFβ and IL-11 (12)] that play crucial roles in decidualization and implantation. Of particular relevance to the present work is the demonstrated ability of RA to regulate VEGF expression in certain cells and tissues. As such, RA-induced down-regulation of VEGF mRNA and protein appears in a number of malignant cell types (13,14,15) although up-regulation of VEGF has also been described (16). Similarly, in normal cells and tissue, both down- and up-regulation of VEGF by RA have been reported (17,18). Thus, the action of RA on VEGF appears to be cell type specific, and the factors and molecular events that control this specificity of action are unknown. We postulate that one role played by retinoids in the endometrium is to spatiotemporally regulate VEGF secretion during the periimplantation phase of the menstrual cycle and in early pregnancy to sustain embryonic development. In support of this hypothesis, our results demonstrate that RA can rapidly promote VEGF secretion from endometrial stromal cells through a posttranscriptional mechanism that involves increased translational efficiency.
Results
RA enhances VEGF secretion from human endometrial stromal cells
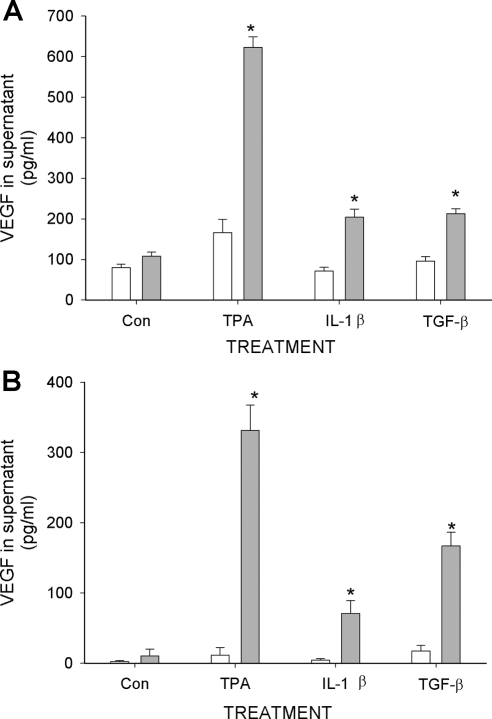
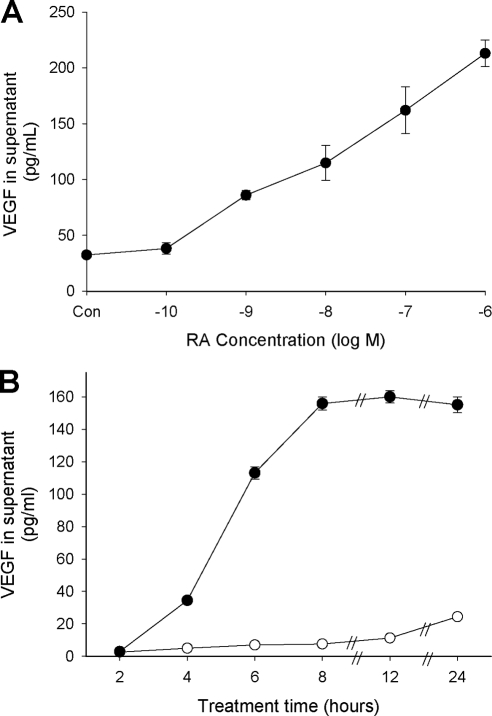
We first examined the effects of RA on VEGF secretion from primary human endometrial stromal cells (ESC) obtained from tissue biopsies. Figure 1A shows that RA alone does not alter the constitutive (control) levels of VEGF secretion into the supernatant of 24-h cultures, but enhances secretion when the cells are co-treated with 12-O-tetradecanoyl-phorbol-13-acetate (TPA), IL-1β, or TGF-β. In contrast to that seen in some other cell systems (19,20,21), these compounds did not, by themselves, significantly affect the concentration of VEGF in the culture supernatant. Identical effects were seen using HESC as a cell line model of human ESC (HESC; Fig. 1B) (22,23). However, the constitutive levels of secreted VEGF, and the enhanced levels detected in the treated HESC cultures, generally were less than seen in the primary cultures. In both cases, concentrations of VEGF were greatest in the presence of RA + TPA. Dose- and time-dependent experiments with RA and TPA indicated that significantly higher levels of VEGF were detected with RA concentrations as low as 1 nm and that increased levels of VEGF could be detected in the culture supernatant after only 4 h of treatment (Fig. 2).
Figure 1.
Stimulation of VEGF secretion from primary ESC (A) and from HESC (B) by RA. Cells were cultured in 12-well culture plates in the absence (Con) or presence of TPA (50 nm), IL-1β (5 ng/ml), or TGF-β (5 ng/ml), and cotreated with 1 μm RA (gray bar) or solvent control (open bar). After 24 h, cultured supernatant was analyzed for VEGF concentration by ELISA. Values represent mean ± sem of at least three independent experiments for each condition. *, Significant difference from corresponding treatment without RA. Con, Control.
Figure 2.
Dose response (A) and time course (B) of RA + TPA treatment on VEGF secretion from HESC. In panel A, cells were treated overnight with 50 nm TPA plus the indicated concentrations of RA. Results showed significant enhancement of VEGF secretion starting at 1 × 10−9 m RA. In panel B, cells were treated with 1 μm RA + 50 nm TPA (•) or solvent control (○) for the times indicated. Increased levels of VEGF were detectable in the supernatant starting at 4 h of treatment and peaked at 8 h. Symbols without error bars indicate that the sem was less than the value represented by the width of the symbol.
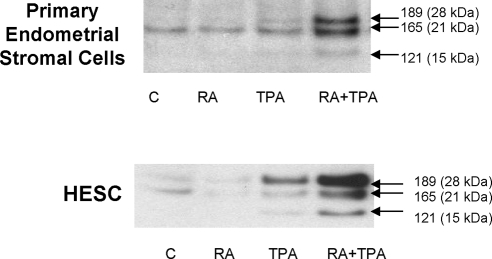
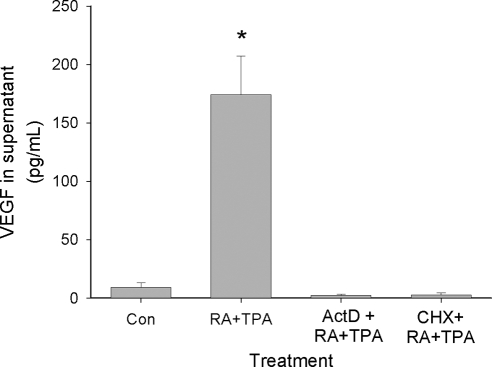
The human VEGF gene is organized in eight exons, separated by seven introns. Alternative exon splicing of this gene results in the generation of multiple isoforms, with those having 121 (VEGF121), 165 (VEGF165), and 189 (VEGF189) amino acids being the predominant species (24). VEGF121 lacks heparin-binding residues encoded by exons 6 and 7 whereas VEGF165 and VEGF189 lack residues encoded by exon 6 or contain products of all eight exons, respectively. Correspondingly, VEGF121 is a freely diffusible protein whereas VEGF189 is almost completely sequestered in the extracellular matrix due to its heparin-binding properties (24). VEGF165 has intermediate properties: although it is moderately diffusible, a significant fraction can remain bound to extracellular matrix. Figure 3 indicates that all three of these VEGF isoforms can be secreted from primary ESC and from HESC, and are markedly enhanced by RA + TPA treatment. The strong increase of the VEGF189 isoform in both primary cells and immortalized HESC suggests that even the VEGF isoforms normally bound to cell surface heparin-containing proteoglycans can be synthesized and secreted (25). Figure 4 shows that the effect of RA + TPA requires new mRNA and protein synthesis because actinomycin D and cycloheximide, respectively, fully inhibit the response. This result argues against the possibility that preformed sequestered protein contributed to enhanced VEGF release by RA + TPA-treated cells. This contention was further supported by observations that soluble heparin or heparinase added to the cultures did not increase VEGF concentration in the supernatant (data not shown).
Figure 3.
Western blot showing stimulation of VEGF in the supernatant of primary ESC and HESC treated overnight with the indicated compounds at the concentrations described in the legend to Fig. 1. The data show that RA + TPA markedly enhanced secretion of all three VEGF isoforms (121, 165, and 189 amino acids) from both primary cells and HESC. C, Control.
Figure 4.
VEGF stimulation by RA + TPA requires new mRNA and protein synthesis. HESC were treated for 4 h with solvent control (Con) or RA (1 μm) + TPA (50 nm) in the absence or presence of 10 μg/ml actinomycin D (inhibitor of mRNA production) or 10 μg/ml cycloheximide (CHX, protein synthesis inhibitor). VEGF protein levels in the supernatant were assessed by ELISA. *, Significant difference from control-treated cells. ActD, Actinomycin D; Con, control.
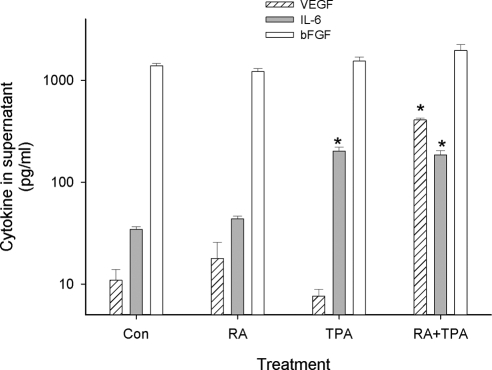
In addition to VEGF, other cytokines known to be secreted from ESC include IL-6 and bFGF (basic fibroblast growth factor) (26,27). To test whether RA + TPA was specific for VEGF up-regulation, supernatants from control and treated cultures were analyzed by ELISA for IL-6, and bFGF as well as for VEGF concentrations (Fig. 5). Levels of IL-6 and bFGF in control cultures of HESC were comparable to those detected in primary ESC (27,28). Results showed no significant alteration of bFGF secretion by any treatment condition. As previously reported for primary cells, TPA by itself enhanced secretion of IL-6 (29), but cotreatment with RA had no additional effect. By contrast, VEGF levels were unaffected by RA or TPA alone, but VEGF was potently up-regulated by treatment with the combination of RA + TPA. These results demonstrate specificity to the action of RA + TPA on VEGF protein secretion and that its up-regulation is not the result of a global effect on culture conditions or cellular activity (e.g. changes in pH, preapoptotic protein release, increase in exocytotic Golgi vesicles) that might result in a general increase in protein production or secretion (30,31).
Figure 5.
RA effect on VEGF secretion is specific. HESC were treated with RA (1 μm), TPA (50 nm), RA (1 μm) + TPA (50 nm), or solvent control (Con) as indicated for 24 h, and the supernatant VEGF concentration was tested by ELISA (patterned bar), IL-6 (gray bar), and bFGF (open bar). VEGF secretion was significantly increased by the combination of RA + TPA but no other treatment. bFGF was constitutively secreted in control cells and was unaffected by any treatement. IL-6 was significantly stimulated by TPA but not further affected by RA. *, Significant difference from controls. Con, Control.
Biological activity of secreted VEGF
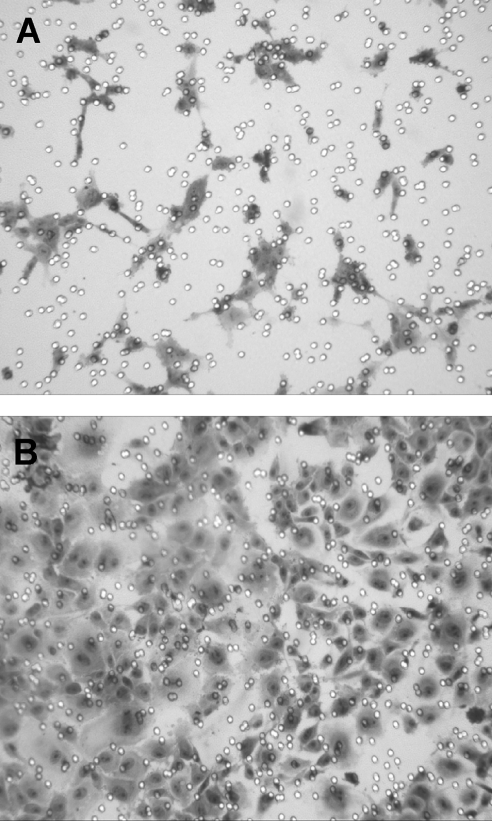
To determine whether the VEGF secreted from HESC was biologically active, we used a migration assay using human umbilical vein endothelial cells (HUVEC) (32). To rule out effects on migration by the treatment compounds themselves, the cells were incubated with RA + TPA or solvent control for 6 h, then washed and refed without added compounds and cultured overnight. Despite the transient exposure and removal of RA + TPA, we measured VEGF concentrations by ELISA and observed that HESCs continue to secrete high levels of VEGF for up to 16 h (data not shown). Figure 6 shows a marked increase in HUVEC migration in chambers containing supernatant from the RA + TPA-treated cells (panel B) reflecting the increased VEGF detected by ELISA and confirming its functional angiogenic activity.
Figure 6.
Migration assay using HUVEC to confirm that VEGF secreted from HESC is biologically active. HESC were treated with RA (1 μm) + TPA (50 nm) or solvent control for 6 h, then washed and refed without added compounds, and cultured overnight. This procedure generated high VEGF-containing supernatant without the continued presence of RA and TPA. The migration assay was performed as described in Materials and Methods. Briefly, 2 × 106 HUVEC were plated on Matrigel-coated transwell filters and conditioned media from vehicle- (panel A) or RA + TPA-pretreated (panel B) HESC were added to the lower chambers. After 24 h, HUVEC migrating toward the conditioned media were stained with Giemsa. The marked increase in HUVEC migration in chambers containing supernatant from the RA + TPA-treated cells reflects the substantial up-regulation of bioactive VEGF secretion.
Up-regulation of VEGF mRNA expression
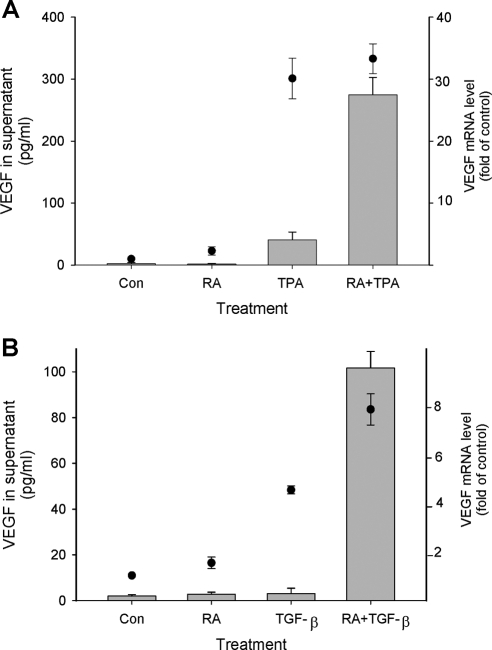
To determine whether stimulation of VEGF secretion from HESC by RA + TPA reflects regulation at the transcriptional level, we assessed mRNA expression by quantitative RT-PCR. As shown in Fig. 7A, the combination treatment of RA + TPA increased steady-state VEGF mRNA concentrations more than 30-fold, which paralleled the marked enhancement of VEGF protein secreted into the culture supernatant. Experiments to assess the decline in VEGF mRNA of control and combination-treated cultures after addition of actinomycin-D to block RNA synthesis indicated a half-life of approximately 3 h under both conditions (Fig. S1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Thus, the up-regulation of VEGF mRNA by the compounds does not involve increased stability of the transcripts. Our calculated VEGF mRNA half-life compares with that determined by other investigators in various cell types (33,34). The lack of treatment-induced changes in this parameter by RA + TPA suggests that up-regulation of VEGF mRNA levels result from direct transcriptional effects. Surprisingly, Fig. 7A shows that a similar increase in VEGF mRNA expression was also induced in TPA-only-treated cells. This finding was consistent with transient transfection experiments involving VEGF promoter reporter constructs into HESC, which showed that TPA and RA + TPA induced VEGF promoter activity to a similar extent; RA alone was without activity (supplemental Fig. S2). However, in stark contrast to that seen with cells cultured with combination RA + TPA, those cultured only with the latter compound demonstrated a blunted increase in secreted protein. Thus, whereas differences in VEGF mRNA levels between TPA and RA + TPA-cultured cells were not significant, combination treatment induced an approximately 7-fold higher concentration of VEGF in the supernatant than that detected in cultures treated with TPA alone.
Figure 7.
Dissociation of VEGF mRNA and protein secretion. HESC were treated with either TPA (A) or TGF-β (B) alone or a combination with RA as indicated. Concentrations of RA, TPA, and TGF-β were as shown in the legend to Fig. 1. VEGF mRNA was assessed by quantitative real-time RT-PCR (black circles) after treatment for 6 h. VEGF protein in the supernatant (bars) was evaluated after 6 h (in experiments with TPA) or overnight treatment (in experiments with TGF-β). VEGF protein levels were only significantly increased in the supernatant of combination-treated cells. By contrast, results showed significant increases in mRNA expression in the presence of TPA or TGF-β alone. Con, Control.
To test whether similar discrepancies between induction of VEGF at the mRNA level and secretion of the protein into the supernatant was a general phenomenon of RA cotreatment with other activators of VEGF gene transcription, parallel experiments were performed with RA and TGF-β. Figure 7B shows a similar phenomenon as determined with RA and TPA. Thus, whereas TGF-β alone caused a significant enhancement of VEGF mRNA, no increase in VEGF secretion was seen unless the cells were cotreated with RA + TGF-β.
Translational regulation of VEGF by RA
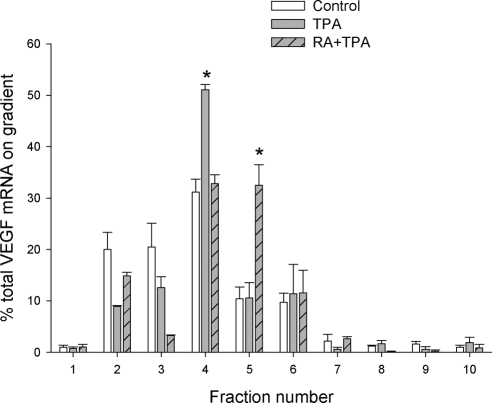
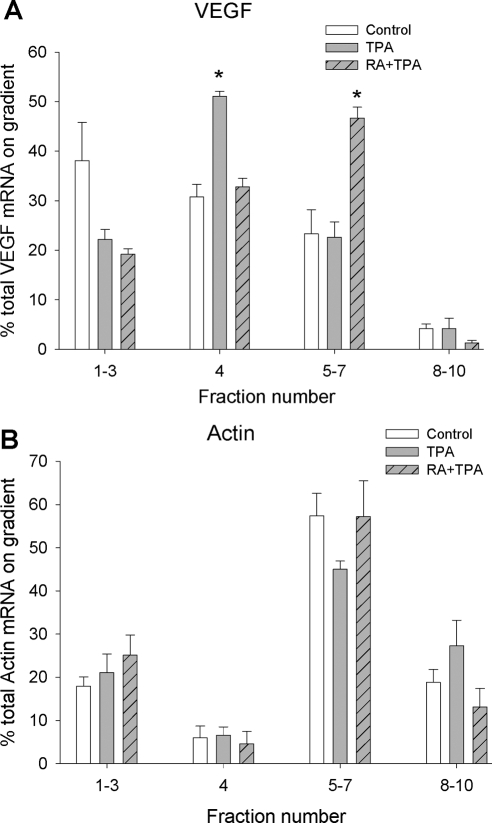
To directly examine whether VEGF mRNA is translationally inhibited under resting conditions in cells undergoing normal growth, and whether RA + TPA may stimulate VEGF translation, we performed linear sucrose gradient fractionation experiments (Fig. 8). This approach assesses translation efficiency by measuring the ability of mRNA transcripts to be incorporated into complexes that carry actively translating polyribosomes (35). Sedimentation of RNA and ribosomal components was monitored by absorption at wavelength 254 nm. Under our fractionation conditions, the top three fractions of the gradient contain complexes that are not involved in active translation, including dormant ribosome-free messenger ribonucleoprotein (mRNP) complexes, ribosome subunits, and 80S monoribosomes. The rest of the fractions contain polyribosomes associated with mRNAs engaged in peptide elongation. Fraction 4 contains short polyribosomes, including those at the initial stage of secretory protein synthesis, which are thus translationally held for docking to the rough endoplasmic reticulum. Complexes sedimenting in fractions 5–10 harbor large numbers of polyribosomes responsible for rapid peptide synthesis. As shown in Fig. 8, untreated (control) cells have greater than 40% of the VEGF mRNA retained in translationally inactive fractions (fractions 1–3). About 30% of the VEGF mRNA carried short polyribosomes sedimenting in fraction 4. Less than 30% of VEGF mRNA was associated with large polyribosomes. Both TPA and RA + TPA treatment reduced the VEGF mRNA in the translationally inactive pool. In addition, TPA alone advanced the VEGF mRNA into the short polyribosome fraction (fraction 4 in Figs. 8 and 9), yet failed to promote the VEGF mRNA to associate with larger polyribosomes that are engaged in active translation. In contrast, RA + TPA treatment promoted the VEGF mRNA to be engaged with larger polyribosome complexes for active translation (fraction 5 in Fig. 8), particularly passing the hurdle of short polyribosomes in fraction 4 that are most likely translationally stalled. Importantly, the effect of TPA and RA + TPA on polyribosome association of the VEGF mRNA is specific, because polyribosome association of the housekeeping gene β-actin was not affected by either TPA or RA + TPA (Fig. 9). The sedimentation of polyribosomes in our fractionation assay was confirmed by EDTA treatment, which dissociated polyribosomes into ribosome subunits and released the translation template mRNA into ribosome-free mRNP complexes (supplemental Fig. S3).
Figure 8.
Polyribosome association of VEGF mRNA in response to TPA and RA + TPA. HESC were incubated with TPA (50 nm), TPA (50 nm) + RA (1 μm), or solvent control for 6 h. The cytoplasmic extracts were subjected to linear sucrose gradient fractionation in parallel and each treatment yielded ten 1-ml fractions. Total RNA was isolated from each fraction and subjected to qRT-PCR as described in Materials and Methods. The percentage of total VEGF mRNA in each gradient fraction was calculated and graphically displayed. *, Significant increase in percent of total VEGF mRNA in the indicated fractions compared with controls.
Figure 9.
The effect of TPA and RA + TPA on polyribosome association of VEGF mRNA is specific. Linear sucrose gradient fractionation was carried out as described in Fig. 8. The percentage of the VEGF mRNA (A) and β-actin mRNA (B) in the translationally inactive complexes (fractions 1–3), short polyribosomes (fraction 4), and large translating polyribosomes (fractions 5–7 and 8–10) were calculated and graphically displayed. In contrast to the altered distribution of VEGF mRNA on the gradient (A), neither TPA nor RA + TPA had any effect on polyribosome association on β-actin mRNA. *, Significant increase in percent of total VEGF mRNA in the indicated fractions compared with controls.
Stimulation of VEGF secretion by RA involves a redox-sensitive mechanism
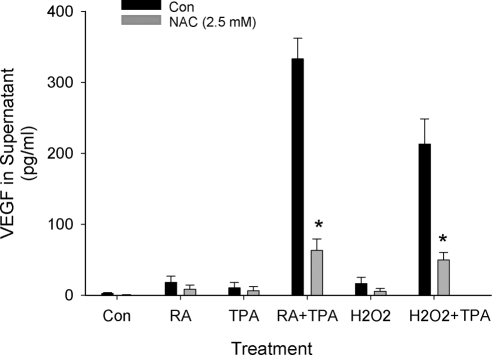
In addition to transcriptional regulation through cellular redox mechanisms, translational control of VEGF synthesis via oxidative processes has also been described (36). This mechanism of action is of particular interest to us because RA has been shown to influence reactive oxygen species (ROS) in a variety of cell types (37) and oxidative processes are proposed to play important roles in ESC decidualization (38). To test the possibility that stimulation of VEGF by RA + TPA involves a redox-sensitive pathway, experiments to assess VEGF secretion by control and treated HESC were performed as before but with the addition of the nonspecific antioxidant N-acetylcysteine (NAC; Fig. 10). Because of its SH group, NAC scavenges H2O2 and therefore acts as a general inhibitor of ROS production, as well as being a precursor of glutathione (39). Results showed that NAC inhibited RA + TPA-stimulated secretion of VEGF by more than 80%. Furthermore, our data indicated that cotreatment of HESC with H2O2 + TPA dramatically increased VEGF secretion into the culture supernatant. This effect was similarly sensitive to inhibition by NAC. By itself, H2O2 did not significantly alter VEGF protein levels.
Figure 10.
RA + TPA stimulation of VEGF requires production of ROS. HESC were treated overnight with the compounds as indicated in the absence or presence of the antioxidant NAC. Concentrations of RA and TPA were as shown in the legend to Fig. 1. Results show that NAC blocked secretion of VEGF by more than 80%. An increase of ROS by H2O2 (200 μm) in combination with TPA was seen to mimic the effect of RA + TPA on VEGF secretion. NAC similarly inhibited the VEGF-stimulating effects of H2O2 + TPA. *, Significant decrease from similarly treated cultures without NAC. Con, Control.
Endogenous RA in human endometrial cells
Previous reports in animal models have indicated that RA is produced in ESC and that its synthesis increases with the degree of decidualization (9). To test for this phenomenon in human cells, we adopted the strategy of in vitro decidualization of primary ESC (1,40,41) using exposure to a decidualization cocktail for a total of 7 d to model this differentiation process. These stimuli induce morphological development of an epithelioid phenotype and are associated with the characteristic, progressive expression and secretion of prolactin (PRL) as a biochemical marker of decidualization (41,42). The results in cocktail-treated cultures showed a characteristic change in morphology from bipolar fibroblast-like to polygonal decidual cells as reported previously (42) whereas the morphology of solvent-treated controls remained unchanged. These morphological changes were associated with biochemical differentiation of the ESCs, manifested as increased PRL in the culture supernatant from undetectable levels in the solvent-treated cultures to 46.0 ng/ml (mean of duplicate cultures) in the supernatant of cocktail-treated cells. Measurement of RA levels in the control and decidualized ESCs showed a significant increase (pmol/g protein) from 96.3 ± 4.3 to 131.5 ± 3.9, respectively (mean ± sem, P < 0.03). In contrast, changes in retinol levels were not significant (61.1 ± 12.8 to 78.7 ± 1.1 nmol/g protein).
To quantify endogenous RA in human endometrial cells in vivo, RA levels were determined in secretory-phase endometrial biopsies (n = 6) by liquid chromatography tandem mass spectrometry. The mean (±sem) RA concentration was 18.07 ± 4.08 pmol/g tissue. In these same tissues, the mean retinol level was determined to be 1.02 ± 0.24 nmol/g tissue.
Discussion
Regulation of VEGF by RA has been demonstrated in both normal and malignant cell types. In human keratinocytes, RA, through its receptor retinoic acid receptor (RAR)α, has been shown to inhibit VEGF protein secretion by interfering with the activator protein transcription factor AP1, resulting in decreased levels of VEGF mRNA (17). In gastric and bronchial carcinoma cells, RA, respectively, down- and up-regulates VEGF mRNA levels, also through transcriptional mechanisms of action (14,16). As such, although up- or down-regulation of VEGF protein production by RA appears to be cell type and malignant phenotype specific, all effects heretofore reported have been shown to be mediated either at the level of transcription or have not been defined. Our results demonstrate that in human ESC, RA can cooperate with other factors to positively regulate VEGF secretion, primarily at the translational level. As shown in Figs. 1 and 7, RA alone showed little effect on mRNA expression or on secretion of VEGF from primary ESC or HESC. However, when combined with known activators of VEGF gene transcription (e.g. TPA, IL-1β, or TGF-β) (19,20,21,24), RA markedly amplified the concentration of VEGF protein detected in the supernatant of both cell types. When we combined TPA or TGF-β with RA as coactivators of VEGF secretion, our results demonstrated that up-regulation of mRNA expression was not sufficient to promote secretion of the protein from the cells. Thus, whereas TPA alone stimulated VEGF promoter activity and up-regulated mRNA expression, significant protein secretion was detected only when RA was added to the cultures. This effect showed specificity in that IL-6 secretion from these cells was enhanced by TPA alone, but was not further up-regulated by RA, and constitutive secretion of bFGF was not affected by any of the treatment conditions.
Translation profile analysis provided further evidence that RA + TPA treatment significantly shifted VEGF mRNA into a pool of actively translating polyribosomes (Figs. 8 and 9). The most robust change of VEGF mRNA in our fractionation assay was the increase in fraction 5 in response to RA + TPA (Fig. 8). Notably, c-myc-enhanced VEGF translation in B cells (43) and mammalian target of rapamycin-dependent VEGF translation in myeloma cells (44) also directed VEGF mRNA into translating polyribosmes with sedimentation rates similar to that observed in RA + TPA-treated HESC and not to the most heavy polyribosome complexes. Interestingly, TPA alone appears to reduce the fraction of VEGF mRNA in translationally dormant mRNP complexes (fractions 2 and 3 in Fig. 8), demonstrating its contribution to enhance the early stages of translation. However, VEGF mRNA was mostly retained in short polyribosomes (fraction 4, Figs. 8 and 9), which likely represent those held for translation elongation, an obvious hurdle that prevents the production of full-length VEGF peptides for secretion. The retention of VEGF mRNA in short polyribosomes by TPA alone suggests that regulation of VEGF protein production involves distinct mechanisms at multiple steps (45), and that TPA alone is not fully activating all such steps. Thus, on top of the transcriptional up-regulation by TPA, RA as a costimulator acts at a later step during VEGF mRNA translation, which helps to overcome the checkpoint that holds VEGF mRNA on short polyribosomes (fraction 4). On the other hand, RA treatment alone is not sufficient to enhance VEGF secretion. Thus, it is apparent that other events unrelated to transcriptional activation or overcoming this checkpoint are supplied by TPA and are necessary for efficient VEGF production. The precise mechanism of the part(s) played by RA in translational regulation of VEGF in this cell type will be the subject of future studies.
Translational control of VEGF synthesis via production of ROS has been described (36). Our experiments demonstrating marked inhibition of RA + TPA-stimulated VEGF secretion by NAC, combined with the ability of H2O2 to mimic the costimulating effects of RA, suggest the involvement of a ROS-mediated pathway in this system. This contention is supported by the fact that RA is reported to influence ROS levels in a variety of cell types (37). Downstream of oxidative stress, other redox-sensitive mechanisms that involve translational regulation of VEGF have been described. These include increased translation of VEGF mRNA transcripts via two internal ribosomal entry sites (IRES) located in the prominent VEGF 5′-untranslated region (46,47). A precedent demonstrating the ability of RA to induce IRES-mediated translation has been shown in the case of mouse TrkB mRNA, which, like VEGF, contains a complex secondary structure in its untranslated region with two independent IRES (48).
Translational regulation of VEGF has also been demonstrated to be influenced by a zinc finger-containing mRNA binding protein, Zfp36l1, which acts as a negative regulator of VEGF translation during mouse embryonic development (49). Although a functional relationship between Zfp36 family members and retinoid signaling has not, to our knowledge, been demonstrated, translational control by RA via RARα acting as an mRNA-binding protein has recently been reported (50,51). In those studies, cytoplasmic RARα was shown to block translation by binding to a subset of mRNAs, including glutamate receptor mRNA, in neuronal dendrites. Ligation of RARα by RA reduced the RNA-binding affinity of the receptor, thereby relieving its translational repression. This mechanism of action is unlikely in our cell system as it requires high levels of cytoplasmic RARα protein (51). In ESC, as in most nonneuronal cell types, RAR is localized primarily in the nucleus with low cytoplasmic levels of the protein (52).
Other mechanisms for regulating VEGF translation have been described that involve ROS-sensitive kinase activity (53,54). For example, c-myc stimulation of VEGF mRNA translation is mediated by phosphatidylinositol 3-kinase activation, which is dependent on ROS production (43,53). Finally, the VEGF chaperone protein oxygen-regulated protein 150 (ORP150), which is up-regulated by inducers of ROS and endoplasmic reticulum stress (e.g. tunicamycin) (55), increases the translation and extracellular release of VEGF (56). However, regulation of ORP150 by RA has not been described, and our initial experiments in HESC (our unpublished data) failed to show that RA + TPA had effects on ORP150 or the endoplasmic reticulum stress-related protein GRP78 (56).
Endocrine regulation of the human VEGF gene during the luteal phase of the menstrual cycle and in early pregnancy appears to be dominated by the ovarian sex hormones, 17β-estradiol and progesterone (57). In this regard, an estrogen response element has been identified in the human VEGF gene promoter through which estrogen regulation of VEGF is conferred (58). Through this estrogen response element, estrogen can rapidly up-regulate VEGF mRNA in endometrial glandular epithelial and stromal cells. On the other hand, reports of the effects of progesterone on endometrial VEGF production are inconsistent, which may reflect complex combinatorial interactions on the gene promoter (59) and distinct regulatory actions on particular VEGF isoforms (60). As such, it is generally accepted that global changes in endometrial VEGF gene transcription during the menstrual cycle reflect alterations in the systemic levels of estrogen and progesterone. After ovulation, the production of VEGF in the endometrium is largely limited to epithelial cells with a dramatic decline in the stromal component (61). However, focal angiogenesis and hyperpermeability around predecidualized stromal cells in the implantation site are needed for successful embryo receptivity. Based on the findings presented in this report, we propose that production of RA at the site of implantation can mediate the requirement for localized VEGF protein secretion to meet the needs of the invading embryo. This hypothesis is supported by reports that endometrial stromal cells and placental cytotrophoblasts can synthesize RA from retinol (9,62,63) during early decidualization and cytotrophoblast invasion. Our findings of increased RA production by endometrial stromal cells decidualized in vitro are consistent with this contention and the demonstration of RA accumulation in secretory endometrium in vivo. Indeed, the endogenous concentrations of RA in endometrial tissue are high enough to be functionally relevant. Assuming an average of 70% water content of the wet-weight biopsy tissue, we can extrapolate endometrial tissue concentrations of RA of approximately 26 nm, a concentration that effectively stimulated VEGF secretion in our in vitro dose-response experiments (Fig. 2). As such, our determination of the endogenous levels of RA in the biopsy specimens, along with the knowledge that retinoid receptors are preferentially expressed at the implantation site in humans (64), adds additional credence to our hypothesis.
As we have indicated, VEGF regulation by RA appears to vary according to cell type and environment. We previously reported, using HL-60 cells as a model for neutrophils, that RA transcriptionally repressed VEGF expression in this cell type. We suggested that this finding may be relevant to the environment of endometriosis, where we observed neutrophilic infiltration of the endometrium (65). This cellular response also may be relevant to pathological decidual inflammation in pregnancy (66). However, although uterine natural killer cells and macrophages coexist in normal decidua, neutrophils are not typically observed unless the pregnancy is compromised (67). Thus, in the setting of normal placentation, we would anticipate that the positive effect of RA on VEGF secretion from ESC observed in the present report would prevail as a contributor to implantation site vascularization.
Taken together, these results suggest a dynamic role for the temporal and spatial modulation of retinoid metabolism in decidualizing human endometrium and predict a focal mechanism for promoting localized VEGF protein secretion critical for early events in the successful establishment of pregnancy.
Materials and Methods
Endometrial tissue and cell cultures
Primary endometrial cells were obtained from normally cycling, reproductive age women patients undergoing surgery for gynecological conditions without evidence of endometrial disease according to protocols approved by the Institutional Review Board, Emory University School of Medicine. Primary cultures of normal ESC were isolated as described in previous work (28,68). In some cases, the epithelial and stromal fractions of the biopsy tissue were separated and the stromal cells were cultured and treated as an in vitro model of endometrial stromal decidualization (40,41). In these cell culture experiments, the ESC were grown to 60–80% confluence in phenol red-free medium (DMEM/Ham’s F-12) supplemented with 5% charcoal-stripped fetal calf serum and then treated with 1 nm 17β-estradiol + 1 μm medroxyprogesterone acetate + 0.5 mm dibutyrl cAMP (decidualization cocktail) or solvent control. After treatment for 5 d, the cultures were refed and allowed to incubate for an additional 48 h, after which the morphology of the cells was assessed, and PRL was measured in the supernatant (ELISA kits from RayBiotech, Inc., Norcross, GA) as a biochemical marker of decidualization (41). The HESC cell line was developed and kindly provided to us by Drs. C. J. Lockwood and Graciela Krikum (Department of Obstetrics, Gynecology, and Reproductive Science, Yale University School of Medicine, New Haven, CT). HESC were immortalized from primary ESC by stable transfection of a gene coding for an essential catalytic protein subunit of human telomerase reverse transcriptase (22,23). These high telomerase-expressing cells show no structural or numerical chromosomal abnormalities and display similar biochemical properties, cytoskeletal markers, and gene expression as their normal counterpart. HESC also demonstrate normal responses to ovarian steroids (i.e. estrogen and progesterone) and, in coculture assays, were found to be as effective as normal primary stromal cells in regulating the proliferation of endometrial epithelial cells (23). All cultures were grown in DMEM/F12 medium containing 10% fetal bovine serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 10 mm of HEPES (complete medium). For treatment, all-trans-RA (Sigma Chemical Co., St. Louis, MO) was diluted in dimethyl sulfoxide to a stock concentration of 50 mmol/liter and then diluted to the indicated concentration in complete medium for the experiments. TPA, IL-1β, and TGF-β (Sigma) were used at the concentrations indicated.
RNA and protein analysis
For VEGF mRNA quantitation, reverse transcription was used to synthesize cDNA from RNA template as described previously (69). For real-time PCR, a total of 25 μl reaction mix was prepared using Omni-beads (TAKARA BIO, Inc., Shiga, Japan), 0.25× Syber Green dye (Fisher Scientific, Pittsburg, PA) and specific primer sets (0.3 μm each). Primer sequences used were as follows: VEGF, sense (5′-GCACCCATGGCAGA-3′), antisense (5′-GCTGCGCTGATAGA-3′); glyceraldehyde-3-phosphate dehydrogenase, sense (5′-CCATGGAGAAAGGCT-3′, antisense (5′-CAAAGTTGTCATGG-3′); actin, sense (5′-GGAGCAATGATCTT-3′), antisense (5′-CCTTCCTGGGCATG-3′). The PCR was set for 40 cycles in a Opticon 2 real-time thermocycler (Bio-Rad Laboratories, Hercules, CA) as described elsewhere (70). The data were analyzed after normalization with glyceraldehyde-3-phosphate dehydrogenase or actin RNA levels, using the formula 2ΔΔct, where ct is the cycle threshold.
Expression of cellular VEGF protein isoforms was assessed by Western blot analysis. Total protein was isolated from cell pellets by homogenization in lysis buffer. Equal amounts of protein from treated and untreated cultures were separated on a denaturing polyacrylamide gel and transferred to a nylon membrane. Nonspecific binding of antibodies was blocked by incubation (overnight, 4 C) in Tris-buffered saline containing 0.1% Triton X-100 and 5% nonfat dry milk. Membranes were then incubated with anti-VEGF antibody (nonisoform specific; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in Tris-buffered saline-Tween 20 and 5% milk overnight at 4 C. Specific binding to VEGF proteins was visualized by chemiluminescent detection and exposure to ECL hyper film (Amersham Pharmacia Biotech, Piscataway, NJ). VEGF, IL-6, and bFGF concentrations in cell culture supernatants were measured using standardized ELISA kits (R & D Systems, Minneapolis, MN).
Transient transfection assays
A 3019-bp human VEGF promoter-firefly luciferase reporter vector was created in pGL2 as described previously (58). HESC were seeded at a density of 4 × 104 cells per well in six-well dishes and grown to 75% confluence. For each well, 1 μg of plasmid DNA of the VEGF construct and 0.4 μg of the internal control plasmid pRL-TK (Renilla luciferase gene) were cotransfected into the cells using 3 μl of Lipofectamine 2000 lipofection reagent (Invitrogen, Carlsbad, CA) as performed in our earlier work (71). After 24 h of incubation, cells were treated with RA, TPA, RA + TPA, or solvent control for another 24 h. The preparation of cell extracts and measurement of luciferase activities were carried out using the Dual-Luciferase Reporter kit according to recommendations of the manufacturer (Promega Corp., Madison, WI). The assays for firefly luciferase activity and Renilla luciferase activity were performed sequentially using one reaction tube in a luminometer with one injector. Changes in firefly luciferase activity were calculated and plotted after normalization with changes in Renilla luciferase activity in the same sample. All of the vehicle controls were considered as 100%. Values of treatment group firefly luciferase-Renilla luciferase ratio were given as percentage of controls.
Endothelial cell migration assay
To confirm that the treatment-induced VEGF secreted from HESC was biologically active, we assessed its capacity to stimulate migration of HUVEC. The migration assay was performed as previously described (32) using 12-well polycarbonate filter (8 μm pore size) Transwells (Corning Costar; eBioscience, Inc., San Diego, CA) coated with 20 μl Matrigel (PharMingen, San Diego, CA) diluted with a 2× volume of complete medium. The lower chamber contained culture supernatant from HESC that had been treated with RA + TPA for 6 h or a similarly manipulated solvent control (without RA + TPA). To avoid direct effects of the compounds, HESC were washed and replenished with media without RA or TPA and cultured overnight. HUVEC (1 to 5 × 104) were seeded in the upper compartment of the transwells and cultured for 24–36 h. Cells remaining on the upper surface (Matrigel side) were removed by cotton swabs. Cells that migrated to the lower side of the filter were fixed in 4% formaldehyde, stained with Giemsa solution, and viewed under a microscope.
Determination of retinoid levels in endometrial tissue
Endometrial biopsy specimens for retinoid determination were obtained from patients undergoing elective gynecological surgery for nonendometrial indications according to protocols approved by the Institutional Review Board, Emory University School of Medicine. The study population included women of ages ranging 36–38 yr with samples acquired in the secretory phase. Immediately after extraction, pipelle samples were washed in saline buffer by gently swirling, protected from ambient light, and stored at −80 C until analyzed for retinoid content. Biopsy specimens or cultured cell pellets were prepared for retinoid analysis under yellow lights. Cell and tissue samples were homogenized by hand in ground-glass homogenizers (Kontes, size 22) on ice in 0.5–1.0 ml saline (0.9% NaCl). Total protein concentration of the samples was determined using the Bradford method (Bio-Rad). Samples were extracted as described elsewhere (72,73,74). Retinoic acid was quantified by liquid chromatography tandem mass spectrometry with atmospheric pressure chemical ionization in positive-ion mode on an API-4000 (Applied Biosystems, Foster City, CA) (72,73). Retinol was quantified by HPLC/UV on an Alliance 2690 (Waters) (71). Cultured stromal cell retinoids are expressed as moles per g total protein; tissue retinoids are expressed as moles per gram of tissue.
Linear sucrose gradient fractionation assay for polyribosome association of mRNAs
To evaluate ribosome binding to mRNA transcripts, linear sucrose gradient fractionation of cytoplasmic extracts was performed based on the procedure described by Feng et al. (35), with modifications. Briefly, cells were incubated with 100 μg/ml cycloheximide in the growth media for 15 min to freeze translating polyribosomes on the mRNAs before harvest in a lysis buffer containing 20 mm Tris-HCl, pH 7, 100 mm NaCl, 5 mm MgCl2, 100 μg/ml cycloheximide, 5 μl/ml RNAsin (Promega), 0.5% Triton X-100 (Sigma), and a cocktail of protease inhibitors in a total volume of 800 μl. Cellular debris was pelleted by centrifugation at 13,000 × g for 30 min. The resulting supernatant (700 μl) was loaded onto a 15–45% (wt/vol) linear sucrose gradient containing 5 mm MgCl2 and centrifuged at 39,000 × g for 60 min at 4 C. For EDTA treatment, cells were not treated with cycloheximide, and the MgCl2 in the lysis buffer was replaced by 10 mm EDTA. The lysates were fractionated on sucrose gradients containing 1 mm EDTA but no MgCl2. Ten 1.2-ml fractions from each gradient were collected into ribonuclease-free microfuge tubes using an Isco gradient fractionator (Isco, Lincoln, NE). Total RNA from each fraction was extracted using a standard phenol-chloroform method. The resulting RNA pellets were reconstituted in ribonuclease-free water and stored at −80 C until reverse transcription to cDNA and subsequent real-time PCR analysis using the primers described above. The percentage of mRNA in each fraction was calculated and graphically and is displayed in the corresponding figures.
Statistics
All experiments shown were performed a minimum of three times. SPSS software was used for data analysis, and the data were expressed as mean ± sem. Differences between treatment groups were analyzed by t test (two tailed) where P < 0.05 was considered statistically significant. The data shown in some figures (e.g. photographs of gels, cell migration experiments) are from a representative experiment, which was qualitatively replicated in at least three independent experiments.
Supplementary Material
Acknowledgments
We thank Zhaoju Shen for her expert technical assistance in many of the experiments. We also thank Daniel Gradinger for his insight and help with some of the theoretical aspects of experiments during the early stages of the work.
Footnotes
This work was supported by The Eunice Kennedy Schriver National Institutes of Child Health and Human Development/National Institutes of Health through Grants HD55379 and HD55787, as part of the Specialized Cooperative Center Program.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 12, 2009
Abbreviations: bFGF, Basic fibroblast growth factor; ESC, endometrial stromal cells; HESC, cell line model of human ESC; HUVEC, human umbilical vein endothelial cells; IRES, internal ribosomal entry sites; mRNP, messenger ribonucleoprotein; NAC, N-acetylcysteine; ORP150, oxygen-regulated protein 150; PRL, prolactin; RA, retinoic acid; RAR, retninoic acid receptor; ROS, reactive oxygen species; TPA, tetradecanoyl-phorbol-13-acetate; VEGF, vascular endothelial growth factor.
References
- Maruyama T, Yoshimura Y 2008 Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55:795–810 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW 1996 Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442 [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ 2006 Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update 12:747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J 1975 Dating the endometrial biopsy. Am J Obstet Gynecol 122:262–263 [DOI] [PubMed] [Google Scholar]
- Dvorak HF 2006 Discovery of vascular permeability factor (VPF). Exp Cell Res 312:522–526 [DOI] [PubMed] [Google Scholar]
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA 2003 Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol 188:177–182 [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA 2004 Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350:672–683 [DOI] [PubMed] [Google Scholar]
- Zheng WL, Ong DE 1998 Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol Reprod 58:963–970 [DOI] [PubMed] [Google Scholar]
- Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE 2000 Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology 141:802–808 [DOI] [PubMed] [Google Scholar]
- Yan ZH, Noonan S, Nagy L, Davies PJ, Stein JP 1996 Retinoic acid induction of the tissue transglutaminase promoter is mediated by a novel response element. Mol Cell Endocrinol 120:203–212 [DOI] [PubMed] [Google Scholar]
- Osteen KG, Bruner-Tran KL, Ong D, Eisenberg E 2002 Paracrine mediators of endometrial matrix metalloproteinase expression: potential targets for progestin-based treatment of endometriosis. Ann NY Acad Sci 955:139–146 [DOI] [PubMed] [Google Scholar]
- Elias JA, Zheng T, Einarsson O, Landry M, Trow T, Rebert N, Panuska J 1994 Epithelial interleukin-11. Regulation by cytokines, respiratory syncytial virus, and retinoic acid. J Biol Chem 269:22261–22268 [PubMed] [Google Scholar]
- Hameed DA, el-Metwally TH 2008 The effectiveness of retinoic acid treatment in bladder cancer: impact on recurrence, survival and TGFα and VEGF as end-point biomarkers. Cancer Biol Ther 7:92–100 [DOI] [PubMed] [Google Scholar]
- Zhang JP, Chen XY, Li JS 2007 Effects of all-trans-retinoic on human gastric cancer cells BGC-823. J Dig Dis 8:29–34 [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Rockenstein A, Ramaswamy A, Celik I, Wunderlich A, Lingelbach S, Hofbauer LC, Zielke A 2007 Retinoic acid inhibits angiogenesis and tumor growth of thyroid cancer cells. Mol Cell Endocrinol 264:74–81 [DOI] [PubMed] [Google Scholar]
- Maeno T, Tanaka T, Sando Y, Suga T, Maeno Y, Nakagawa J, Hosono T, Sato M, Akiyama H, Kishi S, Nagai R, Kurabayashi M 2002 Stimulation of vascular endothelial growth factor gene transcription by all trans retinoic acid through Sp1 and Sp3 sites in human bronchioloalveolar carcinoma cells. Am J Respir Cell Mol Biol 26:246–253 [DOI] [PubMed] [Google Scholar]
- Diaz BV, Lenoir MC, Ladoux A, Frelin C, Démarchez M, Michel S 2000 Regulation of vascular endothelial growth factor expression in human keratinocytes by retinoids. J Biol Chem 275:642–650 [DOI] [PubMed] [Google Scholar]
- Tokuda H, Hatakeyama D, Akamatsu S, Tanabe K, Yoshida M, Shibata T, Kozawa O 2003 Involvement of MAP kinases in TGF-β-stimulated vascular endothelial growth factor synthesis in osteoblasts. Arch Biochem Biophys 415:117–125 [DOI] [PubMed] [Google Scholar]
- Frank S, Hübner G, Breier G, Longaker MT, Greenhalgh DG, Werner S 1995 Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem 270:12607–12613 [DOI] [PubMed] [Google Scholar]
- Ono M 2008 Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 99:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE 2009 Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine 16:573–580 [DOI] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ 2004 A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology 145:2291–2296 [DOI] [PubMed] [Google Scholar]
- Barbier CS, Becker KA, Troester MA, Kaufman DG 2005 Expression of exogenous human telomerase in cultures of endometrial stromal cells does not alter their hormone responsiveness. Biol Reprod 73:106–114 [DOI] [PubMed] [Google Scholar]
- Ferrara N 2004 Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611 [DOI] [PubMed] [Google Scholar]
- Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N 1992 Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267:26031–26037 [PubMed] [Google Scholar]
- Wanichkul T, Han S, Huang R-P, Sidell N 2003 Cytokine regulation by peroxisome proliferator-activated receptor γ in human endometrial cells. Fertil Steril 79:763–769 [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Hori M, Ichigo S, Tamaya T 1997 Ovarian steroids regulate the expression of basic fibroblast growth factor and its mRNA in fibroblasts derived from uterine endometrium. Ann Clin Biochem 34:91–96 [DOI] [PubMed] [Google Scholar]
- Sawatsri S, Desai N, Rock JA, Sidell N 2000 Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril 73:1012–1019 [DOI] [PubMed] [Google Scholar]
- Nasu K, Matsui N, Narahara H, Tanaka Y, Takai N, Miyakawa I, Higuchi Y 1998 MaMi, a human endometrial stromal sarcoma cell line that constitutively produces interleukin-6, interleukin-8, and monocyte chemoattractant protein-1. Arch Pathol Lab Med 122:836–841 [PubMed] [Google Scholar]
- Datta D, Kundu PK, Dasgupta S, Fulzele K, Sewlikar S 2001 Effect of addition of proton carriers in culture medium on growth and secretion of hybridoma cell line OKT3. Indian J Physiol Pharmacol 45:367–372 [PubMed] [Google Scholar]
- Marchok AC, Clark JN, Klein-Szanto A 1982 Modulation of growth, differentiation, and mucous glycoprotein synthesis by retinyl acetate in cloned carcinoma cell lines. J Natl Cancer Inst 66:1165–1174 [PubMed] [Google Scholar]
- Li J, Sidell N 2005 Growth-related oncogene produced in human breast cancer cells and regulated by Syk protein-tyrosine kinase. Int J Cancer 117:14–20 [DOI] [PubMed] [Google Scholar]
- Iervolino A, Trisciuoglio D, Ribatti D, Candiloro A, Biroccio A, Zupi G, Del Bufalo D 2002 Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J 16:1453–1455 [DOI] [PubMed] [Google Scholar]
- Chin K, Kurashima Y, Ogura T, Tajiri H, Yoshida S, Esumi H 1997 Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene 15:437–442 [DOI] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST 1997 FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell 1:109–118 [DOI] [PubMed] [Google Scholar]
- Feliers D, Gorin Y, Ghosh-Choudhury G, Abboud HE, Kasinath BS 2006 Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am J Physiol Renal Physiol 290:F927–F936 [DOI] [PubMed] [Google Scholar]
- Conte da Frota Jr ML, Gomes da Silva E, Behr GA, Roberto de Oliveira M, Dal-Pizzol F, Klamt F, Moreira JC 2006 All-trans retinoic acid induces free radical generation and modulate antioxidant enzyme activities in rat Sertoli cells. Mol Cell Biochem 285:173–179 [DOI] [PubMed] [Google Scholar]
- Sugino N 2007 The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta (Suppl A):S133–S136 [DOI] [PubMed] [Google Scholar]
- Gillissen A, Nowak D 1998 Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med 92:609–623 [DOI] [PubMed] [Google Scholar]
- Irwin JC, de las Fuentes L, Giudice LC 1994 Growth factors and decidualization in vitro. Ann NY Acad Sci 734:7–18 [DOI] [PubMed] [Google Scholar]
- Matsui N, Kawano Y, Nakamura S, Miyakawa I 2004 Changes in vascular endothelial growth factor production associated with decidualization by human endometrial stromal cells in vitro. Acta Obstet Gynecol Scand 83:138–143 [DOI] [PubMed] [Google Scholar]
- Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC 2008 Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 135:2659–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita P, Parghi SS, Brandvold KA, Ruddell A 2005 Myc regulates VEGF production in B cells by stimulating initiation of VEGF mRNA translation. Oncogene 24:889–901 [DOI] [PubMed] [Google Scholar]
- Frost P, Shi Y, Hoang B, Lichtenstein A 2007 AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene 26:2255–2262 [DOI] [PubMed] [Google Scholar]
- Pain VM 1996 Initiation of protein synthesis in eukaryotic cells. Eur J Biochem 236:747–771 [DOI] [PubMed] [Google Scholar]
- Akiri G, Nahari D, Finkelstein Y, Le S-Y, Elroy-Stein O, Levi B-Z 1998 Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene 17:227–236 [DOI] [PubMed] [Google Scholar]
- Huez I, Creancier L, Audigier S, Gensac M-C, Prats A-C, Prats H 1998 Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol 18:6178–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman SL, Pfingsten JS, Kieft JS, Krushel LA 2007 The 5′ leader of the mRNA encoding the mouse neurotrophin receptor TrkB contains two internal ribosomal entry sites that are differentially regulated. PLoS ONE 3:e3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Sanchez MJ, Spasic-Boskovic O, Santalucia T, Gambardella L, Burton GJ, Murphy JJ, Norton JD, Clark AR, Turner M 2006 The RNA binding protein Zfp36l1 is required for normal vascularisation and post-transcriptionally regulates VEGF expression. Dev Dyn 235:3144–3155 [DOI] [PubMed] [Google Scholar]
- Chen N, Onisko B, Napoli JL 2008 The nuclear transcription factor RARα associates with neuronal RNA granules and suppresses translation. J Biol Chem 283:20841–20847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MM, Chen L 2008 Retinoic acid-gated sequence-specific translational control by RARα. Proc Natl Acad Sci USA 105: 20303–20308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaka K, Saito T, Wataba K, Ashihara K, Ito E, Kudo R 2001 Changes in expression and subcellular localization of nuclear retinoic acid receptors in human endometrial epithelium during the menstrual cycle. Mol Hum Reprod 7:437–446 [DOI] [PubMed] [Google Scholar]
- Clerkin JS, Naughton R, Quiney C, Cotter TG 2008 Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett 266:30–36 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Zhao H, Liu K, Luo D, Chen Y, Chen Y, Yang X, Gu Q, Xu X 20 Aug 2009 PEDF inhibits JAK2/STAT3-mediated VEGF upregulation under high glucose condition through a mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci 10.1167/iovs.09-3511 [DOI] [PubMed] [Google Scholar]
- Dukes AA, Van Laar VS, Cascio M, Hastings TG 2008 Changes in endoplasmic reticulum stress proteins and aldolase A in cells exposed to dopamine. J Neurochem 106:333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Tsukamoto Y, Hori O, Kitao Y, Yanagi H, Stern DM, Ogawa S 2001 Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res 61:4206–4213 [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN 1999 Vascular endothelial growth factor in reproductive biology. Curr Opin Obstet Gynecol 11:255–260 [DOI] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN 2000 Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc Natl Acad Sci USA 97:10972–10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MD, Vigne JL, Pritts EA, Chao V, Dreher E, Taylor RN 2003 Progestins activate vascular endothelial growth factor gene transcription in endometrial adenocarcinoma cells. Fertil Steril 79:386–392 [DOI] [PubMed] [Google Scholar]
- Ancelin M, Buteau-Lozano H, Meduri G, Osborne-Pellegrin M, Sordello S, Plouët J, Perrot-Applanat M 2002 A dynamic shift of VEGF isoforms with a transient and selective progesterone-induced expression of VEGF189 regulates angiogenesis and vascular permeability in human uterus. Proc Natl Acad Sci USA 99:6023–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF 2000 Angiogenesis and its control in the female reproductive system. Br Med Bull 56:787–797 [DOI] [PubMed] [Google Scholar]
- Deng L, Shipley GL, Loose-Mitchell DS, Stancel GM, Broaddus R, Pickar JH, Davies PJ 2003 Coordinate regulation of the production and signaling of retinoic acid by estrogen in the human endometrium. J Clin Endocrinol Metab 88:2157–2163 [DOI] [PubMed] [Google Scholar]
- Ulven SM, Gundersen TE, Weedon MS, Landaas VO, Sakhi AK, Fromm SH, Geronimo BA, Moskaug JO, Blomhoff R 2000 Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev Biol 220:379–391 [DOI] [PubMed] [Google Scholar]
- Tarrade A, Rochette-Egly C, Guibourdenche J, Evain-Brion D 2000 The expression of nuclear retinoid receptors in human implantation. Placenta 21:703–710 [DOI] [PubMed] [Google Scholar]
- Tee MK, Vigne JL, Taylor RN 2006 All-trans retinoic acid inhibits vascular endothelial growth factor expression in a cell model of neutrophil activation. Endocrinology 147:1264–1270 [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Paidas M, Murk WK, Kayisli UA, Gopinath A, Huang SJ, Krikun G, Schatz F 2009 Involvement of human decidual cell-expressed tissue factor in uterine hemostasis and abruption. Thromb Res 124:516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC 2006 The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 195:803–808 [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN 1997 Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab 82:1621–1628 [DOI] [PubMed] [Google Scholar]
- Han SW, Greene ME, Pitts J, Wada RK, Sidell N 2001 Novel expression and function of peroxisome proliferator-activated receptor γ (PPARγ) in human neuroblastoma cells. Clin Cancer Res 7:98–104 [PubMed] [Google Scholar]
- Sidell N, Pasquali M, Malkapuram S, Barua AB, Wanichkul T, Wada RK 2003 In vitro and in vivo effects of easily administered, low-toxic retinoid and phenylacetate compounds on human neuroblastoma cells. Br J Cancer 89:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Inoue H, Flowers LC, Sidell N 2003 Control of COX-2 gene expression through peroxisome proliferator-activated receptor γ in human cervical cancer cells. Clin Cancer Res 9:4627–4635 [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli JL 2008 Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem 80:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Chen N, Sparks S, Napoli JL 2005 Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J 388:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Napoli JL 2008 HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem 378:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.