Abstract
Pit-1 is a POU-homeodomain transcription factor that dictates the ontogeny of pituitary somatotrophs, lactotrophs, and thyrotrophs through regulation of their respective protein hormone genes: GH, prolactin (PRL), and TSHβ. Although Pit-1 threonine 220 (T220) and serine 115 are protein kinase phospho-acceptor sites, the transcriptional role of Pit-1 phosphorylation remains unclear. In the rat PRL promoter (rPRL), Ras-stimulated transcription is mediated by binding of Ets-1 and Pit-1 at a composite site (FPIV). Ets-1 and Pit-1 physically interact, and Pit-1 T220 is a major Ets-1 contact point. T220 was mutated to aspartic acid (D, to mimic phosphorylation) or a neutral alanine (A), and DNA binding and transcriptional activity were tested. The Pit-1 T220D mutation reduced binding at monomeric Pit-1 sites (FPIV, PRL-1d), but not dimeric Pit-1 sites (FPI). Pit-1 T220A bound all sites with wild-type (WT) affinity. In transfections of HeLa cells, each Pit-1 mutant transcriptionally activated the −425rPRL promoter and cooperated with Ets-1 to WT levels. In contrast, Pit-1-mediated Ras activation of the −425 rPRL promoter was significantly inhibited by T220D. Finally, Pit-1 synergistic activation of the 2500-bp rPRL promoter with estrogen receptor was reduced by T220D compared with T220A and WT Pit-1. Thus, phosphorylation of Pit-1 T220 reduces binding to monomeric sites blunting Ras and estrogen/estrogen receptor stimulation of the rPRL gene promoter. Consequently, T220 phosphorylation of Pit-1 by protein kinase A, protein kinase C, or cell cycle-dependent kinases appears to serve as a regulatory switch, inhibiting Ras and estrogen/estrogen receptor regulatory pathways, while enhancing the cAMP/protein kinase A response, thus allowing a more precise integration of pituitary responses to distinct signaling stimuli.
Pit-1 phosphorylation integrates lactotroph responses to signaling pathways by regulating Pit-1 binding to monomeric or dimeric binding sites in the prolactin gene promoter.
During development of the anterior pituitary gland, the cells of Rathke’s pouch differentiate into six distinct cell types, each of which selectively synthesizes and secretes a unique peptide hormone. The POU-homeodomain transcription factor, Pit-1, dictates the terminal differentiation of three of these cell lineages: the somatotrophs, lactotrophs, and thyrotrophs, through regulation of their unique protein hormone genes, GH, prolactin (PRL), and TSHβ. Pit-1 achieves this unique cell specification by cooperating with other transcription factors, including the estrogen receptor (ER), thyroid hormone receptor (TR), vitamin D receptor, retinoic acid receptor, Pitx-1 (P-Otx), Zn-15, CREB-binding protein (CBP), Ets-1, CCAAT enhancer binding protein, P-Lim, nuclear receptor corepressor I, GATA-2, Oct-1, and Pit-1 itself (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16). In this manner, Pit-1 can harness ubiquitous signaling pathways [T3, 17β-estradiol, retinoic acid, protein kinase A (PKA), protein kinase C (PKC), and Ras pathways]) in a cell-specific manner by functionally interacting with a variety of signal-dependent transcription factors and targeting them to Pit-1-regulated promoters (4,7,11,17,18,19,20,21,22,23,24,25,26,27,28,29). In the rat PRL (rPRL) gene promoter the binding of Pit-1 and ligand-bound estrogen receptor to adjacent sites in the distal enhancer [(−1576), (4)] of the rPRL gene promoter mediates estrogen stimulation. Although weak physical interaction between Pit-1 and ERα has been shown, neither this, nor cooperative DNA binding, appears to mediate synergy (7). Rather, the ability of Pit-1 to bind to the Prl-1d site (−1565) as a monomer dictates the domains that it utilizes to synergize with ERα and coactivators (7). Similarly, Ras stimulation is mediated by the binding of Ets-1 and Pit-1 at a composite Ets-binding site (EBS)/footprint IV Pit-1 site (FPIV) located at −217 to −190 in the proximal rPRL promoter. Although Ets-1 and Pit-1 physically interact, even in the absence of DNA, their interaction, like that of Pit-1 and ERα, does not appear to mediate either the synergistic activation of the rPRL promoter by Ets-1 and Pit-1, or their Ras responsiveness (30).
In recent studies, we demonstrated that the carboxy-terminal region of the Pit-1 transcriptional activation domain (TAD) [amino acids (AA) 60–80] represents a novel Ras-responsive domain (30,31). This Pit-1 Ras-responsive domain also correlates with the Pit-1 domain required for optimal Pit-1/GATA-2 synergy mediated through Mediator 220/TR-associated protein 220 (5,32), and for synergy with TR and ER through regulation of the repressor/activator activities of receptor interacting protein 140/steroid receptor coactivators (SRCs) (29,33). MAPK phosphorylation of SRC-1 can increase its transcriptional activity (34), and in response to epidermal growth factor activation, SRCs can bind and activate Ets transcription factors (35,36). Similarly, phosphorylation of Ets-1 at a consensus MAPK phosphorylation site has been shown to be critical for Ras stimulation of rPRL by Ets-1 (22). Finally, the ability of the rPRL promoter to respond to Ras stimulation is inhibited by activation of PKA (37) and PKC (38). Thus phosphorylation appears to play an important role in regulating the Pit-1 activation of rPRL gene expression.
Three serine-threonine phosphorylation sites have been identified in Pit 1: serine 115 (S115), threonine 219 (T219), and threonine 220 (T220). Although the T219 site is poorly phosphorylated, the S115 and T220 sites are phosphorylated both in cells treated with cAMP or phorbol esters or by directly phosphorylating purified Pit-1 proteins with PKA and PKC (39). This finding provided a potential mechanism by which PKC and PKA signaling pathways might regulate Pit-1 target genes. In fact, mutation of these serines and threonines to alanines to block phosphorylation reduced the ability of Pit-1 to bind to sites in the TSHβ promoter and mediate cAMP activation of this gene (40). However, these alanine mutations did not appear to affect the ability of Pit-1 to mediate either basal or induced activation of the PRL and GH gene promoters (41,42). The T220 phosphorylation site is conserved among all members of the POU family, and phosphorylation of this site during mitosis inhibits the binding of Oct-1 to DNA sites (43,44). Similarly, phosphorylation of Pit-1 at both T220 and S115 is elevated in mitotic cells and binding of Pit-1 to the PRL-1p, GHF-1p, and GH-1p sites was reduced (45). These data suggest that phosphorylation of Pit-1 may primarily act to alter DNA binding affinity, and thus affect selectivity of DNA binding sites. In addition, we have shown that the phosphorylation or mutation of T220 in the Pit-1 homeodomain alters its ability to physically interact with the Ets-1 RIII Region (AA 190-257) (46).
The current study utilizes aspartic acid mutations to mimic phosphorylation of the S115 and T220 sites in Pit-1. These Pit-1 mutants were used to examine the role of Pit-1 phosphorylation on DNA binding and cooperation with transcriptional partners to mediate transcriptional activation of the rPRL promoter in response to various signaling cascades. We show that a Pit-1 T220D phosphomimic inhibits Pit-1 binding at monomeric DNA-binding sites (FPIV/EBS, Prl-1d), but has no effect on Pit-1 binding to dimeric DNA sites (FPI) in the rPRL promoter. Functionally, this decreased DNA binding serves to inhibit both Ras and E/ER stimulation of the rPRL promoter, but not basal transcriptional activity. Thus, altered DNA site specificity in response to Pit-1 T220 phosphorylation provides an important mechanism for Pit-1 to dynamically integrate cellular responses to multiple signaling pathways.
Results
The Pit-1 T220D homeodomain does not bind Pit-1 sites
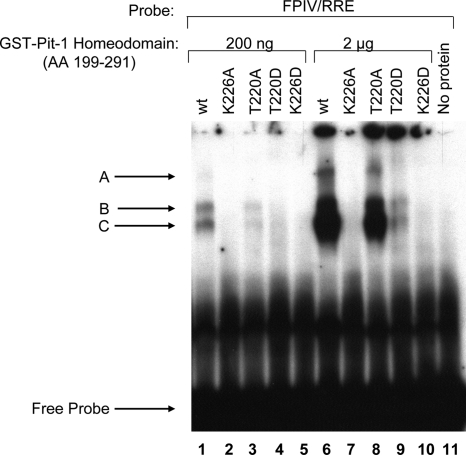
In a previous study, we found that phosphorylation or mutation of the T220 phosphorylation site in the Pit-1 homeodomain altered its affinity for Ets-1 protein (46). Because Ras activation of the rPRL promoter is mediated through a composite Pit-1/Ets-1 binding site located at −217 to −190 in the proximal rPRL promoter, our data suggested that posttranslational modification of Pit-1 T220 by physiologically relevant activation of PKA, PKC, or cell cycle-dependent kinases may serve to regulate the Ets-1/Pit-1 interaction. In the current study, we assessed the ability of wild-type (WT) and mutant (T220D and T220A) Pit-1 glutathione S transferase (GST)-homeodomain fusion constructs to bind to Pit-1-binding sites in the rPRL promoter using EMSA analysis (Fig. 1). This established threonine phosphorylation site, T220, was mutated to either aspartic acid (D) to mimic phosphorylation or a neutral alanine (A) to block phosphorylation. Purified GST-Pit-1 homeodomain proteins (200 ng, lanes 1–5; 2 μg, lanes 6–10) were incubated with a 32P-labeled double-stranded oligonucleotide containing the EBS/FPIV site (−217/−190) that represents the Ras-response element (RRE) in the rPRL promoter. The resulting complexes were resolved on a 6% PAGE gel (Fig. 1). WT Pit-1 (lanes 1 and 6) bound to three specific complexes, A–C, representing the binding of the Pit-1 homeodomain to the DNA sites, as well as likely Pit-1/probe dimers formed by the interactions of the GST fusions (47). The binding of mutant T220A (lane 3, 200 ng), which would prevent any phosphorylation of this site, was less than Pit-1 at low protein concentrations (lane 3, 200 ng), but similar to that of WT Pit-1 at higher protein concentrations (lane 8, 2 μg), whereas T220D, which serves to mimic phosphorylation, nearly eliminated DNA binding at all protein concentrations (lanes 4 and 9). Because K226 has been shown to make base contacts with the Pit-1 DNA-binding site (48), alanine and aspartic acid mutations of this residue should block the binding of the Pit-1 homeodomain to the DNA and were included as negative controls. Consistent with the importance of K226 in direct DNA contact, neither K226A (lanes 2 and 7) nor K226D (lanes 5 and 10) exhibited any DNA binding, even at the highest protein input. These data illustrate that the T220D phosphorylation mimic of Pit-1 inhibited the ability of the Pit-1 homeodomain to bind the Ras-responsive site in the rPRL promoter (Fig. 1).
Figure 1.
T220D Pit-1 homeodomain fails to bind to monomeric Ras Response Element. In each 20-μl reaction, 200 ng or 2 μg of WT and mutant GST-Pit-1 homeodomain (AA 199-291) fusion proteins were incubated with 28,000 cpm of FPIV/RRE probe in binding buffer. After a 30-min incubation at room temperature, 2 μl of loading buffer was added to each sample and ran on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. After electrophoresis, the gel was dried and visualized by autoradiography. Specific GST-Pit-1 homeodomain DNA complexes are labeled A, B, and C.
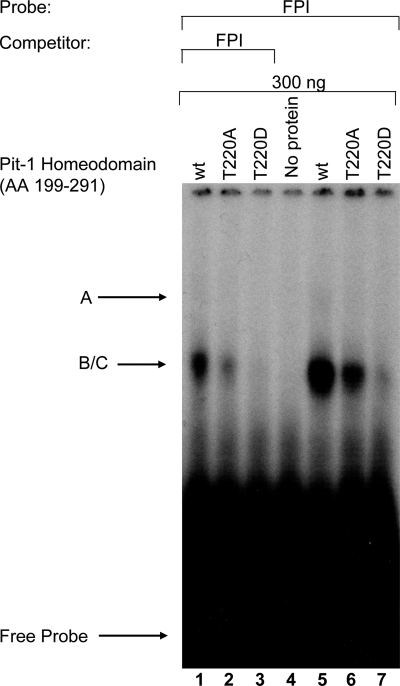
To assess the effect of the phosphorylation mimic on a higher affinity, dimeric Pit-1-binding site, we tested the binding of these same GST-homeodomain constructs for binding to the FPI (PRL-1P) site. In Fig. 2, binding of complexes B and C to WT Pit-1 and T220A is apparent as a single broad band (lanes 5 and 6; B/C), and complex A is only faintly visible (lanes 5 and 6). These differences can be attributed to reduced gel resolution, less efficient labeling of the FP1 probe, and the fact that only lower concentrations of the GST-fusion proteins (300 ng) were used in this experiment. However, the specificity of these bands is verified because binding to these complexes is inhibited by competition with homologous unlabeled probe (lanes 1–3), and these bands are not present in the negative control (lane 4). Finally, similar to the EBS/FPIV oligo, we found that T220D Pit-1 homeodomain exhibited reduced binding (lane 7) to the higher-affinity proximal Pit-1-binding site in the rPRL promoter (Prl-1P, FPI), whereas WT and T220A Pit-1 homeodomain constructs both bound with similar affinities (lanes 5 and 6, Fig. 2).
Figure 2.
T220D Pit-1 homeodomain fails to bind to dimeric FPI (Prl-1P) probe. In each 20-μl reaction, 300 ng of WT and mutant GST-Pit-1 homeodomain (AA 199-291) fusion proteins were incubated with 28,000 cpm of the FPI probe in binding buffer and 1 ng polydeoxyinosinic deoxycytidylic acid. After a 30-min incubation at room temperature, 2 μl of loading buffer was added to each sample and ran on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. After electrophoresis, the gel was dried and visualized by autoradiography. Specific GST-Pit-1 homeodomain DNA complexes are labeled A and B/C.
Full-length Pit-1 T220D binds to dimeric but not monomeric Pit-1 sites in the rPRL promoter
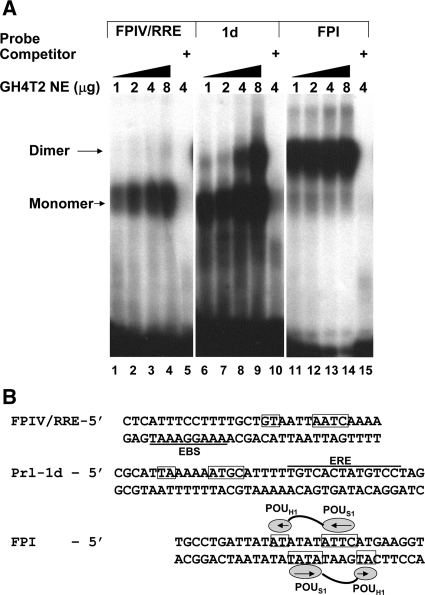
These studies suggested that phosphorylation of T220 inhibited the ability of the Pit-1 homeodomain to bind to DNA-binding sites. However, because the GST-fusion constructs lack the POU-specific domain, which has been shown to be necessary for specific, high-affinity DNA binding and for protein interactions between Pit-1 dimers, we also wanted to test the ability of expressed full-length WT and T220 mutant Pit-1 proteins to bind to Pit-1 monomeric and dimeric sites in the rPRL promoter of varying affinities. Before testing the binding of these expressed Pit-1 proteins to DNA-binding sites, we first conducted a control experiment to establish the binding profile of endogenous Pit-1 from GH4T2 cell nuclear extracts to 32P-labeled oligonucleotides representing the EBS/FPIV site (PRL-4P) that represents the Ras response element, the PRL-1d site from the estrogen-responsive distal enhancer, and the FPI (PRL-1P) site (Fig. 3) (7). We incubated nuclear extracts from GH4T2 cells containing increasing amounts (1–8 μg) of total nuclear protein with each labeled probe (Fig. 3A, lanes 1–4, 6–9, 11–14). To verify specific binding, we added a 500-fold molar excess of unlabeled homologous competitor to incubations containing 4 μg of nuclear extract (Fig. 3A, lanes 5, 10, and 15) or unlabeled heterologous competitor (data not shown). Based on these control studies, we determined that FPIV bound Pit-1 primarily as a monomer (lanes 1–4), and the PRL-1d site bound Pit-1 primarily as a monomer, with apparent dimer binding observed as nuclear extract was increased (lanes 6–9). Finally, FPI preferentially bound Pit-1 as a dimer at all protein concentrations (lanes 11–14). Binding to these probes was specific, because unlabeled homologous competitor was able to displace the radioactively labeled probe (lanes 5, 10, and 15). Moreover, Pit-1 binding as a monomer to the Prl-1D site or as a dimer to FPI is consistent with previously published studies (7,39,41,49,50). Although the FPI site shows primarily dimer binding, when the concentration of nuclear extract is further reduced, dimer binding is lost before monomer binding (data not shown). The comigration of the upper Prl-1d complex with the FPI Pit-1 dimer band suggests that this complex is likely a Pit-1 dimer that is bound to the site at higher Pit-1 concentrations (7); however, it could also represent a Pit-1/ERα complex.
Figure 3.
GH4T2 nuclear extracts reveal binding of Pit-1 as a monomer or dimer to sites in the rPRL promoter. A, In each 30-μl reaction, increasing concentrations of GH4T2 nuclear extracts were incubated with 28,000 cpm of the indicated probe in binding buffer and 800 ng herring sperm DNA. Specificity of binding was assessed by the addition of 500-fold unlabeled homologous competitor DNA. After a 30-min incubation at room temperature, 2 μl of loading buffer was added to each sample and loaded on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. After electrophoresis, the gel was dried and visualized by autoradiography. Monomeric and dimeric Pit-1 binding is indicated. B, Sequence of oligonucleotides is shown with the established POU-specific and POU-homeodomain binding of FP1 indicated (49,56). Putative monomeric binding sites for the POU-specific and homeodomains in the FPIV/RRE and Prl-1d sequences are boxed, and the EBS and estrogen response element (ERE) are indicated with solid lines. NE, Nuclear extract.
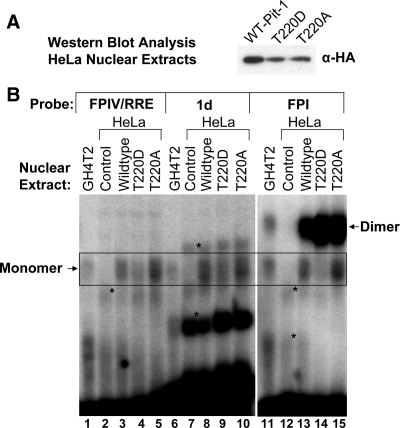
Having validated the binding profiles of endogenous Pit-1 from GH4T2 cells on the different binding sites, we were now prepared to determine whether the full-length WT and T220 mutant Pit-1 proteins exhibited differential binding to these characterized monomeric and dimeric Pit-1 sites. We transiently transfected HeLa cells with mammalian expression vectors for intact hemagglutinin (HA)-tagged WT, T220D, and T220A Pit-1. After 24 h, the cells were harvested and nuclear extracts were isolated for EMSA analysis. We assessed the level of the expressed proteins in the HeLa nuclear extracts by Western blot analysis utilizing a monoclonal antibody directed against the HA epitope (Fig. 4A). Based on the Western blot analysis, we tested equivalent amounts of the expressed Pit-1 proteins (Wild type (WT), T220A, and T220D) for binding to the previously validated 32P-labeled oligonucleotides representing the EBS/FPIV site (PRL-4P) the PRL-1d site, and FPI (PRL-1P) (Fig. 4B). As an additional control, the first lane for each probe contains 0.5 μg of GH4T2 nuclear extract (lanes 1, 6, and 11) as a positive control. HeLa nuclear extracts from transient transfections of the rous sarcoma virus (RSV)-β-globin transfection control were included in these gel shifts as a negative control (lanes 2, 7, and 12). Consistent with the endogenous Pit-1 control experiment, both the GH4T2 nuclear extract and the Pit-1 proteins expressed in HeLa cells bound the FPIV probe only as a monomer (Fig. 3, lanes 1–5; boxed region). Comparison of WT, T220D, and T220A Pit-1 binding (lanes 3–5) reveals approximately equivalent binding by WT and T220A (lanes 3 and 5), but reduced binding by T220D Pit-1 (lane 4) to FPIV. Control HeLa extracts showed slight binding of a faster migrating nonspecific band (lane 2). We also tested the binding of these nuclear extracts to the monomeric Prl-1d site, which binds Pit-1 to synergistically activate the Prl promoter with ER bound to an adjacent site (7). The GH4T2 nuclear extract (at lower concentrations than Fig. 3) bound 1d only as a monomer. Both the WT Pit-1 and the T220A bound as a monomer with equal affinity (lanes 8 and 10). In contrast, the T220D Pit-1 mutant had reduced affinity for this monomeric 1d site (lane 9). The control Hela extract bound two nonspecific bands that are indicated. As a positive control, lanes 11–15 show the binding of each of these nuclear extracts to the FPI probe. The GH4T2 nuclear extract (at lower concentrations than Fig. 3) bound FPI as both a monomer and a dimer (lane 11), and the HeLa transfection control (lane 12) showed low levels of nonspecific binding but no bands that migrated at the location of the monomer or dimer bands. WT HA-Pit-1 bound to FPI as both a monomer and a dimer (lane 13) as did the T220A HA-Pit-1 (lane 15). However, whereas the T220D HA-Pit-1 bound FPI as a dimer with equal or possibly greater affinity than the WT or T220A constructs, T220D binding to FPI as a monomer was markedly reduced (lane 14). The control Hela extract bound two nonspecific bands that are indicated. We included equal amounts of each of the HA-tagged Pit-1 proteins as assessed by Western blot analysis and controlled for total protein by adding additional Hela control extract where necessary. In addition, the high-affinity binding of each of the HA-tagged Pit-1 constructs as a dimer to the FPI probe serves as a positive control for their binding activity. In summary, we found that in each case the WT and T220A Pit-1 proteins recapitulated the binding of the GH4T2 nuclear extracts. In comparison, whereas the T220D mutant bound to the FPI site preferentially as a dimer, it had decreased affinity for monomeric Pit-1 sites including both the FPIV/RRE site and the PRL-1d site. These data suggest that pseudo-phosphorylation of Pit-1 at T220 inhibits its ability to bind to DNA-binding sites as a monomer.
Figure 4.
Differential binding of full-length T220D Pit-1 to monomeric and dimeric Pit-1-binding sites from the rPRL promoter. A, HeLa cells were transiently transfected with control vector, or RSV-HA-tagged Pit-1 full-length expression constructs for WT Pit-1 and mutations T220D and T220A. After 24 h, the cells were harvested and isolated nuclear extracts were analyzed for HA-tagged Pit-1 expression by Western blot analysis with an anti-HA antibody. B, In each 30-μl gel shift reaction, GH4T2 or Hela nuclear extracts from cells transfected with control empty vector, or full-length WT Pit-1 and mutations in 10 μg of total protein (based on densitometry of the Western blot, we added 3.8 6.7 8.1 μl of WT Pit-1, T220D, and T220A, respectively) were incubated with 28,000 cpm of the indicated probe in binding buffer and 800 ng polydeoxyinosinic deoxycytidylic acid. After a 30-min incubation at room temperature, 2 μl of loading buffer was added to each sample and loaded on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. Monomer and Dimer Pit-1 bands are indicated. *, Nonspecific complexes.
Because of the complicated nature of these studies and to verify that HeLa nuclear proteins were not altering the affinity of the Pit-1 proteins, we chose to validate these studies by testing the binding of the WT and mutant Pit-1 proteins generated in a rabbit reticulocyte TNT in vitro transcription and translation system. The use of alternative initiating methionines (M1and M27) for translational initiation results in multiple Pit-1 isoforms (Fig. 5A). Similarly, the DNA binding of these Pit-1 isoforms results in multiple Pit-1 DNA complexes. However, like the Pit-1 proteins expressed in HeLa cells, the WT and T220A Pit-1 proteins expressed in the TNT system bound to the monomeric RRE site (lanes 2 vs. 4, boxed) with similar affinity, whereas T220D Pit-1 revealed decreased binding to the RRE (lane 3). Again, as a positive control, each of the TNT expressed Pit-1 proteins bound to the FP1 probe strongly as a dimer (lanes 7–9), whereas T220D Pit-1 had decreased monomer binding compared with WT and T220A Pit-1 (lanes 7–9, boxed region). Homologous competitor was included to inhibit probe binding in lanes 5 and 11–14. Together these studies used three different expression systems to illustrate that the T220D phosphorylation mimic of Pit-1 inhibits the binding of the Pit-1 homeodomain to DNA. Further, the comparison of DNA binding by full-length Pit-1 vs. homeodomain fusions at different sites in the rPRL gene promoter indicates that the inhibition of DNA binding by T220D only occurs at monomeric Pit-1-binding sites and can be overcome by the protein-protein and protein-DNA interactions that occur upon the binding of full-length Pit-1 at dimeric sites.
Figure 5.
Full-length T220D Pit-1 generated by in vitro transcription and translation also reveals differential binding at monomeric RRE and dimeric FPI sites. The full-length WT Pit-1 and mutant proteins (in pGem4 vectors) were prepared using the TNT coupled transcription-translation reticulocyte lysate system with T7 polymerase (Promega protocol). A, Representative in vitro transcription and translation reaction of Pit-1 incorporating [35S]methionine. The resulting reaction was electrophoresed on a 15% SDS-PAGE gel and visualized by autoradiography. B, In each 20-μl reaction, 2 μl of sample was incubated with 28,000 cpm of the RRE, FPI, or FPIII probe in binding buffer and 0.5 μg poly deoxyinosinic deoxycytidylic acid. To the reaction, specific competitor was added (5.4 pmol RRE, 5.6 pmol FPI). After a 30-min incubation at room temperature, 2 μl of loading buffer was added to each sample and ran on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. After electrophoresis, the gel was dried and visualized by autoradiography. Monomeric and dimeric binding is indicated. MW, Molecular mass; NS, nonspecific binding.
A T220D phosphorylation mimic of Pit-1 inhibits Ras stimulation, but not basal activation, of rPRL promoter
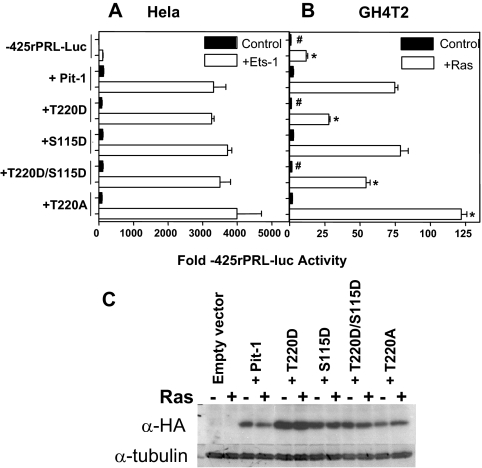
Having shown that phosphorylation or mutation of the T220 phosphorylation site in Pit-1 could alter its ability to interact with both Ets-1 (46) and the FPIV/RRE binding site (Figs. 1, 4, and 5), we next sought to determine whether the phosphorylation site mutants also altered the ability of Pit-1 to mediate basal activity and Ras responsiveness of the rPRL promoter. We generated mutations of the established phosphorylation sites in Pit-1: T220 and S115. We mutated these sites to either aspartic acid (D) to mimic phosphorylation or a neutral alanine (A) to block phosphorylation in the context of N-terminal hemagglutinin (HA)-tagged full-length Pit-1 in a mammalian expression vector under the control of the RSV promoter (T220D, S115D, T220D/S115D, and T220A). First, we transiently cotransfected the WT and phosphorylation site mutant Pit-1 expression constructs with a luciferase reporter construct containing 425 bp of the proximal 5′-flanking sequence from the rPRL gene (pA3-425rPRL-LUC) with and without mammalian expression constructs for Ets-1 into the HeLa cervical carcinoma cell line (Fig. 6A, left panel). Because HeLa cells don’t express either Pit-1 or Ets-1, this reconstitution assay allows us to test the ability of the Pit-1 constructs to both stimulate basal transcriptional activity and synergize with Ets-1 to activate the rPRL promoter. Second, we transiently cotransfected each of these constructs with the pA3-425rPRL-LUC reporter with and without oncogenic V12Ras (pSV-RAS) in the rat pituitary-derived GH4T2 cell line (Fig. 6A, right panel). Transfections of GH4T2 cells, which express both endogenous Pit-1 and Prl, allow us to test the ability of the WT and mutant Pit-1 proteins to mediate Ras responsiveness of rPRL promoter.
Figure 6.
Pit-1 WT and phosphorylation site mutants inhibit Ras responsiveness, but not basal transcriptional activity of rPRL promoter. A, −425 rPRL (3 μg) was cotransfected with WT and mutant pRSV-HA-Pit-1 constructs (5 μg) in HeLa cells ± pSG5-Ets-1 (5 μg). B, −425 rPRL (3 μg) was cotransfected with Pit-1 constructs (5 μg) in GH4T2 cells with V12Ras (2 μg). All electroporations included 100 ng of hrlTK-Renilla as an internal control for transfection efficiency. Data are mean ± sem of triplicate samples representative of at least three different transfections. Data were analyzed by ANOVA with Newman-Keul’s posttest analysis. *, Ras-transfected values that differ from −425rPRL + Pit-1 + Ras P < 0.05. #, Pit-1 only transfected values that differ from −425rPRL + Pit-1 P < 0.05. C, Total protein (50 μg) from transfected cells was analyzed by Western blot with an antibody directed against the HA-tag on each of the expressed Pit-1 constructs. Equivalence of gel loading was assessed by stripping the blot and blotting with an antibody against tubulin.
In the HeLa reconstitution model, all of the Pit-1 constructs induced a similar approximately 100-fold stimulation of basal transcriptional activity of the −425rPRL promoter and synergized with Ets-1 to stimulate the −425rPRL promoter by about 3500-fold (Fig. 6A, left panel). These results are consistent with previous reports (20,30,46) and indicate that the phosphorylation site mutants of Pit-1 are all capable of activating basal transcriptional activity of the rPRL promoter. Furthermore, these Pit-1 mutations had no effect on their ability to functionally synergize with Ets-1, suggesting that these mutants do not interfere with binding to Ets-1 in vivo (46).
In the GH4T2 model, the cotransfection of constitutively active Ras stimulates the activity of the −425rPRL promoter by approximately 10-fold, consistent with previous results (18). The addition of WT HA-tagged Pit-1 or an S115D mutant stimulated the Ras response about 75-fold compared with the basal activity of the −425PRL promoter in the absence of Ras. Conversely, T220D and the combination T220D/S115D mutation inhibited the ability of Pit-1 to stimulate the Ras response, resulting in 28- and 54-fold stimulations, respectively. In contrast, the T220A mutant increased the Ras response to 122-fold. Thus, the phosphomimic Pit-1 T220D exhibits only about 20% of the activity of the phosphorylation-resistant Pit-1 T220A mutant with the “A” mutant showing a gain of function in respect to Ras activation. Comparison of the expression levels of these constructs by Western blot (Fig. 6B) shows that the changes in Ras responsiveness by the T220 mutations were not due to reduced expression. In fact, we have expressed the T220D construct at both slightly higher and slightly lower levels than the other Pit-1 expression constructs and have consistently seen reduction of Ras responsiveness at all levels of T220D expression (data not shown). To control for potential variations in gel loading, we stripped the blot and reprobed it with an antibody against tubulin to show that equivalent amounts of protein were loaded in each well.
The loss of monomeric DNA binding by T220D (Figs. 4 and 5) suggests that the reduced ability of Pit-1 to mediate the Ras response upon phosphorylation or aspartic acid mutation of T220 is due to reduced binding by Pit-1 to the Ras response element. The ability of both WT and mutant Pit-1 constructs to bind to dimeric DNA-binding sites like FPI explains why Pit-1 T220D continues to activate the rPRL promoter and synergizes with Ets-1 in the Hela reconstitution assay. Previous studies have shown that activation of PKA and PKC reduce the ability of Ras to stimulate the rPRL promoter (37,38). Whereas PKA/PKC-mediated phosphorylation and inactivation of Raf has previously been identified as one mechanism for this inhibitory activity (51), our data suggest that the phosphorylation of Pit-1 and inhibition of DNA binding may represent a novel, gene-specific mechanism for inhibiting Ras activation of the rPRL promoter.
The phosphorylation mimic of Pit-1 T220D is less responsive to synergistic interactions with ERα
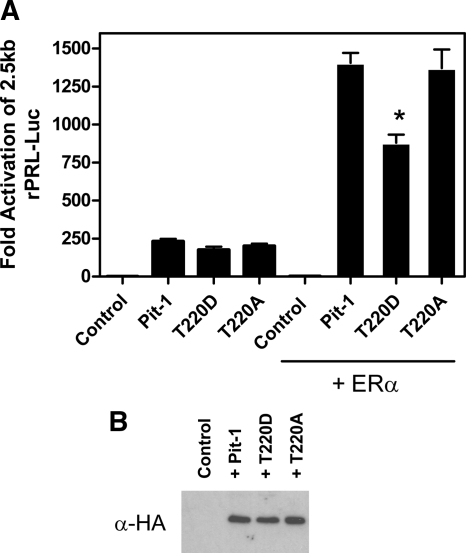
In addition to having reduced affinity for the Ras response element, the gelshift data (Fig. 4B) also indicate that monomeric Pit-1 T220D has lower affinity for the Prl-1d site. This site is adjacent to the estrogen response element in the distal enhancer (−1.5kb) and has been shown to be important for estradiol stimulation of the −2.5-kb rPRL promoter (7,25). The binding of Pit-1 as a monomer at this site also dictates its ability to utilize specific domains for its synergy with the estrogen receptor (7). Consequently, because T220D Pit-1 has lowered affinity for monomeric binding sites, its ability to synergize with estrogen receptor and activate the −2.5-kb rPRL promoter should be decreased. We tested the ability of WT Pit-1 and the T220 mutants to synergize with ER and activate the −2500 bp rPRL promoter by transiently transfecting HeLa cells cultured in serum free Optimem media with −2.5 rPRL-Luc, TK-Renilla, and RSV-βglobin (control) or WT, T220D, or T220A RSV-HA-Pit-1, and a mammalian expression construct for ERα, where indicated. The cells were treated with 10 nm 17β-estradiol. and after 24 h the cells were harvested and assayed for luciferase activity (Fig. 7). The addition of WT Pit-1, T220D, or T220A in the absence of ERα or 17β-estradiol, stimulated −2.5 rPRL-Luc promoter activity to approximately the same extent (264-, 214-, and 230-fold, respectively). The addition of ERα and estradiol in combination with either wild type or T220A Pit-1 resulted in similar synergistic stimulation of the Prl promoter to 1505- and 1575-fold, respectively. In comparison, the combination of ERα, estradiol, and T220D Pit-1 stimulated the rPRL promoter by only 977-fold (40% of WT Pit-1). Thus, these data suggest that phosphorylation of Pit-1 at T220 also blunts estrogen stimulation of the rPRL promoter. Western blot analysis of these constructs expressed in HeLa cells indicates that the loss in transcriptional activity was not due to decreased expression of Pit-1 T220D (Fig. 7B). The EMSA and transcriptional data show that the phosphorylation of Pit-1 at T220 governs its ability to bind to composite elements containing monomeric Pit-1-binding sites and thus regulates Pit-1’s function as an integrator of Ras and estradiol signaling to the rPRL promoter.
Figure 7.
Cooperative activation of the 2.5-kb rPRL promoter by ERα and Pit-1 is reduced by mutation of Pit-1 T220 to aspartic acid (D), but not alanine (A). Hela cells were transiently transfected with −2.5 rPRL-Luc, pcDNA ERα, and RSV-HA-Pit-1 or the T220 mutants and treated with 10 nm 17β-estradiol. Data represent mean ± sem of triplicate samples in three separate experiments. *, Different from + Pit-1 by Dunnett’s P < 0.01. B, Hela cells were transiently transfected by electroporation with 5 μg of RSV-HA-Pit-1, the T220 mutants, or control vector. At 24 h after transfection the cells were harvested. Total protein (100 μg) from transfected cells was analyzed by Western blot with an antibody directed against the HA-tag on each of the expressed Pit-1 constructs.
Discussion
Pit-1 integrates cell signaling by interacting with pathway-specific transcription factors and directing their activity to Pit-1 target genes. The ability of Pit-1 to interact with these transcription factors and synergistically activate transcription is dependent upon the ability to assemble specific coactivator complexes at composite DNA-binding sites. The assembly of these complexes appears to be dependent upon the structural features that are revealed when Pit-1 interacts with transcription factor partners or when it binds to DNA sites. In addition, two critical serine/threonine phosphorylation sites have been identified in Pit-1: S115 and T220. Both in vivo and in vitro studies have been used to show that these sites can be targeted by PKA and PKC, as well as cell cycle-dependent kinases (39,45). Several reports have described an altered affinity for distinct Pit-1-binding sites as a role for phosphorylation of T220. Unfortunately, the functional role of Pit-1 phosphorylation remains unclear (39,40,41,42,45). In the present study, the reduced affinity of the Pit-1 T220 phosphorylation mimic (T220D) for monomeric Pit-1-binding sites in the rPRL promoter results in a loss of Ras and estradiol responsiveness of the rPRL promoter. Furthermore, PKA and PKC activities have both been shown to inhibit Ras stimulation of rPRL promoter activity (37,38). Thus, our data suggest that phosphorylation of Pit-1 at T220 by these kinases inhibits the binding of Pit-1 to monomeric DNA sites in the rPRL gene promoter and regulates the ability of Pit-1 to form synergistic interactions with the transcriptional partners that regulate the integrated development and function of cells in the Pit-1 lineage.
Ras stimulation of the rPRL promoter requires the binding of both Ets-1 and Pit-1 at a composite Ets-1/Pit-1 binding element located at −217 to −190. Mutation of either of these sites inhibits the Ras response indicating that binding of both Ets-1 and Pit-1 is required. The region III TAD (AA 190–257) of Ets-1 can physically interact with the Pit-1 homeodomain (AA 199–291) (2,18,20,30,46), and this physical interaction upon binding of Ets-1 and Pit-1 to the composite DNA element, and the phosphorylation of Ets-1 by MAPK may generate a unique structural face for the binding of coactivator complexes that mediate the Ras response. In addition, T220 in the Pit-1 homeodomain has been identified as an important contact point in the interaction of Pit-1 with Ets-1 (46). Because T220 is an established phosphorylation site in Pit-1 (39,40,41,42,45), we mutated T220 to an alanine (A) to prevent phosphorylation without significantly altering the backbone of the protein, and to aspartic acid (D) to mimic the negative charge of phosphorylation. Because phosphorylation of Oct-1 at an equivalent site in the homeodomain has been shown to block DNA binding by Oct-1 (49) and has been implicated in reduced binding by Pit-1 (39,45), we tested the ability of WT and T220 mutants to bind Pit-1 sites in the rPRL promoter. Using purified WT and mutant GST-Pit-1 homeodomain fusion proteins, we found that the T220D phosphorylation mimic exhibited reduced binding to Pit-1-binding sites compared with either WT or T220A Pit-1 homeodomain. These data suggest that the T220D phosphorylation mimic simply prevents Pit-1 DNA binding; however, because the POU-specific domain is also an important component for high-affinity DNA binding, we examined the ability of full-length WT and mutant Pit-1 proteins to bind to Pit-1-binding sites from the rPRL promoter. We (Fig. 3) and others have shown that the FPIV and Prl-1D sites preferentially bind Pit-1 as a monomer whereas the FPI/PRL-1P site primarily binds a Pit-1 dimer. Using the full-length WT and mutant T220 Pit-1 proteins in EMSA analysis using oligonucleotides for these Pit-1 binding sites (Fig. 4), we found that the T220D mutation inhibited the binding of Pit-1 as a monomer, primarily affecting the Prl-1D and FPIV sites, but bound as a dimer to the FPI/PRL-1p site with near WT activity. Pit-1 exists as a monomer and can bind DNA as a monomer on certain target elements. However, basal activation of most Pit-1 targets is typically regulated by the binding of Pit-1 as a homodimer (7,50). In fact, previous studies have shown that mutation of the dimeric FPI site in the rPRL gene promoter reduces basal Pit-1-stimulated activity in HeLa cells by approximately 90% (52). X-ray crystallographic analysis of a Pit-1 dimer bound to a 28-bp perfect palindromic form of the Prl-1p (FPI) site revealed that the dimer structure of Pit-1 involved the interaction of the POU domain with the homeodomain of the Pit-1 partner (49). These Pit-1 interactions may serve to stabilize Pit-1 dimer binding to the DNA. In addition, binding of Pit-1 as a dimer at this palindromic site places the T220 phosphorylation site much farther away from the DNA backbone than the analogous phosphorylation site in Oct-1 bound to DNA as a monomer (49). This increased spacing is primarily due to DNA bending with the dimer Pit-1 DNA-binding site being held much straighter than the monomeric Oct-1 DNA-binding site. This model supports our finding that the T220D phosphorylation mimic inhibited the binding of Pit-1 as a monomer (Figs. 4 and 5) and consequently decreased transcriptional activity at the FPIV/RRE and Prl-1d sites at which monomeric Pit-1 partners with Ets-1 and ER to mediate Ras (Fig. 6B) and estrogen signaling (Fig. 7A), respectively, to the rPRL gene promoter. In contrast, basal transcriptional activity of Pit-1 mediated primarily through dimeric Pit-1 binding at the FPI site was essentially unaffected by the T220D phosphomimic (Fig. 6A).
Similarly, previous studies exploring the synergism between ER and Pit-1 have indicated that mutation of the monomeric Prl-1D site adjacent to an estrogen response element results in loss of ER/Pit-1 synergism, indicating that the primary synergistic response with estrogen receptor was mediated through this composite element. Furthermore, the structural conformation of Pit-1 when bound as a monomer to the Prl-1D site forces Pit-1 to utilize the amino-terminal TAD (AA 45-72) for the ER-synergistic response (7). When the 1D site is converted to a dimmer-binding site by the introduction of a GH-1 half-site, synergy with ER is retained, but is no longer dependent upon the amino-terminal Pit-1 TAD. These data suggest that the monomeric/dimeric binding of Pit-1 dictates the transcriptional coactivators that mediate the synergistic response through the presentation of distinct TAD domains for coactivator binding.
Because POU-homeodomain transcription factors utilize a bipartite DNA-binding domain that can partially encircle the DNA, the DNA element dictates the configurations of subdomains and subsequent recruitment of specific coregulators to control transcription (53). Recent studies suggest that the activity of Pit-1 at dimeric FPI/Prl-1p sites in the rPRL promoter is determined by a regulated balance of corepressor complexes that contain nuclear receptor corepressor/silencing mediator of retinoid and thyroid hormone receptor, mSin3A/B, and other histone deacetylases, and a coactivator complex that includes the histone acetyltransferase-containing proteins CBP and p300/CBP-associated factor. Activation by cAMP or insulin can switch the balance of these coregulators toward binding increased levels of coactivators. This response is not mediated through phosphorylation of Pit-1; rather hormone/growth factor addition stimulates the phosphorylation of CBP and regulates CBP recruitment to Pit-1/DNA complexes (54). In fact, the homodimerization of Pit-1 may, itself, be an important mechanistic step for the recruitment of CBP (55). In contrast, Ras activation of the rPRL promoter at the monomeric Pit-1 (FPIV)/Ets composite binding site appears to be independent of CBP activity, relying instead on a coactivator complex that includes a member of the p160 SRC family (31). The recruitment of these specific activating transcriptional complexes appears to be dependent upon the specific structural features that are revealed when Pit-1 binds to DNA sites as a monomer or a dimer. Pit-1 binding to the monomeric Prl-1D site, which is juxtaposed to an estrogen response element in the distal enhancer of the rPRL promoter, dictates use of the Pit-1 amino-terminal activation domain (AA 45-72) for synergy with ER (7,33). This amino-terminal TAD region of Pit-1 (AA 45-80) is also required for Ras stimulation of the rPRL promoter (30). This Ras-responsive domain can be further delineated into an activator of basal transcription (AA 50-70) and a basal inhibitory/Ras-stimulatory domain (AA 70-85) that is used by members of the p160 family of steroid receptor coactivators for Ras/ER/TR synergy with Pit-1 (23,31,33). Deletion of this domain in Pit-1 (AA 72-100) converts receptor interacting protein 140, a p160 family member, from a repressor to an activator of transcription on the rPRL, but not rGH, gene promoter (29). In addition, increasing the spacing of the contact points for the POU-specific domain and POU-homeodomain from 4 bp in the PRL promoter 1P site to 6 bp in the GH promoter GH-1 site sufficiently alters the structure of Pit-1 to transform it from a trans-activating factor to a repressor in pituitary lactotrophs (56). Similarly, studies of the human GH (hGH) locus control region (HSI) reveal variant Pit-1-binding sites that generate specific Pit-1/DNA structural conformations to recruit non-CBP-associated histone acetyl-transferase activity to the HSI region of the hGH promoter (31,47). These data indicate that monomeric/dimeric Pit-1 binding, the relative spacing of the Pit-1 POU and homeodomain binding sites, and the phosphorylation of Pit-1 generate unique structural faces for the binding of distinct coactivator/corepressor complexes to regulate the expression of Pit-1 target genes.
Naturally occurring Pit-1 mutations have been identified that interfere with the ability of Pit-1 to activate gene promoter targets by inhibiting interactions with transcriptional partners. One such mutation, K216E (57), is interesting because its localization within the recognition sequence for phosphorylation of T220 may have effects similar to the T220D phosphorylation mimic used in these studies. This mutation was present on a single allele of a patient with combined pituitary hormone deficiency. This patient presented with measurable GH levels that did not respond to stimulation and normal Prl hormone levels that did not respond to stimulation with TRH and developed hypothyroidism within the first 2 yr of life (57). This mutation has been shown to inhibit the interaction of Pit-1 with the retinoic acid receptor in the activation of the Pit-1 promoter (57) and to inhibit monomeric binding of Pit-1 to the GH-1-binding site. Closer examination of the DNA and protein interactions of this mutant indicated that it also increased dimeric Pit-1 binding to the Prl-1P and GH-1 sites, but reduced the affinity of Pit-1 for CBP/p300 (55). Consequently, this naturally occurring mutation, like our Pit-1 phosphorylation mimic, suggests that the phosphorylation of Pit-1 regulates the actions of Pit-1 by altering its affinity for both transcriptional cofactors and DNA-binding sites.
The ability of Pit-1 to direct signaling pathways to its target promoters is conferred by specific structural conformations it assumes upon binding to specific DNA sites and partnering with transcriptional coactivators and repressors. In addition, two critical serine/threonine phosphorylation sites have been identified in Pit-1: S115 and T220. Both in vivo and in vitro studies have been used to show that these sites can be targeted by PKA and PKC, as well as cell cycle-dependent kinases (39,45). We have shown that the T220D phosphorylation mimic of Pit-1 inhibits the ability of Pit-1 to mediate Ras and estradiol signaling to the rPRL promoter. Although, the T220D phosphorylation mimic may also inhibit the recruitment of coactivator complexes, reduced binding of this Pit-1 phosphorylation mimic to the monomeric Pit-1-binding sites (FPIV (RRE), PRL-1d) indicates that this may be the primary mechanism for reduced transcriptional activity in response to these stimuli. Thus, phosphorylation of Pit-1 at T220 serves to negatively regulate the ability of Pit-1 to mediate responses in which the monomeric binding of Pit-1 is required for selective interaction with transcriptional coregulators. The identification of members of the p160 SRC family as coactivators for both Ras and E/ER stimulation of the PRL gene promoter indicates that the monomeric binding of Pit-1 may reveal important contact points within the amino-terminal Pit-1 TAD for interaction with this coactivator complex (7,31). In contrast, this phosphorylation mimic had no effect on dimeric binding of Pit-1 at FPI (PRL-1p) and relatively little effect on basal transcriptional activity of Pit-1. In fact, different coactivator complexes interact with Pit-1 at the PRL-1p site, and Pit-1 dimerization may be a critical step in formation of these complexes. Taken together, these findings suggest that phosphorylation of Pit-1 at T220 serves as a mechanism to integrate the different signaling pathways that impinge on Pit-1 target genes by regulating monomeric and dimeric Pit-1 DNA binding and the assembly of unique transcriptional regulatory complexes.
Materials and Methods
Nuclear extracts
Nuclear extracts of GH4T2 and HeLa cells were isolated utilizing a modification of the method of Dignam et al. (58). Cells were harvested in PBS containing 3 mm EDTA and pelleted by centrifugation at 500 × g for 5 min. Cell pellets were washed in five packed cell volumes of a hypotonic solution containing: 10 mm HEPES buffer, pH 7.9; 1.5 mm MgCl2; 10 mm KCl; 0.5 mm dithiothreitol (DTT); 0.15 mm spermine; 0.5 mm spermidine; 10 mm sodium fluoride; 1 mm sodium vanadate; 25 mm β-glycerophosphate and a 1× concentration of Complete protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN). After centrifugation at 500 × g for 5 min, the cells were resuspended in two packed cell volumes of the same buffer. After incubation on ice for 10 min, the cells were lysed by homogenization in a Dounce homogenizer using pestle B. Lysis was verified by trypan blue staining, and cells were pelleted by centrifugation at 3500 × g for 5 min. Pellets containing nuclei were resuspended in nuclear extract buffer containing 20 mm HEPES buffer, pH 7.9; 0.42 m KCl; 1.5 mm MgCl2; 0.2 mm EDTA; 0.5 mm DTT; 10 mm sodium fluoride; 1 mm sodium vanadate; 25 mm β-glycerophosphate; 25% glycerol; and a 1× concentration of Complete protease inhibitors (Roche Molecular Biochemicals) with several strokes of the Dounce homogenizer and incubated at 4 C with mixing for 30 min. Extracted nuclei were pelleted by centrifugation at 13,000 × g for 20 min at 4 C, and supernatants containing nuclear proteins were adjusted to 100 mm KCl by dialysis in 20 mm HEPES buffer, pH 7.9; 100 mm KCl; 12.5 mm MgCl2; 0.1 mm EDTA; 2 mm DTT; and 17% glycerol. Precipitate was removed by centrifugation at 13,000 × g for 20 min at 4 C. The supernatant containing extracted nuclear proteins was supplemented with a 1× concentration of Complete protease inhibitors (Roche Molecular Biochemicals) and 1 mm sodium vanadate, which were snap frozen in a dry ice ethanol bath and stored at −80 C.
GST fusion protein preparation
Recombinant fusion proteins were prepared from bacterial extracts. Overnight cultures of Escherichia coli BL-21 (DE3) pLysS (Stratagene, La Jolla, CA), transformed with pGex plasmids, were diluted 1:10 in fresh Luria broth supplemented with ampicillin (100 μg/ml) and grown at 30 C to an absorbance of 0.5 at 600 nm. The cultures were induced by addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mm. Cultures were grown for an additional 2 h at 30 C. The bacteria were harvested by centrifugation at 5000 × g for 10 min at 4 C and stored at −80 C until preparation. The bacterial pellets were resuspended in 5 ml Bugbuster Reagent (Novagen, Madison, WI) per gram of bacterial pellet supplemented with a 1× concentration of Complete protease inhibitor mixture (Roche Molecular Biochemicals) and 25 U Benzonase per ml reagent and incubated at room temperature with rocking for 20 min to allow for lysis. After lysis, cellular debris was removed by centrifugation at 16,000 × g for 20 min at 4 C. The supernatant was transferred to a clean tube and bound to glutathione-Sepharose CL-4B (Amersham Pharmacia Biotech, Piscataway, NJ) for 1 h at room temperature. The sepharose was washed extensively with 1× PBS supplemented with 1× Complete Protease inhibitors and 1 mm DTT. Protein concentration was measured by the Bio-Rad assay (Bio-Rad Laboratories, Inc., Hercules, CA). Bound protein was analyzed for intactness and mass by SDS-PAGE in parallel with known amount of BSA and Coomassie blue staining.
TNT transcription and translation
WT and mutant Pit-1 proteins were synthesized, using the TNT coupled transcription-translation reticulocyte lysate system with T7 polymerase and supercoiled plasmids pGem4, pGem4rGHF-1, pGem4rGHF-1T220A, and pGem4rGHF-1T220D according to the manufacturer’s protocol (Promega Corp., Madison, WI).
EMSA
EMSA analysis was conducted as previously described (59). Synthesized oligonucleotides were annealed, and the double-stranded probes were labeled using Klenow DNA polymerase and α-32P-dCTP (19). The sequences of the composite Ets/Pit-1 Ras response element (FPIV), the 1p (FPI), and 1d Pit-1-binding sites from the rPRL promoter of Pit-1 are shown in Fig. 3B. SalI linkers were added to the 5′-ends for use in radiolabeling. In each reaction (20–30 μl), nuclear extracts from GH4T2 cells or transfected HeLa cells, GST-fusion proteins, or TNT-generated proteins were incubated with 28,000 cpm (∼0.1 ng) of the indicated probe in binding buffer [10 mm HEPES, 75 mm KCl, 4% glycerol, 1 mm EDTA, 1 mm DTT, 0.1% Nonidet P-40 (NP-40), and nonspecific DNA]. Where indicated, unlabeled competitor DNA was added to the reaction (5.4 pmol RRE, 5.3 pmol FPIII, 5.6 pmol FPI). After a 30-min incubation at room temperature, 2 μl of loading buffer [25% Ficoll, 0.01% bromophenol blue in 0.25× 22.5 mm Tris borate, 0.5 mm EDTA (TBE)] was added to each sample, and samples were separated on preelectrophoresed 6% nondenaturing polyacrylamide gel in 0.25× TBE buffer at 190 V. After electrophoresis, gels were dried and visualized by autoradiography.
Plasmid construction
pA3-425PRL-Luc containing the proximal 425 bp of 5′-flanking sequence from the rPRL promoter in the pA3Luc reporter has been previously described (37). Mutations mEBS and mFPIV have been previously described (19).
Plasmid pRSV-HA-Pit-1 encoding HA-tagged rat Pit-1 has been previously described (18). Mutant pRSV-HA-Pit-1 plasmids were generated by overlap extension PCR utilizing internal mutant primers and primers directed against the 5′- and 3′-ends of the HA-Pit-1 coding sequence and dual rounds of amplification. Primer sequences were as follows: T220D S, GAGGACAGATATCAGTATCGC; T220D AS, GATACTGATATCTGTCCTCCGT; T220A S, GAGGACAGCCATCAGTATCGC; T220A AS, GATACTGATGGCTGTCCTCCGT; S115D S, AGGCGGAAAGATAAATTGGTGGAAG; S115D AS, CCACCAATTTATCTTTCCGCCTG. PCR products were cloned into PCR2.1, and mutant fragments of Pit-1 were subcloned into the pRSV-HA-Pit-1 vector using internal BstXI and XhoI sites for the T220 mutations and internal PpuMI and XhoI sites for S115 mutations. PGem4rGHF-1 was generated by digesting pRSVrGHF-1 with HindIII and KpnI and ligating into pGem4. T220A and T220D mutations in pGem4rGHF-1 were generated by restriction digestion of pRSV-HA-Pit-1 T220A and T220D with BstXI and XhoI and ligating into digested pGem4rGHF-1. Plasmids were verified by sequencing (University of Colorado Cancer Center Sequencing Core). Plasmid pSV-Ras contains the T24 bladder carcinoma Harvey Ras valine 12 mutant oncogene (V-12 Ras) (18).
Cell culture
HeLa and GH4T2 cells were maintained in DMEM (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10 mm HEPES, nonessential amino acids, glutamine, 15% horse serum, and 2.5% fetal calf serum (Life Technologies, Inc). Cells were grown at 37 C in 5% CO2. Medium was changed 4–16 h before transfection.
Transient transfection
Electroporation.
Cells were harvested with 1× PBS with 3 mm EDTA and resuspended in culture medium. Aliquots of approximately 2–4 × 106 cells in 200 μl of media were added to plasmid DNA as described in figure legends and transfected by electroporation at 220 V and 500 μF using a Bio-Rad Gene Pulser with 4-mm gap cuvettes. After transfection, cells were plated on 60-mm tissue culture plates in culture media and incubated for 24 h. All electroporations included 100 ng of hrlTK-Renilla (Promega) as an internal control for transfection efficiency. Total DNA was kept constant, and nonspecific effects of viral promoters were controlled for by transfecting appropriate control vectors. Approximately 24 h after the electroporation, the cells were harvested in PBS containing 3 mm EDTA, pelleted by centrifugation at 500 × g for 5 min, and incubated for 15 min at room temperature in 40 μl of 1× passive lysis buffer before reading luciferase activity.
Lipid-mediated transfection.
Cells were transiently transfected using Effectene (QIAGEN, Chatsworth, CA) according to manufacturer’s instructions. Briefly, on d 1, HeLa or GH4T2 cells were harvested in 0.05% trypsin with 0.5 mm EDTA and plated on 96-well plates at a concentration of 20,000 or 45,000 cells per well in 100 μl of media, respectively. On d 2, plasmid DNA was prepared for transfection by mixing 0.8 μl/well of Enhancer reagent and 2.5 μl/well Effectin reagent diluted in 30 μl of enterochromaffin buffer per well. Total DNA was held constant, and each well was transfected with 1 ng of hrlTK-Renilla as an internal control for transfection efficiency. We controlled for nonspecific effects of viral promoters by transfecting appropriate control vectors. At 24 h after transfection, the cells were harvested and firefly luciferase and Renilla luciferase activity was determined on a Dynex microtiter plate luminometer using the Dual luciferase reporter assay system (Promega).
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants R01 DK46868 (to A.G.H.) and K01 DK02946 and R56 DK070952 (to D.L.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2009
Abbreviations: AA, Amino acids; CBP, CREB-binding protein; DTT, dithiothreitol; EBS, Ets-binding site; ER, estrogen receptor; GST, glutathione-S-transferase; HA, hemagglutinin; NP-40, Nonidet P-40; PKA, protein kinase A; PKC, protein kinase C; RRE, Ras-response element; RSV, rous sarcoma virus; S115, serine 115; SRC, steroid receptor coactivator; T220, threonine 220; TAD, transcriptional activation domain; TBE, 22.5 mm Tris borate, 0.5 mm EDTA; TR, thyroid hormone receptor; WT, wild type.
References
- Bach I, Rhodes SJ, Pearse II RV, Heinzel T, Gloss B, Scully KM, Sawchenko PE, Rosenfeld MG 1995 P-Lim, a LIM homeodomain factor, is expressed during pituitary organ and cell commitment and synergizes with Pit-1. Proc Natl Acad Sci USA 92:2720–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AP, Brodsky KS, Diamond SE, Kuhn LC, Liu Y, Gutierrez-Hartmann A 2000 The Pit-1 homeodomain and α-domain interact with Ets-1 and modulate synergistic activation of the rat prolactin promoter. J Biol Chem 275:3100–3106 [DOI] [PubMed] [Google Scholar]
- Dasen JS, O'Connell SM, Flynn SE, Treier, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG 1999 Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97:587–598 [DOI] [PubMed] [Google Scholar]
- Day RN, Koike S, Sakai M, Muramatsu M, Maurer RA 1990 Both Pit-1 and the estrogen receptor are required for estrogen responsiveness of the rat prolactin gene. Mol Endocrinol 4:1964–1971 [DOI] [PubMed] [Google Scholar]
- Gordon DF, Woodmansee WW, Black JN, Dowding JM, Bendrick-Peart J, Wood WM, Ridgway EC 2002 Domains of Pit-1 required for transcriptional synergy with GATA-2 on the TSHα gene. Mol Cell Endocrinol 196:53–66 [DOI] [PubMed] [Google Scholar]
- Gordon DF, Lewis SR, Haugen BR, James RA, McDermott MT, Wood WM, Ridgway EC 1997 Pit-1 and GATA-2 interact and functionally cooperate to activate the thyrotropin β-subunit promoter. J Biol Chem 272:24339–24347 [DOI] [PubMed] [Google Scholar]
- Holloway JM, Szeto DP, Scully KM, Glass CK, Rosenfeld MG 1995 Pit-1 binding to specific DNA sites as a monomer of dimer determines gene-specific use of a tyrosine-dependent synergy domain. Genes Dev 9:1992–2006 [DOI] [PubMed] [Google Scholar]
- Jacob KK, Stanley FM 2001 Elk-1, C/EBPα, and Pit-1 confer an insulin-responsive phenotype on prolactin promoter expression in Chinese hamster ovary cells and define the factors required for insulin-increased transcription. J Biol Chem 276:24931–24936 [DOI] [PubMed] [Google Scholar]
- Lira SA, Kalla KA, Glass CK, Drolet DW, Rosenfeld MG 1993 Synergistic interactions between Pit-1 and other elements are required for effective somatotroph rat growth hormone gene expression in transgenic mice. Mol Endocrinol 7:694–701 [DOI] [PubMed] [Google Scholar]
- Sánchez-Pacheco A, Peña P, Palomino P, Güell A, Castrillo JL, Aranda A 1998 The transcription factor GHF-1, but not the splice-variant GHF-2, cooperates with thyroid hormone and retinoic acid receptors to stimulate rat growth hormone gene expression. FEBS Lett 422:103–107 [DOI] [PubMed] [Google Scholar]
- Schaufele F, West BL, Baxter JD 1992 Synergistic activation of the rat growth hormone promoter by Pit-1 and the thyroid hormone receptor. Mol Endocrinol 6:656–665 [DOI] [PubMed] [Google Scholar]
- Steinfelder HJ, Hauser P, Nakayama Y, Radovick S, McClaskey JH, Taylor T, Weintraub BD, Wondisford FE 1991 Thyrotropin-releasing hormone regulation of human TSHB expression: role of a pituitary-specific transcription factor (Pit-1/GHF-1) and potential interaction with a thyroid hormone-inhibitory element. Proc Natl Acad Sci USA 88:3130–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto DP, Ryan AK, O'Connell SM, Rosenfeld MG 1996 P-OTX: A Pit-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc Natl Acad Sci USA 93:7706–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctôt C, Drouin J 1998 The pan-pituitary activator of transcription, Ptx-1, acts in synergy with SF-1 and Pit-1 and is an upstream regulator of the Lim-homeodomain gene Lim-3/Lhx-3. Mol Endocrinol 12:428–441 [DOI] [PubMed] [Google Scholar]
- Voss JW, Wilson L, Rosenfeld MG 1991 POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev 5:1309–1320 [DOI] [PubMed] [Google Scholar]
- Xu L, Lavinsky RM, Dasen JS, Flynn SE, McInerney EM, Mullen TM, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal AK, Rose DW, Glass CK, Rosenfeld MG 1998 Signal-specific co-activator domain requirements for Pit-1 activation. Nature 395:301–306 [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Hagman J, Gutierrez-Hartmann A 1998 Ets transcription factors: nuclear effectors of the Ras/MAP kinase signaling pathway. Trends Biochem Sci 23:213–216 [DOI] [PubMed] [Google Scholar]
- Bradford AP, Conrad KE, Wasylyk C, Wasylyk B, Gutierrez- Hartmann A 1995 Functional interaction of c-Ets-1 and GHF-1/Pit-1 mediates Ras activation of pituitary-specific gene expression: Mapping of the essential c-Ets-1 domain. Mol Cell Biol 15:2849–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AP, Conrad KE, Tran PH, Ostrowski MC, Gutierrez-Hartmann A 1996 GHF-1/Pit-1 functions as a cell specific integrator of Ras signalling by targeting the Ras pathway to a composite Ets-1/GHF-1 response element. J Biol Chem 271:24639–24648 [DOI] [PubMed] [Google Scholar]
- Bradford AP, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A 1997 Interaction of Ets-1 and the POU-homeodomain GHF-1/Pit-1 reconstitutes pituitary specific gene expression. Mol Cell Biol 17:1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe RE, Frazer-Abel AA, Gutierrez-Hartmann A, Bradford AP 1997 Functional components of fibroblast growth factor signal transduction in pituitary cells. J Biol Chem272:30852–30859 [DOI] [PubMed] [Google Scholar]
- Wasylyk C, Bradford AP, Gutierrez-Hartmann A, Wasylyk B 1997 Conserved mechanisms of Ras evolutionary related transcription factors, Ets-1 and pointed P2. Oncogene 14:899–913 [DOI] [PubMed] [Google Scholar]
- Chang W, Zhou W, Theill LE, Baxter JD, Schaufele F 1996 An activation function of Pit-1 required selectively for synergistic transcription. J Biol Chem 271:17733–17738 [DOI] [PubMed] [Google Scholar]
- Schaufele F 1996 CCAAT/enhancer-binding protein alpha activation of the rat growth hormone promoter in pituitary progenitor GHFT1–5 cells. J Biol Chem 271:21484–21489 [DOI] [PubMed] [Google Scholar]
- Nowakowski BE, Maurer RA 1994 Multiple Pit-1 binding sites facilitate estrogen responsiveness of the prolactin gene. Mol Endocrinol 8:1742–1749 [DOI] [PubMed] [Google Scholar]
- Rhodes SJ, Chen R, DiMattia GE, Scully KM, Kalla KA, Lin SC, Yu VC, Rosenfeld MG 1993 A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the Pit-1 gene. Genes Dev 7:913–932 [DOI] [PubMed] [Google Scholar]
- Kim MK, McClaskey JH, Bodenner DL, Weintraub BD 1993 An AP-1-like factor and the pituitary-specific factor Pit-1 are both necessary to mediate hormonal induction of human thryotropinα gene expression. J Biol Chem 268:23366–23375 [PubMed] [Google Scholar]
- Farrow KN, Manning N, Schaufele F, Gutierrez-Hartmann A 1996 The c-Jun ë -domain inhibits neuroendocrine promoter activity in a DNA sequence- and pituitary-specific manner. J Biol Chem 271:17139–17146 [DOI] [PubMed] [Google Scholar]
- Chuang FM, West BL, Baxter JD, Schaufele F 1997 Activities in Pit-1 determine whether receptor interacting protein 140 activates or inhibits Pit-1/nuclear receptor transcriptional synergy. Mol Endocrinol 11:1332–1341 [DOI] [PubMed] [Google Scholar]
- Duval DL, Jean A, Gutierrez-Hartmann A 2003 Ras signaling and transcriptional synergy at a flexible Ets-1/Pit-1 composite DNA element is defined by the assembly of selective activation domains. J Biol Chem 278:39684–39696 [DOI] [PubMed] [Google Scholar]
- Duval DL, Jonsen MD, Diamond SE, Murapa P, Jean A, Gutierrez-Hartmann A 2007 Differential utilization of transcription activation subdomains by distinct coactivators regulates Pit-1 basal and Ras responsiveness. Mol Endocrinol 21:172–185 [DOI] [PubMed] [Google Scholar]
- Gordon DF, Tucker EA, Tundwal K, Hall H, Wood WM, Ridgway EC 2006 MED220/TRAP220 functions as a transcriptional coactivator with Pit-1 and GATA2 on the TSHβ promoter in thyrotropes. Mol Endocrinol 20:1073–1089 [DOI] [PubMed] [Google Scholar]
- Schaufele F 1999 Regulation of estrogen receptor activation of the prolactin enhancer/promoter by antagonistic activation function-2-interacting proteins. Mol Endocrinol 13:935–945 [DOI] [PubMed] [Google Scholar]
- Rowan BG, Weigel NL, O'Malley BW 2000 Phosphorylation of steroid receptor coactivator-1. identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem 275:4475–4483 [DOI] [PubMed] [Google Scholar]
- Goel A, Janknecht R 2004 Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J Biol Chem 279:14909–14916 [DOI] [PubMed] [Google Scholar]
- Myers E, Hill AD, Kelly G, McDermott EW, O'Higgins NJ, Buggy Y, Young LS 2005 Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res 11:2111–2122 [DOI] [PubMed] [Google Scholar]
- Conrad KE, Gutierrez-Hartmann A 1992 The ras and protein kinase A pathways are mutually antagonistic in regulating rat prolactin promoter activity. Oncogene 7:1279–1286 [PubMed] [Google Scholar]
- Oberwetter JM, Conrad KE, Gutierrez-Hartmann A 1993 The Ras and protein kinase C signaling pathways are functionally antagonistic in GH4 neuroendocrine cells. Mol Endocrinol 7:915–923 [DOI] [PubMed] [Google Scholar]
- Kapiloff MS, Farkash Y, Wegner M, Rosenfeld MG 1991 Variable effects of phosphorylation of Pit-1 dictated by the DNA response elements. Science 253:786–789 [DOI] [PubMed] [Google Scholar]
- Steinfelder HJ, Radovick S, Wondisford FE 1992 Hormonal regulation of the thyrotropin β-subunit gene by phosphorylation of the pituitary-specific transcription factor Pit-1. Proc Natl Acad Sci USA 89:5942–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimura Y, Howard PW, Maurer RA 1994 Pit-1 binding sites mediate transcriptional responses to cAMP through a mechanism which does not require inducible phosphorylation of Pit-1. Mol Endocrinol 8:1559–1565 [DOI] [PubMed] [Google Scholar]
- Fischberg DJ, Chen XH, Bancroft C 1994 A Pit-1 phosphorylation mutant can mediate either basal or induced prolactin or growth hormone promoter activity. Mol Endocrinol 8:1566–1573 [DOI] [PubMed] [Google Scholar]
- Roberts SB, Segil N, Heintz N 1991 Differential phosphorylation of the transcription factor Oct1 during the cell cycle. Science 253:1022–1026 [DOI] [PubMed] [Google Scholar]
- Segil N, Roberts SB, Heintz N 1991 Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science 254:1814–1816 [DOI] [PubMed] [Google Scholar]
- Caelles C, Hennemann H, Karin M 1995 M-phase-specific phosphorylation of the POU transcription factor GHF-1 by a cell cycle-regulated protein kinase inhibits DNA binding. Mol Cell Biol 15:6694–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustijn KD, Duval DL, Wechselberger R, Kaptein R, Gutierrez-Hartmann A, van der Vliet PC 2002 Structural characterization of the PIT-1/ETS-1 interaction: PIT-1 phosphorylation regulates PIT-1/ETS-1 binding. Proc Natl Acad Sci USA 99:12657–12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewchuk BM, Ho Y, Liebhaber SA, Cooke NE 2006 A single base difference between Pit-1 binding sites at the hGH promoter and locus control region specifies distinct Pit-1 conformations and functions. Mol Cell Biol 26:6535–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Moye-Rowley S, Maurer RA 1995 In vivo mutational analysis of the DNA binding domain of the tissue-specific transcription factor, Pit-1. J Biol Chem 270:25520–25525 [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Li P, Leon-del-Rio A, Rosenfeld MG, Aggarwal AK 1997 Structure of Pit-1 POU domain bound to DNA as a dimer: unexpected arrangement and flexibility. Genes Dev 11:198–212 [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Flynn SE, Voss JW, Albert VR, Kapiloff MS, Wilson L, Rosenfeld MG 1990 The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell 61:1021–1033 [DOI] [PubMed] [Google Scholar]
- Häfner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W 1994 Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol 14:6696–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnarayan S, Chiono M, Alexander LM, Gutierrez-Hartmann A 1995 Reconstitution of protein kinase A regulation of the rat prolactin promoter in HeLa nonpituitary cells: identification of both GHF-1/Pit-1-dependent and -independent mechanisms. Mol Endocrinol 9:502–512 [DOI] [PubMed] [Google Scholar]
- Phillips K, Luisi B 2000 The virtuoso of versatility: POU proteins that flex to fit. J Mol Biol 302:1023–1039 [DOI] [PubMed] [Google Scholar]
- Zanger K, Radovick S, Wondisford FE 2001 CREB binding protein recruitment to the transcription complex requires growth factor-dependent phosphorylation of its GF box. Mol Cell 7:551–558 [DOI] [PubMed] [Google Scholar]
- Cohen RN, Brue T, Naik K, Houlihan CA, Wondisford FE, Radovick S 2006 The role of CBP/p300 interactions and Pit-1 dimerization in the pathophysiological mechanism of combined pituitary hormone deficiency. J Clin Endocrinol Metab 91:239–247 [DOI] [PubMed] [Google Scholar]
- Scully KM, Jacobson EM, Jepsen K, Lunyak V, Viadiu H, Carrière C, Rose DW, Hooshmand F, Aggarwal AK, Rosenfeld MG 2000 Allosteric effects of Pit-1 DNA sites on long-term repression in cell type specification. Science 290:1127–1131 [DOI] [PubMed] [Google Scholar]
- Cohen LE, Zanger K, Brue T, Wondisford FE, Radovick S 1999 Defective retinoic acid regulation of the Pit-1 gene enhancer: a novel mechanism of combined pituitary hormone deficiency. Mol Endocrinol 13:476–484 [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG 1983 Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe RE, Gutierrez-Hartmann A 2001 Pituitary Ets-1 and GABP bind to the growth factor regulatory sites of the rat prolactin promoter. Nucleic Acids Res 29:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.