Abstract
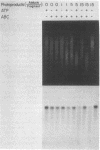
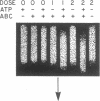
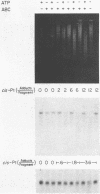
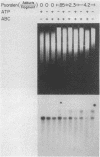
A general method has been developed to measure the formation and removal of DNA adducts in defined sequences of mammalian genomes. Adducted genomic DNA is digested with an appropriate restriction enzyme, treated with Escherichia coli UvrABC excision nuclease (ABC excinuclease), subjected to alkaline gel electrophoresis, and probed for specific sequences by Southern hybridization. The ABC excinuclease incises DNA containing bulky adducts and thus reduces the intensity of the full-length fragments in Southern hybridization in proportion to the number of adducts present in the probed sequence. This method is similar to that developed by Bohr et al. [Bohr, V. A., Smith, C. A., Okumoto, D. S. & Hanawalt, P. C. (1985) Cell 40, 359-369] for quantifying pyrimidine dimers by using T4 endonuclease V. Because of the wide substrate range of ABC exinuclease, however, our method can be used to quantify a large variety of DNA adducts in specific genomic sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. E., Murray A. M. Benzpyrene groups bind preferentially to the DNA of active chromatin in human lung cells. Nucleic Acids Res. 1982 Mar 11;10(5):1547–1555. doi: 10.1093/nar/10.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Ho L., Hanawalt P. C. Characterization of a DNA repair domain containing the dihydrofolate reductase gene in Chinese hamster ovary cells. J Biol Chem. 1986 Dec 15;261(35):16666–16672. [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Brown T. C., Beard P., Cerutti P. A. Preferential repair of N-acetoxy-acetylaminofluorene lesions in the nuclease-hypersensitive region of simian virus 40. J Biol Chem. 1987 Sep 5;262(25):11947–11951. [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Ellis N., Chasin L. A. Structure of the dihydrofolate reductase gene in Chinese hamster ovary cells. Nucleic Acids Res. 1983 Apr 11;11(7):1997–2012. doi: 10.1093/nar/11.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn S. M., Lieberman M. W. The use of antibodies to 5-bromo-2'-deoxyuridine for the isolation of DNA sequences containing excision-repair sites. J Biol Chem. 1984 Oct 25;259(20):12456–12462. [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. N., Beverley S. M., Schimke R. T. Rapid spontaneous dihydrofolate reductase gene amplification shown by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3711–3715. doi: 10.1073/pnas.80.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Leadon S. A. Differential repair of DNA damage in specific nucleotide sequences in monkey cells. Nucleic Acids Res. 1986 Nov 25;14(22):8979–8995. doi: 10.1093/nar/14.22.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Nose K., Nikaido O. Transcriptionally active and inactive genes are similarly modified by chemical carcinogens or X-ray in normal human fibroblasts. Biochim Biophys Acta. 1984 Apr 5;781(3):273–278. doi: 10.1016/0167-4781(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Okumoto D. S., Bohr V. A. DNA repair in the metallothionein gene increases with transcriptional activation. Nucleic Acids Res. 1987 Dec 10;15(23):10021–10030. doi: 10.1093/nar/15.23.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi G. B., Walker I. G. Lack of excision of 4HAQO adducts from DNA by cell extracts that excise pyrimidine dimers. Biochem Biophys Res Commun. 1986 Nov 14;140(3):775–781. doi: 10.1016/0006-291x(86)90701-1. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Barbacid M. Transforming genes in human tumors. J Cell Biochem. 1982;20(1):51–61. doi: 10.1002/jcb.240200106. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sancar A., Smith F. W., Sancar G. B. Purification of Escherichia coli DNA photolyase. J Biol Chem. 1984 May 10;259(9):6028–6032. [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Sancar A. Repair of N-methyl-N'-nitro-N-nitrosoguanidine-induced DNA damage by ABC excinuclease. J Bacteriol. 1987 Feb;169(2):540–545. doi: 10.1128/jb.169.2.540-545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- Zolan M. E., Smith C. A., Hanawalt P. C. Formation and repair of furocoumarin adducts in alpha deoxyribonucleic acid and bulk deoxyribonucleic acid of monkey cells. Biochemistry. 1984 Jan 3;23(1):63–69. doi: 10.1021/bi00296a010. [DOI] [PubMed] [Google Scholar]