Abstract
Estrogen receptors ERα and ERβ, members of the nuclear receptor superfamily, exert profound effects on the gene expression and biological response programs of their target cells. Herein, we explore the dynamic interplay between these two receptors in their selection of chromatin binding sites when present separately or together in MCF-7 breast cancer cells. Treatment of cells (containing ERα only, ERβ only, or ERα and ERβ) with estradiol or ER subtype-selective ligands was followed by chromatin immunoprecipitation analysis with a custom-designed tiling array for ER binding sites across the genome to examine the effects of ligand-occupied and unoccupied ERα and ERβ on chromatin binding. There was substantial overlap in binding sites for these estradiol-liganded nuclear receptors when present alone, but many fewer sites were shared when both ERs were present. Each ER restricted the binding site occupancy of the other, with ERα generally being dominant. Binding sites of both receptors were highly enriched in estrogen response element motifs, but when both ERs were present, ERα displaced ERβ, shifting it into new sites less enriched in estrogen response elements. Binding regions of the two ERs also showed differences in their enrichments for other transcription factor binding motifs. Studies with ER subtype-specific ligands revealed that it was the liganded subtype that principally determined the spectrum of chromatin binding. These findings highlight the dynamic interplay between the two ERs in their selection of chromatin binding sites, with competition, restriction, and site shifting having important implications for the regulation of gene expression by these two nuclear receptors.
The dynamic interplay between estrogen receptors α and β in their selection of chromatin binding sites has important implications for their regulation of gene expression.
Nuclear hormone receptors play key roles in many aspects of reproductive physiology, development, and metabolism, and they are also involved in many disease states, including hormone-regulated cancers such as breast cancer (1,2,3). The effects of estrogens in breast cancer are mediated through two estrogen receptors (ERs), ERα and ERβ, that are encoded by genes on different chromosomes and function as ligand-modulated transcription factors, up- and down-regulating gene expression in a target tissue-selective manner (4,5). The presence of ERα in breast cancer cells is associated with enhanced proliferation in response to estrogens, whereas several studies have implicated ERβ as exerting antiproliferative effects (5,6,7,8,9,10).
ERα and ERβ are highly homologous in their DNA-binding domains (97% amino acid identity), but they are quite different in their ligand-binding domains (56% identity) and transcriptional activation function-1 (AF-1) regions (∼20% identity). The differences in their ligand-binding domains allow the two ER subtypes to bind certain ligands with high selectivity for one or the other ER subtype (11,12,13,14). Although most human breast cancers coexpress both ERs (15,16,17), much less is known about the role of ERβ in breast cancer and how the presence of both ERs might affect cellular responses to hormone, although the presence of ERβ in breast tumors is generally associated with a better patient prognosis (17,18,19,20,21).
We previously used microarray transcriptional profiling to comprehensively study the estrogen-regulated gene expression profiles in breast cancer cells expressing ERα or ERβ (4,5,6,10,22). These studies provided a system-wide view of the actions of these receptors on target genes and also revealed marked transcriptome dynamics in response to 17β-estradiol (E2) and selective ER modulators. Although both ERα and ERβ have been shown to be able to heterodimerize when present in the same cell, the impact of having two ER subtypes in one cell and the potential role of heterodimers on gene regulation is still unclear. Hence, our lab (4,5,23) and others (24,25,26) had performed several gene expression studies aimed at studying the interplay between ERα and ERβ and characterizing the role of ERβ in influencing the transcriptional activity of ERα. These studies revealed that ERβ significantly impacted ERα gene expression, both in an enhancing and a suppressing fashion, and that some genes responded to E2 stimulation only in the copresence of ERβ (4). This raised the possibility that interaction between the two ER subtypes might enable the ER complexes to access new chromatin regions when present together. To compare the activities of ERα and ERβ and understand how they might be modulating each other’s activities, we need to identify the first step in the ERβ signal cascade, namely to define a map of both ERα and ERβ binding sites when the two ERs are present alone or together in breast cancer cells.
Through the studies reported herein, we sought to address a number of these important questions. When ERα and ERβ individually are present alone in cells, what are the ranges of their binding sites and to what extent do they overlap? When both ERα and ERβ are present, in what ways do they interact in terms of binding site selection? Are their binding site ranges extended? Are sites that were occupied by either ERα or ERβ when present alone still accessible to both receptors when both receptors are present? Is the binding site selection of a liganded ER subtype affected by an unliganded ER dimer partner?
To better understand this interplay between ERα and ERβ binding at the genomic level, we have used chromatin immunoprecipitation (ChIP) combined with DNA microarray (ChIP-chip) analysis with a unique custom-designed tiling array and profiled the genome-wide binding events of the two ERs in breast cancer cells containing various complements of ERα and ERβ, treated with the natural hormone E2, the ERα-selective ligand propyl pyrazole triol (PPT) (14), or the ERβ-selective ligand ERB-041 (12). Our findings demonstrate that there is substantial overlap in the chromatin binding sites for E2-liganded ERα and ERβ when they are present alone in cells, but when these ERs are present together, many fewer sites are shared and new sites become occupied. Our findings highlight the dynamic interplay of competition, restriction, and site selection shifting, that occurs between the two ER subtypes in their selection of chromatin binding sites and how this is modulated by their state of ligand occupancy.
Results
Generation of human breast cancer cells containing ERα only, ERβ only, or both ERα and ERβ and their examination using a custom tiled microarray of ER binding sites
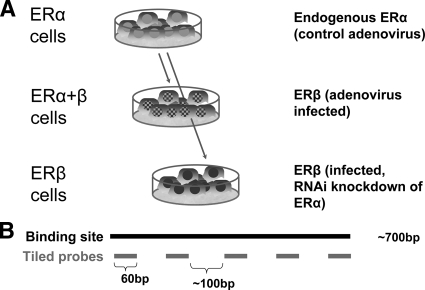
To examine ER-binding site selection by ERα and ERβ in human breast cancer cells, as shown schematically in Fig. 1A, we used adenoviral gene delivery to introduce ERβ into ERα-positive MCF-7 cells, both without and with small interfering RNA (siRNA) directed against ERα. These produced cells with three complements of ERs, namely cells containing endogenous ERα only, or ERα plus ERβ at equal levels, or ERβ only, as previously described (5). We then examined the localization of ERα and ERβ, when present together or separately, in response to different ligand treatments using ChIP-chip with a custom tiling array. We assessed the effects of unliganded and liganded ERs at ER-binding sites, and we compared the endogenous hormonal ligand E2 (dual activation of ERα and ERβ) and the subtype-selective nonsteroidal ligands PPT (ERα preferential activation) (14) and ERB-041 (ERβ preferential activation) (12).
Figure 1.
Generation of MCF-7 cells containing different complements of ERα and ERβ for ChIP-chip studies. A, MCF-7 cells were infected with control β-galactosidase-expressing adenovirus or ERβ-expressing adenovirus to generate cells containing ERα-only and ERα plus ERβ, respectively. Cells containing ERβ only were generated by knockdown of ERα by siRNA transfection of cells containing ERα plus ERβ. B, Schematic diagram showing location of tiled probes in the custom-designed tiling arrays. Each probe is 60 bp in length, and probes are tiled approximately 100 bp from each other. RNAi, RNA interference.
For our ChIP-chip analysis, we hybridized the ChIP chromatin and input DNA onto NimbleGen custom-designed tiling arrays that provide coverage of all known and predicted ER-binding sites across the genome in MCF-7 cells encompassing approximately 61,000 documented and putative ER binding sites (described in detail in Materials and Methods); binding site probe design is shown in Fig. 1B. MCF-7 cells having the three complements of ER (ERα only, ERα plus ERβ, and ERβ only) were treated with control (0.1% ethanol) vehicle or one of the three ER ligands for 45 min, a time that is optimal for ER recruitment to chromatin. Chromatin fragments bound by ER were immunoprecipitated and hybridized onto the tiling arrays. Several examples of ChIP-chip signal intensity peaks for positive and negative ER binding regions are shown in supplemental Fig. S1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Table 1 shows the number of ERα and ERβ binding sites observed under the various experimental conditions. With E2 treatment, in MCF-7 cells expressing ERα only, we identified 4405 ERα-binding sites [ERα(α-cell) sites]; in MCF-7 expressing ERβ only, we identified 1897 ERβ-binding sites [ERβ(β-cell) sites]; and in MCF-7 cells expressing both ERα and ERβ, we identified 3252 ERα-binding sites [ERα(αβ-cell) sites] and 1744 ERβ-binding sites [ERβ(αβ-cell) sites] (Table 1). We do not know whether the antibodies used in ChIP for ERα and ERβ work with equal efficiency or not. Hence, it is possible that some of the differences in the number of binding sites for ERα and ERβ may be due to differences in the affinities of the antibodies for their receptor targets.
Table 1.
Summary of ER binding sites in the three MCF-7 cells expressing ERα only, both ERα and ERβ, or ERβ only
| Cell type | Ligand | Antibodies | Binding sites | |
|---|---|---|---|---|
| 1 | MCF7 (α-cells) | E2 | Anti-ERα | 4405 |
| 2 | MCF7 (α-cells) | PPT | Anti-ERα | 3269 |
| 3 | MCF7 (αβ-cells) | E2 | Anti-ERα | 3252 |
| 4 | MCF7 (αβ-cells) | E2 | Anti-ERβ | 1744 |
| 5 | MCF7 (αβ-cells) | PPT | Anti-ERα | 3466 |
| 6 | MCF7 (αβ-cells) | ERB-041 | Anti-ERβ | 1109 |
| 7 | MCF7 (β-cells) | E2 | Anti-ERβ | 1897 |
| 8 | MCF7 (β-cells) | ERB-041 | Anti-ERβ | 1042 |
Values are the mean from three independent experiments, with each experiment done in duplicate.
A number of mock ChIP-chip experiments were also performed to ensure the fidelity of our ChIP-chip analyses. In addition, we selected a set of random sites (n = 42) and validated ER binding with ChIP-quantitative PCR in 93% (39 of 42) to assess our false discovery rate (FDR). Our FDR of 7% is consistent with other genome-wide ChIP studies (24,27) (see Materials and Methods and supplemental Table S1). We also identified which regions on our designed tiling arrays were actually bound by ER. These showed over 80% of the ER binding sites to be distributed in ChIP-chip (28) and ChIP-paired end ditag (PET) (24) ER binding regions, with the remainder in computationally predicted estrogen response element (ERE) regions (29), and 1% or less in control regions on the array, indicating selectivity in ER binding (supplemental Table S2).
Each ER subtype shifts the binding sites of the other ER, with ERα having a dominant effect
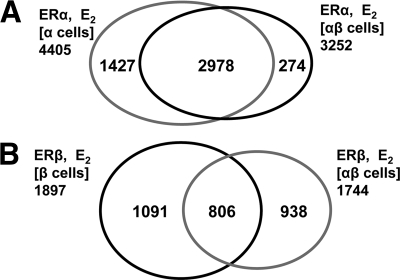
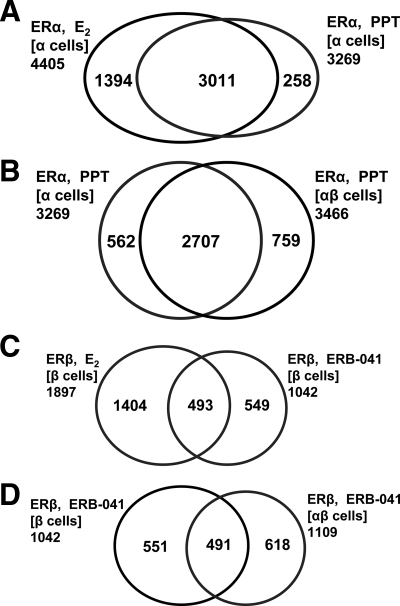
To understand how the presence of the ER-subtype partner might influence the pattern of chromatin binding sites for ERα or ERβ, we compared the pattern of binding site occupancy by these ERs after E2 exposure in the three cell types. Specifically, we compared how ERα binding sites in ERα cells changed when ERβ was also present [i.e. ERα(α-cell) sites vs. ERα(αβ-cell) sites], and conversely, we examined how ERβ binding sites in ERβ cells changed when ERα was also present [ERβ(β-cell) sites vs. ERβ(αβ-cell) sites]. The results are visualized in the Venn diagram in Fig. 2.
Figure 2.
Effect of ER subtype partner on ER binding site distribution with E2 treatment. A, The introduction of ERβ into the cells has a relatively minor effect on the distribution of ERα binding sites. B, ERα has a more pronounced effect on the distribution of ERβ binding sites.
The presence of ERβ had a limited effect on the distribution of ERα binding sites when compared with that seen in ERα-only cells. There was a reduction in the number of ERα binding sites in ERαβ-cells, but the overlap of binding sites remained very high, with 92% of ERα(αβ-cell) binding sites overlapping the ERα(α-cell) binding sites and only 8% being new sites (Fig. 2A). By contrast, ERα had a much more profound effect on the distribution of ERβ binding sites. As shown in Fig. 2B, although the number of ERβ binding sites was essentially unaffected by the presence of ERα, there was a far greater shift in the ERβ sites selected. As a result, the overlap of ERβ binding sites in ERαβ-cells with those in ERβ-only cells was much more limited (806, ∼40%), but in these ERαβ-cells, ERβ occupied 938 new sites, which represent nearly 60% of the sites it occupies in these cells. These findings suggest that when the two ER subtypes are both present, ERα is more dominant in competing for ER binding sites than ERβ, and as a consequence, ERβ binding is shifted into many new sites.
Mutual competition between ERα and ERβ binding site occupancy restricts the number of potential ERα/ERβ heterodimer sites
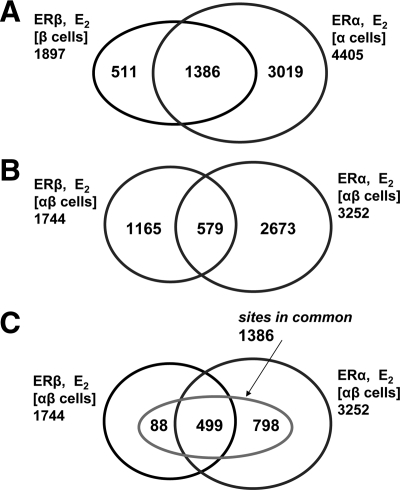
To further evaluate the influence of each ER subtype partner on chromatin binding, we examined the occupancy of ER binding sites by ERα and ERβ when they were present either separately or together in cells (Fig. 3, A and B, respectively) after E2 treatment. When present separately, ERα bound to about twice as many sites as ERβ, but both occupied many of the same sites, with 73% of ERβ(β-cell) binding sites also being ERα(α-cell) binding sites (Fig. 3A). This is consistent with current knowledge that ERα and ERβ can recognize the same estrogen response element motif (30) but that ERα binds to DNA with higher affinity than ERβ (31,32,33). Notably, a significant fraction of ERα(α-cell) and ERβ(β-cell) sites represent sites that are in common to both ERs (i.e. can be occupied by either ERα or ERβ when they are present alone). A different pattern emerges when both receptors are present in the cells (ERαβ-cells, Fig. 3B). The ERα and ERβ binding sites were much more distinct from one another in the ERαβ-cells than they were in ERα-only and ERβ-only cells. Thus, when both ERα and ERβ were present, there was a marked restriction in the number of binding sites that can be shared; in fact, more than 800 of the 1386 sites in common were no longer accessible to both ERs in ERαβ-cells, with fewer than one third of the sites in common (579) being ones that can be shared.
Figure 3.
Venn diagrams comparing the occupancy of ER binding sites by ERα and ERβ when they are present either separately or together in cells treated with E2. A, ERα or ERβ can each occupy many of the same sites when the other ER subtype is not present in the cells. B, When both receptors are present, ERα and ERβ share a more limited number of sites. C, Diagram showing the intersection of the intersections from A (sites in common) and B (shared sites) and how sites in common that are not shared are allocated predominantly to ERα.
The relationship between sites in common and shared sites is illustrated in Fig. 3C, which shows the intersection of the two intersections in Fig. 3, A and B. Nearly all the shared sites were also sites in common (499 of 579); however, of the approximately 900 sites in common that are not shared sites, almost 95% are commandeered by ERα in ERαβ-cells, another illustration of the dominance of ERα over ERβ in site selection. The number of possible ERα/ERβ heterodimer binding sites also appears to be smaller (579 shared sites) than might have been expected from the overlap of sites bound by the individual subtypes (1386 sites in common). This suggests that heterodimerization is not a favored state for ERα and ERβ.
Sequence analysis of ERα vs. ERβ binding sites
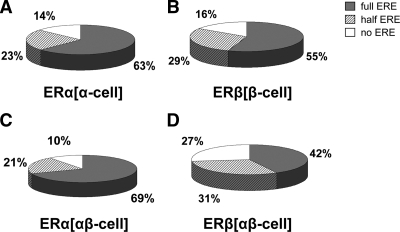
We next examined whether the genomic sequences to which ERα and ERβ bind contained a recognizable ERE motif by performing a DNA-binding motif search. We considered a 13-bp site with up to two positions varying from the canonical ERE (GGTCAnnnTGACC) as a putative full-ERE motif. It is of interest to note that about 80% of the sites identified as containing full EREs have only one ERE motif within the binding region, with about 15% containing two EREs and less than 5% containing three or more EREs (see supplemental Fig. S2). Among the ERα(α-cell) binding regions (Fig. 4A), 63% contained full-ERE sequences, 23% had ERE half-sites, and 14% had no ERE-like sequences. The ERE motif distribution in the ERβ(β-cell) binding regions (Fig. 4B) was very similar to that of the ERα(α-cell) regions, supporting the notion that ERα and ERβ, in the absence of the other ER subtype, bind predominantly to similar recognition motifs (31,32,33). In addition, the presence of ERβ along with ERα did not change this motif recognition profile of ERα (Fig. 4C). However, the ERβ binding sites in ERαβ-cells (Fig. 4D) contained a much lower percentage of ERE sequences than did those in ERβ-only cells (Fig. 4B), with almost one third not containing any ERE-like sequences. Thus, the new sites occupied by ERα and ERβ when the other subtype is present were less enriched in EREs, with the 938 new ERβ sites being only 38% enriched and the 274 new ERα sites only 45% enriched. In this respect, the dominance of ERα over ERβ was again evident by the larger number of ERβ proteins that were shunted to sites less enriched in ERE motifs.
Figure 4.
Presence of ERE sequences in ERα or ERβ binding sites with E2 treatment. Binding sites were probed for the presence of full ERE, half ERE, and no ERE motifs. A, ERα binding sites in ERα-only cells; B, ERβ binding sites in ERβ-only cells; C, ERα binding sites in cells containing both ERα and ERβ; D, ERβ binding sites in cells containing both ERα and ERβ.
Analysis of enrichment of transcription factor binding sites
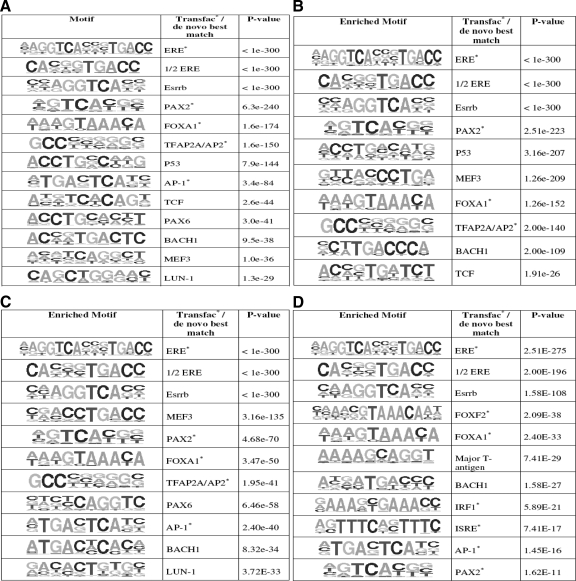
To determine the presence of motifs for other transcription factors that might play a part in the binding of ERα and ERβ to chromatin, we searched for enriched transcription factor binding site (TFBS) motifs by both de novo and TRANSFAC candidate scanning approaches in the DNA sequences corresponding to ERα(α-cell), ERα(αβ-cell), ERβ(β-cell), and ERβ(αβ-cell) binding sites relative to genomic background. These statistically enriched TFBS motifs are listed in Fig. 5.
Figure 5.
Analysis of enrichment of TFBSs. A, Transcription factor binding motifs that are enriched in ERα (α-cell) binding sites; B, transcription factor binding motifs that are enriched in ERα (αβ-cell) binding sites; C, transcription factor binding motifs that are enriched in ERβ (β-cell) binding sites; D, transcription factor binding motifs that are enriched in ERβ (αβ-cell) binding sites.
As expected, ERE and ERE half-site motifs were the most enriched motifs in all the sets of ER binding sites. In addition, Forkhead transcription factor motifs (FOXA1), and AP1, BACH1, Esrrb, and PAX2 motifs were also highly enriched in both ERα and ERβ binding sites, suggesting that chromatin binding of both ERα and ERβ might be assisted by a core set of transcription factors. However, there were some noteworthy differences in the enriched TFBS motifs found for ERα vs. ERβ binding sites. For example, p53 and T-cell factor (TCF) motif enrichment was observed in only ERα binding sites (Fig. 5, A and B). Also, although PAX2 and PAX6 motifs were found to be enriched in ER binding sites, the PAX2 motif was found in all four binding sets, whereas the PAX6 motif was enriched only in ERα(α-cell) and ERβ(β-cell) binding sites, that is, only when these receptors were present alone. Interferon regulatory factor 1 (IRF1) and interferon-stimulated response element (ISRE) motifs were also found to be associated only with ERβ(αβ-cells) binding sites and not with the three other binding sets.
Correlation between ER binding sites and gene regulation by hormone
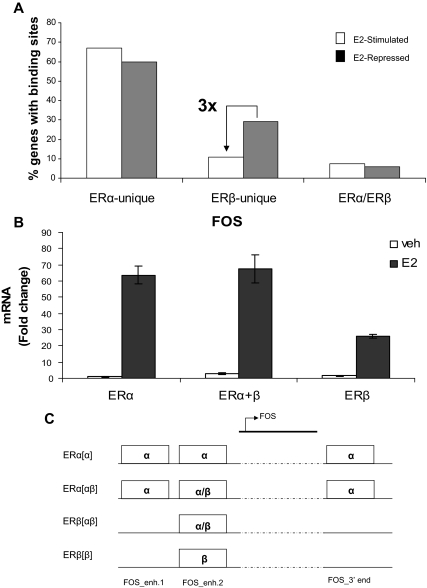
Having shown the competitive nature of ERα and ERβ recruitment to the chromatin binding sites in cells expressing both receptors, we next investigated the association between ERα and ERβ recruitment to cis-regulatory sites and E2-mediated transcriptional responses in ERαβ-cells. For this, we compared the potential regulatory regions (50 kb upstream and 50 kb downstream of the transcription start site) of 467 genes that are either E2 stimulated or repressed (4 h E2 treatment) in these cells (5) and that have at least one site bound specifically by only one of the receptors (i.e. ERα unique sites or ERβ unique sites) or sites shared by both receptors (i.e. ERα/ERβ shared sites) (Fig. 6A). Our analysis showed that the enhancer regions of E2-repressed genes in ERαβ-cells were three times more likely to have binding sites unique to ERβ than to ERα, suggesting that ERβ homodimers might be recruited more strongly than ERα homodimers to the enhancer regions of some E2-repressed genes.
Figure 6.
Correlation between ER binding and transcriptional output in response to E2. A, Correlation between E2-regulated genes and binding of ERα-unique (only ERα binds), ERβ-unique (only ERβ binds), or ERα/ERβ sites (sites shared by both ERs) within ±50 kb of the transcription start site of the genes. B, FOS mRNA levels were assessed by quantitative PCR after 4 h treatment of MCF-7 cells differentially expressing ERα and/or ERβ. Data represent average fold change ± sd for three independent experiments. C, ERα and ERβ chromatin binding (by conventional ChIP assays) were measured by quantitative PCR after 45 min E2 treatment of MCF-7 cells expressing ERα and/or ERβ. ERα and ERβ occupancy of three different ER binding sites (FOS enhancer 1, FOS enhancer 2, and FOS 3′ region) that are closest to the FOS gene are presented graphically. Enh, Enhancer.
To further characterize ERα and ERβ functional mechanisms and possible comodulatory effects on gene regulation, we monitored ERα and ERβ recruitment to chromatin target sites both upstream and downstream of the transcription start site of the well known E2-regulated gene FOS in the three cell types after E2 treatment. We first measured the transcript level of FOS by quantitative RT-PCR in response to E2 treatment (Fig. 6B). In both ERα-only and ERαβ-cells, the E2-stimulated expression of FOS was very similar; however, we saw a reduced (∼40%) expression of FOS in cells expressing only ERβ, suggesting that ERβ might be a weaker transcriptional activator of this gene.
We then examined both ERα and ERβ recruitment to three potential ER binding sites (identified by our ChIP-chip data) by ChIP-quantitative PCR. The sites are denoted as FOS_enh1, FOS_enh2, and FOS_3′end. The first two sites are located approximately 20 kb upstream, whereas the third is located approximately 5 kb downstream of the FOS transcription start site. The receptor binding data are shown in Fig. 6C. We observed that ERα and ERβ could bind to the FOS_enh2 site in all three types of cells, and this was not affected by the presence of the other ER subtype; however, FOS_enh1 and FOS_3′end sites were bound exclusively by ERα. We hypothesize that either one or both of these sites (FOS_enh1 and FOS_3′end) might be responsible for the enhanced transcription of FOS seen in ERα-only and in ERαβ-cells.
Binding site distribution with ERα- or ERβ-selective ligands vs. E2
To assess the effect of the ERα-selective ligand PPT on ERα chromatin binding, we examined binding site occupancy after PPT exposure in ERα-only or ERαβ-cells (Table 1). In ERα-only cells, the sites to which PPT-liganded ERα bound were almost all the same as those bound by E2-liganded ERα (Fig. 7A). Thus, the ERα-PPT complex was similar to the ERα-E2 complex in its selection of chromatin binding sites. Also, although E2-liganded ERα and ERβ acted as competitors in binding site selection when both were copresent in cells (cf. Fig. 2B), unoccupied ERβ had little impact on PPT-liganded ERα chromatin binding (Fig. 7B).
Figure 7.
Venn diagrams comparing ER binding site occupancy after cell treatment with the ERα-selective ligand (PPT) or ERβ-selective ligand (ERB-041) vs. E2. A, ERα binding sites in ERα-only cells (E2 vs. PPT treatment); B, ERα binding sites in cells containing ERα only or both ERα and ERβ (PPT treatment); C, ERβ binding sites in ERβ-only cells (E2 vs. ERB-041 treatment); D, ERβ binding sites in cells containing ERβ only or both ERα and ERβ (ERB-041 treatment).
A different pattern emerged in cells treated with ERB-041, an ERβ-selective ligand (12). In contrast to the similarity of E2- and PPT-liganded ERα sites (Fig. 7A), ERB-041-liganded ERβ sites were much more distinct from E2-liganded ERβ sites, with only half of the ERβ-ERB-041 sites overlapping with ERβ-E2 sites (Fig. 7C), indicating that E2 and ERB-041 form complexes with ERβ that differ in their chromatin binding site selection. Also, unoccupied ERα did shift the binding site distribution of ERB-041-liganded ERβ (Fig. 7D).
Overlap of ERα and ERβ binding sites with subtype-selective ligands
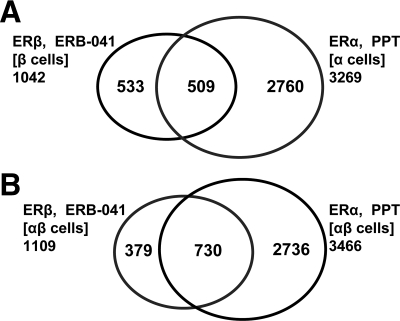
We also examined the relationship between ERα-PPT and ERβ-ERB-041 chromatin binding sites, in ERα-only and ERβ-only cells, and then in ERαβ-cells (Fig. 8). Previously, we had defined the overlap of binding sites in E2-liganded ERs in ERα-only and ERβ-only cells as sites in common, i.e. sites that could be occupied by either ER subtype, provided that the other subtype was not present. Intriguingly, there were fewer ERα-PPT(α-cells) and ERβ-ERB-041(β-cells) sites in common (509 sites, 13%, Fig. 8A) than were in common when ERα and ERβ were liganded with E2 in these cells (1386 sites, 28%, Fig. 3A). This suggests that the conformations induced by the binding of a subtype-selective ligand to its respective ER subtype are more distinctive than those induced by the binding of E2, and based on the findings presented above, the major difference is in the range of sites bound by ERB-041-liganded ERβ compared with E2-liganded ERβ.
Figure 8.
Venn diagrams showing the overlap of ERα binding sites (PPT treatment) and ERβ binding sites (ERB-041 treatment). A, ERβ binding sites in ERβ-only cells (ERB-041 treatment) and ERα binding sites in ERα-only cells (PPT treatment); B, ERβ and ERα binding sites in cells containing both ERα and ERβ (ERB-041 vs. PPT treatment).
When we investigated ERα and ERβ binding sites in ERαβ-cells after ER subtype-specific ligand activation, we found a somewhat larger number of sites shared by ERα and ERβ (730, Fig. 8B) than when both ERs were co-occupied by E2 (579, Fig. 3B). Because PPT and ERB-041 are ER subtype-selective ligands, when ERαβ-cells are treated with one or the other of these ligands, only one of the ER subtypes will be liganded. The greater number of shared sites and the lack of binding site restriction observed when the subtype-selective ligands are used indicate that an unliganded ER has a more limited ability to compete with a liganded ER for the same binding sites.
Discussion
Previous studies done by our group and others have shown that ERβ has a significant impact in modulating the expression of genes regulated by ERα in breast cancer cells (4,5,23,25). However, understanding how these transcriptional programs are orchestrated by both of these receptors requires an examination of the chromatin targets of ERα and ERβ when they are present, either separately or together, under various treatment conditions, aspects investigated in this study. We have made several novel observations. First, the selection of chromatin binding sites is remarkably dynamic, with each ER subtype being affected by the other and by their state of ligand occupancy. Second, when each is present alone, ERα and ERβ bind many of the same sites (sites in common). Third, when ERα and ERβ are both present, there is a mutual competition that greatly restricts the number of sites that both can occupy (shared sites). Fourth, this mutual competition also shifts the binding of each ER uniquely to new sites, that is, to sites that were not occupied when the receptors were present alone. In this restriction and shift, ERα dominates over ERβ by capturing the great majority of sites in common lost to restriction, with the result that it causes a much greater shift in ERβ to new sites, sites that are less enriched in ERE motifs. Finally, when subtype-selective ligands were used, the liganded ER subtype had a dominant effect over the unliganded ER subtype in the selection of binding sites. These findings highlight the dynamic interplay and competition between the two ER subtypes in their selection of chromatin binding sites, and they point to an ERα dominance model that results, when both receptors are present, in only a limited number of sites to which ERα/ERβ heterodimers might be binding and a distinctive and expanded set of binding sites for ERα and ERβ homodimers.
Dynamics and competition in ERα and ERβ binding site selection leads to distinctiveness and limits the range of potential ER heterodimer binding sites
The DNA-binding domains of ERα and ERβ differ by only one amino acid, and ERα and ERβ homodimers and ERα/ERβ heterodimers can bind to similar ERE-containing motifs both in vitro and in reporter gene constructs (30,31,32,33). ERα homodimers bind EREs with higher affinity than ERα/ERβ heterodimers, and ERβ homodimers bind most weakly (31,32,33,34). This is consistent with our findings that after E2 treatment, there are more ERα(α-cell) binding sites than ERβ(β-cell) sites. Our whole-genome ChIP-chip studies show, however, that the binding sites for ERα and ERβ when present alone were more distinctive than might be presumed based on the receptor structure and motif binding preference, because although there were many common binding sites for ERα and ERβ (1386), there were also many sites that could be occupied by only ERα (3019), and some sites that could be occupied by only ERβ (511). Thus, in the context of chromatin, factors other than the structure of the ER subtype DNA-binding domains appear to enforce a specificity to their binding site selection that gives a distinctiveness to the sites they occupy. When both ERs were present, competition between them made their binding site distribution even more distinct, restricting the number of sites that could be occupied by either ER much further, to only 579 shared sites. These are the only possible sites to which ERα/ERβ heterodimers could bind, and this number is quite small.
A model for ERα dominance
There are equal levels of ERα and ERβ in our ERαβ-cells (5), and if homo- and heterodimers formed with equal stability, one would expect a 1:2:1 statistical distribution for ERα homodimers to ERα/ERβ heterodimers to ERβ homodimers. However, because ERα homodimers are more stable than ERα/ERβ heterodimers and ERβ homodimers less stable (31,32,33,35,36,37), the fraction of heterodimers will be reduced and that of ERα homodimers increased. Thus, ERα dominance arises both from the higher DNA binding affinity of ERα homodimers than heterodimers and the preferred formation of ERα homodimers (at the expense of heterodimers). Thus, it is not surprising that of the total number of ER binding sites we find in ERαβ-cells (∼4400), only 13% (∼600) are sites to which ERα/ERβ heterodimers might bind. Also, of the approximately 900 sites in common that were lost when both ERs are present, nearly 90% were claimed by ERα; none of these 800 sites were available as heterodimer binding sites. These findings illustrate again the dynamic and competitive nature of chromatin binding site selection by ERα and ERβ, with ERα being the dominant subtype. It further suggests that the biological effects of ERβ that reduce estrogen activity through ERα might be accounted for by factors beyond the formation of ERα/ERβ heterodimers that have reduced activity, because these heterodimers would be present at only a small fraction of the total number of ER binding sites.
The chromatin binding sites for ERα had been mapped previously on a genome-wide scale using ChIP-chip or ChIP-PET analysis (24,27,38,39), and these studies have provided an unprecedented view of the broad distribution of ER binding sites throughout the genome. More recently, two studies (39,40) examined binding sites in cells having ERα or ERα plus ERβ treated with the ER subtype nonselective ligand, E2. Our studies have expanded upon previous studies and examined the characteristics of ERβ binding sites, when present alone and with ERα, and whether ERα and ERβ collaborate and/or compete for these binding sites in the presence of E2 and also ER subtype-specific ligands. In studies by Dahlman-Wright and colleagues (39,40), the addition of ERβ was found to cause a shift in the binding sites for ERα, as we also observed. However, because these studies did not include ERβ-only cells, or any investigations with ER subtype-selective ligands, it is not possible to make other comparisons (i.e. common vs. shared sites and shift of ERβ sites when present with ERα vs. present alone or influence of unoccupied receptor subtype).
New binding sites become occupied by ERα and ERβ homodimers when both ERs are present
Although the fraction of sites that can be occupied by both ERα and ERβ when both are present together is small, the presence of both subtypes had another pronounced effect: it shifted the site selection such that each ER bound to new sites. The shift for ERα was relatively modest, but the shift for ERβ was pronounced. When ERα was added, about half of ERβ sites were relinquished, but a nearly equal number of new sites were gained. This shift to new binding sites is intriguing, because it implies that ERα and ERβ bind, presumably as homodimers, to nearly 300 and to more than 900 new sites, respectively, when they are copresent.
It is intriguing to consider whether the moderating effect that ERβ has on ERα gene regulatory activity (4,5,26) might be the result of the extension of estrogen action through these many new sites to which ERα and ERβ bind only when both ERs are present. Thus, it is still an open question whether the effect of ERβ on the activity of ERα arises from ERα/ERβ heterodimer formation, from the new sites with which the ER homodimers interact when both are present, or from both processes.
Sequence motifs in chromatin binding sites for ERα and ERβ
From our sequence analysis, we found that when present alone, both ERα and ERβ bound mostly to chromatin targets containing ERE motifs. This observation fits well with studies showing that ERα and ERβ recognize the same ERE motif (30,31,32,33), yet our ChIP-chip findings suggest that there are other factors enforcing the selectivity and range of ER subtype binding, because there were many sites to which only ERα (in ERα-cells) or only ERβ (in ERβ-cells) bind as homodimers. The ERE motif distribution was even more interesting in the ERαβ-cells; here, ERα sites had nearly the same enrichment of ERE motifs as in the ERα cells, whereas ERβ bound to sites that contained a lower percentage of ERE sequences. This is another reflection of ERα preferential binding to sites with good EREs, thereby shifting ERβ to new sites less enriched in EREs when both ERs are present.
A number of studies have previously examined the transcriptional activities of ERα and ERβ, with the bulk of evidence implying that ERβ has growth-suppressive activities (6,7,8,24). Thus, it is of interest that we found the enhancer regions of E2-repressed genes were three times more likely to have binding sites occupied by ERβ than by ERα. Thus, although ERα may be the generally dominant ER subtype, within the enhancer regions of these E2-repressed genes, ERβ competes effectively to preferentially exclude ERα from binding.
Liganded ERα dominates over unliganded ERβ in chromatin binding site selection
A unique feature of this study is our use of ER subtype-selective ligands to achieve differential occupancy of ERα or ERβ, even when both are present in cells. This is not possible using the nonselective ligand E2 that binds well to both ERα and ERβ. The ERα complex with PPT selected almost all of the same binding sites as did the ERα-E2 complex, suggesting that both receptor complexes are very similar. Although there are no published x-ray structures of ERα-PPT complexes, they appear very similar to ERα-E2 complexes in terms of receptor conformation, dimer stability, and interaction with coregulators (36,37,41,42,43,44). By contrast, ERβ complexed with ERB-041 or E2 bound to mostly different sites. This difference in accessing chromatin binding sites is not reflected in the x-ray crystal structures of ERβ ligand-binding domain complexes with these two ligands [PDB entry 2j7x for ERβ with E2 (unpublished) and 1x7b for ERβ with ERB-041 (45)]. Thus, differences outside of the ligand-binding domain and/or differences in cofactor recruitment (5) might account for the differences in their chromatin binding.
When both ERs were liganded by E2, we found that the number of sites that could be occupied by both ERs present together (shared sites) was less than those that could be occupied by one or the other when present separately (sites in common). This competition between ER subtypes, however, did not occur uniformly when a subtype-selective ligand was used. The overlap of binding sites for PPT-ERα in ERα-only or in ERαβ-cells was essentially the same, indicating that unliganded ERβ did not restrict the binding of liganded ERα. By contrast, binding site selection by ERB-041-ERβ in ERβ only or in ERαβ-cells was different, indicating that unliganded ERα did affect the binding site selection of liganded ERβ. ER dimerization studies, both in vitro and in cells, have demonstrated that ER homo- and heterodimer formation is favored by ligand occupancy of both partners in the dimer, with occupancy of ERα being the more important (36,37,46).
The cartography of ERα and ERβ chromatin binding sites and the biology of ERα and ERβ
There is abundant evidence that in addition to ERα, ERβ plays an important role in regulating biological responses of diverse target tissues and cells to estrogens. Normal breast tissue contains both ERα and ERβ, and ER-positive human breast cancers usually contain both ERα and ERβ, with ERβ levels typically declining relative to ERα with disease progression (17,18,19,20,21). Increasing evidence indicates that ERβ has a restraining effect on the pro-proliferative activities of ERα in estrogen-responsive breast cancer cells and in breast tumors (6,7,8,9,10). ERβ also modulates the genome-wide gene expression profiles induced by E2 through ERα (4,5,26). Our observation of differences in ERα and ERβ binding to ER binding regions near the FOS gene highlight that binding site selection by these ERs may underlie their differences in regulation of this gene (5). Our studies also showed enrichment of some different transcription factor binding motifs in ERα vs. ERβ binding regions that may enable coassociations of ERα and ERβ with distinct transcription factors that may support different gene-selective and tissue-selective activities of these two ERs. In this regard, a previous study has shown direct interaction between E2-activated ERα (but not activated ERβ) and TCF isoforms on EREs contained in the osteopontin promoter (47). Likewise, recent studies show important interrelationships between p53 and ERα in breast cancer and their copresence at ERα-regulated genes (48). Both of these reports are in agreement with our observations of TCF and p53 motif enrichment only in ERα binding site regions.
Taken together, our studies reveal the dynamic interplay between ERα and ERβ in their selection of chromatin binding sites and reveal a novel process, expansion of binding sites exclusively for ERα or for ERβ when both ERs are present, that may operate in addition to ERα/ERβ complex formation as a mechanism by which ERβ might moderate ERα activity in target cells. These findings on binding site selection dynamics may apply more broadly to other nuclear hormone receptors, especially other steroid hormone receptors (such as progesterone and glucocorticoid receptors), that also have two closely related receptor forms that can bind as homo- and heterodimers and impact the biology of each other.
Materials and Methods
Ligands, cell culture, adenovirus infection, and siRNA transfection
MCF-7 cells were cultured in MEM (Sigma Chemical Co., St. Louis, MO), supplemented with 5% calf serum (HyClone, Logan, UT), and 100 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA). For estrogen-free experiments, the cells were maintained in phenol red-free MEM plus 5% charcoal-dextran-treated calf serum for at least 3 d and were then seeded at a density of 3 × 105 cells per 10-cm tissue culture dish (Corning, Corning, NY) for 2 d before adenovirus infection. Recombinant adenoviruses were constructed and prepared as described (4). Cells were infected with either control adenovirus expressing β-galactosidase (Ad) or adenovirus expressing ERβ (AdERβ) for 72 h. Conditions used were those described previously (4,5,10) to generate MCF-7 cells expressing levels of ERβ equal to that of the endogenously expressed ERα. siRNA experiments for knockdown of the endogenous ERα in MCF-7 cells were performed as previously described and resulted in knockdown of ERα mRNA and protein by greater than 95% (5). siERα sequences (Dharmacon, Lafayette, CO) were forward, 5′-UCAUCGCAUUCCUUGCAAAdTdT-3′, and reverse, 5′-UUUGCAAGGAAUGCGAUGAdTdT-3′ (5). Because ERα knockdown did not affect ERβ levels, the level of ERβ obtained in the ERβ-only cells (5) was similar to that of ERα in the original MCF-7 cells. Estradiol was from Sigma. The ER subtype-selective ligands PPT and ERB-041 were synthesized as described (14,45). Studies used 10 nm E2, 50 nm PPT, and 500 nm ERB-041, concentrations that reflect their relative binding affinities, and give maximal occupancy of receptors by these ligands.
ChIP assays
ChIP for ERα and ERβ were carried out as described (49) and used the ERα antibody HC-20 (Santa Cruz Biotechnology, Santa Cruz, CA); ERβ antibodies were a combination with equal parts of CWK-F12 produced by our lab (50), GTX70182 (GeneTex, San Antonio, TX), GR40 (Calbiochem, La Jolla, CA), and PA1-311 (Affinity Bioreagents, Golden, CO). The ChIP DNA was used for ChIP-chip analysis and quantitative real-time PCR.
ChIP-chip analyses
We used a custom-designed tiling array, produced by NimbleGen, that contains approximately 77,000 genomic regions consisting of about 61,000 ER-binding sites and about 16,000 negative/control regions. The ER-binding sites were selected based on 1) published ERα ChIP-chip data (27), accounting for 10,599 sites; 2) published ERα ChIP-PET data (n =1234 sites) (24); and 3) computational predicted ERE sites using an optimized algorithm (29) (n =37,499 sites). These were compared against control probes from both nonbinding regions and nonmammalian sequences. The probes in our arrays are approximately 60 bp in length, and they are tiled at a distance of about 100 bp from each other within a binding site. In validation studies, we tested a total of 42 sites and validated ER binding in 93% (39 of 42) of the selected sites (see supplemental Table S1). Our ChIP-chip experiments thus had false-positive error rates of approximately 7%, which are similar to those reported in other genome-wide ChIP-chip or ChIP-PET studies (24,27). We performed three biological replicates (each biological replicate being from an independent experiment with two separate hybridizations onto tiling arrays) to identify enriched binding sites. The raw intensity signals of the ChIP-chip experiments were normalized and averaged across the three replicates. The binding sites were identified by the intersection of peaks detection (four or more probes whose intensity signals are above a specific threshold) and default FDR score in the NimbleGen software. Both the peak cutoff threshold and FDR values were calculated using NimbleScan software (all settings were left as the default in the software). The detailed algorithm on how NimbleGen software calculates the FDR and determines the peaks can be found at the NimbleGen ChIP-on-chip web site (http://www.nimblegen.com/products/chip/index.html). The locations of all binding sites will be deposited and publicly available. Raw signal intensity values of several random sites (both ER binding and nonbinding regions) are shown in supplemental Fig. S1 to illustrate the distinct intensity differences between regions binding ER vs. regions not binding ER.
Computational motif analyses
Motif analysis was performed using the program HOMER (http://biowhat.ucsd.edu/homer/) (51). DNA sequences corresponding to ERα(α-cell), ERα(αβ-cell), ERβ(β-cell), and ERβ(αβ-cell) binding sites were used. HOMER will search for enriched motifs by two different methods: de novo and TRANSFAC (52). In the TRANSFAC approach, HOMER will search for enrichment of motifs (TRANSFAC known transcription factor matrices), and the enriched motifs found were scored using the hypergeometric distribution relative to genomic background (24). In the de novo approach, an exhaustive search for all n-mers (6 < n < 13) was performed, and each n-mer was scored for its enrichment in the ER binding sites using the hypergeometric distribution relative to background genomic sequence. The enriched n-mer sequences were subsequently identified by matching them to known transcription factor consensus sequences.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R37DK015556 (J.A.K.), 5R01CA018119 (B.S.K.), and P01AG024387 (J.A.K. and B.S.K.) and a grant from The Breast Cancer Research Foundation (B.S.K.). T.H.C. was supported by an A*STAR graduate fellowship from The Singapore Agency for Science, Technology, and Research. E.C.C. received support from NIH T32 ES07326. E.T.L. is supported by the Agency for Science Technology and Research (A*STAR) of Singapore and EU Grant CRESCENDO (FP6-018652).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 6, 2009
Abbreviations: AF-1, Activation function-1; ChIP, chromatin immunoprecipitation; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; FDR, false discovery rate; PET, paired end ditag; PPT, propyl pyrazole triol; siRNA, small interfering RNA; TCF, T-cell factor; TFBS, transcription factor binding site.
References
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS 2006 Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA 2001 Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS 2006 Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 147:4831–4842 [DOI] [PubMed] [Google Scholar]
- Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS 2008 Estrogen receptors α and β as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol 22:1032–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC 2004 Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 64:423–428 [DOI] [PubMed] [Google Scholar]
- Ström A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA 2004 Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101:1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA 2008 A genome-wide study of the repressive effects of estrogen receptor β on estrogen receptor α signaling in breast cancer cells. Oncogene 27:1019–1032 [DOI] [PubMed] [Google Scholar]
- Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, Miller LD, Smeds J, Bergh J, Katzenellenbogen BS 2006 Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res 66:7334–7340 [DOI] [PubMed] [Google Scholar]
- Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS 2000 Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-α and estrogen receptor-β: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology 141:3534–3545 [DOI] [PubMed] [Google Scholar]
- Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith Jr JC, Harris HA 2004 Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-β ligands. J Med Chem 47:5021–5040 [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA 1999 Estrogen receptor subtype-selective ligands: asymmetric synthesis and biological evaluation of cis- and trans-5,11-dialkyl-5,6,11, 12-tetrahydrochrysenes. J Med Chem 42:2456–2468 [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2000 Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Frasor J 2004 Therapeutic targeting in the estrogen receptor hormonal pathway. Semin Oncol 31:28–38 [DOI] [PubMed] [Google Scholar]
- Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H 2000 Expression levels of estrogen receptor-α, estrogen receptor-β, coactivators, and corepressors in breast cancer. Clin Cancer Res 6:512–518 [PubMed] [Google Scholar]
- Saji S, Hirose M, Toi M 2005 Clinical significance of estrogen receptor beta in breast cancer. Cancer Chemother Pharmacol 56(Suppl 1):21–26 [DOI] [PubMed] [Google Scholar]
- Shaaban AM, O'Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS 2003 Declining estrogen receptor-β expression defines malignant progression of human breast neoplasia. Am J Surg Pathol 27:1502–1512 [DOI] [PubMed] [Google Scholar]
- Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM 2004 Oestrogen receptor β: what it means for patients with breast cancer. Lancet Oncol 5:174–181 [DOI] [PubMed] [Google Scholar]
- Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Wärri A, Weihua Z, Van Noorden S, Wahlstrom T, Coombes RC, Warner M, Gustafsson JA 2002 Estrogen receptor β in breast cancer. Endocr Relat Cancer 9:1–13 [DOI] [PubMed] [Google Scholar]
- Roger P, Sahla ME, Mäkelä S, Gustafsson JA, Baldet P, Rochefort H 2001 Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res 61:2537–2541 [PubMed] [Google Scholar]
- Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 64:1522–1533 [DOI] [PubMed] [Google Scholar]
- Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER)α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145:3473–3486 [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET 2007 Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC 2004 Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Movérare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C 2003 Estrogen receptor (ER)-β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol Endocrinol 17:203–208 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Vega VB, Lin CY, Lai KS, Kong SL, Xie M, Su X, Teh HF, Thomsen JS, Yeo AL, Sung WK, Bourque G, Liu ET 2006 Multiplatform genome-wide identification and modeling of functional human estrogen receptor binding sites. Genome Biol 7:R82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M 2004 Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Mol Cell Biol 24:7681–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG 1997 Estrogen receptors α and β form heterodimers on DNA. J Biol Chem 272:19858–19862 [DOI] [PubMed] [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S 1997 Human estrogen receptor β binds DNA in a manner similar to and dimerizes with estrogen receptor α. J Biol Chem 272:25832–25838 [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA 1997 Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 11:1486–1496 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Loven MA, Melvin VM, Edwards DP, Nardulli AM 2002 Differential modulation of DNA conformation by estrogen receptors α and β. J Biol Chem 277:8702–8707 [DOI] [PubMed] [Google Scholar]
- Jisa E, Jungbauer A 2003 Kinetic analysis of estrogen receptor homo- and heterodimerization in vitro. J Steroid Biochem Mol Biol 84:141–148 [DOI] [PubMed] [Google Scholar]
- Paulmurugan R, Tamrazi A, Katzenellenbogen JA, Katzenellenbogen BS, Gambhir SS 2008 A human estrogen receptor (ER)α mutation with differential responsiveness to nonsteroidal ligands: novel approaches for studying mechanism of ER action. Mol Endocrinol 22:1552–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell E, Xu W 2008 Intermolecular interactions identify ligand-selective activity of estrogen receptor α/β dimers. Proc Natl Acad Sci USA 105:19012–19017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL 2007 Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao H, Marstrand TT, Ström A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K 2008 The genome landscape of ERα- and ERβ-binding DNA regions. Proc Natl Acad Sci USA 105:2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi Z, Zhao C, Putnik M, Gustafsson JA, Dahlman-Wright K 2009 Binding of estrogen receptor α/β heterodimers to chromatin in MCF-7 cells. J Mol Endocrinol 43:65–72 [DOI] [PubMed] [Google Scholar]
- Tamrazi A, Carlson KE, Daniels JR, Hurth KM, Katzenellenbogen JA 2002 Estrogen receptor dimerization: ligand binding regulates dimer affinity and dimer dissociation rate. Mol Endocrinol 16:2706–2719 [DOI] [PubMed] [Google Scholar]
- Tamrazi A, Carlson KE, Katzenellenbogen JA 2003 Molecular sensors of estrogen receptor conformations and dynamics. Mol Endocrinol 17:2593–2602 [DOI] [PubMed] [Google Scholar]
- Hurth KM, Nilges MJ, Carlson KE, Tamrazi A, Belford RL, Katzenellenbogen JA 2004 Ligand-induced changes in estrogen receptor conformation as measured by site-directed spin labeling. Biochemistry 43:1891–1907 [DOI] [PubMed] [Google Scholar]
- Kim SH, Tamrazi A, Carlson KE, Katzenellenbogen JA 2005 A proteomic microarray approach for exploring ligand-initiated nuclear hormone receptor pharmacology, receptor selectivity, and heterodimer functionality. Mol Cell Proteomics 4:267–277 [DOI] [PubMed] [Google Scholar]
- Manas ES, Unwalla RJ, Xu ZB, Malamas MS, Miller CP, Harris HA, Hsiao C, Akopian T, Hum WT, Malakian K, Wolfrom S, Bapat A, Bhat RA, Stahl ML, Somers WS, Alvarez JC 2004 Structure-based design of estrogen receptor-β selective ligands. J Am Chem Soc 126:15106–15119 [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Labrie F, Giguère V 1999 Dominant activity of activation function 1 (AF-1) and differential stoichiometric requirements for AF-1 and -2 in the estrogen receptor α-β heterodimeric complex. Mol Cell Biol 19:1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tanani M, Fernig DG, Barraclough R, Green C, Rudland P 2001 Differential modulation of transcriptional activity of estrogen receptors by direct protein-protein interactions with the T cell factor family of transcription factors. J Biol Chem 276:41675–41682 [DOI] [PubMed] [Google Scholar]
- Shirley SH, Rundhaug JE, Tian J, Cullinan-Ammann N, Lambertz I, Conti CJ, Fuchs-Young R 2009 Transcriptional regulation of estrogen receptor-α by p53 in human breast cancer cells. Cancer Res 69:3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DH, Sheng S, Charn TH, Waheed A, Sly WS, Lin CY, Liu ET, Katzenellenbogen BS 2008 Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res 68:3505–3515 [DOI] [PubMed] [Google Scholar]
- Choi I, Ko C, Park-Sarge OK, Nie R, Hess RA, Graves C, Katzenellenbogen BS 2001 Human estrogen receptor β-specific monoclonal antibodies: characterization and use in studies of estrogen receptor β protein expression in reproductive tissues. Mol Cell Endocrinol 181:139–150 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK 2005 Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Münch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E 2003 TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31:374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.