Abstract
We previously demonstrated that bovine epiphyseal chondrocytes separated by density gradient centrifugation differ in proliferative response to IGF-I and IGF-I receptor number. To identify novel modifiers of IGF-I action at the growth plate, we used microarray analyses to compare bovine hypertrophic and reserve zones and identified several receptors differentially expressed across the growth plate: NTRK2 [receptor for brain-derived neurotrophic factor (BDNF)], KIT [receptor for stem cell factor (SCF)], and MER and AXL [two receptors for growth arrest-specific 6 (Gas6)]. The corresponding ligands were tested for their ability to stimulate either proliferation of isolated chondrocytes or differentiation in ATDC5 cells. Each factor inhibited IGF-I-mediated proliferation in isolated chondrocytes by attenuating ERK1/2 activation. SCF, BDNF, Gas6, and C-type natriuretic peptide promoted differentiation in ATDC5 cells, each factor producing different expression patterns for collagen X, collagen 2, aggrecan, and lysyl oxidase. Whereas multiple factors stimulated ATDC5 differentiation, only IGF-I and high-dose insulin, out of several factors implicated in chondrocyte maturation, stimulated proliferation of isolated chondrocytes. IGF-I appears to be the primary proliferative signal in growth plate chondrocytes, whereas multiple factors including SCF, BDNF, and Gas6 regulate the pace of differentiation at the growth plate.
SCF, BDNF, Gas6 and CNP each inhibits IGF-I-stimulated growth plate chondrocyte proliferation by attenuating ERK1/2 activation and also stimulates chondrocyte differentiation.
The growth plate is a specialized and highly organized cartilaginous tissue wherein chondrocytes undergo a tightly regulated progression from proliferation to maturation, hypertrophy, matrix synthesis and mineralization, and finally apoptosis. The basic structure of the mammalian growth plate is maintained throughout the growth phase of the animal, including scattered small reserve zone (RZ) cells at the metaphyseal side, proliferating chondrocytes that divide along the long axis of the bone to form regular columns of cells, larger pre-hypertrophic chondrocytes, and the fully differentiated large hypertrophic zone (HZ) cells at the epiphyseal side. Whereas the major collagen of mature trabecular bone is type 1, epiphyseal chondrocytes initially secrete type 2 collagen and then switch to type X collagen synthesis as they cease proliferating and mature into pre-hypertrophic chondrocytes (1). The low-molecular-weight type X collagen is the main collagen of mature postmitotic hypertrophic chondrocytes, which are responsible for mineralization of the surrounding matrix before yielding to apoptosis.
This process of organized chondrocyte maturation is governed by many circulating factors including GH, IGF-I, glucocorticoids, estrogen, thyroid hormone, and vitamin D (2). However, preservation of growth plate architecture during linear growth, which requires a continual progression of chondrocytes through the differentiation program from proliferation to hypertrophy, is also likely to be maintained by longitudinal gradients of locally produced soluble factors and their cell surface receptors. Local paracrine factors are important to the regulation of chondrocyte differentiation, because many human skeletal dysplasias are caused by interruptions in finely tuned paracrine differentiation programs. Several paracrine systems including the Indian hedgehog (IHH)/PTHrP system, fibroblast growth factors (FGF) such as FGF18, and bone morphogenic proteins display distinct patterns of expression across the growth plate (3,4,5,6,7,8).
By using chondrocytes isolated from prepubertal cattle and separated by density gradient centrifugation, we previously demonstrated gradients of expression of COLX (encoding collagen X), IGF-I proliferative response, and IGF-I receptor number across the bovine growth plate (9). Our goal in the present study was to identify additional factors that regulate chondrocyte development by modulating IGF-I activity at the growth plate. The relatively large numbers of cells obtained allowed us to study the proliferation of isolated chondrocytes in primary culture and to compare expression profiles of the RZ and HZ by microarray. Several candidate factors corresponding to cell surface receptors identified by microarray analysis of bovine chondrocyte fractions were examined for their ability to directly stimulate chondrocyte proliferation or modulate IGF-I-stimulated proliferation. Three of these factors, stem cell factor (SCF), brain-derived neurotrophic factor (BDNF), and growth arrest-specific 6 (Gas6), each inhibit bovine chondrocyte proliferation and stimulate differentiation in ATDC5 cells.
Results
Chondrocyte proliferation
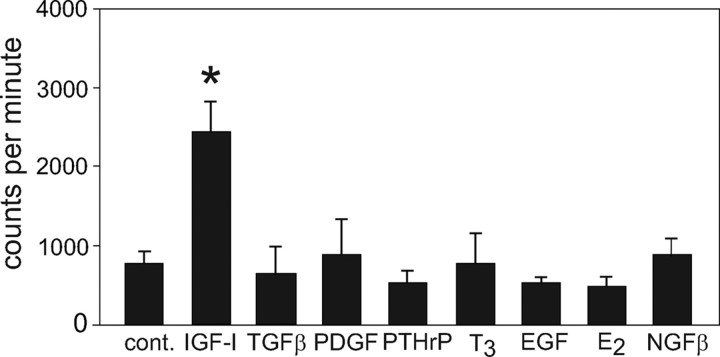
We tested the ability of several factors to increase [3H]thymidine incorporation into bovine chondrocytes isolated from density gradient fraction 4, which contains both reserve and proliferative zone cells. As seen previously, 100 ng/ml IGF-I increased [3H]thymidine uptake almost 3-fold after 24 h in culture. No other factor assayed, including TGFβ, PTHrP, T3, estradiol (E2), platelet-derived growth factor (PDGF), or epidermal growth factor (EGF), stimulated proliferation (Fig. 1). Nerve growth factor-β (NGFβ) was tested because it was highly expressed in both reserve and hypertrophic cells by microarray (not shown). In contrast, PDGF and EGF increased [3H]thymidine incorporation into HeLa cells by 2.5- and 3-fold, respectively (positive control for factor activity, not shown). The only individual factor other than IGF-I that stimulated chondrocyte proliferation was high-dose insulin: 1600 nm insulin increased [3H]thymidine uptake almost 3-fold at 24 h; 100 nm insulin increased uptake almost 2-fold, whereas 10 and 1 nm insulin did not significantly affect proliferation (data not shown). When used in combination with IGF-I, none of the factors increased [3H]thymidine uptake above that seen with IGF-I alone; both TGFβ and PTHrP actually inhibited IGF-I-mediated proliferation of the bovine chondrocytes (not shown).
Figure 1.
Proliferation of bovine chondrocytes in response to various factors. Proliferation of isolated RZ chondrocytes was determined by [3H]thymidine uptake after 24 h factor addition and expressed as counts per minute. *, P < 0.01. cont, Control.
Previous reports indicate that IGF-I stimulation of chondrocyte proliferation is mediated by the MAPK ERK1/2 as well as by phosphatidylinositol 3-kinase (PI3K) activation (10,11). We incubated the isolated faction 4 chondrocytes in the presence of 100 ng/ml IGF-I and either U0126, an inhibitor of MAPK kinase, the kinase directly upstream of ERK1/2, or the PI3K inhibitor wortmannin. Figure 2 shows that IGF-I stimulation of isolated bovine chondrocyte proliferation is dependent on ERK1/2 activation but is unaffected by PI3K inhibition.
Figure 2.
Effect of U0126 or wortmannin on chondrocyte proliferation. Either the MAPK kinase inhibitor U0126 at 10 μm or the PI3K inhibitor wortmannin (Wort) at 100 nm was added to isolated RZ chondrocytes in the presence or absence of 100 ng/ml IGF-I for 24 h and [3H]thymidine uptake used to assess inhibition of proliferation by the two kinase inhibitors. *, P < 0.01.
Phenotyping of fractionated chondrocytes using microarrays
We sought to identify potential regulators of growth plate development by using microarrays to compare gene expression patterns of low-density HZ cells (fraction 1) to high-density RZ and proliferative zone cells from fraction 4.
Transcripts enriched in the HZ
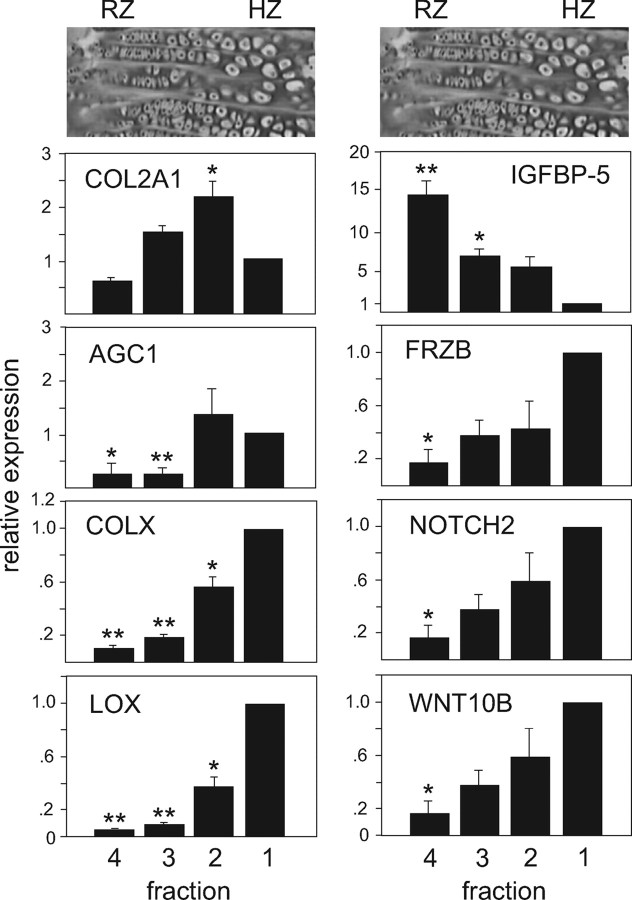
The microarray analysis yielded 87 transcripts that met criteria of 2-fold or greater expression in the HZ (fraction 1) as compared with the RZ on all six HZ/RZ pairs, with a false discovery rate of 0.05 or lower (selected transcripts are listed in Table 1, and the entire list of transcripts is included in supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals. org). Secreted proteins included Wnt10b and frizzled- related protein 3 (FRZB, a Wnt antagonist), adrenomedullin, and CXC chemokine ligand 12, also known as stromal cell-derived factor 1 (SDF-1). Cell surface receptors such as NOTCH2, MER tyrosine kinase (which binds Gas6), the receptor for SDF-1 (chemokine orphan receptor 1, CMKOR1), and the vascular endothelial growth factor receptors (NRP-1 and VEGFR) were also enriched in the HZ as well as the C-type natriuretic peptide (CNP) decoy receptor NPR3. The transcript with highest relative expression in the HZ, lysyl oxidase (LOX), and transcripts with lesser degrees of relative expression in the HZ (FRZB, NOTCH2, and Wnt10B) were chosen for verification of relative expression in bovine chondrocyte fractions by RT-PCR (Fig. 3).
Table 1.
Selected transcripts enriched in either the HZ or the RZ
| Gene symbol | Gene name | Fold change | P value | Ref. no. |
|---|---|---|---|---|
| Selected transcripts enrichedin fraction 1 (HZ) | ||||
| Cell surface receptors | ||||
| ADRB2 | Adrenergic, β-2 receptor | 9.96 | 2.5 × 10−4 | NM_174231 |
| NPR3 | Natriuretic peptide receptor 3 | 6.28 | 1.5 × 10−4 | Bt.15298.1 |
| CXADR | Coxsackie and adenovirus receptor | 4.00 | 3.9 × 10−5 | Bt.13744.1 |
| MERTK | Mer tyrosine receptor kinase | 3.98 | 1.8 × 10−4 | XM_580552 |
| CMKOR1 | Chemokine orphan receptor 1 (CXCR7) | 3.34 | 1.0 × 10−6 | XM_586581 |
| NOTCH2 | Notch homolog 2 | 2.99 | 1.7 × 10−4 | Bt.9169.1 |
| VEGFR | Vascular endothelial growth factor receptor | 2.74 | 7.0 × 10−4 | XM_876610 |
| LRP2 | LDL receptor-related protein 2 | 2.69 | 6.0 × 10−5 | Bt.11704.1 |
| Secreted factors | ||||
| ADM | Adrenomedullin | 4.39 | 1.0 × 10−4 | NM_173888 |
| BTC | Betacellulin | 4.30 | 2.6 × 10−5 | NM_173896 |
| SMOC2 | Secreted modular calcium-binding protein 2 | 2.78 | 1.5 × 10−3 | XM_867313 |
| FRZB | Frizzled-related protein | 2.56 | 2.1 × 10−3 | NM_174059 |
| WNT10B | Wingless-type MMTV integration site family, 10B | 2.30 | 1.8 × 10−4 | Bt.6354.1 |
| Selected transcripts enrichedin fraction 4 (RZ) | ||||
| Cell surface receptors | ||||
| NTRK2 | Neurotrophic tyrosine kinase receptor 2 | 2.95 | 4.6 × 10−4 | Bt.12217.3 |
| ACVR1 | Activin receptor, type 1 | 2.86 | 1.7 × 10−3 | NM_176663 |
| KIT | Kit tyrosine kinase receptor | 2.78 | 5.7 × 10−4 | Bt.26445.1 |
| EDNRA | Endothelin receptor | 2.63 | 1.7 × 10−3 | Bt.25346.1 |
| AXL | Axl tyrosine receptor kinase | 2.17 | 9.7 × 10−5 | Bt.6865.1 |
| Secreted factors | ||||
| IGFBP5 | IGF-binding protein 5 | 3.40 | 1.8 × 10−4 | Bt.21465.1 |
| SRPX2 | Sushi-repeat containing protein, X-linked 2 | 2.70 | 6.5 × 10−4 | NM_001014926 |
| SFRP5 | Secreted frizzled-related protein 5 | 2.40 | 1.9 × 10−3 | NM_174461 |
| TGFB1 | TGF-β1 | 2.40 | 5.6 × 10−5 | XM_588272 |
| CHRD | Chordin | 2.22 | 1.3 × 10−4 | CA035043 |
| SCF | Stem cell growth factor | 2.22 | 5.4 × 10−4 | XM_600895 |
| STC1 | Stanniocalcin 1 | 2.08 | 1.4 × 10−3 | Bt.6853.1 |
Figure 3.
Relative expression in bovine chondrocyte fractions of COL2A1, AGC1, and COLX as well as of selected differentially expressed transcripts identified by microarray: LOX, IGFBP-5, FRZB, NOTCH2, and WNT10B. Real-time RT-PCR was used to quantify mRNA levels of the marker proteins in the four density fractions of bovine growth plate chondrocytes. Data points were calculated using the ΔΔCt method and represent the mean ± se of real-time data from at least five separate chondrocyte preparations, expressed as fold difference from fraction 1 (the calibrator). Typical hematoxylin-eosin stains of bovine growth plate sections are aligned with the corresponding chondrocyte fraction. Note that y-axis scales are not identical. **, P < 0.001; *, P < 0.02 (compared with fraction 1).
Transcripts enriched in the RZ
Forty-seven transcripts were enriched in the RZ (fraction 4) as compared with the HZ (selected transcripts are listed in Table 1; supplemental Table S2 includes the complete list of transcripts). Cell surface receptors included ACVR1 (an activin receptor), KIT (the receptor for SCF), NTRK2 (or TrkB, a neurotrophin receptor that binds BDNF), EDNRA (an endothelin receptor), and AXL (a receptor for Gas6). Secreted factors included IGF-binding protein (IGFBP)-5, chordin, TGFβ1, and SCF. IGFBP-5 expression in bovine chondrocyte RZ was verified by RT-PCR (Fig. 3).
Identifying potential modulators of chondrocyte proliferation
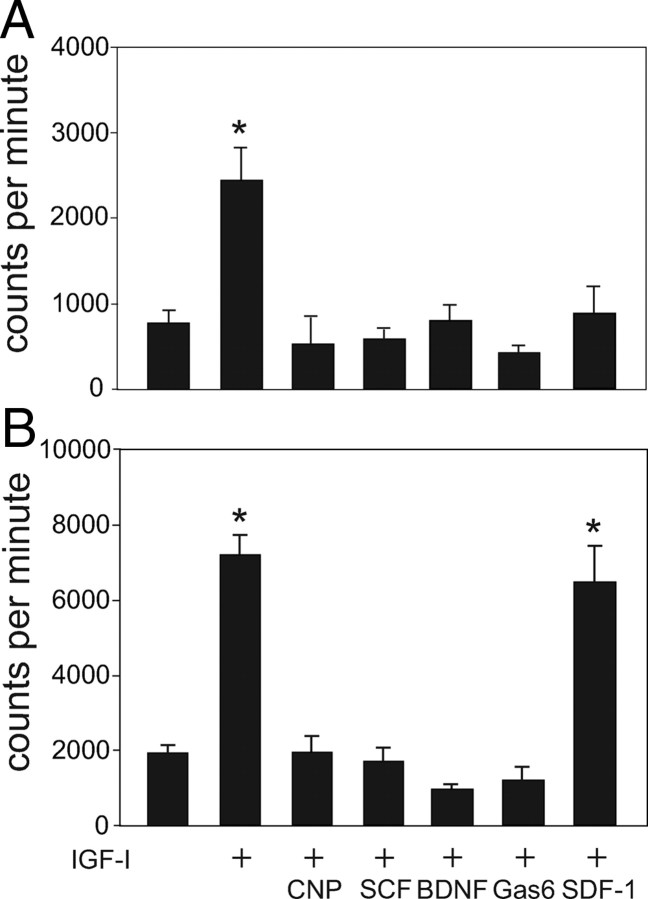
None of the ligands corresponding to the cell surface receptors identified by microarray increased RZ proliferation (Fig. 4). Therefore, we asked whether any of these factors might affect IGF-I-stimulated proliferation. Fraction 4 cells were incubated with IGF-I alone or in combination with SCF, BDNF, Gas6, CNP, or SDF-1. Whereas SDF-1 had no effect on proliferation, SCF, BDNF, and Gas6 each completely abrogated IGF-I-stimulated [3H]thymidine uptake at 24 h. CNP, a known regulator of chondrocyte differentiation, similarly inhibited IGF-I-stimulated proliferation.
Figure 4.
Effects of IGF-I, CNP, SCF, BDNF, Gas6, and SDF-1 on chondrocyte proliferation. A, Isolated bovine RZ cells were incubated with each of the factors and [3H]thymidine uptake measured after 24 h. Proliferation is expressed as counts per minute. B, RZ cells were incubated for 24 h with either IGF-I alone or IGF-I in combination with each of the factors. *, P < 0.01.
Because IGF-I-stimulated chondrocyte proliferation was dependent on ERK1/2 activation (Fig. 2), we questioned whether the factors CNP, SCF, BDNF, and Gas6 might inhibit proliferation in these cells by interfering with ERK activation. Indeed, as shown in Fig. 5A, 1 h incubation with each factor attenuated ERK1/2 activation by IGF-I. ATDC5 cells exposed to insulin for 24 h before being subjected to a time course of stimulation with either IGF-I or IGF-I plus BDNF or CNP showed a similar pattern of ERK1/2 attenuation (Fig. 5B).
Figure 5.

A, Western immunoblotting of active and total ERK1/2 in RZ cells stimulated with IGF-I, CNP, SCF, BDNF, or Gas6. Isolated RZ cells were serum starved for 1 h and then preincubated with or without each of the four factors for 1 h, followed by stimulation with 100 ng/ml IGF-I for 10 min. Cell lysates for immunoblotted for active with anti-phospho-ERK1/2 antisera as well as total ERK1/2. B, After 24 h incubation with insulin (to induce differentiation), ATDC5 cells were changed to serum-free, insulin-free medium and given 100 ng/ml BDNF, 200 ng/ml CNP, or no treatment for 1 h. IGF-I (100 ng/ml) was added for the indicated times and lysates blotted for active and total ERK1/2.
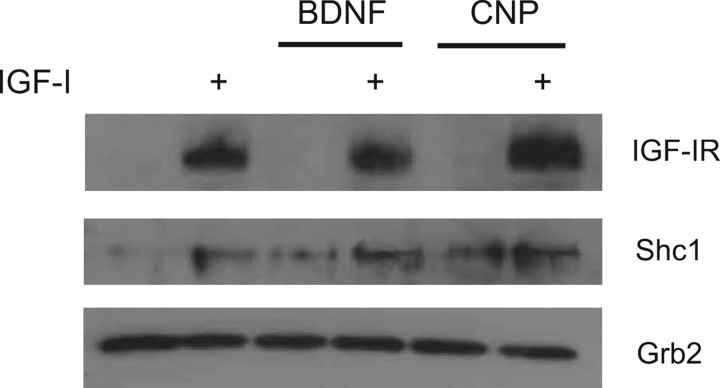
To determine whether the observed attenuation of MAPK activation was the result of reduced IGF-I receptor activation, tyrosine-phosphorylated proteins from lysates of fraction 4 cells were immunoprecipitated and analyzed by Western blotting for the presence of various signaling molecules known to be activated by IGF-I. We focused on two of the factors, BDNF [which activates a receptor tyrosine kinase (RTK)] and CNP (which activates a receptor guanylate cyclase). IGF-I receptor (IGF-IR) is an RTK that exists as a heterodimer and undergoes autophosphorylation upon IGF-I binding; phosphorylation of tyrosine residues in the kinase domain is necessary for full kinase activation (12). Activated IGF-IR then phosphorylates and recruits the adaptor molecules insulin receptor substrate (IRS)-1 and Shc1, which in turn recruit the adaptor molecule Grb2, a major upstream regulator of the ERK MAPK pathway. Grb2 may be recruited by IRS-1 or may bypass IRS-1 via recruitment by Shc1 binding to IGF-IR (13,14). In Fig. 6, tyrosine phosphorylation of IGF-IRβ in response to IGF-I binding is not appreciably reduced by either BDNF or CNP preincubation. The SH3-containing adaptor molecule Shc appears to be present in greater amounts in lysates from IGF-I-treated cells, whereas Grb2 is present in equal amounts in all lysates. Similar results were seen when ATDC5 lysates were used (not shown). We were unable to detect IRS-1 in any of the immunoprecipitates, despite using two different antisera (from Santa Cruz Biotechnology, Santa Cruz, CA, and Sigma-Aldrich, St. Louis, MO) as well as anti-phosphotyrosine (Y632) IRS-1 (Santa Cruz) (not shown). Of note, all three probe sets for IRS-1 on the bovine microarray detected very low levels of IRS-1 RNA in both RZ and HZ cells (not shown).
Figure 6.
Immunoprecipitation of tyrosine-phosphorylated signaling proteins in chondrocyte lysates. Isolated RZ cells were serum starved for 1 h and then given BDNF (100 ng/ml), CNP (200 ng/ml), or no treatment for 1 h. Cells were then treated with IGF-I (100 ng/ml) for 10 min or no treatment; lysates from treated cells were immunoprecipitated with anti-phosphotyrosine antisera, and the resulting precipitates were analyzed by Western blotting for the presence of IGF-IR, Shc1, or Grb2.
Identified modulators of IGF-I action stimulate chondrocyte differentiation
When cultured with insulin, the murine chondrogenic cell line ATDC5 recapitulates many features of the growth plate chondrocyte differentiation program (15,16). We measured the relative expression of the early markers Col2a1 and Agc1 and the later markers ColX and Lox as well as the receptors for SCF, Gas6, BDNF, and CNP in ATDC5 cells grown in the presence of insulin for 2 wk with samples harvested every 2 d (see Fig. 8). Expression of Col2a1 was highest at approximately d 11, whereas ColX expression was greatest at d 14. Note that for each receptor tested, the expression pattern in developing ATDC5 cells is consistent with the expression pattern seen in bovine chondrocyte fractions; for example, the Gas6 receptors AXL and MER have opposite expression patterns both in bovine chondrocyte fractions and in developing ATDC5 cells (see Fig. 7).
Figure 8.
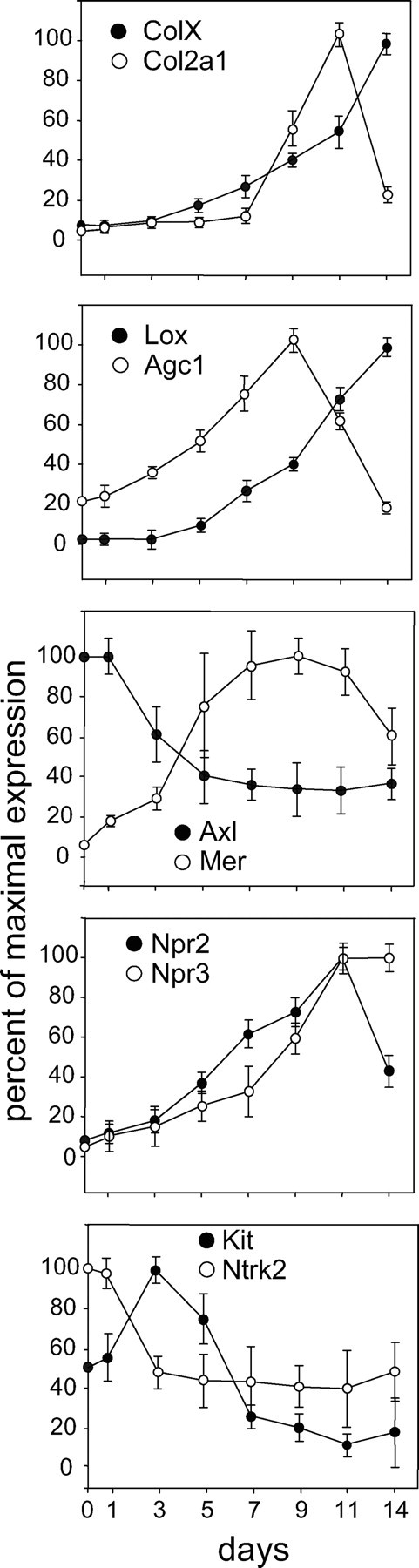
Relative expression of differentiation markers ColX, Col2a1, Lox, and Agc1 and receptors Axl, Mer, Npr2, Npr3, Kit, and Ntrk2 in ATDC5 cells incubated with insulin for 14 d (compare with expression pattern in bovine chondrocytes shown in Figs. 2 and 4). Real-time RT-PCR was used to quantify mRNA levels. Data points were calculated using the ΔΔCt method, represent the mean ± se of real-time data from at least six samples, and are expressed as percentage of maximal expression for each marker.
Figure 7.
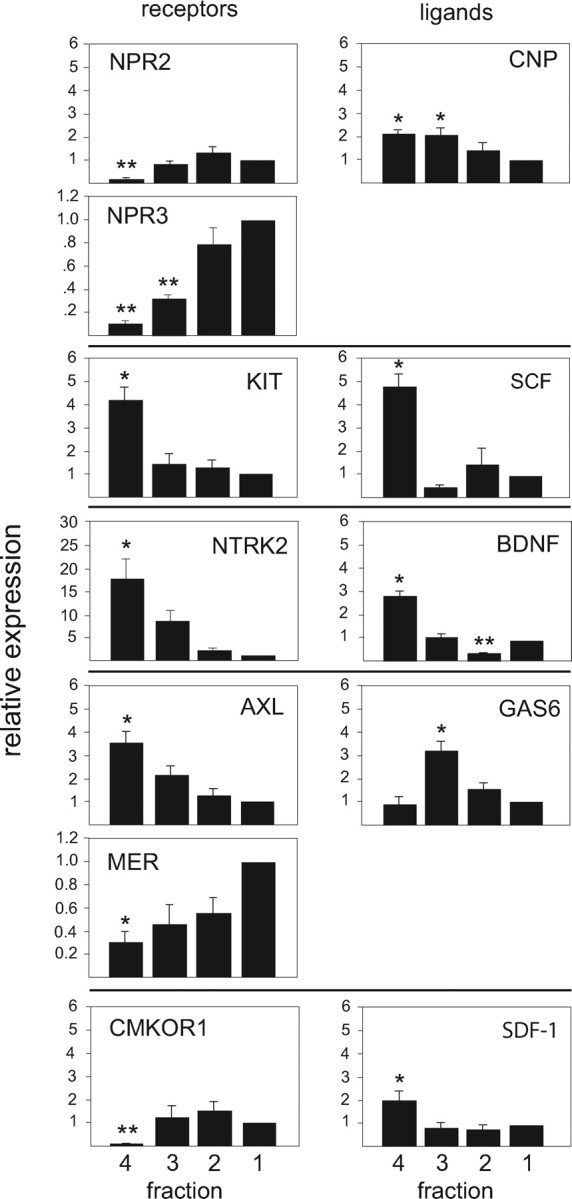
Relative expression in bovine chondrocyte fractions of cell surface receptors and their respective ligands identified by microarray. CNP and Gas6 each interact with two receptors; horizontal lines between panels delineate corresponding receptors and ligands. Real-time RT-PCR was used to measure relative mRNA levels in the four density fractions of bovine growth plate chondrocytes. Data points were calculated using the ΔΔCt method and represent the mean ± se of real-time data from at least five separate chondrocyte preparations, expressed as fold difference from fraction 1 (the calibrator). Note that y-axis scales are not identical. **, P < 0.001; *, P < 0.04 (compared with fraction 1).
To see whether SCF, BDNF, Gas6, or SDF-1 would affect growth plate development, confluent ATDC5 cells were cultured with insulin alone or in combination with each of the various factors for 3 d. The early chondrocytic marker Col2a1 was best stimulated by SCF, whereas the other early marker Agc1 was increased by both SCF and BDNF. Gas6, on the other hand, suppressed both early markers during the first 3 d of ATDC5 cell development. SCF, CNP, Gas6, and BDNF each increased expression of the late marker ColX approximately 2- to 4-fold over insulin alone, whereas SDF-1 had no effect (see Fig. 9).
Figure 9.
Relative expression of markers Col2a1, Agc1, ColX, and Lox in ATDC5 cells during insulin-induced differentiation. Left, ATDC5 cells at confluence were incubated with insulin (INS) alone or in combination with each of the factors for 72 h (d 1–3). Real-time RT-PCR was used to quantify mRNA levels of the marker proteins. Data points were calculated using the ΔΔCt method and represent the mean ± se of real-time data from five sample pairs, expressed as fold difference from insulin alone (the calibrator). Right, ATDC5 cells at confluence were given insulin for 6 d, at which time cells were cultured for an additional 72 h in the presence of either insulin alone or in combination with each factor (d 6–9). **, P < 0.01; *, P = 0.03 (compared with insulin alone).
Overall, SCF was the most potent stimulator of differentiation during the first 3 d of ATDC5 cell differentiation, increasing both early and late markers in ATDC5 cells. In contrast, BDNF and Gas6 each significantly inhibited Col2a1 expression, and Gas6 also suppressed Agc1. SCF and Gas6 were the most robust stimulators of Lox, another late marker identified by microarray (see Fig. 9). Note that the activity of SCF and BDNF during the early phase of ATDC5 differentiation corresponds with the expression patterns for their respective receptors, KIT and NTRK2, as shown both in bovine chondrocyte fractions (Fig. 7) and in developing ATDC5 cells (Fig. 8).
To ascertain whether these factors might enhance later stage chondrocyte development, confluent ATDC5 cells were cultured with insulin for 6 d, at which time factors were added for an additional 3 d. Whereas SCF had been the most potent stimulator of differentiation through d 3, only Gas6 increased the expression of the four differentiation markers in the later period. Moreover, whereas Gas6 suppressed Col2a1 and Agc1 expression on d 1–3, it increased the expression of both markers on d 6–9 (Fig. 9). Note that this difference in Gas6 activity corresponds with the reciprocal expression patterns of the Gas 6 receptors AXL and MER, seen in both bovine cells (Fig. 7) and developing ATDC5 cells (Fig. 8).
Studies employing ATDC5 cells to examine patterns of chondrocyte differentiation use high-dose insulin to initiate and sustain the differentiation program. To ensure that the factors CNP, SCF, Gas6, and BDNF were affecting chondrocyte development via their modulation of IGF-I action, and not in fact insulin action, confluent ATDC5 cells were cultured exactly as done for the previous experiments, but 100 ng/ml IGF-I was substituted for insulin. For each of the factors CNP, SCF, Gas6, and BDNF, the change in expression of the four markers Col2a1, Agc, ColX, and Lox was qualitatively that same as that seen with high-dose insulin in Fig. 9 (data not shown).
Discussion
Only IGF-I stimulates RZ proliferation, and its activity requires ERK activation
Several factors have been reported to support growth plate chondrocyte proliferation in other assay systems, including TGFβ1 and -β2, FGF1, FGF2, and FGF18 in human growth plate chondrocytes (17) and PTHrP in avian sternal cartilage (18) and fetal mouse limb explant cultures (19). In immature bovine chondrocytes separated from other cells of the growth plate, physiological concentrations of IGF-I and pharmacological concentrations of insulin (presumably acting through the IGF-IR) were the only stimulators of proliferation. The differences between these studies probably reflects the fact that our use of isolated RZ chondrocytes eliminated potential paracrine effects from either perichondrium or more differentiated growth plate zones. Many factors act in a paracrine fashion at the growth plate; for example, TGFβ regulates chondrocyte growth indirectly via PTHrP from the perichondrium (20,21,22). Such paracrine effects as might be seen in whole-limb explants or mixed cell populations would not be apparent in our isolated chondrocytes, allowing us to assess only direct effects of various factors on chondrocytes.
In other models such as mouse primary chondrocytes and the chondrogenic cell line RCJ3.IC5.18, proliferation of growth plate chondrocytes is both ERK1/2 and PI3K dependent (10,11). In various cell types, the p110γ subunit of PI3K is activated by Gβγ subunits of G protein-coupled receptors; activation of PI3K by receptor tyrosine kinases such as IGF-IR is still a matter of investigation and is thought to involve the phosphorylation and recruitment of IRS-1 (23). In whole mouse tibias, PI3K was necessary for hypertrophic differentiation, but inhibition of PI3K had little effect on IGF-I-stimulated cell cycle progression once the cells had entered the proliferative zone (24). In our isolated bovine chondrocytes, PI3K inhibition had no effect on IGF-I-stimulated proliferation, which instead was dependent on ERK1/2 activation. The factors corresponding to the receptors identified by microarray, CNP, SCF, Gas6, and BDNF, each inhibits IGF-I-dependent proliferation by at least partially attenuating ERK1/2 activation, supporting the idea that IGF-I, via activation of the traditional MAPK pathway, is the primary promoter of growth plate chondrocyte proliferation. The attenuation of MAPK activation seen by BDNF and CNP is not due to interference with IGF-IR activation by IGF-I or subsequent signaling molecule recruitment, because Shc1 and Grb2 association with IGF-IR is not affected by either BDNF binding to the RTK TrkB or CNP binding to the receptor guanylate cyclase GC-B. Also, IRS-1 did not appear to be involved in IGF-I signaling in the bovine chondrocytes, which is consistent with the observation that IGF-I action is not PI3K dependent in these cells.
Differential gene expression in growth plate zones
Relative levels of RNA transcripts in growth plate chondrocyte zones have previously been studied using microarrays. Approaches have included the use of laser microdissection to divide rat growth plate material into two equal sections (25), the comparison of RNA expression levels before and after differentiation of whole limb buds from mouse embryos (26), and the use of microdissection to separate the epiphyseal cartilage of 7-d-old rats into several zones to examine the differential expression of bone morphogenetic protein signaling molecules across the growth plate (27).
Our results are largely consistent with these previous microarray studies but have allowed the identification of additional regulators of chondrocyte maturation. The ability to isolate large numbers of RZ and HZ chondrocytes from bovine tissue enabled us to generate replicates relatively easily, thus providing statistical power to identify differentially expressed genes that would likely have been missed had fewer replicates been analyzed.
Differentially expressed growth factor receptors mediate chondrocyte differentiation
The observation that ligands corresponding to receptors identified in bovine chondrocytes inhibited IGF-I-stimulated proliferation led us to question whether these ligands could affect chondrocyte differentiation. The factors we tested in the murine chondrocytic cell line ATDC5, SCF, BDNF, Gas6, SDF-I, and CNP, differed in their ability to alter expression of four differentiation markers, Col2a1, Agc1, ColX, and Lox. Moreover, each factor displayed differing activities at different developmental stages corresponding to the expression patterns of the respective receptors. For example, KIT and NTRK2 expression are highest in bovine RZ chondrocytes and undifferentiated ATDC5 cells, suggesting that SCF and BDNF act early in the chondrocyte maturation program to attenuate proliferation and stimulate differentiation. Similarly, the differential activity of Gas6 between early- and later-phase differentiation is likely explained by differential expression of two tyrosine kinase receptors for Gas6, AXL and MER. Gas6 was recently reported to suppress Col2a1 and Agc1 expression after 3 d in C3H10T1/2 cells, another in vitro model of chondrogenesis (28). AXL, enriched in the bovine RZ, might mediate the Gas6-induced suppression of certain transcripts during early-phase differentiation, whereas Gas6 binding to MER in the HZ might support differentiation in later developmental stages.
CNP acts through GC-B (NPR2) (29,30). In ATDC5 cells GC-B expression is highest in the pre-hypertrophic cells (31), and CNP stimulates expansion of the HZ in mouse tibia explants (32), implicating CNP in later chondrocyte maturation stages. However, we found that CNP augmented differentiation in ATDC5 cells during early-phase differentiation. Expression of the CNP decoy receptor NPR3 is relatively low in both the bovine RZ cells and undifferentiated ATDC5 cells. The decoy receptor Npr3 modulates the natriuretic peptide system locally (33,34). These results suggest that the activity of CNP is influenced by the relative levels of its receptor and decoy, such that CNP might stimulate differentiation early in the chondrocytic program when NPR3 levels are low.
It is unclear at this point why SCF, BDNF, and Gas6, having receptors enriched in the same cell population, produced different patterns of expression of the differentiation markers Col2a1 and Agc1 in early-phase ATDC5 cell differentiation. One possibility is that attenuation of ERK1/2 activity is required for promotion of differentiation in these cells, and varying degrees of ERK activity in chondrocytes modulate the proliferation-promoting signal of IGF-I to a pro-differentiation signal. Further work is needed to explore this possibility.
Physiological significance based on animal models
If the receptors and ligands identified in the present study are important for growth plate chondrocyte differentiation in vivo, then mice lacking these proteins should grow abnormally. SCF, presumably acting through the tyrosine kinase receptor Kit, was the most potent stimulus for early-phase differentiation. Sl/Sl mice (homozygous for mutations in the Steel locus encoding SCF itself) die perinatally (35). However, SCF is normally expressed in both secreted and membrane-bound forms (36), and Sl/Sld mice expressing low levels of soluble SCF and no membrane-bound SCF have decreased bone mineral density and are significantly smaller than their wild-type littermates (37).
BDNF, presumably acting through NTRK2 (TrkB), supported early-phase ATDC5 differentiation. Mice lacking either BDNF or its receptor, TrkB, are significantly dwarfed, with weights as low as 25–30% of their wild-type littermates (38,39,40,41).
AXL and MER belong to the TAM (Tyro-3, AXL, MER) family of tyrosine kinase receptors that bind the product of Gas6 (42,43). Mice homozygous for deletions in any single member of the TAM family showed no gross anatomical defects, implying functional overlap between the three receptors. Double mutants were normally grown, although males showed abnormal germ cell development. The Tyro-3/Axl/Mer triple-mutant mice were small and growth retarded but also displayed multiple major organ defects and severe immune system dysfunction (44).
Finally, as previously reported, mice with targeted disruptions of either CNP or NPR2 develop severe dwarfism (45,46), and animals overexpressing CNP are overgrown (47,48).
Conclusion
In some developmental models, such as 3T3-L1 preadipocytes and chondrogenic ATDC5 cells, IGF-I drives both proliferation and differentiation. Our results suggest that in epiphyseal chondrocytes, IGF-I is the major factor that directly stimulates proliferation but that several factors, including SCF, BDNF, and Gas6 modulate the IGF-I signal from one that is proliferative to one that favors differentiation, at least partly by attenuation of MAPK activation. Thus, this report adds SCF, BDNF, and Gas6 to the growing list of positive regulators of growth plate development.
Materials and Methods
Growth factors and hormones
Recombinant human IGF-I, human SDF-1, TGFβ, E2, PTHrP, PDGF, EGF, NGFβ, and T3 were purchased from Sigma-Aldrich; human SCF, mouse Gas6, and human BDNF were purchased from R&D Systems (Minneapolis, MN); CNP was from Bachem Bioscience, Inc. (Torrance, CA). Calf serum, insulin, transferrin, and selenium were from GIBCO, Inc. (Grand Island, NY).
Harvesting and separation of chondrocytes
Growth plate chondrocytes were obtained from metacarpals of male dairy cattle aged 6–10 months and fractionated by continuous density gradient centrifugation as described (9). Cells were removed from the gradient in four fractions of equal volume, with fractions 1 and 4 representing the lowest- and highest-density cells, respectively. Fraction 4 cells were analyzed by real-time RT-PCR to ensure that collagen 1 (COL1A1) and decorin (DCN) message levels were not present, ruling out perichondrial contamination.
Cell proliferation assays
Cells taken directly from Percoll gradients were plated in serum-free DMEM/F12 medium on 60-mm plastic plates at a density of 1 × 105 cells per well and maintained in a humidified incubator at 37 C and 5% CO2. All cell samples were plated and assayed in triplicate. At 12 h, various factors were added at the following concentrations: 100 ng/ml IGF-I, 5 ng/ml TGFβ, 10 ng/ml PDGF, 100 nm PTHrP, 10 ng/ml T3, 20 ng/ml EGF, 20 ng/ml NGFβ, 10 ng/ml E2, 200 ng/ml CNP, 50 ng/ml SCF, 100 ng/ml BDNF, 400 ng/ml Gas6, and 100 ng/ml SDF-1. Eight hours after growth factor addition, [3H]thymidine (Amersham, Piscataway, NJ; 95 Ci/mmol) was added at 1 μCi per well. Sixteen hours later, cells were rinsed once with PBS, scraped into 5% trichloroacetic acid, transferred to 24-mm Whatman glass filters, and rinsed three times with 5 ml 5% trichloroacetic acid on a vacuum manifold. Filters were rinsed once with 70% ethanol and dried completely; incorporated radioactivity was measured with a scintillation counter. For experiments including kinase inhibitors, the inhibitors were added at the same time as the above factors and were used at the following concentrations: 10 μm U0126 (Promega, Madison, WI) and 100 nm wortmannin (Sigma).
Western blotting for ERK1/2 activation
Isolated fraction 4 cells were plated at 1 × 106 cells per 100-mm plate in serum-free DMEM/F12 1:1 supplemented with 1 mg/ml BSA and allowed to rest overnight. Cells were preincubated with either 200 ng/ml CNP, 50 ng/ml SCF, 400 ng/ml Gas6, 100 ng/ml BDNF, or no treatment. After 1 h pretreatment, cells received either 100 ng/ml IGF-I or no further treatment. Alternatively, ATDC5 cells at confluence were changed to medium plus insulin (see below) for 24 h, after which they were changed to serum-free, insulin-free medium for 1 h. Cell were pretreated with 100 ng/ml BDNF or 200 ng/ml CNP for 1 h, and then IGF-I was added at 100 ng/ml for various lengths of time. At the indicated times, medium was removed and cells scraped into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of total protein were separated on 10% SDS-polyacrylamide gels, and transferred to Hybond-P polyvinylidene difluoride membranes (Amersham). Membranes were blocked in StartingBlock (Thermoscientific, Rockford, IL). The primary antibody anti-phospho-ERK1/2 (Invitrogen, Carlsbad, CA) was used at a dilution of 1:5000 for 2 h at room temperature, or anti-total-ERK1/2 (Sigma) was used at 1:20,000. After washing with Tris-buffered saline (TBS) plus 0.05% Tween 20, the membranes were incubated with an horseradish peroxidase-labeled secondary antibody at 1:5000 for 1 h. After washing with TBS/Tween 20 followed by TBS, bands were visualized by chemiluminescence and Hyperfilm (Amersham).
Immunoprecipitation
Isolated fraction 4 cells were serum starved for 1 h before use. ATDC5 cells at confluence (as described below) were incubated in the presence of insulin for 24 h, after which time they were rinsed and changed to serum-free, insulin-free medium for 1 h. Cells received either BDNF (100 ng/ml), CNP (200 ng/ml), or no treatment for 1 h, after which they were then given IGF-I (100 ng/ml) or no treatment. Cells were rinsed once in cold PBS and then lysed in 0.5 ml lysis buffer -1% Triton X-100, 20 mm Tris (pH 7.4), 137 mm NaCl, 1 mm EDTA, 1 mm MgCl2, 10% glycerol] with protease and phosphatase cocktails added (Sigma). Insoluble material was removed by centrifugation and the lysates precleared with 30 μl protein A-agarose (Invitrogen) for 1 h at 4 C. Anti-phospho-tyrosine antisera (Cell Signaling Technology, Beverly, MA) was added at 1:100 and lysates incubated at 4 C with rocking overnight. Fresh protein A-agarose was added for 1 h, after which pellets were washed three times with 1 ml lysis buffer, and 50 μl sample buffer was added to each sample and equal volumes separated on 10% SDS-polyacrylamide gels. Western blotting was performed as above with either anti-IGF-IR, anti-shc1, anti-Grb2, or anti-IRS-1 (Santa Cruz), each at 1:1000 dilution, and processed as above.
ATDC5 cell culture
ATDC5 cells were obtained from Riken (Tsukuba, Japan) and maintained in DMEM/F12 1:1 with 10% fetal bovine serum (Invitrogen) and penicillin/streptomycin/amphotericin B (Invitrogen). To induce differentiation, cells were plated at 1 × 105 cells per 100-mm plate. Once at confluence (generally d 3), the medium was changed to DMEM/F12 with 5% fetal bovine serum plus 10 μg/ml insulin, 10 μg/ml human transferrin, and 10 ng/ml Na selenite (ITS), following which the medium was changed every other day. To test the ability of various factors to stimulate chondrocyte differentiation, each was used in addition to ITS for 3 d at the same concentrations used in the proliferation assays.
RNA isolation, cDNA synthesis, and real-time RT-PCR
RNA was extracted from cells using RNA STAT-60 (Tel-Test, Inc., Friendswood, TX) per the manufacturer’s instructions. For each sample, 10 μg total RNA was subjected to deoxyribonuclease I treatment to remove genomic DNA using the DNA-free kit (Ambion, Austin, TX), and 5 μg deoxyribonuclease I-treated RNA was then reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions.
Real-time RT-PCR was performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) following the protocol supplied by the manufacturer. 18S, DCN, COL1A1, and COLX detection was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) and 100 nm of each primer and probe. The remainder of the bovine targets, as well as all mouse sequences, were analyzed using double-stranded DNA dye SYBR Green I with SYBR Green Universal PCR Master Mix (Applied Biosystems) and 200 nm of each primer. Wherever possible, primers spanned an intron/exon boundary. All RT-PCR were confirmed to produce only a single PCR product. Primer and probe sets are displayed in supplemental Table S3, except for those previously published for bovine targets DCN, COL1A1, COLX (9), and mouse target ColX (49).
Relative gene expression for each mRNA was calculated by the ΔΔCT method using fraction 1 as the calibrator. The ΔΔCT method is more fully described in User Bulletin 2 of the ABI Prism 7700 Sequence Detection system.
Microarray analyses
Six independent sample pairs of RNA from fresh fraction 1 and fraction 4 cells were prepared using RNA STAT 60. Samples were analyzed with an Agilent 2100 Bioanalyzer to confirm RNA integrity. To rule out contamination by either perichondrial tissue or lymphocytes before microarray analysis, all fraction 4 cDNA preparations were confirmed by real-time RT-PCR to have unappreciable levels of the T cell receptor-α subunit (TCRα), an Ig-specific sequence (IgJ), and perichondrial-specific transcripts COL1A1 and DCN.
Hybridization was performed according to standard Affymetrix (Santa Clara, CA) protocols (http://www.affymetrix. com/support/technical/manuals/affx). The Bovine Genome Array from Affymetrix contains 24,072 probe sets representing approximately 23,000 bovine transcripts. The arrays were scanned at high resolution using Affymetrix GeneChip Scanner 3000. Results were analyzed using GeneSpring GX version 7.2 software (Agilent Technologies, Redwood City, CA). Data, chip, and genes were normalized in three stages (log-transformed raw data <0.01 set to 0.01, global normalization to the 50th percentile, followed by gene-level normalization) using the recommended GeneSpring default settings for data transformation. Default values of the tunable parameters as provided in Microarray Suite 5.0 were used to filter for present or marginal signals. Approximately 50% of the genes on each array survived the filter. Each transcript that survived the filter on at least six of the 12 chips was considered for further analysis. The log-transformed intensity data for these transcripts were analyzed by one-way parametric testing (Welch t test) to filter for genes differentially expressed between the two cell fractions. Type I errors were controlled using the Benjamini-Hochberg false discovery rate with a filter of P < 0.05. Of the transcripts surviving these filters, differentially expressed transcripts were defined as having a 2-fold or greater difference between fraction 1 and fraction 4 on all six microarray pairs.
Other statistical methods
For real-time RT-PCR verification of microarrays, pooled results from at least five sets of fraction 1–4 cells, each performed in duplicate, were analyzed by one-way ANOVA with Student-Newman-Keuls multiple-comparison methods using SigmaStat version 3.0 (SPSS, Inc., Chicago, IL). For all real-time RT-PCR analyses in ATDC5 differentiation experiments, results were pooled from three separate experiments, each performed in triplicate, analyzed by RT-PCR in duplicate, and then analyzed by Student’s t tests using SigmaPlot version 10.0 (Systat Software, Inc., Chicago, IL). For cell proliferation studies, results from at least three separate experiments, all performed in triplicate, were analyzed by one-way ANOVA with Student’s t tests using SigmaPlot.
Supplementary Material
Acknowledgments
We thank David Meyers, Alan Rubin, and their employees at Dallas City Packing Co. for supplying calf forelegs.
Footnotes
This work was supported by National Institutes of Health Grant DK073447 (to M.R.H.). P.C.W. is the Audry Newman Rapoport Distinguished Chair in Pediatric Endocrinology.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 6, 2009
Abbreviations: BDNF, Brain-derived neurotrophic factor; CNP, C-type natriuretic peptide; E2, estradiol; EGF, epidermal growth factor; FGF, fibroblast growth factor; FRZB, frizzled-related protein 3; Gas6, growth arrest-specific 6; HZ, hypertrophic zone; IGFBP, IGF-binding protein; IGF-IR, IGF-I receptor; IRS, insulin receptor substrate; NGFβ, nerve growth factor-β; PDGF, platelet-derived growth factor; PI3K, phosphatidylinositol 3-kinase; RTK, receptor tyrosine kinase; RZ, reserve zone; SCF, stem cell factor; SDF-1, stromal cell-derived factor 1; TBS, Tris-buffered saline.
References
- Stevens DA, Williams GR 1999 Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol 151:195–204 [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM 2003 Systemic and local regulation of the growth plate. Endocr Rev 24:782–801 [DOI] [PubMed] [Google Scholar]
- Weisser J, Riemer S, Schmidl M, Suva LJ, Pöschl E, Bräuer R, von der Mark K 2002 Four distinct chondrocyte populations in the fetal bovine growth plate: highest expression levels of PTH/PTHrP receptor, Indian hedgehog, and MMP-13 in hypertrophic chondrocytes and their suppression by PTH (1-34) and PTHrP (1-40). Exp Cell Res 279:1–13 [DOI] [PubMed] [Google Scholar]
- Kindblom JM, Nilsson O, Hurme T, Ohlsson C, Sävendahl L 2002 Expression and localization of Indian hedgehog (Ihh) and parathyroid hormone related protein (PTHrP) in the human growth plate during pubertal development. J Endocrinol 174:R1–6 [DOI] [PubMed] [Google Scholar]
- MacLean HE, Kronenberg HM 2005 Localization of Indian hedgehog and PTH/PTHrP receptor expression in relation to chondrocyte proliferation during mouse bone development. Dev Growth Differ 47:59–63 [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE 2000 Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem 48:1493–1502 [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Matsunaga S, Onishi T, Nagamine T, Origuchi N, Yamamoto T, Ishidou Y, Imamura T, Sakou T 1998 Immunohistochemical localization of bone morphogenetic proteins and the receptors in epiphyseal growth plate. Anticancer Res 18:2339– 2344 [PubMed] [Google Scholar]
- Liu Z, Lavine KJ, Hung IH, Ornitz DM 2007 FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol 302:80–91 [DOI] [PubMed] [Google Scholar]
- Hutchison MR, Bassett MH, White PC 2007 Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology 148:3122–3130 [DOI] [PubMed] [Google Scholar]
- Kiepe D, Ciarmatori S, Hoeflich A, Wolf E, Tönshoff B 2005 Insulin-like growth factor (IGF)-I stimulates cell proliferation and induces IGF binding protein (IGFBP)-3 and IGFBP-5 gene expression in cultured growth plate chondrocytes via distinct signaling pathways. Endocrinology 146:3096–3104 [DOI] [PubMed] [Google Scholar]
- Ciarmatori S, Kiepe D, Haarmann A, Huegel U, Tönshoff B 2007 Signaling mechanisms leading to regulation of proliferation and differentiation of the mesenchymal chondrogenic cell line RCJ3.1C5.18 in response to IGF-I. J Mol Endocrinol 38:493–508 [DOI] [PubMed] [Google Scholar]
- Baserga R 1999 The IGF-I receptor in cancer research. Exp Cell Res 253:1–6 [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J 1990 Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212 [DOI] [PubMed] [Google Scholar]
- Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S 1991 Oncogenes and signal transduction. Cell 64:281–302 [DOI] [PubMed] [Google Scholar]
- Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y 1997 Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J Bone Miner Res 12:1174–1188 [DOI] [PubMed] [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y 1996 Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol 133:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RC, Wang J, Sylvester JE, Mougey EB 2004 Growth factor regulation of human growth plate chondrocyte proliferation in vitro. Biochem Biophys Res Commun 317:1171–1182 [DOI] [PubMed] [Google Scholar]
- Harrington EK, Roddy GW, West R, Svoboda KK 2007 Parathyroid hormone/parathyroid hormone-related peptide modulates growth of avian sternal cartilage via chondrocytic proliferation. Anat Rec (Hoboken) 290:155–167 [DOI] [PubMed] [Google Scholar]
- Mau E, Whetstone H, Yu C, Hopyan S, Wunder JS, Alman BA 2007 PTHrP regulates growth plate chondrocyte differentiation and proliferation in a Gli3 dependent manner utilizing hedgehog ligand dependent and independent mechanisms. Dev Biol 305: 28–39 [DOI] [PubMed] [Google Scholar]
- Serra R, Karaplis A, Sohn P 1999 Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J Cell Biol 145:783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateder DB, Rosier RN, Schwarz EM, Reynolds PR, Puzas JE, D'Souza M, O'Keefe RJ 2000 PTHrP expression in chondrocytes, regulation by TGF-β, and interactions between epiphyseal and growth plate chondrocytes. Exp Cell Res 256:555–562 [DOI] [PubMed] [Google Scholar]
- Alvarez J, Horton J, Sohn P, Serra R 2001 The perichondrium plays an important role in mediating the effects of TGF-β1 on endochondral bone formation. Dev Dyn 221:311–321 [DOI] [PubMed] [Google Scholar]
- Hirsch E, Braccini L, Ciraolo E, Morello F, Perino A 2009 Twice upon a time: PI3K’s secret double life exposed. Trends Biochem Sci 34:244–248 [DOI] [PubMed] [Google Scholar]
- Ulici V, Hoenselaar KD, Gillespie JR, Beier F 2008 The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev Biol 8:40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Middleton F, Horton JA, Reichel L, Farnum CE, Damron TA 2004 Microarray analysis of proliferative and hypertrophic growth plate zones identifies differentiation markers and signal pathways. Bone 35:1273–1293 [DOI] [PubMed] [Google Scholar]
- James CG, Appleton CT, Ulici V, Underhill TM, Beier F 2005 Microarray analyses of gene expression during chondrocyte differentiation identifies novel regulators of hypertrophy. Mol Biol Cell 16:5316–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Parker EA, Hegde A, Chau M, Barnes KM, Baron J 2007 Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol 193:75–84 [DOI] [PubMed] [Google Scholar]
- Motomura H, Niimi H, Sugimori K, Ohtsuka T, Kimura T, Kitajima I 2007 Gas6, a new regulator of chondrogenic differentiation from mesenchymal cells. Biochem Biophys Res Commun 357:997–1003 [DOI] [PubMed] [Google Scholar]
- Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, Imura H 1992 Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130:229–239 [DOI] [PubMed] [Google Scholar]
- Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV 1991 Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252:120–123 [DOI] [PubMed] [Google Scholar]
- Suda M, Tanaka K, Yasoda A, Komatsu Y, Chusho H, Miura M, Tamura N, Ogawa Y, Nakao K 2002 C-type natriuretic peptide/guanylate cyclase B system in ATDC5 cells, a chondrogenic cell line. J Bone Miner Metab 20:136–141 [DOI] [PubMed] [Google Scholar]
- Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F 2007 C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol 7:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O 1999 The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 96:7403–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert J, Jaubert F, Martin N, Washburn LL, Lee BK, Eicher EM, Guénet JL 1999 Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3). Proc Natl Acad Sci USA 96:10278–10283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JH, Gresham GA 1956 A gene for eyelids open at birth in the house mouse. Nature 178:272–273 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Nocka KH, Buck J, Besmer P 1992 Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell 3:349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotinun S, Evans GL, Turner RT, Oursler MJ 2005 Deletion of membrane-bound steel factor results in osteopenia in mice. J Bone Miner Res 20:644–652 [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T 1995 Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92:8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Fariñas I, Backus C, Reichardt LF 1994 Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76:989–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R 1994 Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368:147–150 [DOI] [PubMed] [Google Scholar]
- Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B 1999 Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci 19:8919–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ 1996 Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem 271:9785–9789 [DOI] [PubMed] [Google Scholar]
- Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K 1996 Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271:30022–30027 [DOI] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G 1999 Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728 [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, Pfeifer A, Hofmann F, Nakao K 2002 Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology 143:3604– 3610 [DOI] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K 2001 Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98:4016–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K 1994 Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest 93:1911–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K 2004 Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med 10:80–86 [DOI] [PubMed] [Google Scholar]
- Huang Z, Xu H, Sandell L 2004 Negative regulation of chondrocyte differentiation by transcription factor AP-2α. J Bone Miner Res 19:245–255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.