Abstract
Background and Aims
Myrmecochory is a conspicuous feature of several sclerophyll ecosystems around the world but it has received little attention in the semi-arid areas of South America. This study addresses the importance of seed dispersal by ants in a 2500-km2 area of the Caatinga ecosystem (north-east Brazil) and investigates ant-derived benefits to the plant through myrmecochory.
Methods
Seed manipulation and dispersal by ants was investigated during a 3-year period in the Xingó region. Both plant and ant assemblages involved in seed dispersal were described and ant behaviour was characterized. True myrmecochorous seeds of seven Euphorbiaceae species (i.e. elaiosome-bearing seeds) were used in experiments designed to: (1) quantify the rates of seed cleaning/removal and the influence of both seed size and elaiosome presence on seed removal; (2) identify the fate of seeds dispersed by ants; and (3) document the benefits of seed dispersal by ants in terms of seed germination and seedling growth.
Key Results
Seed dispersal by ants involved one-quarter of the woody flora inhabiting the Xingó region, but true myrmecochory was restricted to 12·8 % of the woody plant species. Myrmecochorous seeds manipulated by ants faced high levels of seed removal (38–84 %) and 83 % of removed seeds were discarded on ant nests. Moreover, seed removal positively correlated with the presence of elaiosome, and elaiosome removal increased germination success by at least 30 %. Finally, some Euphorbiaceae species presented both increased germination and seedling growth on ant-nest soils.
Conclusions
Myrmecochory is a relevant seed dispersal mode in the Caatinga ecosystem, and is particularly frequent among Euphorbiaceae trees and shrubs. The fact that seeds reach micro-sites suitable for establishment (ant nests) supports the directed dispersal hypothesis as a possible force favouring myrmecochory in this ecosystem. Ecosystems with a high frequency of myrmecochorous plants appear not to be restricted to regions of nutrient-impoverished soil or to fire-prone regions.
Key words: Caatinga, Euphorbiaceae, directed dispersal hypothesis, myrmecochory, north-east Brazil, seed dispersal, seed germination, seedling growth
INTRODUCTION
Myrmecochory involves plants bearing elaiosomes (van der Pijl, 1982), which encompasses a large set of seed appendages originating from seed or fruit tissue including those referred to in the literature as the aril, ariloide and caruncle (see review by Gorb and Gorb, 2003). Ants are attracted to the elaiosomes, which are used as a handle for seed transportation (Beattie, 1985). During transport, some seeds are lost by the ants and potentially germinate and establish in new sites (Horvitz, 1981; Beattie, 1985). The seeds that reach the nests have their elaiosomes eaten by the ants and are then discarded, normally intact, into nest galleries, or outside into the refuse dump, or in the vicinity of nest entrances (Horvitz and Beattie, 1980; O'Dowd and Hay, 1980).
Over 3000 angiosperm species from more than 80 families have been classified as myrmecochores and they inhabit different ecosystems throughout the world (see reviews by Beattie, 1983; Cowling et al., 1994). Myrmecochory is especially common among herbs of the temperate forests in the Northern Hemisphere (Beattie and Culver, 1981) and woody shrubs in dry sclerophyll vegetation growing in the unfertile soils of Australia, South Africa and the Mediterranean region (Berg, 1975; Milewski and Bond, 1982; Bond and Slingsby, 1983; Westoby et al., 1991). According to the ‘nutrient enrichment’ theory (sensu Beattie, 1985), myrmecochory has evolved more frequently in ecosystems with poor soils because ants deposit the seeds in or around their nests, which are nutrient-enriched micro-sites and therefore more suitable for seed germination than random sites. The evolution of myrmecochory has also been associated with reduced parent–offspring conflict via distance dispersal (Andersen, 1988; Higashi et al., 1989); fire- and predator-avoidance via seed burial by ants (Heithaus, 1981; Hughes and Westoby, 1992); and the production of potassium-poor diaspores in environments with nutrient limitation (Westoby et al., 1991; Cowling et al., 1994). All these mechanisms have received some support in the literature and their relative importance is believed to be variable among ecosystems and plant life forms (see reviews by Gorb and Gorb, 2003; Giladi, 2006).
The Caatinga ecosystem consists of patches of seasonally dry forest (sensu Mooney et al., 1995; Pennington et al., 2000) and sclerophyll vegetation that covers a semi-arid region of 730 000 km2 in north-east Brazil (IBGE, 1985; Sampaio, 1995). Such variation in vegetation structure is conditioned by topography, human disturbance and, most importantly, by a combination of average annual rainfall and soil attributes (Sampaio, 1995; Prado, 2003). Rainfall ranges from 240 to 900 mm per year throughout the Caatinga and soils range from moderately fertile and shallow to impoverished deep sandy soils at both landscape and regional level (IBGE, 1985; Sampaio, 1995). The Caatinga ecosystem therefore presents some of the attributes found in those ecosystems showing high scores of myrmecochory, but like other semi-arid ecosystems of South America, this seed dispersal mode remains scarcely investigated (Leal, 2003a). By addressing myrmecochory in the Caatinga it is possible to test the generality of current ideas regarding both the role played by this dispersal mode and its selective forces.
This study assesses the importance of seed dispersal by ants in the Caatinga and investigates the benefits of true myrmecochory in terms of seed removal and fate, seed germination, and seedling growth. A detailed account is given of the natural history of myrmecochory in this ecosystem. First, the assemblage of ant species involved in seed dispersal is described and details of ant behaviour toward seeds are presented. Second, manipulation and dispersal by ants, the fate of myrmecochorous seeds for a set of Euphorbiaceae species and some properties of the soil from ant nests are documented. Finally, the benefits from seed dispersal by ants are addressed in terms of seed germination and seedling growth. The role of myrmecochory in the Caatinga is speculated, the main hypotheses concerning the benefits from seed dispersal by ants are discussed, and possible selective forces favouring myrmecochory in this semi-arid ecosystem are identified.
MATERIALS AND METHODS
Study site
The study was carried out in the region of Xingó (Fig. 1), located along the valley of the São Francisco river, north-east Brazil. The region stretches over sedimentary basins, mountains, plateaus and ravines that surround the São Francisco river (IBGE, 1985). Predominant soils are lithosols, cambisols, eutrophic podzols, non-calcic brown soils and planosols (Sampaio, 1995). The climate is typically semi-arid with a marked annual dry season (<60 mm per month) lasting 7–11 months. Annual rainfall is around 500 mm, with the wettest period between April and August, but long-lasting (2–3 years) periods of severe drought are frequent (Sampaio, 1995). The vegetation is a mosaic of physiognomic types ranging from patches of seasonally tropical dry forests (sensu Pennington et al., 2000) to scrub vegetation (Sampaio, 1995; Prado, 2003). The Xingó region houses over 120 woody plant species, and the Leguminosae, Euphorbiaceae and Cactaceae are the most species-rich plant families (Silva et al., 2003). Sixty-one ant species have been recorded in the Xingó region, and the Myrmicinae, Formicinae and Dolichoderinae are the richest ant subfamilies (Leal, 2003b). Detailed information on the flora, fauna, ecological interactions and conservation status of the Xingó region is available in Leal et al. (2003).
Fig. 1.
The area covered by the Caatinga ecosystem (A), and location of the Xingó region, north-east Brazil (B).
Ant–diaspore interactions
In order to characterize both the plant and the ant assemblages involved in ant–seed interactions at the study site a pool of 70 plots of 0·1 ha (10 × 100 m) were used. These plots were haphazardly located within a 2500-km2 area covered by a mosaic of vegetation stretching over flat lands, canyons, ravines and outcrops. Plots housed together 101 shrub and tree species (Silva et al., 2003), comprising approx. 10 % of the whole woody flora of the Caatinga ecosystem (MMA, 2002). Plant vouchers are deposited at the UFP Herbarium of the Universidade Federal de Pernambuco, Brazil (vouchers 30·444–30·875). From March 1999 to December 2000 each plot was surveyed once during a 6-h period for any diaspore (i.e. fruits or seeds) that had been manipulated by ants on the ground. Whenever ants were found exploiting a diaspore (i.e. contacting the surface of the diaspore for the apparent purpose of collecting liquids, or removing portions of it), the interaction was registered and both ant and plant species were identified. Additional data included the number of ant individuals and their behaviour toward the diaspore (see Leal and Oliveira, 1998; Pizo and Oliveira, 1998).
Manipulation, dispersal and fate of myrmecochorous seeds
In order to characterize seed manipulation and dispersal by ants, experiments were carried out using caruncle-bearing seeds of seven tree and shrub species of Euphorbiaceae that have been recorded in the study site: Cnidoscolus quercifolius, Cnidoscolus urens, Croton campestris, Jatropha gossypifolia, Jatropha mollissima, Jatropha ribifolia and Manihot glaziovii. Caruncle-bearing Euphorbiaceae species represent the largest group of true myrmecochores in the Caatinga (Leal, 2003a). A diplochorous dispersal system, in which autochory (ballistic discharge of seeds from explosively dehiscent capsules) is followed by myrmecochory, is a common feature in many euphorbs, particularly among Neotropical species (Webster, 1994; Passos and Ferreira, 1996).
A 100-m transect at the Fazenda Miramar was randomly established, where four of the 70, 0·1-ha plots were situated. One hundred seeds of each species were measured and placed along a transect on the ground in groups of ten, at intervals of 10 m to permit seed removal by different ant colonies (cf. Leal and Oliveira, 1998). Seeds were set up at 0700 h and checked at 2-h intervals, from 0800 to 1800 h. Ant behaviour toward seeds was recorded and seed-carrying ants were followed until they entered their nests or disappeared in the leaf litter. Seeds were assigned to two non-exclusive categories: (1) cleaned – in which case ants removed part of, or the whole, elaiosome; and (2) dispersed – when seeds were transported. In the latter case, the distance of seed removal was measured. The information obtained from this transect-based experiment was used to analyse the relationship between seed length and (1) percentage of seeds cleaned, (2) percentage of seeds removed and (3) average distance of seed removal. Additionally, to quantify ballistic dispersal, the distance between the trunk of fruiting individuals and seeds with the elaiosome below them early in the morning was measured to ensure that seeds were not previously removed by the ants. Ten individuals per plant species and 30 seeds per species (n = 210 seeds) were used. Although the plant individuals were at least 10 m apart it was not possible to guarantee that seeds at the tail of the ballistic dispersal distribution were recorded.
To test the hypothesis that the elaiosome attracts ants and facilitates seed transportation, 100 elaiosome-bearing seeds and 100 seeds that had their elaiosome experimentally removed (elaiosome-free seeds) were placed along a 1000-m transect, one transect for each of the seven Euphorbiaceae species used. Seeds were arranged in pairs (with and without elaiosome) that were set 10 m apart in order to guarantee independent discovery events by different ant colonies. Seeds were set up at 0700 h and checked after 24 h to count the number of seeds with and without the elaiosome that had been removed. Removal experiments were performed on different occasions from May 2000 to February 2001; the general procedure follows that given in Leal and Oliveira (1998).
Seed germination and seedling growth
In order to examine whether elaiosome removal by ants improves the germination percentage of myrmecochorous seeds, germination tests were conducted in the greenhouse of the Universidade Federal de Pernambuco during June and November 2001. Again, the caruncle-bearing seeds from the seven Euphorbiaceae species found at the study site were used. Seeds of Cnidoscolus quercifolius (n = 140), C. urens (192), Croton campestris (200), Jatropha gossypifolia (192), J. mollissima (140), J. ribifolia (192) and Manihot glaziovii (140) were divided into two groups of equal sample sizes: intact seeds, and seeds that had their elaiosome removed by ants. For this second treatment seeds in which the elaiosome had been experimentally removed were again used in order to accomplish minimum sample sizes (see Horvitz, 1981). Germination tests were performed in the greenhouse under ambient temperature and uniform light conditions (Pizo and Oliveira, 1998). Seeds in each category were placed in separate plastic boxes (40 × 40 cm), buried 1 cm into soil from the study sites and 5 cm apart from each other. Germination boxes were regularly watered, and checked for germination events at 3-d intervals over a period of 4 months.
The hypothesis that germination would be increased in soils from ant nests was tested for the same Euphorbiaceae species. Seeds without elaiosome of Cnidoscolus quercifolius (80 seeds), C. urens (120), Croton campestris (160), Jatropha gossypifolia (120), J. mollissima (80), J. ribifolia (120) and Manihot glaziovii (80) were divided into two groups of equal sample sizes, and planted in soils of different origins: one group in ant-nest soils (i.e. a mix of soils collected from nests of Camponotus blandus, Ectatomma muticum, Dorymyrmex brunneus, Pheidole spp., Solenopsis spp. and Trachymyrmex spp.), and the second group in soil randomly collected at sites away from ant nests. Germination tests in the greenhouse followed the procedure described above. The C. quercifolius seedlings grew unexpectedly rapidly in terms of stem diameter, and therefore measurements of this variable were opportunistically included to test whether growth rate is higher in ant-nest vs. nest-free soil (general procedure follows those of Leal and Oliveira, 1998; Pizo and Oliveira, 1998).
In order to verify if ant nests modify soil properties, which may affect seed germination and seedling growth, ten 500-g soil samples were collected from nests of Camponotus blandus, Dorymyrmex brunneus, Dinoponera quadriceps, Pheidole spp., Solenopsis spp. and Trachymyrmex spp. (three different nests were collected per sample totalling 30 nests). Soil samples were not analysed separately by ant species owing to low availability of soils for several species. As a control, a soil sample approx. 2 m apart from each ant nest was collected (n = 10 control samples). Samples were air-dried and soils were analysed for micro- and macronutrients, nitrogen, carbon, total organic matter and granulometry according to EMBRAPA (1997). Finally, soil penetrability in refuse piles of nests and random adjacent spots was evaluated (n = 15 per treatment). At each location, a sharpened wire stake (30 cm long) was released from the inside top of a 1·5-m-high plastic PVC tube. The depth reached by the stake into the ground was the estimate of soil penetrability for that location (sensu Passos and Oliveira, 2004).
Statistical analysis
Differences between ballistic seed dispersal and seed dispersal by ants were compared via Mann–Whitney tests and the average rates of seed cleaning vs. seed removal was compared by using a Wilkoxon test. Simple linear regressions were used to analyse the relationships between seed size and (1) number of seeds cleaned, (2) number of seeds removed and (3) removal distances. Differences in (1) removal rate of seeds with and without elaiosome, (2) seed germination with and without elaiosome and (3) seed germination in ant-nest soil vs. random Caatinga soil were analysed with chi-square tests. Average stem diameter of Cnidoscolus quercifolius seedlings growing in ant nest soil vs. Caatinga soil was compared with a t test. Both chemical and physical soil variables were compared by t and Wilcoxon tests. Normality was verified with the Lilliefors test. The statistical procedure follows Sokal and Rohlf (1995). Analyses were carried out using BioEstat 2·0 (Ayres et al., 2000).
RESULTS
Ant–diaspore interactions
A single inspection of 70, 1-ha plots in the study area revealed 577 ant–diaspore interactions on the ground involving 27 tree and shrub species and 18 ant species. This means that 26·7 % of the woody flora recorded in these plots had seeds manipulated by ants during the study. However, we did not observe ants manipulating seeds of herbs, epiphytes and climbers, even though this study covered the wet season, when most annual herbs in the Caatinga are fruiting.
Plant species manipulated by ants comprised two groups of species (Table 1). One group was formed by 14 species with elaiosome-free or non-myrmecochorous seeds (i.e. with seeds not specially adapted for dispersal by ants) from the Anacardiaceae, Annonaceae, Boraginaceae, Cactaceae and Sapotaceae families, which bear fleshy fruits such as drupes and berries. The second group consisted of 13 elaiosome-bearing seed species, including seeds bearing caruncles, true arils and sarcotestas (sensu Gorb and Gorb, 2003). True myrmecochory was thus restricted to 12·8 % of the flora recorded in our plots and caruncle-bearing seeds referred exclusively to Euphorbiaceae species. In fact, the Euphorbiaceae presented the highest number of species with seeds manipulated by ants (11 species belonging to five genera), and seeds of Jatropha mollissima and Cnidoscolus quercifolius attracted most ant species (ten species). Seed species manipulated by ants were 1·2–28 mm in length but dispersal services provided by ants varied among plant species. More precisely, 66·7 % of the seed species were both cleaned and removed (3–14 mm in size), 25·9 % were only removed (1·2–5 mm in size) and two (Annona coriacea and Spondias tuberosa) were merely cleaned (28 mm in size).
Table 1.
Plant species with diaspores manipulated by ants on the ground in the Xingó region, north-east Brazil
| Plant family and species | Fruit type | Fruit length (mm) | Seed length (mm) |
Elaiosome type | Seed dispersal service | |
|---|---|---|---|---|---|---|
| Cleaning | Removal | |||||
| Anacardiaceae | ||||||
| 1. Myracrodruon urundeuva Allemão | drupe | 14 | 4 | – | X | |
| 2. Schinus terebinthifolius Raddi | drupe | 6 | 5 | – | X | |
| 3. Spondias tuberosa Arruda | drupe | 48 | 28 | – | X | |
| Anonaceae | – | |||||
| 4. Annona coriacea Mart. | symcarp | 130 | 28 | – | X | |
| Boraginaceae | – | |||||
| 5. Cordia globosa (Jacq.) H.B.K. | drupe | 5 | 3 | – | X | X |
| 6. Cordia leucocephala Moric. | drupe | 5 | 3 | – | X | X |
| Cactaceae | ||||||
| 7. Cereus jamacaru DC. | berry | 50 | 1·5 | – | X | |
| 8. Melocactus bahiensis (Br. Et Rose) Werderm | berry | 15 | 1·2 | – | X | |
| 9. Opuntia palmadora Britton & Rose | berry | 30 | 2 | – | X | |
| 10. Pilosocereus gounellei (F. A. C. Weber) Byles & G. D. Rowley | berry | 50 | 1·5 | – | X | |
| 11. Pilosocereus pachycladus F. Ritter | berry | 45 | 1·3 | – | X | |
| Capparaceae | ||||||
| 12. Capparis flexuosa L. | capsule | 50 | 10 | sarcotesta | X | X |
| Celastraceae | ||||||
| 13. Maytenus rigida Mart. | capsule | 8 | 5 | aril | X | X |
| Euphorbiaceae | ||||||
| 14. Cnidoscolus obtusifolius Pohl. | capsule | 24 | 12·5 | caruncle | X | X |
| 15. Cnidoscolus quercifolius Pohl. | capsule | 25 | 13·5 | caruncle | X | X |
| 16. Cnidoscolus urens (L.) Arthur | capsule | 16 | 7·2 | caruncle | X | X |
| 17. Croton campestris A.St.-Hil. | capsule | 10 | 4·3 | caruncle | X | X |
| 18. Croton micans Sw. | capsule | 10 | 4 | caruncle | X | X |
| 19. Croton sonderianus Müll. Arg. | capsule | 10 | 5 | caruncle | X | X |
| 20. Jatropha gossypifolia L. | capsule | 14 | 7·3 | caruncle | X | X |
| 21. Jatropha mollissima Pohl. Ex. Baill. | capsule | 25 | 12 | caruncle | X | X |
| 22. Jatropha mutabilis L. | capsule | 24 | 12 | caruncle | X | X |
| 23. Jatropha ribifolia (Pohl) Baill. | capsule | 13 | 7 | caruncle | X | X |
| 24. Manihot glaziovii Müll. Arg. | capsule | 22 | 11 | caruncle | X | X |
| Malpighiaceae | ||||||
| 25. Byrsonima vaccinifolia A. Juss. | drupe | 10 | 7 | – | X | X |
| Ramnaceae | ||||||
| 26. Zizyphus joazeiro Mart. | drupe | 18 | 14 | – | X | X |
| Sapotaceae | ||||||
| 27. Bumelia sartorum Mart. | berry | 13 | 10 | – | X | X |
Fruit type, fruit and seed size according van Roosmalen (1985), Andrade-Lima (1989), Lorenzi (1998) and Barroso et al. (1999). Elaiosome type sensu Gorb and Gorb (2003).
Ants of the subfamily Myrmicinae were the most commonly involved in ant–seed interactions and accounted for more than 63 % (364/577) of the records (Table 2). Four species of Pheidole accounted for 31 % (182/577) of the records. Other frequent ant species were Dorymyrmex spp. (94 records), Ectatomma muticum (48) and Trachymyrmex sp. 1 (33). Ants manipulated diaspores on the ground and were never observed climbing the vegetation to access fruits or seeds. Diaspores were manipulated by different ant species in different ways: (1) individual diaspore transport to nests was recorded for Camponotus, Ectatomma, Dinoponera and Odontomachus species; (2) recruitment of nest-mates and cooperative transport of diaspores or parts of elaiosome/fruit pulp to nests as in Cyphomyrmex, Crematogaster, Dorymyrmex, Pheidole and Trachymyrmex species; and (3) recruitment of nest-mates and removal of elaiosome/fruit pulp (i.e. no seed transport) as typical for Solenopsis and rarely for Pheidole species. Manipulated diaspores reached the ground by being passively dropped by parent plants or by vertebrate fugivores, or via ballistic dispersal.
Table 2.
Ant species recorded manipulating diaspores on the ground in the Xingó region, north-east Brazil
| Ant subfamily and species | No. of plant species used (no. of records) | Ant behaviour | Plant species |
|---|---|---|---|
| Dolichoderinae | |||
| 1. Dorymyrmex bruneus (Forel) | 11 (41) | RT | 1, 6, 8, 13, 14, 15, 16, 17, 19, 25 |
| 2. Dorymyrmex thoracicus (Gallardo) | 17 (53) | RT | 2, 3, 6, 7, 10, 11, 12, 13, 15, 16, 17, 20, 21, 22, 24, 26, 27 |
| Ectatomminae | |||
| 3. Ectatomma muticum (Mayr) | 13 (48) | IT | 4, 6, 8, 9, 10, 12, 13, 14, 15, 16, 17, 21, 24, 25 |
| Formicinae | |||
| 4. Camponotus blandus (Fr. Smith) | 10 (20) | IT | 9, 10, 11, 12, 13, 15, 20, 21, 25, 26 |
| Myrmicinae | |||
| 5. Crematogaster sp. 1 | 9 (33) | RT | 1, 2, 3, 6, 7, 11, 21, 27 |
| 6. Crematogaster sp. 2 | 7 (17) | RT | 1, 8, 16, 17, 22, 26, 27 |
| 7. Cyphomyrmex rimosus (Spinola) | 4 (11) | RT | 5, 6, 15, 17, 19 |
| 8. Pheidole sp. 1 | 15 (54) | RT | 2, 3, 6, 7, 9, 11, 13, 15, 17, 18, 19, 20, 21, 22, 26, 27 |
| 9. Pheidole sp. 2 | 11 (38) | RT | 2, 3, 6, 10, 11, 12, 17, 18, 20, 21, 23 |
| 10. Pheidole sp. 3 | 16 (67) | RT | 2, 3, 7, 8, 10, 11, 13, 14, 15, 16, 19, 21, 23, 24, 24, 26 |
| 11. Pheidole sp. 5 | 6 (23) | RT | 3, 10, 16, 17, 18, 21 |
| 12. Solenopsis sp. 1 | 8 (30) | RC | 2, 3, 6, 7, 8, 9, 11, 15, 17 |
| 13. Solenopsis sp. 3 | 5 (21) | RC | 11, 15, 16, 20, 21 |
| 14. Solenopsis sp. 5 | 5 (27) | RC | 3, 11, 20, 21, 23 |
| 15. Trachymyrmex sp. 1 | 9 (33) | RT | 2, 6, 13, 16, 20, 21, 23, 26 |
| 16. Trachymyrmex sp. 2 | 4 (10) | RT | 1, 4, 6, 21, 22 |
| Ponerinae | |||
| 17. Odontomachus haematodus (Linnaeus) | 10 (22) | IT | 1, 4, 13, 14, 15, 16, 17, 21, 24, 25 |
| 18. Dinoponera quadriceps (Kempf) | 8 (29) | IT | 1, 4, 7, 15, 17, 22, 23, 27 |
Ant behaviour: IT = individual transport of diaspores to nest sites; RT = recruitment of nest-mates and transport of diaspores or parts of it to the nest; and RC = recruitment of workers and removal of the fruit pulp or seed elaiosome on the spot (without seed transport). Plant species according to the numbers given in Table 1.
Manipulation, dispersal and fate of myrmecochorous seeds
From the plant perspective, two interaction categories emerged. Seed cleaning occurred particularly when Solenopsis ants were involved in the interaction, but this behaviour was also observed in some species of Pheidole. The rate of seed cleaning varied from 7 % in Croton campestris to 43 % in Jatropha mollissima (Table 3). By contrast, a large array of ants (15 species) were involved in seed transportation, with Camponotus blandus, Ectatomma muticum, Odontomachus haematodus, Dorymyrmex spp. and Pheidole spp. being the most frequent. Seed removal varied from 38 % in Manihot glaziovii to 85 % in Croton campestris, and the average rate of seed removal (66 %) was almost three-fold higher than that of seed cleaning (24 %; Z = 2·36, n = 7, P = 0·018). Eighty-three per cent (±10·7 %) of removed seeds were transported to ant nests, whereas 10·9 ± 7·9 % of seeds were dropped on the litter during seed transportation (Table 3). Ants removed the elaiosome and maintained the cleaned seeds inside the nest or deposited them in the vicinity of the nest entrance, including refuse piles and nest mounds. Finally, dispersal distances by ants varied from a few centimetres to more than 11 m, and the mean dispersal distance of ant-dispersed seeds ranged from 409·2 to 538 cm among the seven species. These values exceeded by far those obtained from ballistic dispersal (Table 4).
Table 3.
Ant seed cleaning and removal of seven Euphorbiaceae species in the Xingó region, north-east Brazil
| Plant species | Percentage cleaned seeds | Percentage removed seeds | Percentage removed seeds in deposition sites |
Ant species | ||
|---|---|---|---|---|---|---|
| Ant nest | Litter | Unknown | ||||
| Cnidoscolus quercifolius | 33 | 49 | 89·7 | 10·3 | 0·0 | 2, 5, 9, 10, 14, 15, 17 |
| Cnidoscolus urens | 32 | 84 | 88·1 | 9·5 | 2·4 | 1, 3, 8, 9, 10, 13, 16 |
| Croton campestris | 7 | 85 | 90·6 | 9·4 | 0·0 | 2, 3, 8, 10, 11, 12, 13, 17 |
| Jatropha gossypifolia | 10 | 76 | 73·7 | 26·3 | 0·0 | 3, 8, 9, 13, 14, 16 |
| Jatropha mollissima | 35 | 55 | 92·7 | 7·3 | 0·0 | 2, 3, 5, 8, 10, 13, 14 |
| Jatropha ribifolia | 43 | 79 | 63·3 | 13·9 | 27·8 | 2, 3, 6, 8, 10, 13, 14, 16 |
| Manihot glaziovii | 15 | 38 | 84·2 | 0·0 | 15·8 | 3, 10, 11, 12, 13, 16 |
| Mean ± s.d. | 24 ± 14·04 | 66 ± 19·8 | 83·1 ± 10·7 | 10·9 ± 7·9 | 2·0 ± 3·3 | |
Ant species numbers as in Table 1 (n = 100 seeds per plant species). Removed seed category may include some cleaned seeds.
Table 4.
Average distances of Euphorbiaceae seeds from ballistic and ant dispersion (mean ± s.d.) in the Xingó region, north-east Brazil (n = 30 seeds per species for ballistic dispersal)
| Species | Ballistic dispersal distance (cm) | Ant dispersal distance (cm) | Mann–Whitney test | Maximum ant dispersal distance (cm) |
|---|---|---|---|---|
| Cnidoscolus quercifolius | 44·2 ± 36·7 | 510·0 ± 290·3 | P < 0·001 | 880 |
| Cnidoscolus urens | 12·8 ± 8·1 | 487·4 ± 220·9 | P < 0·0001 | 720 |
| Croton campestris | 13·6 ± 9·9 | 418·3 ± 317·4 | P < 0·0001 | 1130 |
| Jatropha gossypifolia | 14·9 ± 8·7 | 409·2 ± 207·2 | P < 0·0001 | 822 |
| Jatropha mollissima | 38·8 ± 26·6 | 538·5 ± 274·05 | P < 0·001 | 968 |
| Jatropha ribifolia | 15·0 ± 8·5 | 487·0 ± 262·9 | P < 0·0001 | 849 |
| Manihot glaziovii | 32·07 ± 19·3 | 522·0 ± 257·9 | P < 0·001 | 1064 |
See Table 3 for the number of ant-removed seeds per plant species.
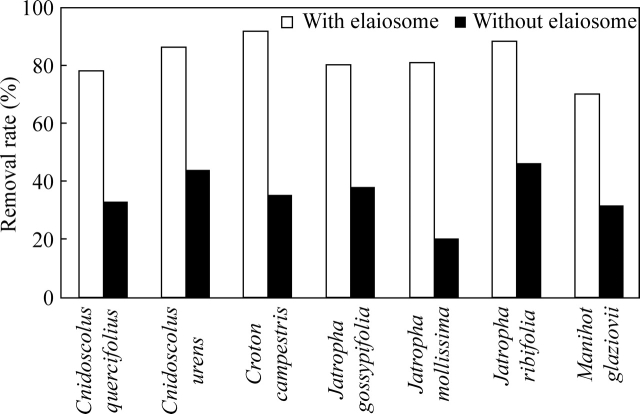
Seed cleaning was not affected by seed size (Fig. 2A). By contrast, seed removal was negatively influenced by seed size (y = –51·05x + 111·58, r2 = 0·77, F = 16·95, P = 0·0098), which accounted for almost 80 % of the variation in the proportion of seeds carried by ants (Fig. 2B). The removal distance was also positively influenced by seed size (y = 120·03x + 374·63, r2 = 0·62, F = 8·26, P = 0·0345), as larger dispersal distances were observed among larger seeds (Fig. 2C). Dispersal of large seeds was carried out preferentially by large ants (e.g. Ectatomma, Dinoponera and Odontomachus species) that foraged individually and carried diaspores to nests, and subsequently discarded intact seed on the ground (high-quality dispersers sensu Giladi, 2006). As predicted, ants preferentially removed elaiosome-bearing seeds and the removal percentage was twice as high as that among seeds without elaiosomes (Cnidosculos quercifolius: χ2 = 40·99, d.f. = 1, P < 0·0001; C. urens: χ2 = 38·77, d.f. = 1, P < 0·0001; Croton campestris: χ2 = 70·09, d.f. = 1, P < 0·0001; Jatropha gossyipfolia: χ2 = 36·46, d.f. = 1, P < 0·0001; J. mollissima: χ2 = 74·43, d.f. = 1, P < 0·0001; J. ribifolia: χ2 = 39·89, d.f. = 1, P < 0·0001; and Manihot glaziovii: χ2 = 30·42, d.f. = 1, P < 0·0001; Fig. 3). It appeared that the elaiosome served as a handle, without which the hard and smooth seed coat of the Euphorbiaceae seeds would not allow seed transport by ants.
Fig. 2.
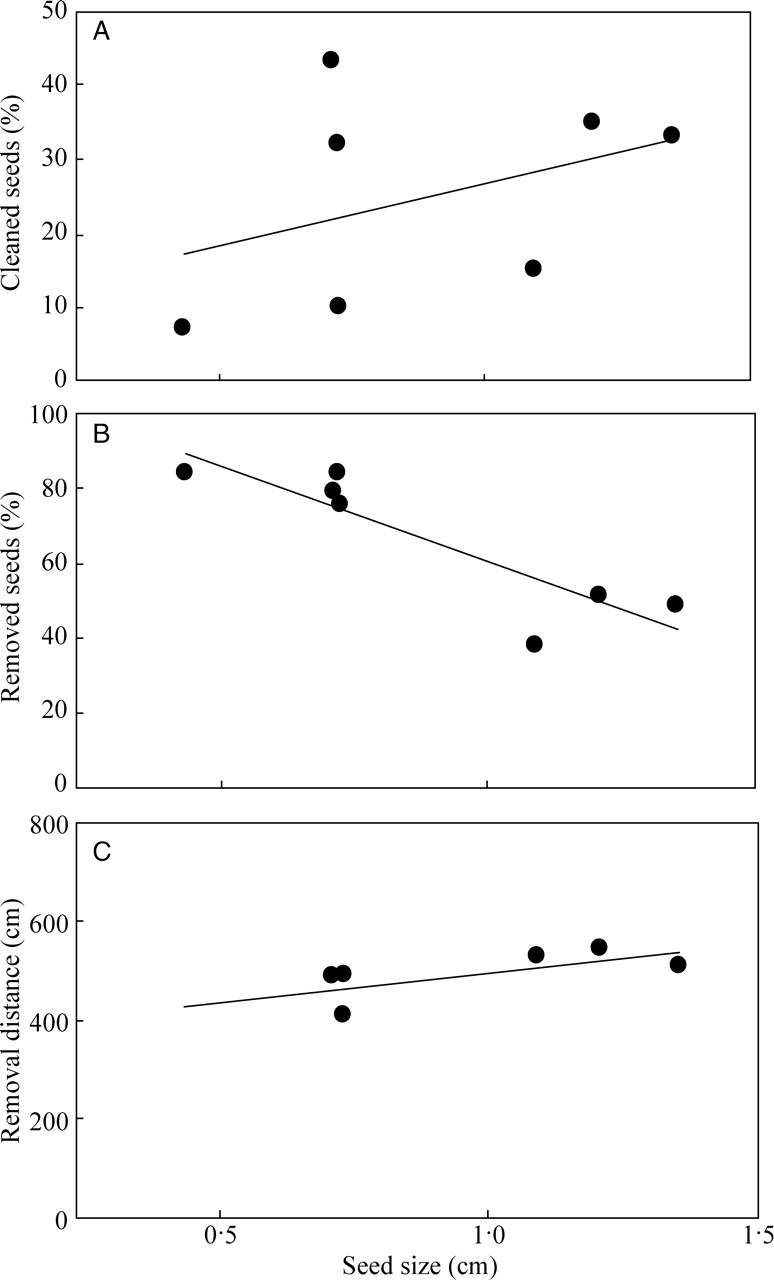
Relationships between seed size of seven Euphorbiaceae species and the percentage of cleaned seeds (A), percentage of removed seeds (B) and average distance of seed removal (C) in the Xingó region, north-east Brazil (n = 100 seeds per plant species; each point represents one plant species).
Fig. 3.
Seed removal rate (%) of seven Euphorbiaceae species comparing seeds with and without elaiosome. Within-species comparisons; chi-square tests; P < 0·0001; n = 100 seeds per species per treatment.
Seed germination and seedling growth
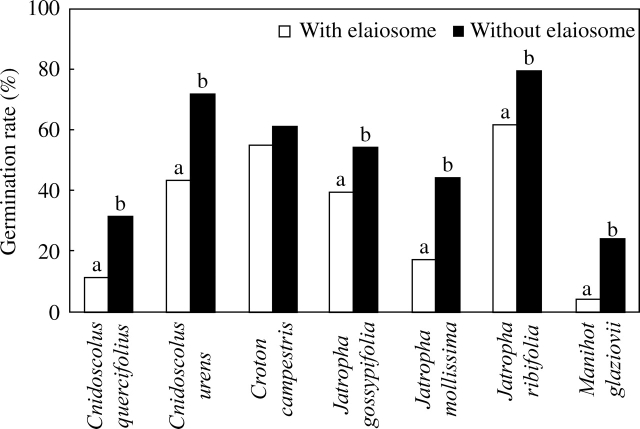
Experiments in the greenhouse revealed that elaiosome removal increased seed germination by at least 30 %. More specifically, differences in terms of germination percentage were significant for Cnidoscolus quercifolius (χ2 = 8·31, d.f. = 1, P < 0·01), C. urens (χ2 = 15·57, d.f. = 1, P < 0·001), Jatropha gossyipfolia (χ2 = 4·10, d.f. = 1, P < 0·05), J. mollissima (χ2 = 12·12, d.f. = 1, P < 0·001), J. ribifolia (χ2 = 7·21, d.f. = 1, P < 0·01) and Manihot glaziovii (χ2 = 10·55, d.f. = 1, P < 0·05), but not for Croton campestris (Fig. 4).
Fig. 4.
Seed germination rate (%) of seven Euphorbiaceae species comparing seeds with vs. without elaiosome. Bars with different letters are significantly different (within-species comparisons; chi-square tests; P < 0·05; n per treatment: Cnidoscolus quercifolius = 70, Cnidoscolus urens = 96, Croton campestris = 100, Jatropha gossypifolia = 96, Jatropha mollissima = 70, Jatropha ribifolia = 96 and Manihot glaziovii = 70).
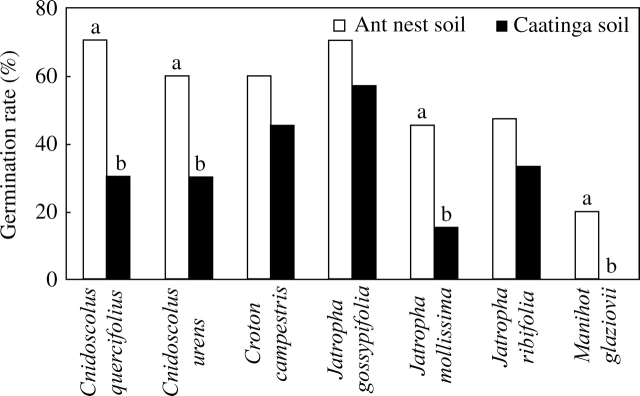
The results from the germination experiments were consistent with the hypothesis of increased germination for seeds on ant nests as compared with random sites. Seeds on ant-nest soil germinated at significantly higher levels (at least 50 % higher) in the case of Cnidoscolus quercifolius (χ2 = 6·41, d.f. = 1, P < 0·05), C. urens (χ2 = 5·46, d.f. = 1, P < 0·05), Jatropha mollissima (χ2 = 4·28, d.f. = 1, P < 0·05) and Manihot glaziovii (χ2 = 4·44, d.f. = 1, P < 0·05; Fig. 5). The remaining seed species showed similar, although no significant, trends. In the case of Cnidoscolus quercifolius a higher germination in nest soil was followed by an increased growth rate of the corresponding seedlings, as indicated by their stem diameter: 8·08 ± 1·01 mm in ant-nest soil vs. 4·95 ± 1·07 mm in nest-free soil (t = 2·57, d.f. = 5, P < 0·004). Soil from ant nests and random spots had similar nutrient contents with the exception of calcium and magnesium (higher at ant nests). However, ant nests had higher cation exchange capacity (CEC), sum of cations and clay content (Table 5). Soil penetrability was three times higher in ant nests than in random sites.
Fig. 5.
Seed germination rate (%) of seven Euphorbiaceae species comparing seeds in soil samples taken from ant nest sites vs. random Caatinga sites. Bars with different letters are significantly different (within-species comparisons; chi-square tests; P < 0·05; n per treatment: Cnidoscolus quercifolius = 40, Cnidoscolus urens = 60, Croton campestris = 80, Jatropha gossypifolia = 60, Jatropha mollissima = 40, Jatropha ribifolia = 60 and Manihot glaziovii = 40).
Table 5.
Soil variables (mean ± s.d.) from ant nests (n = 10) and randon spots (n = 10) with their respective units in the Xingó region, north-east Brazil
| Soil variables | Units | Ant-nest soil | Control soil | Statistical results |
|---|---|---|---|---|
| Nitrogen | g kg−1 | 0·7 ± 0·5 | 0·7 ± 0·2 | t = –0·18, gl = 9, P = 0·85 |
| Carbon | g kg−1 | 9·9 ± 6·9 | 9·0 ± 2·5 | t = 0·41, gl = 9, P = 0·69 |
| Phosphorus | mg kg−1 | 85·6 ± 59·0 | 77·5 ± 80·8 | Z = 0·51, T = 23, P = 0·61 |
| Potassium | cmol kg−1 | 1·1 ± 0·8 | 0·9 ± 0·3 | Z = 0·18, T = 21, P = 0·86 |
| Calcium | cmol kg−1 | 9·4 ± 3·0 | 7·7 ± 1·8 | t = 2·32, gl = 9, P = 0·05 |
| Magnesium | cmol kg−1 | 4·2 ± 1·7 | 2·1 ± 1·1 | Z = 2·29, T = 5, P = 0·02 |
| Zinc | ppm | 1·4 ± 0·3 | 1·4 ± 0·3 | t = 0·19, gl = 9, P = 0·85 |
| Manganese | ppm | 78·2 ± 24·4 | 86·5 ± 7·4 | Z = 1·17, T = 16, P = 0·24 |
| Copper | ppm | 1·0 ± 0·3 | 0·9 ± 0·3 | t = 0·92, gl = 9, P = 0·38 |
| Iron | ppm | 88·0 ± 19·9 | 96·0 ± 15·0 | t = 0·85, gl = 9, P = 0·42 |
| Organic matter | g kg−1 | 15·4 ± 11·9 | 15·6 ± 4·4 | Z = 0·31, T = 25, P = 0·76 |
| CEC | cmol kg−1 | 16·1 ± 3·7 | 12·7 ± 2·7 | Z = 1·99, T = 8, P = 0·04 |
| Sum of cations | cmol kg−1 | 15·3 ± 3·7 | 11·9 ± 2·5 | Z = 1·99, T = 8, P = 0·04 |
| pH | 6·9 ± 0·2 | 6·9 ± 0·2 | t = 0·82, gl = 9, P = 0·43 | |
| Coarse sand | g kg−1 | 336·1 ± 69·3 | 344·8 ± 82·4 | t = 0·49, gl = 9, P = 0·63 |
| Fine sand | g kg−1 | 309·2 ± 31·7 | 328·4 ± 30·3 | t = 1·72, gl = 9, P = 0·12 |
| Silt | g kg−1 | 171·9 ± 47·2 | 165·7 ± 41·7 | t = 0·54, gl = 9, P = 0·60 |
| Clay | g kg−1 | 178·0 ± 25·7 | 157·0 ± 40·7 | t = 2·40, gl = 9, P = 0·04 |
| Penetrability | cm | 3·7 ± 1·4 | 1·0 ± 0·5 | t = 6·98, gl = 14, P < 0·0001 |
Significant diferences are highlighted in bold type.
DISCUSSION
The results presented here indicate that seed dispersal by ants involves approximately one-quarter of the woody flora inhabiting the Xingó region, but true myrmecochory is most frequent among shrubs and trees of the Euphorbiaceae. The Caatinga houses approx. 930 vascular plant species (MMA, 2002), most of which are primarily dispersed by abiotic means or by vertebrates (Machado et al., 1997; Tabarelli et al., 2003). The Euphorbiaceae is one of the richest families in this ecosystem in terms of woody plant species (Rodal and Melo, 1996), and at least 73 species, namely trees and shrubs from the genera Cnidoscolus, Croton, Jatropha and Manihot, can be assigned as myrmecochores given that their caruncle-bearing seeds are apparently exclusively dispersed by ants (see Barroso et al., 1999, for dispersal syndrome within these genera). Moreover, the Caatinga houses a large pool of primarily vertebrate-dispersed plant species (non-myrmecochorous diaspores), which are secondarily dispersed by ants after the seeds reach the ground, e.g. the Anacardiaceae, Annonaceae, Boraginaceae and Cactaceae species found in the study site. All these findings suggest that ants play a relevant role as seed dispersers in the Caatinga.
Plants are expected to obtain several advantages from myrmecochory. According to a recent review by Giladi (2006) advantages can be assigned into five categories or hypotheses: directed dispersal, distance dispersal, predator avoidance, nutrient limitation and fire avoidance. In the scope of this paper the first two categories are more relevant as only a small fraction of the Caatinga is covered by nutrient-impoverished soils and natural fires are rare or absent (Rizzini, 1979; Prado, 2003). The directed dispersal hypothesis states that ants discard seeds at or near their nests, which represent nutrient-enriched sites that improve seed germination and seedling recruitment. These plant benefits have been recognized as the main explanation for the evolution of myrmecochory, particularly in the case of shrubs and trees (Beattie, 1985; Passos and Oliveira, 2002, 2004; Giladi, 2006).
The present results provide valuable information regarding the nature and the benefits involved in the directed dispersal hypothesis. At the study site, manipulation of caruncle-bearing Euphorbiaceae seeds by ants can be characterized by: (1) the high rates of seed removal, and seed deposition around nest entrances or inside ant nests; (2) the positive correlation between the rate of seed removal by ants and the presence of the elaiosome; and (3) the increased germination and better seedling growth in ant-nest soils. In contrast to other studies (Passos and Oliveira, 2002, 2004), no marked differences in terms of nutrient content in ant-nest soils were detected. However, this microhabitat may provide a deep, soft, moist substratum (see Table 5) that promotes increased seed germination and better seedling performance such as that found here, particularly in those patches covered by shallow and rocky soils (i.e. impenetrable spots).
The distance dispersal hypothesis assumes that seed dispersal reduces parent–offspring conflict and seedling competition beneath the parent plants (Giladi, 2006). In the experiments described here the distance of Euphorbiaceae seed dispersal by ants was greater than by the ballistic mode, and seeds on the tail of the dispersal curve were recorded over 11 m away from parents. Although ant-dispersed seeds have previously been reported 180 m away from parents (Whitney, 2002), seeds in the tail of curves are usually located only 10–15 m away (see Gómez and Espadaler, 1998). Additionally, the rate of seed removal was negatively correlated with seed size whereas removal distance was positively correlated with this variable. As previously reported in the literature, both the presence of an elaiosome and seed size affect diaspore attractiveness and determine which ants are physically able to lift and remove the seed from the spot (e.g. Mark and Olesen, 1996; Pizo and Oliveira, 2001; Passos and Oliveira, 2003). Given that the studied ant assemblage in the Caatinga is largely composed of small ants (68 % smaller than 5 mm; Leal, 2003b), the removal of large seeds was executed by a subset of the whole assemblage, large ant species such as Ectatomma muticum, Dinoponera quadriceps and Odontomachus haematodus. By contrast, small seeds faced higher rates of removal but for shorter distances by a more complete subset of the entire ant fauna. As Giladi (2006) has argued, myrmecochory can be expected to reduce parent–offspring conflict and seedling competition (i.e. dispersal distance hypothesis) if the range of seed dispersal by ants exceeds the spatial scale of parent–offspring conflicts. Although the spatial distribution of seedlings was not mapped, higher seed dispersal distance by ants compared with ballistic dispersal clearly indicates that seedlings from ant-dispersed seeds are expected neither to recruit beneath parents nor to compete with ballistically dispersed seeds. This is particularly valid for large seeds as they appear to be dispersed further than the small seeds.
Finally, a myrmecochory-driven plant benefit was documented that has not been addressed in recent reviews (e.g. Giladi, 2006). Elaiosome removal by ants was shown to result in increased germination percentage. This may be a peculiarity of Euphorbiaceae and other caruncle-bearing seed species as their elaiosome (also referred to as micropylar aril) covers the micropyle (Webster, 1994; Gorb and Gorb, 2003), the structure responsible for seed imbibition (Kigel and Galili, 1995). In other words, caruncle removal by ants may be crucial for increased or faster seed imbibition and, consequently, seed germination. Based on these findings we suggest that the ant-mediated increase in seed germination adds an additional benefit to myrmecochores of the Caatinga, although it may especially apply for the removal of micropylar arils in the Euphorbiaceae. It is important to mention that several studies have failed to demonstrate this benefit of seed manipulation by ants (see Horvitz, 1981; Passos and Ferreira, 1996).
In summary, myrmecochory appears to play a relevant role as a seed dispersal mode in the Caatinga, and it is particularly frequent among woody Euphorbiaceae. Dispersal services provided by ants have the potential of improving seed germination and seedling growth, particularly in the case of large seeds of several euphorbs that are discarded on ant-nest soils following elaiosome removal. The finding that seeds reached localized sites suitable for establishment supports the directed dispersal hypothesis as one of the possible forces to explain the selective advantage of myrmecochory in this ecosystem. Ecosystems with a high frequency of myrmecochorous plants appear not to be restricted to regions of nutrient-impoverished soils or to fire-prone regions, but can evolve where physically soils represent a harsh spot for seed germination and seedling growth.
Supplementary Material
ACKNOWLEDGEMENTS
We thank C. R. F. Brandão for ant identification, M. Sales and D. C. A. Barbosa for plant identification, and P. S. Oliveira for valuable criticism on a first draft of the manuscript. A. Powell and two anonymous referees also provided improvements to the final version of the manuscript. Programa Xingó and CNPq provided logistical support and a grant for I.R.L. (process 300582/98-6).
LITERATURE CITED
- Andersen A. Dispersal distance as a benefit of myrmecochory. Oecologia. 1988;75:507–511. doi: 10.1007/BF00776412. [DOI] [PubMed] [Google Scholar]
- Andrade-Lima D. Plantas da Caatinga. Rio de Janeiro: Academia Brasileira de Ciências; 1989. [Google Scholar]
- Ayres M, Ayres M, Jr, Ayres DL, Santos AS. BioEstat 2·0: Aplicações estatísticas nas áreas das ciências biológicas e médicas. Manaus: Sociedade Civil Mamirauá, MCT – CNPq; 2000. [Google Scholar]
- Barroso GM, Morim MP, Peixoto AL, Ichaso CLF. Frutos e sementes: morfologia aplicada à sistemática de dicotiledôneas. Viçosa: Universidade Federal de Viçosa; 1999. [Google Scholar]
- Beattie AJ. Distribution of ant-dispersed plants. Sonderbänd des Naturwissenschaftlichen Vereins in Hamburg. 1983;7:249–270. [Google Scholar]
- Beattie AJ. The evolutionary ecology of ant–plant mutualisms. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Beattie AJ, Culver DC. The guild of myrmecochores in the herbaceous flora of West Virginia forests. Ecology. 1981;62:107–115. [Google Scholar]
- Berg RY. Myrmecochorous plants in Australia and their dispersal by ants. Australian Journal of Botany. 1975;62:714–722. [Google Scholar]
- Bond W, Slingsby P. Seed dispersal by ants in Cape shrublands and its evolutionary implications. South African Journal of Science. 1983;79:231–233. [Google Scholar]
- Cowling RM, Pierce SM, Stock WD, Cocks M. Why are there so many myrmecochorous species in the Cape fynbos? In: Arianoutsou M, Groves RH, editors. Plant–animal interactions in mediterranean-type ecosystem. Dordrecht: Kluwer Academic Publishers; 1994. pp. 159–168. [Google Scholar]
- EMBRAPA. Manual de métodos de análises de solo. 2nd edn. Rio de Janeiro: Ministério da Agricultura e do Abastecimento; 1997. [Google Scholar]
- Giladi I. Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos. 2006;112:481–492. [Google Scholar]
- Gómez C, Espadaler X. Myrmecochorous dispersal distance: a world survey. Journal of Biogeography. 1998;25:573–580. [Google Scholar]
- Gorb E, Gorb S. Seed dispersal by ants in a deciduous forest ecosystem. Mechanisms, strategies, adaptation. Dordrecht: Kluwer Academic Publishers; 2003. [Google Scholar]
- Heithaus ER. Seed predation by rodents on three ant-dispersed plants. Ecology. 1981;62:136–145. [Google Scholar]
- Higashi S, Tsuyuzaki S, Ohara IF. Adaptive advantages of ant-dispersed seeds in the myrmecochorous plant Trillium tschonoskii (Liliaceae) Oikos. 1989;54:389–394. [Google Scholar]
- Horvitz CC. Analysis of how ant behavior affects germination in a tropical myrmecochore Calathea microcephala (P. & E.) Koernicke (Maranthaceae): microsite selection and aril removal by neotropical ants, Odontomachus, Pachycondyla, and Solenopsis (Formicidae) Oecologia. 1981;51:47–52. doi: 10.1007/BF00344651. [DOI] [PubMed] [Google Scholar]
- Horvitz CC, Beattie AJ. Ant dispersal of Calathea (Maranthaceae) seeds by carnivorous ponerines (Formicidae) in a tropical rain forest. American Journal of Botany. 1980;67:321–326. [Google Scholar]
- Hughes L, Westoby M. Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology. 1992;73:1285–1299. [Google Scholar]
- IBGE. Atlas Nacional do Brasil: Região Nordeste. Rio de Janeiro: IBGE; 1985. [Google Scholar]
- Kigel J, Galili G. Seed development and germination. New York: Marcel Dekker Inc; 1995. [Google Scholar]
- Leal IR. Dispersão de sementes por formigas na caatinga. In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e conservação da caatinga. Recife: Editora Universitária da UFPE; 2003a. pp. 593–624. [Google Scholar]
- Leal IR. Diversidade de formigas em diferentes unidades de paisagem da caatinga. In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e conservação da caatinga. Recife: Editora Universitária da UFPE; 2003b. pp. 435–461. [Google Scholar]
- Leal IR, Oliveira PS. Interactions between fungus-growing ants (Attini), fruits and seeds in cerrado vegetation in Southeast Brazil. Biotropica. 1998;30:170–178. [Google Scholar]
- Leal IR, Tabarelli M, Silva JMC. Ecologia e conservação da caatinga. Recife: Editora Universitária da UFPE; 2003. [Google Scholar]
- Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Nova Odessa: Editora Plantarum; 1998. [Google Scholar]
- Machado ICS, Barros LM, Sampaio EVSB. Phenology of Caatinga species at Serra Talhada, PE, Northeastern Brazil. Biotropica. 1997;29:57–68. [Google Scholar]
- Mark S, Olesen JM. Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia. 1996;107:95–101. doi: 10.1007/BF00582239. [DOI] [PubMed] [Google Scholar]
- Milewski AV, Bond WJ. Convergence of myrmecochory in mediterranean Australia and South Africa. In: Buckley RC, editor. Ant–plant interactions in Australia. Dordrecht: Dr. W. Junk Publishers; 1982. pp. 89–98. [Google Scholar]
- MMA. Biodiversidade brasileira: avaliação e identificação de áreas e ações prioritárias para conservação, utilização sustentável e repartição de benefícios da biodiversidade brasileira. Brasília: Ministério do Meio Ambiente; 2002. (Série Biodiversidade no. 5). [Google Scholar]
- Mooney HA, Bullock SH, Medina E. Introduction. In: Bullock SH, Mooney HA, Medina E, editors. Seasonally dry tropical forests. Cambridge: Cambridge University Press; 1995. pp. 1–8. [Google Scholar]
- O'Dowd DJ, Hay ME. Mutualism between harvester ants and a desert ephemeral: seeds escape from rodents. Ecology. 1980;61:531–540. [Google Scholar]
- Passos L, Ferreira SO. Ant dispersal of Croton priscus (Euphorbiaceae) seeds in a tropical semideciduos forest in southeastern Brazil. Biotropica. 1996;28:697–700. [Google Scholar]
- Passos L, Oliveira PS. Ants affect the distribution and performance of Clusia criuva seedlings, a primarily bird-dispersed rainforest tree. Journal of Ecology. 2002;90:517–528. [Google Scholar]
- Passos L, Oliveira PS. Interactions between ants, fruits and seeds in a restinga forest in south-eastern Brazil. Journal of Tropical Ecology. 2003;19:261–270. [Google Scholar]
- Passos L, Oliveira PS. Interaction between ants and fruits of Guapira opposite (Nyctaginaceae) in a Brazilian sandy plain rainforest: ant effects on seeds and seedlings. Oecologia. 2004;139:376–382. doi: 10.1007/s00442-004-1531-5. [DOI] [PubMed] [Google Scholar]
- Pennington RT, Prado DE, Pendry CA. Neotropical seasonally dry forests and Quaternary vegetation changes. Journal of Biogeography. 2000;27:261–273. [Google Scholar]
- van der Pijl L. Principles of dispersal in higher plants. Berlin: Springer-Verlag; 1982. [Google Scholar]
- Pizo MA, Oliveira PS. Interactions between ants and seeds of a nonmyrmecochorous neotropical tree, Cabralea canjerana (Meliaceae), in the Atlantic forest of southeast Brazil. American Journal of Botany. 1998;85:669–674. [PubMed] [Google Scholar]
- Pizo MA, Oliveira PS. Size and lipid content of nonmyrmecochorous diaspores: effects on the interaction with litter foraging ants in the Atlantic rain forest of Brazil. Plant Ecology. 2001;157:37–52. [Google Scholar]
- Prado DE. As Caatingas da América do Sul. In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e conservação da caatinga. Recife: Editora Universitária da UFPE; 2003. pp. 3–74. [Google Scholar]
- Rizzini CT. Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. Cidade: Âmbito Cultural Edições Ltda; 1979. [Google Scholar]
- Rodal MJN, Melo AL. Levantamento preliminar das espécies lenhosas da Caatinga de Pernambuco. In: Araújo FA, Prendergast HDV, Mayo SJ, editors. Anais do I Workshop Geral do programa Plantas do Nordeste. Recife: Associação Plantas do Nordeste; 1996. pp. 53–62. [Google Scholar]
- van Roosmalen MGM. Fruits of the Guianan flora. Utrecht: Institute of Systematic Botany; 1985. [Google Scholar]
- Sampaio EVSB. Overview of the Brazilian Caatinga. In: Bullock SH, Mooney HA, Medina E, editors. Seasonal dry tropical forests. Cambridge: Cambridge University Press; 1995. pp. 35–63. [Google Scholar]
- Silva RA, Santos AMM, Tabarelli M. Riqueza de plantas lenhosas em cinco unidades de paisagem da Caatinga. In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e conservação da caatinga. Recife: Editora Universitária da UFPE; 2003. pp. 337–366. [Google Scholar]
- Sokal RR, Rolf FJ. Biometry. W. H. Freeman and Company; 1995. New York. [Google Scholar]
- Tabarelli M, Vicente A, Barbosa DCA. Variation of seed dispersal spectrum of woody plants across a rainfall gradient in northeastern Brazil. Journal of Arid Environments. 2003;53:197–210. [Google Scholar]
- Webster GL. Classification of Euphorbiaceae species. Annals of the Missouri Botanical Garden. 1994;81:3–32. [Google Scholar]
- Westoby M, French K, Hugdes L, Rice B, Rodgerson L. Why do more plant species use ants for dispersal on infertile compared with fertile soils? Australian Journal of Ecology. 1991;16:445–455. [Google Scholar]
- Whitney KD. Dispersal for distance? Acacia ligulata seeds and meat ants Iridomyrmex viridiaeneus. Austral Ecology. 2002;27:589–595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.