Abstract
Background
The covalent attachment of ubiquitin to a substrate protein changes its fate. Notably, proteins typically tagged with a lysine48-linked polyubiquitin chain become substrates for degradation by the 26S proteasome. In recent years many experiments have been performed to characterize the proteins involved in the ubiquitylation process and to identify their substrates, in order to understand better the mechanisms that link specific protein degradation events to regulation of plant growth and development.
Scope
This review focuses on the role that ubiquitin plays in hormone synthesis, hormonal signalling cascades and plant defence mechanisms. Several examples are given of how targeted degradation of proteins affects downstream transcriptional regulation of hormone-responsive genes in the auxin, gibberellin, abscisic acid, ethylene and jasmonate signalling pathways. Additional experiments suggest that ubiquitin-mediated proteolysis may also act upstream of the hormonal signalling cascades by regulating hormone biosynthesis, transport and perception. Moreover, several experiments demonstrate that hormonal cross-talk can occur at the level of proteolysis. The more recently established role of the ubiquitin/proteasome system (UPS) in defence against biotic threats is also reviewed.
Conclusions
The UPS has been implicated in the regulation of almost every developmental process in plants, from embryogenesis to floral organ production probably through its central role in many hormone pathways. More recent evidence provides molecular mechanisms for hormonal cross-talk and links the UPS system to biotic defence responses.
Key words: Ubiquitin, E3 ligase, RING, U-Box, SCF, CRL, ubiquitylation, regulated proteolysis, plant defence, hormonal signalling, biotic stress, pathogen response
INTRODUCTION
Plants, as sessile organisms, rely on proteomic plasticity to remodel themselves during periods of developmental change, to endure varying environmental conditions, and to respond to biotic and abiotic stresses. Regulated ubiquitin- and proteasome-mediated degradation, therefore, plays a crucial role in enabling plants to alter their proteome to maximize their chances of survival under many different circumstances.
In plants, as in all eukaryotes, the ubiquitin/proteasome system (UPS) targets proteins for degradation (note that all abbreviations mentioned in this review are listed and defined in Appendix 1). In this system, the 76-amino-acid protein ubiquitin acts as a covalent molecular tag and its attachment requires three distinct enzymatic activities. Catalysed by E1 [ubiquitin-activating enzyme (UBA)], the ubiquitin C-terminal carboxyl group is first activated by adenylation, and then forms a thioester bond with a cysteinyl sulfhydryl residue on the E1 protein itself. Ubiquitin is then transferred to a cysteinyl sulfhydryl on another protein, the E2 [ubiquitin-conjugating protein (UBC)], preserving the thioester bond. Finally, ubiquitin is typically transferred to the substrate with the help of an E3 (ubiquitin ligase, Fig. 1; reviewed in Pickart and Eddins, 2004).
Fig. 1.
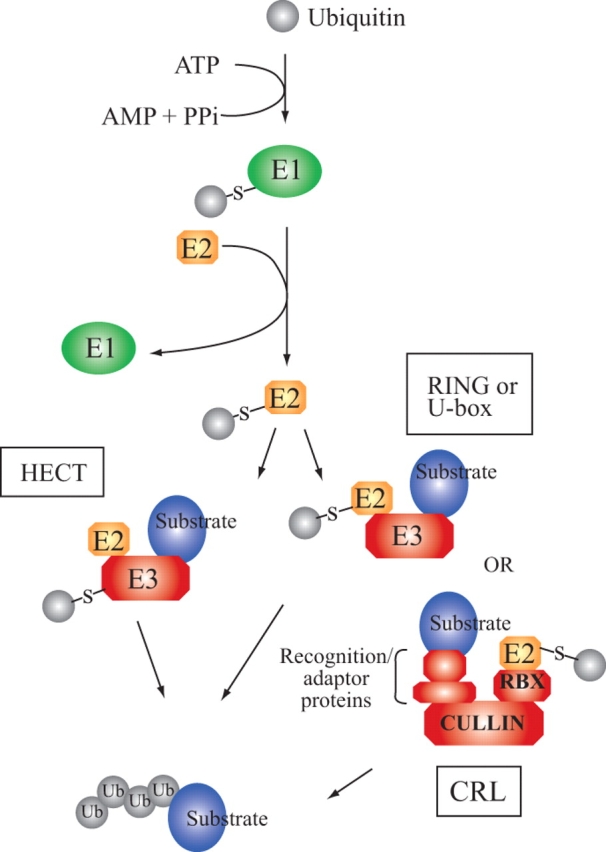
An overview of the ubiquitylation process. RING- and U-box-domain-containing E3 ligases facilitate direct transfer of ubiquitin from the E2 to the substrate, as shown on the right branch of the pathway. In the case of CRLs, the E2 binds to the RBX RING domain-containing subunit that is linked to the substrate through a cullin and various adaptor proteins. HECT domain-containing E3 ligases form a thioester bond with the ubiquitin delivered by the E2 before passing the ubiquitin to the substrate, as shown on the left branch of the pathway.
The first ubiquitin normally forms an isopeptide linkage with a lysyl ε-amino group on the substrate, although a small subset of substrates are modified at the N-terminal amino group (reviewed in Ciechanover and Ben-Saadon, 2004), and at least one substrate has been reported to bind ubiquitin on a cysteinyl sulfhydryl group (Cadwell and Coscoy, 2005). If additional ubiquitins are added, they may be attached to different substrate lysyl ε-amino groups or to one of seven lysyl ε-amino groups on the bound ubiquityl moiety. All seven lysyl residues can be used to form ubiquitin–ubiquitin linkages in budding yeast (Saccharomyces cerevisiae; Peng et al., 2003). Previous work on model substrates established that for a substrate to be recognized and then degraded by the proteasome, a chain of at least four ubiquitins linked via their lysyl residue 48 must be generated on a single substrate lysyl ε-amino group (Chau et al., 1989; Finley et al., 1994; Thrower et al., 2000). But experiments in mammalian cell extracts using an E2 that forms polyubiquitin chains connected through ubiquitin lysyl residue 11 (Baboshina and Haas, 1996) or assays performed with purified proteasomes using an endogenous substrate, yeast cyclin B, ubiquitylated in vitro with lysine 11 and 63 linkages (Kirkpatrick et al., 2006) suggest that other ubiquitin–ubiquitin linkages can serve as proteolytic signals, even in the complete absence of lysine 48 linkages. In addition, the attachment of a lysine-63-linked tetraubiquitin chain to lysine 48 of the ubiquitin portion of the UbDHFR fusion protein permits degradation of this model substrate by purified proteasomes (Hofmann and Pickart, 2001). Furthermore, multiple short chains of mixed lysyl linkages are formed on cyclin B in vitro by the APC (anaphase-promoting complex) E3 ubiquitin ligase. These ubiquitylated forms of cyclin B interact with substrate recognition proteins and are substrates for in vitro degradation by purified proteasomes (Kirkpatrick et al., 2006), suggesting that ubiquitin chain formation may be more complex, and that the requirements for recognition by proteasomes and associated proteins may be more relaxed than previously appreciated.
After delivery to the proteasome mediated in part by ubiquitin binding proteins, the polyubiquitylated substrate can then be deubiquitylated by the proteasome's regulatory cap or associated proteases. The free ubiquitin becomes available again for attachment, i.e. it is not degraded concomitant with the substrate. The deubiquitylated substrate is fed into the proteolytic core of the proteasome where it is cleaved into small peptides. After release, these peptide fragments are further hydrolysed to free amino acids by multiple separate proteases and/or proteolytic complexes. One of the few characterized examples is tripeptidyl peptidase II, a homooligomeric complex that hydrolyses larger peptides to tripeptides and could function to process proteasome products (Book et al., 2005).
Although the best-characterized function of ubiquitylation is to target substrates for proteolysis by the proteasome, ubiquitylation has also been linked to non-proteasomal functions, currently mostly in fungi and vertebrates. Ubiquitin chains linked via ubiquitin lysine 63 (UbK63) are involved in several processes including DNA repair, protein activation (reviewed in Schnell and Hicke, 2003), endolysosomal degradation (Duncan et al., 2006) and ribosomal regulation (Spence et al., 2000), and there is some evidence that UbK6-linked chains synthesized by the heterodimeric E3 ligase BRCA1/BARD1 mediate regulation of DNA replication and repair (Morris and Solomon, 2004; Nishikawa et al., 2004). Although there is also evidence for the formation of chains using lysines 11, 27, 29 and 33 in vivo (Peng et al., 2003), knowledge of the functional importance of these alternative polyubiquitin chains is lacking. Meanwhile, monoubiquitylation is required for receptor endocytosis, transcriptional regulation and intracellular sorting (reviewed in Schnell and Hicke, 2003). The non-degradative functions of ubiquitylation are ripe areas for future research in plants.
Components of the UPS have been identified in many species of plants through mutant screens and bioinformatic searches (reviewed in Moon et al., 2004). Strikingly, over 6 % of the predicted arabidopsis (Arabidopsis thaliana ecotype ‘Columbia’) genome encodes proteins involved in the UPS (Downes and Vierstra, 2005; with selected examples in Table 1), and analyses of other plant genomes demonstrate a similar abundance of UPS-related genes. Not surprisingly, components of the UPS have been implicated in countless processes including organ initiation and patterning (Samach et al., 1999; Shen et al., 2002; Imaizumi et al., 2005), light signalling (reviewed in Hoecker, 2005) and circadian clock regulation (Han et al., 2004), but this review will focus on the role of UPS in hormone production, perception and signal transduction, and in plant defence. Although the relationship between the UPS and plant signalling became evident about 20 years ago, when the light-signalling protein phytochrome A was shown to be ubiquitylated (Shanklin et al., 1987), the broad scope of ubiquitin's involvement in plant biology was not predicted. On-going discovery, annotation and functional characterization of UPS genes continues to provide insight into the mechanisms that plants use to regulate their growth and development through targeted protein degradation.
Table 1.
Putative E3 ubiquitin ligase components that recognize substrates involved in hormonal signalling pathways and plant–pathogen interactions
| E3 ligase substrate specificity factor | Organism | Pathway | Predicted substrate(s) | References |
|---|---|---|---|---|
| SCF F-box | ||||
| AFB1, AFB2, AFB3, AFB5 | A. thaliana | Auxin signalling | Aux/IAA proteins with Domain II | (Dharmasiri et al., 2005; Walsh et al., 2006) |
| ACRE189 | N. tabacum | Plant defence | (Rowland et al., 2005) | |
| CEGENDUO | A. thaliana | Lateral root formation | (Dong et al., 2006) | |
| CLINK | Faba bean necrotic yellow virus | Viral pathogenesis | (Aronson et al., 2000) | |
| COI1 | A. thaliana/S. lycopersicum | JA signalling | HDAC6?, RUBISCO small subunit 1b? | (Devoto et al., 2002; Xu et al., 2002; Li et al., 2004) |
| EBF1, EBF2 | A. thaliana | Ethylene signalling | EIN3, EIL1 and homologues | (Guo and Ecker, 2003; Potuschak et al., 2003; Gagne et al., 2004) |
| SLY1/GID2 | A. thaliana/O. sativa | GA signalling | DELLA proteins RGA, GAI, RGL1, RGL2, RGL3 (At); SLR1 (Os); SLN1 (Hv) | (reviewed in Fleet and Sun, 2005) |
| SNE | A. thaliana | GA signalling | DELLA proteins RGA, GAI, RGL1, RGL2, RGL3 | (Strader et al., 2004) |
| SON1 | A. thaliana | Plant defence | (Kim and Delaney, 2002) | |
| TIR1 | A. thaliana | Auxin signalling | Aux/IAA proteins with Domain II | (Gray et al., 1999) |
| TLP9 | A. thaliana | ABA signalling | (Lai et al., 2004) | |
| VirF | A. tumefaciens | Bacterial infection | VirE2, VIP1 | (Tzfira et al., 2004) |
| BTB | ||||
| ARIA | A. thaliana | ABA signalling | ABF2 | (Kim et al., 2004) |
| ETO1, EOL1, EOL2 | A. thaliana | Ethylene synthesis | ACC synthase 5 (ACS5) | (Wang et al., 2004) |
| GMPOZ | H. vulgare | GA and ABA signalling | (Woodger et al., 2004) | |
| NPR1/NIM1 | A. thaliana | Plant defence | (reviewed in Dong, 2004) | |
| U-box | ||||
| ACRE276/PUB17 | N. tobacum, S. lycopersicum/A. thaliana | Plant defence | (Yang et al., 2006) | |
| AvrPtoB | P. syringae | Plant defence | (Abramovitch et al., 2006; Janjusevic et al., 2006) | |
| CHIP | A. thaliana | ABA signalling | protein phosphatase 2A subunit | (Luo et al., 2006) |
| CMPG1/ELI17 CMPG1/ACRE74 Cmpg1 PUB20/PUB21 | P. crispum, N. tabacum S. lycopersicum, A. thaliana | Plant defence | (Kirsch et al., 2001; Gonzalez-Lamothe et al., 2006) | |
| PHOR1 | S. tuberosum | GA signalling | (Monte et al., 2003) | |
| PUB5, PUB12 | A. thaliana | Plant defence | (Navarro et al., 2004) | |
| PUB27, PUB28, PUB29 | A. thaliana | GA signalling? | (Monte et al., 2003) | |
| SPL11 | O. sativa | Plant defence | (Zeng et al., 2004) | |
| HECT | ||||
| UPL3 | A. thaliana | GA signalling | (Downes et al., 2003) | |
| APC | ||||
| HOBBIT | A. thaliana | Auxin signalling | Aux/IAA protein IAA17/AXR3? | (Blilou et al., 2002) |
| RING | ||||
| ACRE132 | N. tabacum | Plant defence | (Durrant et al., 2000) | |
| AIP2 | A. thaliana | ABA signalling | ABI3 | (Zhang et al., 2005) |
| ATL2 | A. thaliana | Plant defence | (Salinas-Mondragon et al., 1999) | |
| ATL6 | A. thaliana | Plant defence | (Salinas-Mondragon et al., 1999) | |
| ATL43 | A. thaliana | ABA signalling | (Serrano et al., 2006) | |
| BRH1 | A. thaliana | BR signalling/plant defence | (Molnar et al., 2002) | |
| EL5 | O. sativa | Plant defence | (Takai et al., 2002) | |
| RHA1b, RHA3b, RMA1, At2g44410, At2g42360 (ATL41), At4g26400, At2g35000 (ATL9), At2g42350 (ATL40), | A. thaliana | Plant defence | (Navarro et al., 2004) | |
| RIN2/RIN3 | A. thaliana | Plant defence | RPM1? | (Kawasaki et al., 2005) |
| SINAT5 | A. thaliana | Auxin signalling | NAC1 | (Xie et al., 2002) |
| XBAT32 | A. thaliana | Auxin signalling | (Nodzon et al., 2004) | |
| XERICO | A. thaliana | ABA synthesis | (Ko et al., 2006) | |
| Interacting proteins | ||||
| SIP | A. thaliana | Plant defence | (Kim et al., 2006) |
There are other excellent reviews covering the UPS in plants (Moon et al., 2004; Smalle and Vierstra, 2004), hormone signalling (auxin: Schwechheimer and Schwager, 2004; Woodward and Bartel, 2005; GA: Sun and Gubler, 2004; Fleet and Sun, 2005; ABA: Himmelbach et al., 2003; BR: Vert and Chory, 2006; Z-Y Wang et al., 2006; ethylene: Chen et al., 2005; Etheridge et al., 2006; JA: Turner et al., 2002; Devoto and Turner, 2005; Lorenzo and Solano, 2005) and plant defence mechanisms (Dangl and Jones, 2001; Thordal-Christensen, 2003; Nurnberger et al., 2004; Schulze-Lefert and Bieri, 2005; Zeng et al., 2006), which cogently describe the experimental underpinnings of our current understanding of these processes. But, with the rapid pace of scientific progress in these exciting areas, new information continues to emerge. This review seeks to build upon the foundation of the previous reviews by drawing together information concerning both internally and externally regulated plant developmental processes that depend upon the UPS both to highlight some common themes and to present the most recent published findings.
OVERVIEW OF UPS COMPONENTS
Before discussing the specific function of the UPS in various hormone signal transduction and plant defence pathways, it is useful to highlight some of the many components that are required to regulate properly the attachment and removal of ubiquitin from potential substrates. A website dedicated to the ubiquitin pathway in arabidopsis lists the identified components (http://plantsubq.genomics.purdue.edu). In the genome of arabidopsis only two genes encode an E1, the first enzyme in the ubiquitylation pathway (Hatfield et al., 1997), whereas the E2 gene family is predicted to contain 37 members (Kraft et al., 2005). The precise function of individual members of this expanded family of ubiquitin-conjugating enzymes remains to be determined. Eight additional genes code for putative UEV (ubiquitin-conjugating E2 enzyme variant) proteins in arabidopsis (Kraft et al., 2005). UEVs resemble ubiquitin-conjugating enzymes, but lack the catalytic cysteine (Broomfield et al., 1998; Sancho et al., 1998). Not surprisingly, human UEV1 fails to promote polyubiquitylation in the presence of an E1 and cellular fractions containing E3s (Sancho et al., 1998), but UEVs do appear to function in UPS-related processes. For instance, Mms2p, a UEV from budding yeast, interacts with a canonical E2, Ubc13p, to direct the formation of UbK63-linked polyubiquitin chains (Hofmann and Pickart, 1999), whereas Tsg101 in humans binds ubiquitin and participates in endosomal sorting and HIV budding (Garrus et al., 2001; Katzmann et al., 2001; Sundquist et al., 2004). By contrast, the COP10 UEV, first identified in arabidopsis, appears to interact with a number of E2s and enhances the formation of UbK48- and UbK63-linked polyubiquitin chains in vitro, perhaps through its ability to increase E2/ubiquitin thioester formation (Yanagawa et al., 2004). Additionally, COP10 forms complexes with components of Cullin4-based E3 ligases described below (Chen et al., 2005).
The vast bulk of the arabidopsis UPS-related genes encode components of E3 ubiquitin ligases (Smalle and Vierstra, 2004). Two mechanistic classes of E3 ligases facilitate the transfer of ubiquitin from the E2 to the substrate. In the case of the HECT domain E3 ligases, ubiquitin forms a covalent thioester linkage with a cysteinyl sulfhydryl group on the HECT protein before being transferred to a lysine on the substrate. Using the conserved domain containing this cysteinyl residue, seven HECT proteins have been identified in arabidopsis (Downes et al., 2003).
The other E3 ligase class does not covalently link with ubiquitin, but rather non-covalently interacts with an E2 protein carrying ubiquitin. Currently, there are two groups within this class, the U-box domain- and RING domain-containing proteins and both domains are thought to be structurally and functionally similar. The U-box and RING domains are required for E2 interaction in their respective proteins, utilizing H-bonds or zinc chelation, respectively, to build a platform and expose the appropriate interface (Zheng et al., 2000; Ohi et al., 2003; Andersen et al., 2004). There are approximately 61 predicted U-box proteins in arabidopsis (http://www.arabidopsis.org/info/genefamily/pub.html). By contrast, over 450 proteins in arabidopsis contain one or more RING domains (Stone et al., 2005).
Cullin RING ligases in plants
RING proteins may act alone, as homo- or heterodimers, or in complex assemblies. Two RING family members, RBX1a and RBX1b (in arabidopsis; Gray et al., 2002), function redundantly to play an essential role in promoting ubiquitylation as part of the multi-subunit E3 ligases called cullin RING ligases (CRLs) (reviewed in Petroski and Deshaies, 2005) – a type universally present in eukaryotes (Willems et al., 2004; Petroski and Deshaies, 2005). Each CRL includes a cullin (CUL in arabidopsis) protein that contains a C-terminal RBX1 binding site. Distinct cullin proteins that define the different types of CRLs bind different adaptors and substrate-recruiting subunits at the cullin N-terminus (see figure in Appendix 2). Eleven CUL-like proteins have been identified in arabidopsis based on identity to vertebrate cullins (Shen et al., 2002) and six have been implicated in CRLs.
Arabidopsis CUL1 (Gray et al., 1999) forms a CRL called an SCF complex (named for the subunits Skp1, cullin1 and F-box). In arabidopsis cells, CUL1 co-immunoprecipitates with HA-tagged ASK1, one of 21 predicted arabidopsis SKP family members (called ASKs for Arabidopsis SKP1-like) (Farras et al., 2001). Both ASK1 and ASK2 interact with several F-box proteins in yeast-two-hybrid assays, including COI1, EID1, TIR1 and UFO (Gray et al., 1999; Samach et al., 1999; Dieterle et al., 2001; Xu et al., 2002) and many of these interactions have been confirmed in vitro or by co-immunoprecipitation assays using arabidopsis extracts. The phenotype of plants with single mutations in these family members suggests that ASK1 and ASK2 perform overlapping but not totally redundant functions. ask2-1 null mutants grow quite normally (Liu et al., 2004). ask1-1 mutants display some defects in vegetative and floral development, such as smaller rosette size, reduced filament length and male sterility (Zhao et al., 1999) owing to a defect in homologous chromosome separation in meiosis (Yang et al., 1999). Meanwhile, ask1 ask2 double mutants exhibit severe defects in embryogenesis and die as seedlings, plus they have higher levels of cyclin D3 (Liu et al., 2004). D-type cyclins are degraded via an SCF in humans (Yu et al., 1998) and cyclin D3 levels rise in arabidopsis seedlings with lower level of RBX1 (Lechner et al., 2002), further implicating ASK1 and ASK2 in SCF-mediated degradation. The biochemical function of the other ASKs has not been elucidated, but the varying RNA expression patterns (Zhao et al., 2003; Takahashi et al., 2004) and RNAi-induced phenotypes observed for different ASK family members suggests that they could regulate particular processes in different organs or tissues (Zhao et al., 2003), although there is also evidence that single knock-outs are often insufficient to cause phenotypic abnormalities (Takahashi et al., 2004).
The SKP/ASK proteins in turn serve as adaptor proteins by interacting with one or more of the 700-plus F-box (substrate-recruiting) proteins encoded by the arabidopsis genome (Gray et al., 1999; Gagne et al., 2002; Kuroda et al., 2002; Risseeuw et al., 2003; Takahashi et al., 2004). The F-box domain derives its name from the SKP/ASK interaction motif found in mammalian cyclin F (Bai et al., 1996). F-box proteins bind an SKP/ASK protein and a UPS substrate simultaneously, thus bringing the substrate in proximity to the CUL1-RBX1-tethered E2 enzyme with an attached activated ubiquityl group. The close proximity presumably facilitates ubiquitin transfer to the substrate, although E2 binding to the RING domain may additionally allosterically activate the E2 for ubiquitin transfer (Ozkan et al., 2005). Another protein, SGT1b, can also associate with this complex (Azevedo et al., 2002; Y. Liu et al., 2002; Gray et al., 2003), and has been shown to reduce the accumulation of at least one SCF substrate in arabidopsis (Gray et al., 2003), but its full role in plant SCFs and proteolysis in general (Holt et al., 2005; Azevedo et al., 2006) is not completely clear.
The other arabidopsis cullin most closely related to CUL1, CUL2, also shows enhanced interaction with one F-box protein when ASK1 is over-expressed in a yeast-two-hybrid assay (Risseeuw et al., 2003), and so probably forms a CRL similar to that of CUL1, but there are as yet no in planta data.
Another class of CRL utilizes the CUL3a or CUL3b protein as the primary scaffold component. Like CUL1, CUL3a and CUL3b associate with the RBX1 E2-recruiting protein, as demonstrated through yeast-two-hybrid (Dieterle et al., 2005; Gingerich et al., 2005; Weber et al., 2005), in vitro pull-down (Weber et al., 2005) and plant extract-based co-immunoprecipitation experiments (Figueroa et al., 2005). But, unlike CUL1, CUL3a and CUL3b do not interact with ASK1; instead they interact in yeast-two-hybrid and pull-down assays with BTB domain-containing proteins (Dieterle et al., 2005; Figueroa et al., 2005; Gingerich et al., 2005; Weber et al., 2005). These interactions depend on residues within the N-terminus of CUL3 and for BTB proteins, residues within the BTB domain of several family members that have been studied (Figueroa et al., 2005; Weber et al., 2005). BTB proteins associate directly with targets of the CUL3-based CRLs, combining adaptor and substrate-recruiting functions in one protein (Pintard et al., 2003b). Thus, it is not surprising that the 80 putative BTB proteins found in arabidopsis contain a number of additional domains such as ankyrin repeats (reviewed in Michaely and Bennett, 1992) and the MATH domain (Xu et al., 2003) implicated in protein–protein interactions (Dieterle et al., 2005; Figueroa et al., 2005; Gingerich et al., 2005; Weber et al., 2005) that are predicted to tether substrates to the CRL for ubiquitylation.
Forward genetic identification of CUL1 (Hellmann et al., 2003; Quint et al., 2005) and reverse genetic studies on CUL1 (Shen et al., 2002; Hellmann et al., 2003) and CUL3a and b (Dieterle et al., 2005; Figueroa et al., 2005; Gingerich et al., 2005; Thomann et al., 2005) have established their essential nature. cul1 loss-of-function mutants are early embryo lethals (Shen et al., 2002; Hellmann et al., 2003), suggesting that CUL2 cannot functionally replace CUL1. By contrast, CUL3a and CUL3b appear to be redundant as mutation of either CUL3 gene does not obviously compromise plant growth (Figueroa et al., 2005) and only mild phenotypic changes such as slightly delayed flowering and reduced hypocotyl response to far red light are reported for the cul3a-1 mutant (Dieterle et al., 2005). However, CUL3 activity is required for viability given that cul3a/cul3b double mutants are early embryonic lethals (Dieterle et al., 2005; Figueroa et al., 2005; Gingerich et al., 2005; Thomann et al., 2005). These findings suggest that both CUL1 and CUL3a/b broadly influence a wide range of essential pathways. By contrast, based on phenotypes of mutants, the individual F-box and BTB family members that potentially bring substrates to CUL1 and CUL3, respectively, appear to regulate more specific aspects of plant development and growth responses during the plant life cycle, ranging from seedling blue-light-mediated phototropism (Motchoulski and Liscum, 1999; Inada et al., 2004) to leaf senescence (Woo et al., 2001; also see below and reviewed in Moon et al., 2004; Gingerich et al., 2005), and these phenotypes are consistent with their functioning in a specific CRL. It is worth mentioning that non-CRL functions, although not documented in plants, are possible for F-box and BTB proteins. Two F-box proteins in budding yeast, Rcy1p and Ctf13p (Kaplan et al., 1997; Russell et al., 1999; Galan et al., 2001), and one BTB protein in Caenorhabditis elegans, MEL-26 (Luke-Glaser et al., 2005), appear to perform CRL-independent functions. However, the stability of the Ctf13p (Kaplan et al., 1997) and MEL-26 (Pintard et al., 2003b) proteins still depends on CRLs, suggesting that the biological function of these proteins remains influenced by the UPS.
The most recently characterized plant CRL uses CUL4 and is similar to animal CUL4 CRLs (McCall et al., 2005; Bernhardt et al., 2006; Chen et al., 2006). Two proteins, AtCUL4-L (long) and AtCUL4-S (short), encoded by the arabidopsis CUL4 gene differ in length by 50 amino acids, but both contain the N- and C-terminal cullin functional domains (Chen et al., 2006). To date, studies have focused on the long version of CUL4. AtCUL4-L interacts with RBX1 at its C-terminus, binds directly to DDB1a (from human Damaged DNA Binding-1) at its N-terminus (Bernhardt et al., 2006; Chen et al., 2006), and connects indirectly with DDB2 or DET1 via DDB1a (Bernhardt et al., 2006). One reasonable interpretation of these observations is that DDB1 serves as the adaptor subunit (analogous to ASK/SKP in CUL1 CRLs) and DDB2 and DET1 are alternative substrate-recruiting subunits (analogous to F-box proteins). However, there is also evidence for the formation of larger CUL4-based complexes. In humans, degradation of the c-Jun transcription factor requires a complex containing CUL4, ROC1 (RBX1 homologue), DDB1, DET1 and COP1, a RING E3 ligase initially identified in plants for its role in regulating photomorphogenesis (Deng et al., 1991; Wertz et al., 2004). In arabidopsis, CUL4-L appears to associate more directly with the CDD complex, composed of the UEV protein COP10, along with DDB1 and DET1, but lacking COP1 (Yanagawa et al., 2004) as indicated by yeast-two-hybrid, in vitro and plant-based co-immunoprecipitation experiments (Chen et al., 2006). However, the CDD complex shows signs of associating with COP1 in arabidopsis plants, and may regulate COP1's ubiquitylation activity. Although the DET1, COP1 and COP10 proteins all contribute to light-regulated signalling, the varying phenotypes of arabidopsis plants with reduced levels of CUL4 protein suggest that it may participate in CRLs with other substrate adaptor units to regulate the degradation of additional proteins (Chen et al., 2006).
Interestingly, there was some early evidence that DDB1 could bind either directly to a substrate (like a BTB protein) or function as an adaptor (like SKP1), but recent work favours the latter interpretation. Tandem affinity purification followed by mass spectrometry has shown interaction between human DDB1 and a large family of proteins termed DCAFs (DDB1-Cullin4-Associated Factors) (Angers et al., 2006; Jin et al., 2006). The majority of these proteins contain WD40 repeats, including DDB2, CSA and COP1 previously shown to function in CUL4-based E3 ligases (Wertz et al., 2004; Groisman et al., 2006; H. Wang et al., 2006). Mutational studies demonstrate that two conserved WDXR motifs in DCAFs are important for their binding to varying portions of a double beta propeller fold in DDB1 (Jin et al., 2006). A limited blastp search through the NCBI database using several human DCAF proteins reveals the existence of WD40-repeat-containing proteins with two or more WDXR motifs in a number of plant species, including arabidopsis, rice (Oryza sativa) and Medicago truncatula, suggesting that plant homologues of DCAFs may exist (Madden et al., 1996). In addition to binding DDB1, DCAFs presumably recruit substrate proteins and bring them into proximity with the CUL4-ROC1-tethered Ub-charged E2. For instance, the DCAF Cdt2 binds to Spd1 in fission yeast (Schizosaccharomyces pombe) and promotes its degradation (Liu et al., 2005). As previously observed for some F-box proteins (Zhou and Howley, 1998) and BTB proteins (Wee et al., 2005), DCAF proteins may also become victims of their own CRL, as ubiquitylation of the DCAF human DDB2 can be promoted by a CUL4A-DDB1-based E3 ligase (Matsuda et al., 2005). To date, it remains unknown whether DDB1 is the only adaptor protein to function in CUL4-based E3 ligases. Two human proteins, CPSF160 and SAP130, show structural similarity to DDB1 (Martinez et al., 2001; Li et al., 2006), suggesting that other CUL4-based ligases may exist. Defining the nature of CUL4 CRL complexes is currently an active area of investigation in plants and in other eukaryotes.
Humans also possess a CRL that uses the adaptor proteins elongin B/C instead of SKP to bring substrates to human CUL2 (not homologous to arabidopsis CUL2) and human CUL5 (Kamura et al., 2004; reviewed in Willems et al., 2004). One arabidopsis protein is reported to have similarity to elongin C (Risseeuw et al., 2003) but no clear homologues of the elongin B adaptor protein, nor human CUL2/CUL5 orthologues have been discovered in plants to date (Shen et al., 2002).
Finally, the APC, among its 11 or more subunits, contains a cullin-like protein, APC2, and a small RING-domain-containing protein, APC11. This CRL, found in plants (Capron et al., 2003), most likely helps to control the stability of cell-cycle regulatory proteins (Fulop et al., 2005).
Regulation of CRL activity
Cullin-based ubiquitin ligases are subject to regulation by a cycle of covalent addition and removal of the 76-amino-acid ubiquitin-like protein called RUB (Related to Ubiquitin; known as Nedd8 in fission yeast and animals). RUBs/Nedd8 attach to a conserved lysyl e-amino group near the C-terminus of all six human cullin family members (Hori et al., 1999). To date the same modification has been directly demonstrated for arabidopsis CUL1 (del Pozo and Estelle, 1999), CUL3a and CUL3b (Figueroa et al., 2005) and CUL4 is present in two electrophoretic forms, including a slower migrating form that increases in abundance in COP9 signalosome mutants (see below for relevance), strongly suggesting that it is also modified by RUB (Chen et al., 2006). RUB attachment, similar to ubiquitin attachment, occurs through the activities of E1-, E2- and E3-like enzymes. The E1-like protein is heterodimeric, composed of AXR1 and ECR1 subunits that activate RUB and pass it to the RCE1 RUB E2 conjugating enzyme. Finally, RBX1 appears to act as the RUB E3 ligase that facilitates RUB attachment to the cullin (Gray et al., 2002), although in Caenorhabditis elegans and budding yeast, an additional protein, DCN-1/Dcn1p, facilitates rubylation/neddylation in vitro and in vivo (Kurz et al., 2005).
Arabidopsis contains two closely related (RUB1 and RUB2) and one more divergent (RUB3) RUB family members (Rao-Naik et al., 1998); their precise functional roles are currently under investigation. RUB conjugation appears to stimulate CRL-based activity in vitro (Read et al., 2000; Wu et al., 2000). Curiously, de-rubylation catalysed by the COP9 signalosome (CSN) (Lyapina et al., 2001; Schwechheimer et al., 2001; Cope et al., 2002) also appears to be required for in vivo robust CRL activity in the degradation of Aux/IAA (auxin/indole-3-acetic acid) proteins (Schwechheimer et al., 2001) and HY5 (long hypocotyl5) (Osterlund et al., 2000) in plants, Spd1 in fission yeast (Liu et al., 2003), MEI-1 in C. elegans (Pintard et al., 2003a), and cyclin E in mice (Mus musculus) (Lykke-Andersen et al., 2003) and Drosophila melanogaster (Doronkin et al., 2003). By contrast, in vitro work with yeast and mammalian proteins demonstrates that the CSN effect is inhibitory rather than stimulatory on SCF activity (Lyapina et al., 2001; Zhou et al., 2001; Yang et al., 2002; Groisman et al., 2003).
These seemingly contradictory results have led to the hypothesis that a dynamic cycling of RUB/Nedd8 attachment and removal is needed for robust CRL activity (Lyapina et al., 2001). Recently, one specific mechanism was proposed to explain this observation. The CSN may be required for CRL activity in vivo by preventing proteolysis of CRL components when substrates are not present (reviewed in Wu et al., 2006).
CRL activity is further impacted by the CAND1 protein that binds to both the N- and C-termini of cullin homologues (Min et al., 2003; Goldenberg et al., 2004; Hu et al., 2004). By such interactions it interferes with the binding of substrate recruiting modules (J. Liu et al., 2002; Zheng et al., 2002; Min et al., 2003; Lo and Hannink, 2006) and attachment of RUB/Nedd8, respectively (Hwang et al., 2003; Goldenberg et al., 2004), thus functioning as a negative regulator of SCF activity in vitro. Therefore, one would expect in vivo loss of CAND1 to hyperactivate SCF activity, but the opposite is observed. Elimination of CAND1 from arabidopsis stabilizes the transcriptional regulators HY5 (Feng et al., 2004), RGA (repressor of ga1-3) (Feng et al., 2004) and IAA7/AXR2 (indole-3-acetic acid7/auxin resistant2) (Chuang et al., 2004) that are normally slated for proteasome-mediated degradation, suggesting that CAND1 acts as a positive regulator of ubiquitin-mediated degradation in vivo through its impact on CRL activity. Therefore, the precise molecular events involved in cycles of CAND1/cullin/RUB/SKP association and dissociation require further characterization. For instance, in vitro, once human CAND1 binds to unmodified human CUL1, even a suprastoichiometric amount of the Nedd8/RUB E2 enzyme in the presence of the Nedd8/RUB E1 cannot attach Nedd8 to CUL1 nor promote the displacement of CAND1 (Goldenberg et al., 2004), leaving open the question of how a new round of CAND1 release and CRL activation can be achieved. Additional recent in vitro studies demonstrate that the F-box protein Skp2 together with Skp1 can promote CAND1 dissociation from CUL1, while a small complex including the p27 substrate recognized by Skp2 protein can inhibit deneddylation of CUL1 by the CSN (Bornstein et al., 2006). These two processes can both promote SCF activity and provide an attractive explanation for increased p27 degradation as Skp2 levels rise near the end of G1. However, the authors point out that additional factors may be required for this process of CAND1 displacement and acknowledge that more work will be required to determine if these findings are broadly applicable to other CRLs (Bornstein et al., 2006).
While the biochemical characterization of CAND1 proceeds, insights into the biological function of CAND1 in plants continue to arise from the identification and characterization of cand1 mutant alleles in arabidopsis. Aberrant phenotypes observed include altered venation patterns in vegetative leaves and cotyledons (Alonso-Peral et al., 2006), modest auxin and ethylene resistance and increased ABA sensitivity compared with wild-type in roots (Cheng et al., 2004; Chuang et al., 2004; Feng et al., 2004), dark-grown hookless hypocotyls, and enhanced sensitivity to low-fluence red light in hypocotyl elongation (Chuang et al., 2004; Feng et al., 2004). Both a missense allele and T-DNA insertion alleles exhibit dwarfing as adult plants with an increased number of rosette leaves with wrinkly blades (Chuang et al., 2004; Feng et al., 2004). T-DNA insertion alleles are almost completely sterile with reduced apical dominance and delayed flowering (Chuang et al., 2004; Feng et al., 2004). These diverse phenotypes are not surprising given CAND1's ability to bind to multiple cullins and consequently to regulate the activities of many CRLs.
Proteasomal recognition and substrate degradation
Clearly, many proteins co-operate to accomplish the regulated attachment of ubiquitin to a substrate, but the story does not end there. Once the protein has been polyubiquitylated, it must be brought to the proteasome, recognized as a proteolytic substrate, deubiquitylated, threaded into the 20S core particle and hydrolysed therein. Selective and regulated deubiquitylation of proteins may play a counteracting role prior to or coincident with proteasomal docking. For instance, deubiquitylation of the human p53 protein by HAUSP inhibits p53 degradation (Li et al., 2002). Although plants contain many putative ubiquitin hydrolases (reviewed in Smalle and Vierstra, 2004), their in vivo roles remain largely uncharacterized. Mechanisms governing substrate recognition, deubiquitylation and degradation by the proteasome also provide important points of regulatory control that require further research.
Ongoing identification of the components of the UPS and characterization of their biochemical activities enhances our understanding of targeted protein degradation in plants. At the same time, a combination of forward genetic, reverse genetic and biochemical techniques enables us to examine the relationship between the UPS and various aspects of plant growth and development, including hormonal signalling and plant defence.
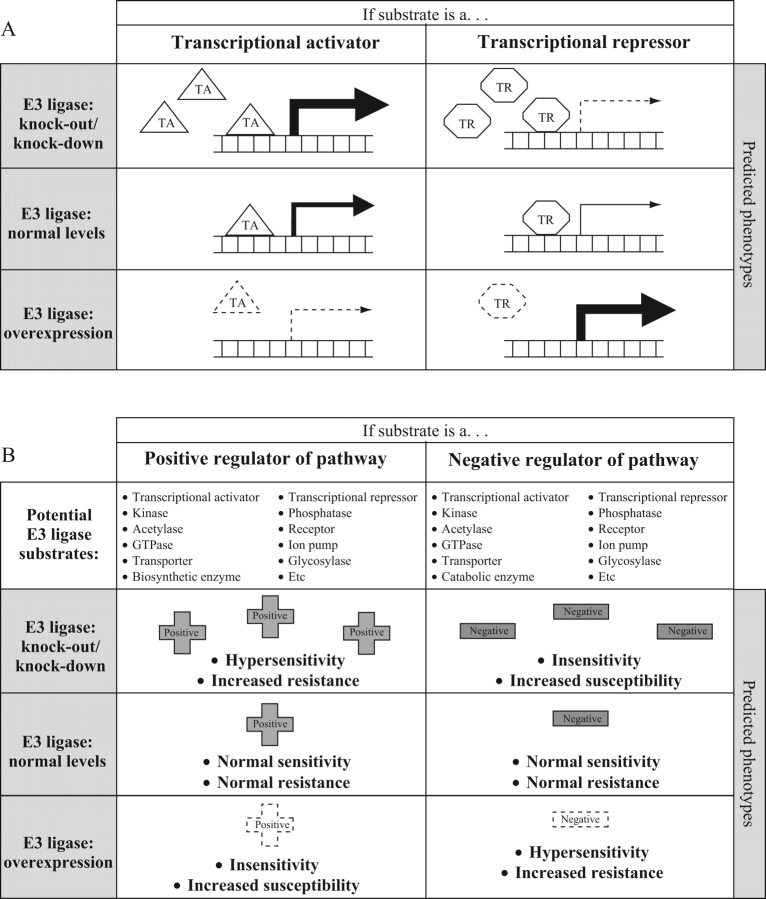
During functional characterization of hormone or defence pathways (discussed in detail here relative to UPS), scientists often monitor the consequences of altered levels of specific UPS components in vivo or in vitro. The molecular and phenotypic consequences depend on the nature of the UPS component (whether it promotes degradation or inhibits degradation) and on the function of the substrate in the pathway under investigation. When a particular UPS component that promotes degradation, such as an E3 ligase, is down-regulated, e.g. through mutation or transcriptional silencing, its substrate(s) should accumulate. So, in the case of a transcriptional activator, this should lead to increased expression of downstream genes (Fig. 2A). However, if the E3 ligase under consideration normally targets a transcriptional repressor for degradation, plants lacking that E3 ligase would have elevated levels of the repressor, and hence reduced transcription of downstream genes (Fig. 2A). The opposite effects would be observed upon over-expression of the E3 ligase, i.e. reduced transcription if the substrate is a transcriptional activator, owing to increased degradation and hence lower steady-state levels, but increased transcription if the substrate is a repressor (Fig. 2A).
Fig. 2.
Predicted phenotypic consequences of altering the levels of E3 ubiquitin ligases at the molecular level (A) and from the perspective of an overall pathway (B). The figure displays the predicted effects of altering the quantity of the E3 ligase components, but its predictions can be extended to mutations that affect the activity of the E3 ligase as well. The effects of mutations that decrease the activity of the E3 ligase, for example by impairing its ability to bind to the substrate or to the E2 enzyme, should resemble the effects of reducing the levels of the E3 protein. And similar outcomes might be expected when an E3 ligase is expressed at a higher level, or exhibits higher activity, for instance owing to an enhanced affinity for its substrate.
Similar predictions of expected phenotypes can be developed for other potential E3 ligase substrates such as a kinase, phosphatase, hormone biosynthetic enzyme or hormone catabolic enzyme (Fig. 2B). Additionally, the effects of perturbations in the degradative components of the UPS can be examined at the level of the downstream pathway. For instance, a transcriptional activator can be either a positive or a negative regulator of a signalling pathway (e.g. in response to hormones, light, drought, pathogen attack, etc.) depending on the types of genes it turns on. Similarly, kinases, phosphatases, receptors, transporters, GTPases, acetylases, ion pumps, etc., can all function as either negative or positive regulators of pathways. If one of these proteins that acts as a positive regulator of a pathway (such as the EIN3 transcription factor in ethylene signalling – see below) is subject to UPS-mediated degradation, then a plant lacking the E3 ligase responsible for degradation of the positive signalling component should be hypersensitive to the stimulus (e.g. ethylene) or should be more disease resistant if the protein positively regulates plant defence (Fig. 2B).
In searching for and analysing novel E3 ligase/substrate interactions, these predictable phenotypes (Fig. 2) can be used to make and test hypotheses about the regulatory roles of the UPS and the substrates in the signalling pathways under investigation. Similar molecular and pathway-level predictions can be made for many components of the UPS. For example, altered levels of other proteins that promote degradation, such as 20S proteasome core subunits, AXR1 and E2 ubiquitin-conjugating enzymes, would be expected to cause similar effects to altered levels of E3 ligases. By contrast, changes in the levels of components of the UPS that act to stabilize substrates, such as deubiquitylating enzymes, would most likely promote opposite outcomes.
Auxin and the UPS
In plants, the hormone auxin appears indispensable for plant survival. Often present as indole-3-acetic acid (IAA), auxin plays a critical role in regulating cell growth, division and differentiation, and on a gross morphological scale, auxin clearly impacts apical dominance, root elongation, lateral root formation and many other processes (reviewed in Davies, 2004; Paciorek and Friml, 2006).
Many, but not all, of auxin's effects are linked to auxin-mediated alterations of the transcriptome and the UPS appears to be involved in allowing these transcriptional changes. Multiple members of the Aux/IAA family of proteins act as repressors of auxin-mediated transcription in transient transfection assays using carrot (Daucus carota) or arabidopsis protoplasts (Ulmasov et al., 1997; Tiwari et al., 2001, 2004). These proteins are subjected to rapid degradation and stabilized versions disrupt normal plant growth and auxin signalling, indicating the biological significance of rapid Aux/IAA proteolysis (reviewed in Reed, 2001). Although there is no published report of ubiquitylated forms of these extremely short-lived proteins, evidence for their degradation via the UPS is compelling. The TIR1 F-box protein (Ruegger et al., 1998), and three additional related F-box proteins called AFB1, 2 and 3 (Dharmasiri et al., 2005a, b) interact with Aux/IAA family members in vitro, suggesting that these proteins are substrates of a CUL1-based CRL, either an SCFTIR1 or an SCFAFBx. Two additional family members, AFB4 and AFB5, remain to be characterized biochemically, but the recent identification of afb5 mutants with reduced sensitivity to the structurally distinct picolinate class of synthetic auxins suggests that these proteins could act in a related pathway (Gagne et al., 2002; Dharmasiri et al., 2005b; Walsh et al., 2006).
Evidence for Aux/IAA ubiquitylation also comes from the observations that in vivo degradation of Aux/IAA fusion proteins such as IAA1:LUC (firefly luciferase) (Ramos et al., 2001), AXR2(IAA7):GUS (β-glucuronidase) and AXR3(IAA17)NT:GUS (a fusion with the N-terminal 102 amino acids of IAA17) (Gray et al., 2001) is slowed by the proteasome inhibitor MG132 and both tagged and endogenous Aux/IAA proteins are stabilized by mutations in several components that affect CRL activity in particular, including AXR1, CAND1, CUL1 and CSN component CSN5 (Gray et al., 2001; Schwechheimer et al., 2001; Zenser et al., 2003; Chuang et al., 2004; Quint et al., 2005).
How do auxin signalling and Aux/IAA proteolysis intersect? Surprisingly, increased levels of auxin promote accelerated degradation of these Aux/IAA transcriptional repressors (Gray et al., 2001; Zenser et al., 2001) by increasing their interactions with TIR1 and related AFBs (Gray et al., 2001; Kepinski and Leyser, 2004; Dharmasiri et al., 2005b). Attempts to uncover the signalling steps between auxin perception and accelerated degradation led to the exciting discovery that auxin itself directly promotes the interaction between TIR1 and tagged forms of Aux/IAA proteins and through this increased interaction probably prompts accelerated proteolysis (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005; and reviewed in Parry and Estelle, 2006). In all experiments, auxin binding occurs in the presence of both a TIR1/AFB protein and its Aux/IAA substrate, leaving open the possibility that both proteins are required to bind this crucial hormone (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005). This mechanism of proteolytic regulation represents a new paradigm for regulation of SCF activity and suggests that small-molecule non-covalent modulation of CRL function may occur in other contexts.
A recent study shows that auxin directs the movement of IAA17:GFP into nuclear protein bodies (NPBs) along with components of SCFTIR1, the COP9 signalosome and the 26S proteasome core (Tao et al., 2005). In arabidopsis cells, this relocalization depends on the RAC1 GTPase, a small signalling protein. RAC1's interactions with various downstream effectors is regulated by rounds of GTP binding and hydrolysis. Expression of a dominant negative form of AtRAC1 suppresses auxin-mediated formation of these NPBs and decreases loss of IAA17:GFP protein, whereas IAA17:GFP levels drop more in cells expressing a constitutively active form of RAC1 (Tao et al., 2005). In addition, work performed in tobacco (Nicotiana tabacum) demonstrates that induction of auxin-responsive genes occurs while GFP-NtRac1 localizes predominantly to the plasma membrane, pointing to the existence of an unknown membrane-associated protein linking RAC GTPases to auxin-mediated acceleration of Aux/IAA degradation (Tao et al., 2002).
There is also evidence that mutation of the HOBBIT Cdc27-like component of the APC stabilizes the IAA17/AXR3 protein. This does not result from a global deceleration of proteolysis as the HY5 transcription factor that participates in light signalling is not similarly stabilized in hobbit mutants (Blilou et al., 2002). It is unclear if the stabilization of IAA17 in hobbit mutants results from any direct interaction between the APC and Aux/IAA proteins, but suggests a link between cell cycle regulation and Aux/IAA degradation.
Although several experiments demonstrate that auxin does not promote a global acceleration of proteolysis (Zenser et al., 2003; H. Li et al., 2004), its effects on proteolysis are not limited to the Aux/IAA proteins. For example, degradation of a phosphorylated E2FB transcription factor that helps regulate cell cycle progression is dramatically slowed in arabidopsis cultured cells incubated with exogenous auxin [(1-NAA)1-naphthalene-acetic acid] (Magyar et al., 2005). By contrast, levels of the NAC1 transcription factor, a positive regulator of auxin-responsive lateral root formation (Xie et al., 2000), drop following treatment with auxin even though its transcript levels are initially induced by auxin (Xie et al., 2000, 2002). Post-translational modification must be the cause of the lowered protein levels because plants expressing 35S:6 × Myc:NAC1 show steady levels of transcript following auxin treatment even though 6 × Myc:NAC1 protein levels drop. The authors found that the SINAT5 RING E3 ligase is capable of ubiquitylating NAC1 in vitro and affecting its accumulation in transgenic plants (Xie et al., 2002). SINAT5 is transcriptionally up-regulated by auxin more slowly than NAC1, but prior to observed decreases in NAC1 protein levels, suggesting that increased levels of SINAT5 cause increased degradation of NAC1 protein in the presence of auxin. It is not clear whether auxin has any additional role in promoting the degradation of NAC1 (e.g. by stimulating post-translational modification that increases its affinity for SINAT5) (Xie et al., 2002).
The auxin signal transduction pathway demonstrates the power of the UPS to control plant behaviour tightly in response to transient or changing signals. For instance, a pulse of auxin will rapidly trigger increased Aux/IAA degradation and turn on genes required for auxin signalling. In roots, this might lead to lateral root formation, mediated in part by the NAC1 protein. But to prevent an over-proliferation of lateral roots, auxin also promotes degradation of the NAC1 protein through increased levels of an E3 ligase that catalyses NAC1 ubiquitylation. Other hormonal and defence signalling pathways could also employ the UPS both to initiate and then to dampen a biological response.
Like SINAT5, two other components of the UPS involved in lateral root development are also transcriptionally up-regulated by auxin, namely the RING E3 ligase, XBAT32 (Nodzon et al., 2004), and the F-box protein CEGENDUO (Dong et al., 2006); however, no substrates for these proteins have been identified. It is interesting to note that the former is a positive regulator of lateral root formation while the latter inhibits lateral root development, again suggesting a potential dual stimulatory and inhibitory role for the UPS in this developmental process.
Several years ago, scientists also uncovered a link between the UPS and auxin transport. AtPIN2/EIR1, an auxin efflux carrier (Petrasek et al., 2006), does not appear to be transcriptionally regulated by auxin, but the levels of a PIN2/EIR1:GUS fusion protein drop following treatment with the auxin 1-NAA or a protein synthesis inhibitor, suggesting that the protein is short-lived and its degradation is regulated by auxin (Sieberer et al., 2000). NAA-mediated loss of EIR1:GUS is slowed by the axr1-3 mutation, intimating that degradation of this protein is regulated by a CRL (Sieberer et al., 2000). This could be through a direct mechanism, or the stability of PIN2/EIR1 could be controlled by a component of the UPS under AXR1-mediated control.
A recent report verified and extended that study by demonstrating that inhibition of proteasome activity results in accumulation of PIN2 protein and by visualizing ubiquitylated forms of PIN2 (Abas et al., 2006). Gravistimulation leads to asymmetric distribution of both endogenous PIN2 and a PIN2:GFP reporter protein that can be blocked by treatment with a proteasome inhibitor. Internalization of the PIN2 protein also appears to be affected by a proteasome-dependent process (Abas et al., 2006). Thus, the UPS affects steady-state levels of PIN2 at the plasma membrane and consequently influences the amount and direction of auxin transport within a plant, but then auxin itself appears to affect the stability of PIN2. This may constitute an important feedback loop to localize and then limit auxin in specific cells.
Gibberellins and the UPS
The gibberellins (GAs), a group of diterpenoid compounds with phytohormone activity, affect various stages of plant development, including seed germination, stem elongation, root growth, flowering and pollen tube elongation (reviewed in Davies, 2004; Swain and Singh, 2005) and like auxin signalling, gibberellin-induced changes in transcription are an important part of the response pathway (Fleet and Sun, 2005). Several features of the GA/UPS-mediated signalling cascade resemble characteristics of the Aux/IAA branch of the auxin signalling pathway. The DELLA proteins, named for highly conserved amino acids at their N-termini, act, like Aux/IAA proteins, as negative regulators of GA signalling. There are five proteins in this family in arabidopsis [GAI (GA insensitive), RGA (Repressor of ga1-3), RGL1 (RGA-like), RGL2 and RGL3 (Dill et al., 2001; Peng and Harberd, 2002)]. SLENDER RICE1 in rice (SLR1) and SLENDER1 in barley (Hordeum vulgare) (SLN1) are single DELLA proteins in these plants (Ikeda et al., 2001) and various other species also have homologous proteins (Thomas and Sun, 2004). As for Aux/IAA proteins, DELLA proteins are subject to hormone-mediated acceleration of degradation. Levels of both endogenous RGA and a GFP:RGA fusion protein drop following GA treatment of arabidopsis seedlings (Silverstone et al., 2001). Protein loss depends on an SCF complex containing the AtSLY1 (SLEEPY) F-box protein (Swain and Singh, 2005). sly1 mutants contain elevated levels of RGA and can no longer eliminate this protein following GA treatment (McGinnis et al., 2003). DELLA proteins (RGA and GAI) and SLY1 interact directly through both yeast-two-hybrid assays and in vitro pull-down experiments using GST-SLY1 (Dill et al., 2004). A single arabidopsis homologue, SNEEZY (SNE), when overexpressed, can compensate for loss of SLY1 suggesting that it also acts as a substrate-recruiting protein for DELLA proteins (Strader et al., 2004). The rice DELLA protein SLR1 also appears to be ubiquitylated through the activity of an SCF complex using the GID2 (Gibberellin-Insensitive Dwarf2) F-box protein (Itoh et al., 2003) in a GA-dependent manner, indicating a conserved function for rice GID2 and its arabidopsis orthologue SLY1.
How gibberellin promotes DELLA protein degradation is unclear. One possibility is that GA-induced post-translational modification of the DELLA proteins enhances their interactions with the F-box component of the SCF E3 ligase. The DELLA proteins do appear to become phosphorylated in the presence of elevated levels of GA in rice (Itoh et al., 2003) and only phosphorylated SLR1 can interact with the GID2 F-box protein in vitro (Gomi et al., 2004). In arabidopsis, there is also evidence for binding of phosphorylated GAI by the F-box protein SLY1 (Fu et al., 2004). But the steps connecting GA and DELLA phosphorylation have not been elucidated.
Recent identification of the first bona fide gibberellin receptors in rice (Ueguchi-Tanaka et al., 2005) and arabidopsis (Nakajima et al., 2006) indicates that the receptor plays a role in GA-induced DELLA degradation. The soluble GA receptors, called GID1 in rice and GID1a, b and c in arabidopsis, bind bioactive GAs. gid1 rice mutants exhibit classic GA insensitivity phenotypes such as dwarfed stature, dark green leaves and lack of alpha-amylase induction in seeds. Sequence homology gives little insight into the molecular mechanisms that underlie GID1-mediated signalling. These proteins resemble hormone-sensitive lipases (HSLs), but lack one of the three conserved residues in the catalytic triad, and rice GID1 cannot hydrolyse an artificial HSL substrate (Ueguchi-Tanaka et al., 2005). However, it is clear that GID1 can bind to the DELLA proteins in a GA-dependent manner recapitulated in yeast and in vitro (Ueguchi-Tanaka et al., 2005; Nakajima et al., 2006). In the rice gid1 mutant, the SLR1 DELLA protein accumulates to higher levels and SLR1 levels fail to drop following treatment with GA, demonstrating that GA-mediated SLR1 degradation depends upon GID1 (Ueguchi-Tanaka et al., 2005). Interestingly, the DELLA proteins RGA and GAI can actually enhance GID1c's affinity for 16,17-dihydro-GA4, suggesting that this DELLA/GID1 two-protein complex may actually be the biological binding target of the hormone (Nakajima et al., 2006). If this model is correct, formation of the GA-GID1-DELLA complex may be required for productive SCFSLY1/GID2-mediated ubiquitylation of the DELLA proteins, and it is unclear how phosphorylation affects this process (Nakajima et al., 2006).
In addition to contributing to GA-mediated regulation of germination, growth and flowering (Wen and Chang, 2002; Macmillan et al., 2005; reviewed in Peng and Harberd, 2002), recent studies demonstrate the importance of the DELLA signalling pathway for plant survival under salt stress. Arabidopsis seedlings grown on 100 mm salt accumulate lower levels of bioactive GA1 and GA4, which should lead to increased DELLA stability. Indeed, a 1-h treatment with 50 mm NaCl is sufficient to increase the levels of a GFP:RGA reporter protein. Salt-induced stabilization of DELLA proteins can then inhibit plant growth to enhance stress tolerance (Achard et al., 2006).
Interestingly, three other hormones have been implicated in regulation of DELLA stability. Depletion of auxin from arabidopsis seedlings through decapitation leads to reduced GA-mediated loss of GFP:RGA from root nuclei whereas application of auxin to the shoot restores rapid GA-induced reduction in GFP:RGA levels (Fu and Harberd, 2003). Ethylene exerts an opposite effect; seedlings grown in ethylene-rich air show slower loss of GFP:RGA from nuclei following GA treatment (Achard et al., 2003). This implies that a ctr1-1 mutant exhibiting a constitutive ethylene response phenotype should accumulate more DELLA proteins capable of promoting stress tolerance. In keeping with this model, ctr1-1 mutants display enhanced survival under high salt conditions. This improved salt tolerance depends at least partially on DELLA proteins as a triple mutant ctr1-1 gai-t6 rga-24 that lacks two DELLA proteins is more susceptible to salt stress than ctr1-1 (Achard et al., 2006). Abscisic acid (ABA) levels also affect DELLA accumulation. Roots treated with ABA show enhanced accumulation of a GFP:RGA fusion protein, both under basal conditions and following treatment with exogenous GA. But, plants mutant for abi1, a serine/threonine phosphatase that positively regulates ABA signalling, no longer accumulate GFP:RGA following ABA treatment (Achard et al., 2006). Intriguingly, this regulation may be cell-type-, organism- or family member-specific as SLN1 protein levels are not affected by ABA in barley aleurone cells (Gubler et al., 2002). It is unclear how these changes in auxin, ethylene and ABA levels cause differential effects on DELLA protein stability, but it does provide an intriguing example of hormonal cross-talk in which multiple pathways can converge to influence the stability of a single family of transcriptional regulators.
GA and ABA also play well-characterized antagonistic roles in regulation of hydrolase production in the aleurone layer of germinated cereal seeds through their effects on another transcription factor, GAMYB. This positive regulator of alpha-amylase expression is transcriptionally up-regulated by GA (Gubler et al., 1995) and repressed by ABA (Gomez-Cadenas et al., 2001). The SLN1 DELLA protein is implicated in the GA-mediated transcriptional regulation of GAMYB (Gomez-Cadenas et al., 2001; Gubler et al., 2002), but a more recent yeast-two-hybrid assay pulled out another potential regulator of GAMYB in barley, named GMPOZ (Woodger et al., 2004). This GAMYB interacting protein derives part of its name from its POZ domain, also known as a BTB domain. This provides a potential link between GAMYB and a CUL3-based CRL. Knock-down of GMPOZ in aleurone cells decreases GA-mediated transcription of an alpha-amylase reporter construct, suggesting that GMPOZ normally positively regulates GA signalling. At the same time, the cells show enhanced ABA-mediated stimulation of a dehydrin reporter, arguing that GMPOZ normally negatively regulates ABA signalling in the barley aleurone layer. As GAMYB protein levels were not assayed, it is unclear how GMPOZ directly affects transcription of GA- and ABA-responsive genes. But, based on the observed functions of BTB proteins in other organisms, it could potentially interact directly with elements of the basal transcription machinery, as seen for BAB1 and BAB2 in Drosophila (Pointud et al., 2001), regulate the stability of GAMYB and/or other transcription factors as seen in Keap1-mediated destabilization of the Nrf2 transcription factor in humans (Furukawa and Xiong, 2005), or even be degraded itself by a CUL3-based CRL as observed for MEL-26 in C. elegans (Pintard et al., 2003b).
Genetic evidence also suggests that a HECT-type E3 ubiquitin ligase, UPL3, influences the GA-regulated process of trichome development as upl3-1 and upl3-2 mutants possess trichomes with elevated numbers of branches, similar to some GA-signalling mutants. upl3-1 and upl3-2 mutants are moderately hypersensitive to GA in a hypocotyl elongation assay, suggesting that some positive potentiator of GA signalling is more abundant in the absence of the UPL3 protein (Downes et al., 2003).
PHOR1, a predicted E3 ligase initially identified in potato (Solanum tuberosum), also appears to participate in GA signalling (Amador et al., 2001). This protein contains a U-box domain (Hatakeyama et al., 2001; Monte et al., 2003), and a series of ARM repeats. An ARM repeat, first identified in the Drosophila protein ARMadillo, is approximately 40 amino acids in length and is typically present in a variable number of tandem copies and functions in protein–protein interactions (reviewed in Hatzfeld, 1999). Plants expressing lowered levels of PHOR1 have shorter, GA-insensitive stems, whereas plants constitutively over-expressing PHOR1 develop longer stems that are hypersensitive to treatment with low levels of GA. Both sets of experiments argue that PHOR1 is normally a positive regulator of GA-stimulated growth. Increased levels of bioactive (GA20) and inactive (GA8, GA29) gibberellins accumulate in PHOR1 antisense lines consistent with a role for PHOR1 in regulation of GA metabolism, and indicative of a lack of normal feedback inhibition in this genetic background. Although a PHOR1:GFP fusion protein can be found in the cytoplasm or nucleus of tobacco BY cells following transient transfection, the majority of PHOR1:GFP signal is localized to the nucleus following treatment with exogenous GA (Amador et al., 2001). Three arabidopsis proteins, AtPUB27, AtPUB28 and AtPUB29, are more than 50% identical to PHOR1 and may link the UPS to GA signalling in arabidopsis through degradation of unidentified substrate(s) (Monte et al., 2003).
Although there is no evidence for UPS-mediated regulation of GA transport, a recent paper presents an exciting link between light signalling, targeted protein degradation and regulation of GA biosynthesis prior to seed germination (Oh et al., 2006). For many years it has been known that light signalling through the phytochrome family of photoreceptors can promote seed germination (Shinomura et al., 1994; Hennig et al., 2002), and more recently it was discovered that this occurs through light-induced transcriptional up-regulation of GA biosynthetic enzymes (Yamaguchi et al., 1998). The PIL5 (PIF3-Like 5) transcription factor represses seed germination by regulating the abundance of transcripts for GA biosynthetic and catabolic enzymes to decrease GA levels in seeds kept in the dark. Consistent with this finding, over-expression of PIL5 blocks up-regulation of the GA biosynthetic enzymes GA3ox1 and GA3ox2 and simultaneously promotes expression of the GA2ox2 oxidase that inactivates GA4 and other biologically active GAs (Oh et al., 2006). Now there is evidence that PhyA and PhyB-mediated signalling can prompt degradation of Myc-tagged PIL5 to increase GA levels and promote germination in response to light. Higher levels of the PIL5 fusion protein accumulate in phyA mutants than in wild-type plants following a pulse of red light, and when examined over a longer period, phyB mutants also show elevated levels of Myc-tagged PIL5 relative to wild-type seedlings following a pulse of red or far-red light. The proteasome inhibitor MG132 blocks light-stimulated loss of Myc-PIL5 following a red or far-red light pulse, arguing that the UPS is required for the normal reduction of PIL5 protein levels in plants exposed to light (Oh et al., 2006). As PIL5 is degraded, GA biosynthetic genes are up-regulated while a GA catabolic gene is repressed, leading to the overall accumulation of GA required for seed germination.
Abscisic acid and the UPS
The hormone abscisic acid (ABA) is another prominent regulator of seed germination that also enables plants to respond to abiotic stresses such as drought. ABA can directly affect ion transport in guard cells to alter stomatal aperture rapidly in response to changing water availability (reviewed in Roelfsema et al., 2004), but other slower ABA responses require changes in transcription. And, as observed for other phytohormones, the UPS is implicated in regulation of ABA-responsive transcription.
The first evidence of ABA-mediated changes in protein stability was observed for the ABI5 (abscisic acid insensitive5) protein in arabidopsis. Unlike the Aux/IAA and DELLA proteins, this basic leucine zipper transcription factor acts as a positive regulator of ABA responses. Therefore, it might not seem surprising that rather than promoting degradation of ABI5, abscisic acid is required for the maintenance of high levels of the ABI5 protein. This was discovered using transgenic arabidopsis plants expressing an HA:ABI5 fusion protein under the control of the constitutive 35S promoter. Although the levels of transcript for this fusion protein remain unaltered following ABA treatment, HA:ABI5 protein levels increase substantially. ABA treatment also blocks loss of the ABI5 protein from cycloheximide-treated seedlings incapable of performing de novo protein synthesis (Lopez-Molina et al., 2001), and treatment of seedlings with proteasome inhibitors leads to the accumulation of ubiquitylated HA:ABI5 (Lopez-Molina et al., 2003), implicating the UPS in ABI5 degradation. This hypothesis is strengthened by the observation that ABI5 is stabilized in the rpn10-1 mutant that has a defect in one of the nine RPN (Regulatory Particle, Non-ATPase) subunits of the 26S proteasome lid. Interestingly, two other proteasome substrates, PhyA and HY5, are not stabilized in this mutant background, suggesting that RPN10 may promote degradation of a subset of proteasome substrates including ABI5 (Smalle et al., 2003). It is unclear how ABA stabilizes ABI5, but several experiments demonstrate that the ABI5-interacting protein (AFP) plays a role in down-regulating ABI5 protein levels in the absence of ABA (Lopez-Molina et al., 2003). Upon removal of ABA from the media, seedlings that are over-expressing AFP show a much more rapid loss of ABI5 than wild-type seedlings or afp mutants. ABI5 and AFP were initially shown to interact in a yeast-two-hybrid assay and this association was confirmed in planta through co-immunoprecipitation experiments. Fluorescently tagged AFP and ABI5 proteins also co-localize in nuclear protein bodies in transgenic arabidopsis seedlings treated with ABA. These nuclear protein bodies also include the COP1 RING E3 ligase when all three proteins are bombarded into onion epidermal cells (Lopez-Molina et al., 2003). However, to date there is no evidence that COP1 ubiquitylates ABI5, so the search is still on for the E3 ligase that acts upon ABI5, and for the abscisic acid receptor and/or other signalling components that link altered ABA levels to changes in ABI5 proteolysis.
The degradation pathway of another ABA-responsive transcription factor, ABI3, has also been studied. This B3-domain-containing transcription factor plays a prominent role in ABA-signalling. Like ABI5, an ABI3:6Myc fusion protein was recently found to be relatively short-lived under basal conditions, but to be long-lived in the presence of proteasome inhibitors (Zhang et al., 2005). It appears that ABI3 degradation is mediated, at least in part, by the AIP2 (ABI3-Interacting Protein2) RING-type E3 ubiquitin ligase. ABI3 and AIP2 interact in a yeast-two-hybrid assay and tagged versions of these proteins co-immunoprecipitate in arabidopsis extracts. Moreover, ABI3 is a substrate of AIP2-mediated ubiquitylation in an in vitro assay. Not surprisingly, over-expression of AIP2:3 × HA reduces the accumulation of ABI3 in arabidopsis seedlings, whereas levels of the endogenous ABI3 protein increase in aip2-1 mutants. ABA regulates the stability of ABI3, but unlike for ABI5, elevated levels of ABA reduce ABI3 accumulation. Similar to the NAC1/SINAT5 signalling system in the auxin pathway, it seems that ABA-mediated transcriptional up-regulation of the AIP2 RING-E3 ligase allows it to degrade more ABI3, providing a partial mechanistic explanation for the link between ABA levels and ABI3 proteolysis. Nevertheless, Zhang et al., (2005) conclude that AIP2 might target other proteins for ubiquitin-mediated degradation, and that ABI3 may also be ubiquitylated by other E3 ligases.
Yet another positive regulator of ABA transcription, the ABF2 (ABRE Binding Factor2) transcription factor, also interacts with a potential component of an E3 ligase, ARIA (ARM protein Repeat Interacting with ABF2), in both yeast-two-hybrid assays and in vitro pull-down experiments (Kim et al., 2004). Over-expression or knock-out of ARIA affects a subset of ABA-regulated processes and indicates that ARIA is a positive regulator of ABA signalling. For example, aria mutants show reduced sensitivity to ABA in root elongation assays. In addition to its ARM repeats, ARIA possesses a BTB domain. Although a GST:ARIA fusion protein can pull down ABF2 in vitro (Kim et al., 2004), it could also potentially mediate interactions with a CUL3 protein and thereby link ABF2-regulated processes to the UPS. As in the case of the GMPOZ BTB-protein involved in negative regulation of ABA signalling in barley (see above), there is no evidence of whether ARIA or its potential substrates undergo UPS-mediated degradation.
Several other sets of experiments highlight potential relationships between ABA signalling and the UPS. First, a characterization of the ATL [Arabidopsis Toxico para Levadura (toxic to fungi)] family of RING E3 ligases included a phenotypic analysis of lines with T-DNA insertions in various ATL genes. A mutation in ATL43 renders seedlings insensitive to ABA in a seed germination assay (Serrano et al., 2006). The U-box E3 ligase AtCHIP may also be involved in ABA signalling. Over-expression of the protein makes plants hypersensitive to the effects of ABA in a germination assay and in a stomatal aperture assay. AtCHIP seems capable of attaching a single ubiquitin moiety to the A subunit of protein phosphatase 2A, but it is unclear whether this is functionally related to the ABA signalling pathway (Luo et al., 2006).
The possible involvement of an SCF-complex in ABA signalling was uncovered through characterization of TLP9 (Lai et al., 2004). This member of the TUBBY-LIKE family of genes in arabidopsis contains a C-terminal tubby domain [named for a protein identified in obese mutant mice (Kleyn et al., 1996)] and an N-terminal F-box domain. Yeast-two-hybrid assays confirm that this F-box protein can interact with arabidopsis ASK1. Phenotypic analyses of two tlp9 insertional mutants indicate that this protein is required for normal ABA signalling. The mutant plants show reduced sensitivity to ABA in seedling germination assays whereas plants over-expressing TLP9 under the control of the 35S promoter exhibit ABA hypersensitivity and lower rates of germination. These phenotypes lead to the prediction that TLP9's substrate(s) are negative regulators of ABA signalling or levels (Fig. 2B), but none has been identified to date. It is interesting to note that in the abi1 ABA-insensitive mutants, TLP9 transcript levels are reduced (Lai et al., 2004).
Finally, there is new evidence that the XERICO RING E3 ligase contributes to regulation of ABA levels in arabidopsis, potentially in response to osmotic and salt stress. Transcription levels of this gene rise in response to high salt and osmotic stress – conditions that normally increase ABA levels (Ko et al., 2006; reviewed in Zhu, 2002). Over-expression of XERICO prompts an increase in ABA levels in transgenic plants relative to wild-type plants when both are grown in well-watered conditions; drought conditions further increase ABA accumulation even in the 35S:XERICO plants. Transcriptional profiling demonstrates an up-regulation of the NCED ABA biosynthetic gene in both wild-type and transgenic plants following treatment with ABA, but the level of induction is greater in the 35S:XERICO plants. XERICO interacts with the E2 ubiquitin-conjugating enzyme, UBC8, in yeast-two-hybrid assays. Unexpectedly, the TLP9 F-box protein also interacts with XERICO in this experiment. Although both appear to function in ABA signalling and show a high degree of co-expression at the transcriptional level, the functional significance of this novel RING/F-box protein interaction has not been discerned (Ko et al., 2006).
Brassinosteroids and the UPS
Brassinosteroids (BRs), a class of polyhydroxylated steroid hormones, also have pleiotropic effects on plant development and regulate processes such as cell division, cell elongation, photosynthesis, vascular differentiation and stem elongation (reviewed in Bishop and Koncz, 2002).
In the BR signal transduction pathway, the first hint that the UPS is involved in BR-mediated transcriptional responses came from analyses of the bes1-D (bri1-EMS-suppressor 1 Dominant) and bzr1-1D (brassinazole-resistant 1-1Dominant) mutants that display BR hypersensitivity (Wang et al., 2002; Yin et al., 2002). These dominant mutants harbour identical proline to leucine missense substitutions in two related proteins that are transcription factors. Analysis of bzr1-1D:CFP and BZR1:CFP fusion proteins in arabidopsis seedlings demonstrates that the mutant protein accumulates to substantially higher levels, indicating that it is stabilized relative to its wild-type counterpart. This suggests that these positive regulators of BR-mediated transcriptional changes must normally be degraded to prevent constitutive BR signalling. Subsequent work identified additional regulatory components that led to the development of a model of BR signalling with some similarity to the Wnt/GSK3/β-catenin signalling pathway found in metazoans (reviewed in Vert et al., 2005; Cadigan and Liu, 2006). New findings, such as those regarding subcellular localization of BR signalling components, highlight the potential for significant differences between these signal transduction cascades (reviewed in Vert et al., 2005; Vert and Chory, 2006), but some similarities may exist. For instance, in the absence of BR, the BIN2 (Brassinosteroid insensitive2) GSK3-like kinase is hypothesized to phosphorylate and thereby destabilize BES1 and BZR1. BIN2 can interact directly with BZR1 in a yeast-two-hybrid assay and can phosphorylate it in vitro (He et al., 2002). Treatment with proteasome inhibitors stabilizes the BZR1 protein, suggesting that it is degraded by the UPS. In addition, the phosphorylated form of BZR1 appears to be the substrate for proteolysis as its abundance is affected more significantly by the proteasome inhibitor treatment than the hypo/un-phosphorylated form of the protein (He et al., 2002). But when BR levels rise, the membrane-bound BRI1 (Brassinosteroid insensitive1) and BAK1 (BRI1-associated receptor kinase) leucine-rich repeat receptor kinases somehow cause an accumulation of hypophosphorylated BES1 and BZR1 (He et al., 2002; Yin et al., 2002). Two mechanisms may contribute to this outcome. First, the receptors might signal to BIN2 to decrease its phosphorylation activity toward BES1 and BZR1. Second, the activity of the nuclear-localized BSU1 (bri1 Suppressor1) phosphatase may also increase. BSU1 can dephosphorylate BES1 in vitro and an activation-tagged line that expresses elevated levels of the BSU1 protein shows an accumulation of the hypophosphorylated form of BES1, demonstrating that this phosphatase also acts on BES1 in vivo (Mora-Garcia et al., 2004). As the levels of dephosphorylated BZR1 and BES1 proteins rise within the nucleus, they can turn on BR-responsive genes (reviewed in Vert et al., 2005). Although their phosphorylation status was initially suggested to be a prime regulator of BES1 and BZR1 protein accumulation, recent evidence suggests that phosphorylation has a much more dramatic effect on BES1's ability to interact with other transcription factors and to bind to and activate transcription from BR-sensitive promoters (Vert and Chory, 2006). So, although BES1 and BZR1 may be relatively short-lived proteins, it does not seem that BR-mediated stabilization of these proteins is required for BR signalling. Nevertheless, identification of the E3 ubiquitin ligases responsible for BES1 and BZR1 degradation will contribute to a fuller understanding of the BR signal transduction cascade.
Although the stability of the BIN2 kinase (Vert and Chory, 2006) and BSU1 phosphatase (Mora-Garcia et al., 2004) are reported to be unaffected by brassinosteroid levels, other components of the BR pathway may be regulated by the UPS. As seen in the auxin and abscisic acid signalling pathways, BR appears to regulate transcriptionally a RING E3 ubiquitin ligase. The BRH1 (Brassinosteroid-responsive RING-H2) transcript is down-regulated by addition of brassinolide in a BRI1-dependent manner. No putative substrates have been identified for this E3 ligase, but it is interesting to note that it is transcriptionally up-regulated by the elicitor chitin, suggesting a potential and novel antagonistic link between BR and pathogen signalling that involves the UPS (Molnar et al., 2002).
Ethylene and the UPS
The small gaseous hormone ethylene regulates several aspects of the plant life cycle including seed germination, flower development, flower senescence and fruit ripening. Ethylene is also produced in response to several types of abiotic and biotic stresses, including pathogen attack (reviewed in Klee, 2004; Chen et al., 2005; van Loon et al., 2006). Thus, ethylene is a regulator both of endogenous developmental programmes, and externally stimulated stress adaptations and plant defences. The UPS appears to function in all of these ethylene-mediated processes.
Transcriptional changes resulting from altered ethylene levels play a vital role in the ethylene signal transduction pathway. The EIN3 (Ethylene-insensitive 3) transcription factor and its homologues act as positive regulators of ethylene responses by turning on downstream transcriptional regulators such as the ERF1 (Ethylene Response Factor) gene (Solano et al., 1998). Ethylene-dependent stabilization of EIN3 thus initiates an important transcriptional cascade (reviewed in Alonso and Stepanova, 2004). EIN3 abundance is controlled by an SCF E3 ubiquitin ligase containing one of two related F-box substrate specificity factors: EBF1 or EBF2 (EIN3-Binding F-box) (Potuschak et al., 2003; Gagne et al., 2004; Guo and Ecker, 2004). These proteins interact with EIN3 and the related EIL1 protein in a yeast-two-hybrid experiment, and EIN3 is pulled down by both GST:EBF1 and GST:EBF2 in vitro (Potuschak et al., 2003). The ethylene precursor ACC increases the stability of an EIN3:FLAG fusion protein (Yanagisawa et al., 2003) and endogenous EIN3 (Guo and Ecker, 2003) in arabidopsis seedlings, but it is unclear exactly how ethylene regulates this process.
Several upstream components in the ethylene signalling pathway, including the ETR/ERS receptor, and EIN2, EIN5 and EIN6 all need to be functional for EIN3 to accumulate. In addition, the CTR1 kinase also contributes to EIN3 proteolytic regulation, because constitutively active ctr1 mutants show elevated levels of EIN3. But CTR1 cannot be the sole regulator of EIN3 stability, as addition of exogenous ethylene still promotes EIN3 accumulation even in a ctr1-1 mutant (Guo and Ecker, 2003). One possible mode of regulation would be at the level of EBF abundance. This model would predict a decreased level of EBF protein as ethylene levels rise. But, interestingly, activation of the ethylene signalling cascade, through EIN3, actually induces EBF2, but not EBF1, transcription, perhaps to promote a negative feedback loop to prevent excessive ethylene response (Potuschak et al., 2003). This differential transcriptional regulation of EBF1 and EBF2 fits with the hypothesis that EBF1 and EBF2 play slightly different roles in regulating EIN3 stability. Analysis of mutant phenotypes suggests that EBF1 may be sensitive to lower levels of ethylene and act as a constitutive damper on the ethylene pathway, whereas EBF2 may play a more significant role at higher ethylene levels, and when the plant wants to shut down an acute ethylene response (Gagne et al., 2004). As the abundance of the EBFs does not seem to be inversely correlated with EIN3 stability, post-translational modification of one or the other could influence their binding abilities. Given that these proteins interact both in yeast-two-hybrid assays and in vitro, post-translational modification of EIN3 or the F-boxes is unlikely to be a requirement for the interactions. However, it is possible that ethylene-mediated modification of EIN3 or the EBFs could disrupt their binding and thereby slow EIN3 degradation.
Although there is much interest in characterizing ethylene's ability to modulate EIN3 stability, efforts are also underway to ascertain the relationship between sugar signalling and EIN3 proteolysis. EIN3 stability is subject to regulation by glucose through the hexokinase signalling pathway. Because ethylene and glucose often have antagonistic effects on plant growth, it makes sense that glucose actually promotes proteolysis of EIN3, which was demonstrated using Flag-, Myc- and GFP- tagged EIN3 proteins in protoplasts and transgenic arabidopsis seedlings (Yanagisawa et al., 2003). Although glucose-mediated loss of EIN3 is severely impaired by treatment with proteasome inhibitors, the specific components of the UPS that regulate EIN3 stability in response to glucose have not been identified. Glucose could act through the EBF1/2-based system, or through a completely novel ubiquitin ligase.
EIN3 may not be the only transcription factor involved in the ethylene response that is regulated at the level of proteolysis. The ERF3 gene of tobacco encodes a transcription factor capable of repressing ethylene responses (Koyama et al., 2003). The mRNA level of its homologue in Nicotiana sylvestris, NsERF3, is increased by ethylene (Kitajima et al., 2000). Yeast-two-hybrid experiments demonstrate an interaction between the ERF3 protein and an E2 ubiquitin-conjugating enzyme NtUBC2 that depends on the region found between the conserved ERF domain and the EAR-motif-containing repressor domain in ERF3. Interestingly, two ERFs from different subfamilies that possess transcriptional activation, as opposed to repressive, capabilities do not interact with the UBC. The yeast-two-hybrid truncated NtUBC2 clone capable of interacting with ERF3 was used to identify full-length NtUBC2 and a closely related protein designated NtUBC1. There is some evidence that NtUBC2 might regulate ERF3. Transient co-expression of ERF3 and a putative dominant-negative form of NtUBC2 in tobacco cells increase ERF3's ability to repress transcription, presumably owing to stabilization of ERF3 by the mutant NtUBC2 (Koyama et al., 2003). To date, it is unknown whether this process is affected by ethylene or whether an E3 is required to bring UBC2 and ERF3 together in vivo.
Although there is evidence that ethylene is capable of modulating the degradation of components of its own signalling pathway, the identification of the hss1 mutant demonstrated that ethylene can also regulate the stability of proteins functioning in the auxin signalling pathway, providing another example of cross-talk occurring through the UPS. Both ethylene and auxin play roles in regulating apical hook development in dark-grown seedlings. One protein required for this developmental programme is HOOKLESS1 (HLS1). HLS1 encodes a putative N-acetyltransferase that is transcriptionally up-regulated by ethylene and is required for apical hook maintenance (Lehman et al., 1996). It also appears to mediate cross-talk with the auxin signalling pathway as a mutation in ARF2 (Auxin response factor2) can suppress many aspects of the hls1 phenotype. ARF2 is a member of a family of transcriptional regulators that bind to auxin response elements (AuxREs) in gene promoters (reviewed in Guilfoyle et al., 1998; Okushima et al., 2005), and recent studies posit that ARF2 is a negative regulator of cell division and organ growth (Schruff et al., 2006). Although ARF2 transcript levels do not change in the hls1 mutants, the level of the ARF2 protein rises, suggesting that HLS1 normally negatively regulates ARF2 accumulation. Consequently, increased levels of ethylene sufficient to increase HLS1 levels cause a concomitant drop in ARF2 protein levels (H. Li et al., 2004). Ethylene's ability to modulate ARF2 levels depends on the presence of the HLS1 protein, and on a functional proteasome as treatment with the MG132 proteasome inhibitor blocks ACC-mediated loss of ARF2.
Light also affects the accumulation of HLS1 and thus ARF2. Even in the presence of exogenous ACC, light can down-regulate the levels of the HLS1 protein, which is correlated with a subsequent rise in ARF2 protein levels. It is unclear both how light affects HLS1 protein abundance, and how HLS1 lowers the stability of ARF2. The transfer of an acetyl group to a substrate by HLS1 could affect ARF2 proteolysis, but based on several experiments, ARF2 does not appear to be its substrate (H. Li et al., 2004). However, other components of the degradation machinery could be regulated by this post-translational modification and their identification should lend insight into UPS-mediated integration of light, ethylene and auxin signalling in apical hook maintenance.
Rising ethylene levels certainly lead to UPS-mediated changes in transcription, but several lines of evidence demonstrate that ethylene production itself is governed in part by the UPS. Evidence for CRL-based regulation of ethylene production arose from two distinct lines of research. From the UPS side of the story, post-transcriptional gene silencing of the closely related RUB1 and RUB2 proteins, which modify CRLs (see above) and regulate their activity, causes overproduction of ethylene in dark-grown arabidopsis seedlings (Bostick et al., 2004). And mutation of the RUB-conjugating enzyme RCE1 also increases ethylene production in dark-grown seedlings (Larsen and Cancel, 2004), again suggesting that RUB conjugation to one or more CRLs negatively regulates some aspect of ethylene production. Despite the existence of hundreds of unique CRLs in arabidopsis that could be affected by the rubylation pathway, one candidate emerged from independent studies of plants with defects in regulation of ethylene synthesis, namely Ethylene Overproduction (ETO) mutants. The eto1-1 mutation disrupts a protein with a BTB domain capable of binding to CUL3 and acting as the substrate recognition subunit in a CUL3-based E3 ubiquitin ligase (Wang et al., 2004).
ACC synthase (ACS) isozymes are probable substrates for this CUL3ETO1 E3 ligase. The abundance of ACS biosynthetic enzymes largely governs ethylene production levels (reviewed in Kende, 1993; Chae et al., 2003). Changes in ACS transcript levels have been shown to affect ethylene levels (Bleecker and Kende, 2000), but post-translational regulation is also evident for at least some ACS family members found in tomato (Solanum lycopersicum) and other plants (Kim and Yang, 1992). ETO1 and related family members ETO-LIKE1 and 2 (EOL1, 2) can bind to ACS5 in yeast-two-hybrid assays. Two other ethylene overproduction mutants, eto2 and eto3, bear mutations in the ACS genes ACS5 and ACS9, respectively (Vogel et al., 1998; Chae et al., 2003). Both eto2-1/acs5 and eto3-1/acs9 mutants possess mutations within their C-termini. Mutant eto2-1/acs5 protein is longer-lived than wild-type ACS5. This increased stability depends upon the ETO1 protein (Chae et al., 2003). Whereas wild-type HA:ACS5 can be pulled down from plants by wild-type RGS:His-tagged ETO1, a mutant form of HA:acs5/eto2-1 does not interact with this protein, suggesting that this mutant protein remains stable because it escapes detection and ubiquitylation mediated by ETO1. In addition, ETO1 interaction with ACS5 inhibits ACS activity, suggesting an additional mode of regulation (Wang et al., 2004).
ACS5 appears to be another point of convergence for two hormone signalling pathways because cytokinin treatment of arabidopsis etiolated seedlings leads to ethylene overproduction and to an increase in the half-life of an Myc:ACS5 fusion protein (Chae et al., 2003). These findings allow the development of a general model in which a CUL3-based ubiquitin ligase containing the ETO1 adaptor protein binds to the C-terminus of ACS5 and targets it for ubiquitylation and degradation. However, at least two major questions remain to be answered.
First, ethylene levels change during many developmental processes and in response to external stimuli. This implies that ETO1-based ACS5 degradation should be slower under conditions that promote ethylene synthesis, but there is no definitive explanation for this phenomenon. Post-translational modification of ACS5 may regulate its interactions with ETO1 or affect components of the UPS. A tomato ACC synthase, ACS2 (Tatsuki and Mori, 2001), undergoes phosphorylation in its C-terminus by a wound-inducible calcium-dependent protein kinase. If this disrupted interactions between the ACS and ETO1, thereby stabilizing the enzyme, it could contribute to wound-induced ethylene production (Tatsuki and Mori, 2001). Although neither tomato ACS2 nor arabidopsis ACS5 have predicted consensus CDPK phosphorylation sites, experiments performed using synthetic peptides demonstrate that they both contain a non-canonical CDPK target site in their C-termini. Furthermore, a comparison of peptides derived from the C-terminus of arabidopsis ACS5/9 (which are identical in this region) containing the wild-type sequence or the equivalent of an eto3 mutation showed that the latter is a better substrate for CDPKI from maize (Zea mays) (Hernandez Sebastia et al., 2004), providing some support for a model in which increased CDPK-dependent phosphorylation of acs mutants blocks ETO1 binding, and hence ubiquitylation. However, there is no definitive evidence of ACS5 or ACS9 phosphorylation in arabidopsis to date.
There is evidence that other kinases can play a role in regulating the stability of different ACS family members. SIPK, a tobacco MAPK that is induced by numerous stimuli including drought, osmotic stress and UV irradiation, also increases ethylene signalling by increasing ACS activity (Kim et al., 2003). Examination of AtMPK6, the SIPK orthologue, indicates that this MAPK contributes to increased ethylene production in arabidopsis, as well. No putative MAPK phosphorylation sites exist in ACS5 or ACS9, although they are found in the C-terminus of ACS2 and ACS6. Accordingly, plant-derived MPK6 can phosphorylate recombinant ACS2 and ACS6, but not ACS5. Conditions that activate MPK6 increase the levels of FLAG-tagged ACS6 in arabidopsis seedlings, and a phosphomimic ACS6 mutant (with serine to aspartate substitutions) accumulates even in the absence of elevated MPK6 activity (Liu and Zhang, 2004). It will be interesting to determine whether specific kinase/ACS combinations that regulate ethylene production all go through ETO1 and its homologues, or if other specific E3 ligase components are involved.
Another outstanding question concerns cytokinin's influence on ACS5 stability. Although the C-termini of the ACSs appear to be hot spots for regulatory modifications, cytokinin-mediated stabilization of ACS5 cannot be solely explained by post-translational modification of the C-terminus. The mutant eto2 protein lacks the proper final 12 amino acids of the protein, including the conserved serine phosphorylated by CDPK (Chae et al., 2003). Nevertheless, eto2 is still stabilized by cytokinin, prompting a search for some novel mode of cytokinin-mediated changes in ACS5 accumulation. One of the first priorities might be to determine whether an ETO1-based E3 ligase or other components of the UPS system are involved in cyokinin's control of ACS5 longevity.
Once a plant produces an appropriate amount of ethylene based on its developmental context and ambient conditions, the hormone needs to be perceived to regulate downstream signalling steps, such as gene transcription (see above). In arabidopsis, there is a family of five receptors that contribute to ethylene perception (reviewed in Alonso and Stepanova, 2004). Analysis of several mutant forms of the ethylene receptors suggested that their stability might also be regulated in a post-transcriptional manner (Zhao et al., 2002). For example, four dominant mutations in ETR1 that confer ethylene insensitivity cause higher accumulation of the ETR1 receptor in dark-grown seedlings. Further analysis of etr1-1 revealed that its mRNA levels are not elevated relative to wild-type ETR1 mRNA, indicating that the elevated levels of etr1-1 protein result from a post-transcriptional change. ETR1 protein levels are not augmented in ein2 or ein3 mutants, suggesting that reduced ethylene sensitivity per se is not the causative agent of ETR1 stabilization. Intriguingly, addition of silver nitrate, which renders the receptors insensitive to ethylene, also increases the accumulation of the wild-type ETR1 protein, but does not further increase the stability of the ethylene-insensitive forms of ETR (Zhao et al., 2002). Given that some animal hormone receptors show accelerated degradation in the presence of their binding hormone, it is possible that the ethylene-insensitive forms of ETR1 that cannot bind the hormone have a lower rate of degradation.
Jasmonates and the UPS
Jasmonates (JAs), derived from linolenic acid (reviewed in Turner et al., 2002), like ethylene, function in normal developmental pathways, but also play a crucial role in allowing plants to mount a defence to biotic challenges. JA affects processes such as pollen development and fruit ripening, and also promotes resistance to insects and pathogens (reviewed in Creelman and Mullet, 1997).
The first indication of UPS involvement in jasmonate signalling came with the identification of the coronatine insensitive1 (coi1) mutant. coi1 plants are insensitive to the JA-like compound coronatine, as well as to methyl jasmonate (MeJA) (Feys et al., 1994). COI1 encodes an F-box protein and both tagged and native forms of COI1 can interact with ASK1, ASK2, RBX1 and CUL1 in planta, suggesting that COI1 assembles into an SCF-type E3 ubiquitin ligase (Devoto et al., 2002; Xu et al., 2002). Four subunits of the CRL-modifying COP9 signalosome can also be co-immunoprecipitated with FLAG-tagged COI1 (Feng et al., 2003). JA-related phenotypes observed in plants with mutations in UPS components bolster the claim of its involvement in JA signalling. For instance, RNAi-mediated knock-down of RBX1 reduces MeJA-dependent induction of genes such as Allene Oxide Synthase (AOS) (Xu et al., 2002), and a mutation in AXR1 impairs MeJA-dependent inhibition of root growth (Tiryaki and Staswick, 2002). In addition, the jai4 mutant obtained through a screen for JA-insensitive mutants in an ein3 background (Lorenzo et al., 2004) has a mutation in the SGT1b protein (Lorenzo and Solano, 2005). As homologues of SGT1 in barley (Azevedo et al., 2002) and tobacco (Y Liu et al., 2002) interact with components of the SCF and the COP9 signalosome, the loss of JA sensitivity in a jai4/sgt1b mutant further links JA signalling to SCF-mediated processes.
Despite the demonstrated importance of COI1 and UPS-related proteins in JA signalling, no substrates of the SCF-COI1 E3 ligase have been conclusively identified to date. The RPD3b/HDAC6 histone deacetylase interacts with COI1 in yeast-two-hybrid assays and in planta, suggesting that it could be a substrate for COI1-mediated ubiquitylation and degradation (Devoto et al., 2002), although further work will be required to substantiate this hypothesis. Over-expression of the related HDAC19 in arabidopsis enhances expression of the JA-responsive PR (Pathogenesis-Related) genes Basic Chitinase and Beta-1,3-glucanase (Zhou et al., 2005). This would imply that if HDAC6 and/or HDAC19 are substrates of COI1-mediated proteolysis, then JA should reduce the interaction between COI1 and the deacetylases to promote JA-mediated chromatin modification and induce PR gene expression. Another potential substrate for COI1-dependent ubiquitylation, the small subunit 1b of Rubisco, was also pulled out of a yeast-two-hybrid screen for COI1-interactors (Devoto et al., 2002). Previous studies demonstrated MeJA-stimulated degradation of Rubisco, and indicated that this might be part of MeJA-induced leaf senescence (reviewed in Parthier, 1990), but no further work has been done to determine whether the Rubisco subunit is subject to COI1-mediated degradation.
Plant defence and the UPS
Plants consistently face challenges from a wide array of biotic stresses, including insect herbivoury, and fungal, bacterial and viral attacks. Both specific and general, and often overlapping defences are mounted in response to each type of biological onslaught, and increasing evidence points to the participation of the UPS in these varied forms of defence.
Regulated proteolysis of endogenous or pathogen-produced proteins can contribute to multiple levels of plant defence. First, the ‘basal defence’ system allows plants to recognize and respond to a broad range of potentially pathogenic (non-host) organisms based on the pathogen-associated molecular patterns (PAMPs) they present to the plant. For instance, the fungal cell wall component chitin and bacterial flagellin proteins both stimulate several protective measures, such as callose deposition (Gomez-Gomez et al., 1999) and oxidative bursts (Felix et al., 1999) in many plant species (for references see M.G. Kim et al., 2005). However, plants also have heightened abilities to respond to particular (host) pathogens through the gene-for-gene, or R-mediated resistance pathways. In this system, a pathogen may evolve a factor, often a protein or peptide, that specially enhances its pathogenesis on specific ‘host’ plants. If a plant protein evolves to counteract this pathogenic factor, the plant will be able to prevent pathogenesis of this specific invader by initiating resistance responses often accompanied by localized cell death through the hypersensitive response (HR). In this case, the pathogenic factor is called an avirulence (Avr) factor and the plant defence protein is termed an R (resistance) protein. If a plant targeted by the pathogen with a particular Avr lacks the cognate R gene, the plant will be susceptible to attack.
In addition to the immediate and local consequences of basal and R-mediated defence signalling, pathogen attack also prompts the development of systemic acquired resistance (SAR), which renders a plant less susceptible to subsequent attacks by a variety of organisms, including fungi, bacteria and viruses (reviewed in Durrant and Dong, 2004). Typically, tissue damage caused by necrotrophic pathogens or the induction of an HR triggers increases in the levels of salicylic acid (SA). This prompts local and distal signalling events, largely through the activity of the NPR1 (non-expresser of PR genes) regulatory protein, as it stimulates expression of many Pathogenesis-Related (PR) genes. A similar NPR1-dependent but SA-independent suite of responses, termed induced systemic resistance (ISR), is stimulated by non-pathogenic root-colonizing bacteria, but also heightens a plant's ability to fend off future attacks from fungi and bacteria (reviewed in Feys and Parker, 2000; Durrant and Dong, 2004).
Ongoing studies of plant defence continue to uncover complex interactions between basal defence, R-mediated defence and systemic defence responses. Some recent experiments have specifically led to interesting modifications of the original gene-for-gene (R-protein-based) model and highlight new links between it and basal defence. First, the ‘guard’ hypothesis (Van Der Biezen and Jones, 1998; Dangl and Jones, 2001) posits that R-mediated resistance is not necessarily initiated through a direct interaction between an Avr factor and its cognate R protein. Rather, R proteins may survey the status of the direct targets of the Avr factor. For example, Pseudomonas syringae directly secretes the AvrRpm1 protein into plant cells, where AvrRpm1 induces phosphorylation of the RIN4 protein. The plant RPM1 R-protein functions as the guard. It does not directly interact with AvrRpm1, but rather recognizes RIN4 phosphorylation as a sign of P. syringae attack and stimulates the hypersensitive response (Mackey et al., 2002). Second, mounting evidence points to cross-talk and shared signalling between R-mediated and basal defences. For example, several studies highlight the ability of pathogens to try to subvert basal defence mechanisms (Jakobek et al., 1993; Hauck et al., 2003; Keshavarzi et al., 2004) and some R proteins may recognize and fight back against this tactic (e.g. M.G. Kim et al., 2005). For basal, R-mediated and systemic defence mechanisms, there is a growing recognition that UPS-mediated events may be important factors both in plant defence and in pathogen virulence programmes (reviewed in Zeng et al., 2006).
Convergence of hormone signalling, plant defence, and the UPS
As previously mentioned, both the ethylene and the jasmonic acid signalling pathways contribute to plant defence. As highlighted above, the UPS appears to be required for various steps in these signal transduction cascades that impact plant defence signalling. For example, ethylene synthesis increases in response to several types of biotic challenges [e.g. bacteria, fungi (reviewed in Broekaert et al., 2006), and insects (Kahl et al., 2000)]. It is interesting to note that ethylene does not always improve disease resistance and actually seems to be correlated with enhanced disease progression in many circumstances (reviewed in Broekaert et al., 2006; van Loon et al., 2006), but in either case, the level of ethylene produced can affect plant susceptibility, and ethylene biosynthesis is subject to UPS-mediated regulation.
Furthermore, ethylene affects the transcription of numerous defence-related genes. For instance, in arabidopsis, ethylene up-regulates many genes encoding cell-wall-modifying enzymes or protein components, oxidative burst regulators, and PR proteins such as β-1, 3-glucanase and osmotin (Zhong and Burns, 2003). All known ethylene signalling to date has been shown to proceed through the EIN3 family of transcription factors (van Loon et al., 2006) and as these are substrates of the UPS, proteolytic regulation must affect transcription of these defence-related genes. Interestingly, many of these genes, especially those in the PR class, are also regulated by JA (reviewed in van Loon et al., 2006) as both JA and ethylene can modulate the transcription of ERF/AP2 transcription factors that function downstream of the EIN3 family (reviewed in Guo and Ecker, 2004). Initial identification of this co-ordinated regulation of ERF1 in arabidopsis demonstrated that over-expression of ERF1 could restore defence responses in a coi1 (F-box protein) mutant, indicating that perturbation of UPS function in the JA signalling pathway could also compromise defence gene expression (Lorenzo et al., 2003). The observation that plants with reduced signalosome levels (fus6/csn1-11) lose JA-stimulated induction of PR genes such as PDF1·2 further substantiates the link between JA, the UPS and defence-related gene expression (Feng et al., 2003).
In tomato, additional evidence of the requirements for COI1 in defence against herbivores arose from the identification of the jai1/coi1 mutant. Methyl jasmonate (MeJA) is no longer able to induce expression of the anti-herbivory serine proteinase inhibitor (PI-II) in jai1/coi1 plants, but gene induction is restored by introduction of a 35S:SlCOI1 transgene. Glandular trichome development and monoterpene production are also compromised in the jai1/coi1 mutants and most likely contribute to the severely reduced resistance to spider mites. Fecundity of female spider mites, elevated on jai1/coi1 leaves, is also restored to wild-type levels in 35S:COI1-complemented mutants (L. Li et al., 2004).
General UPS components in plant defence
Clues as to the importance of targeted protein degradation in defence mechanisms can be gleaned from an analysis of general UPS components that display altered expression levels in response to biotic stresses prompted both by non-host and by host-specific pathogens. However, thorough explanations of underlying mechanisms and causal relationships are lacking. At a very gross level, the finding that tomato mosaic virus (ToMV), tobacco mosaic virus (TMV), wounding, ACC (ethylene precursor), JA and SA all up-regulate two ubiquitin-activating enzymes (NtUBA1 and NtUBA2) in tobacco suggests that plants increase their ubiquitylation capacity when faced with an attack (Takizawa et al., 2005). Up-regulation of additional general components of the UPS has been observed in other plant/pathogen interactions. For instance, sunflower (Helianthus annuum) hypocotyls accumulate higher levels of ubiquitin transcripts following challenge with an incompatible (avirulent) strain of powdery mildew, but not with a compatible strain (Mazeyrat et al., 1999). A glucan fungal elicitor from Phytophthora megasperma also causes up-regulation of ubiquitin transcripts in soybean cells (Levine et al., 1994). A ubiquitin-conjugating enzyme OsUBC5b (but not its homologue OsUBC5a) also shows elevated levels of transcripts following treatment of rice cells with the fungal elicitor N-acetylchitoheptaose (Takai et al., 2002). Transcript levels for two UBCs are also up-regulated in Nicotiana attenuata during herbivorous attack by the insect Manduca sexta (Hui et al., 2003). And, at the proteolytic end of the UPS pathway, three specific 20S proteasome subunits are transcriptionally induced by the proteinaceous fungal elicitor cryptogein in tobacco (Dahan et al., 2001). Although changes in levels of ubiquitin, E2 enzymes and proteasome subunits might be predicted to have broad effects on a plant cell's level of ubiquitin-mediated proteolysis, scientists seek to identify how these changes affect specific host–pathogen interactions.
Specific E3 ligase components in plant defence
Efforts to find pathogen-responsive genes have uncovered several E3 ligases with mRNA levels affected by a number of different general elicitors and avirulence factors (reviewed in Zeng et al., 2006) and genes encoding UPS components have surfaced in screens for mutants with aberrant pathogenesis-related phenotypes. Both lines of research support a role for the UPS in the convergence of both basal and specific defence strategies, as well as systemic responses.
CRL components
NPR1 emerged from a screen for mutants lacking SA-responsive induction of a PR reporter gene (Cao et al., 1994). The initial npr1 mutant failed to achieve SAR following treatment with SA, or an analogue, INA (2,6-dichloroisonicotinic acid), and remained highly susceptible to P. syringae pv. maculicola. This npr1 mutant could still mount an HR following treatment with an avirulent strain of P. syringae but showed defects in avirulent pathogen-mediated induction of SAR as well (Cao et al., 1994). Additional alleles of npr1/nim1/sai1, as well as further molecular and physiological experiments using NPR1/NIM1/SAI1, demonstrate the crucial role played by this protein in SAR, ISR and other defence pathways (reviewed in Dong, 2004). The NPR1 protein contains ankyrin repeats, implicated in protein–protein interactions (reviewed in Michaely and Bennett, 1992), as well as a BTB/POZ domain (Aravind and Koonin, 1999), implying that it could potentially interact with a CUL3-based CRL as well as other proteins, such as substrates for ubiquitylation, simultaneously. Yeast-two-hybrid assays do not point to strong binding between NPR1 and CUL3a or CUL3b (Dieterle et al., 2005), but some NPR1 interactions observed in planta cannot be recapitulated through this experimental technique (Despres et al., 2003). Several studies focusing on NPR1's effect on pathogen-responsive transcription suggest that in response to SA, and redox-mediated conformational changes, NPR1 moves into the nucleus to interact with TGA transcription factors to turn on PR genes (reviewed in Dong, 2004). An analysis of several TGA transcription factors indicates that they undergo differential, proteasome-dependent proteolysis (Pontier et al., 2002). There is some precedent for ubiquitin-mediated activation, followed by degradation of transcription factors in other systems (Salghetti et al., 2001), and it will be interesting to determine if NPR1 participates in a similar process or affects SAR and plant defence in any other UPS-dependent manner, for example by degrading repressors of SAR induction.
SAR-related investigations have also identified the SON1 F-box protein through a mutant screen for suppressors of nim1/npr1-related susceptibility in arabidopsis (Kim and Delaney, 2002). SON1 is hypothesized to act as a negative regulator of plant defence responses, because son1 nim1-1 mutants are less susceptible to inoculation with the Peronospora parasitica oomycete (downy mildew) or P. syringae than nim1-1 alone, and because susceptibility, as measured by downy mildew sporulation, can be restored by constitutive expression of SON1. Interestingly, the increased resistance conferred by mutation of son1 in an npr1/nim1-1 background is not achieved through restoration of PR protein induction, nor does it depend on salicylic acid. And it also does not result from up-regulation of the JA-responsive defensin gene, PDF1·2. However, son1 mutants express constitutively several PR transcripts in the presence of the wild-type NPR1/NIM1 protein. This gives rise to the hypothesis that the SON1 F-box protein normally suppresses NPR/NIM1-mediated induction of PR genes in the absence of an elicitor, but that it may also take part in some sort of SA-independent plant defence response (Kim and Delaney, 2002). SON1 transcript levels do not appear to change in response to pathogenic attack nor after treatment with a salicylic acid analogue, but it is possible that post-translational modifications to SON1 or its substrates reduce SON1's activity following a biotic challenge, thereby enabling the plant to mount a successful defence (Kim and Delaney, 2002).
In an effort to understand R-mediated/gene-for-gene resistance pathways better, up-regulated mRNA sequences were identified in tobacco cells carrying the Cf9 resistance (R) gene after inoculation with the Avr9 elicitor of the biotrophic fungus Cladosporum fulvum. cDNA clones for several of these ACRE (Avr/Cf9 Rapidly Elicited) genes were used for further analysis (Durrant et al., 2000). ACRE189 encodes an F-box protein, providing evidence that SCFCOI1 and SCFEBF1/EBF2 may not be the only the SCF complexes involved in pathogen defence (Rowland et al., 2005). Viral-induced gene silencing of ACRE189 diminishes the ability of tobacco leaves to generate a Cf4- and a Cf9-mediated hypersensitive response. These data suggest that SCFACRE189 may act downstream of at least two different R proteins during incompatible interactions (Rowland et al., 2005).
Additional components of multiple CRLs, or factors that modulate CRL activity, have surfaced as contributors to plant defence. Increased transcription of SKP1 and SGT1, two potential components of many SCF-type E3 ligases (see above), occurs in pepper (Capsicum annuum ‘Bukang’) following inoculation with an incompatible strain of Xanthomonas bacteria (Chung et al., 2006). In fact, sgt1b mutants were found based on their compromised disease resistance (Austin et al., 2002; Tor et al., 2002). Arabidopsis plants with two different mutations in axr1, which functions to activate many SCFs and perhaps all CRLs in vivo (see above), show reduced resistance to the opportunistic fungus Pythium irregulare (Tiryaki and Staswick, 2002). All these data together reinforce the model that CRLs contribute to plant defence.
Interestingly, the F-box proteins that function in auxin signalling are also subject to transcriptional regulation during pathogen attack via induction of a microRNA (Navarro et al., 2006). The P. syringae general flagellin elicitor induces accumulation of the miR393a transcript in arabidopsis. This microRNA then promotes cleavage of TIR1, AFB2 and AFB3 transcripts, causing reduced levels of these F-box proteins. An AXR3NT:GUS reporter protein is stabilized under these conditions, and the accumulation of Aux/IAA repressors following flg22 inoculation presumably contributes to the reduced induction of several auxin-responsive genes. This inhibition of auxin signalling appears to enhance resistance to virulent pathogens as overexpression of AFB1:Myc, which should not be cleaved by the microRNA, can increase susceptibility of arabidopsis plants to a virulent strain of P. syringae. But the auxin response probably does not impact R-mediated signalling, as overexpression of AFB1:Myc does not influence interactions with an avirulent strain of P. syringae carrying the AvrRpt2 gene. These studies reveal that bacterially induced suppression of TIR1/AFB-mediated degradation and auxin signalling increases plant resistance to virulent P. syringae by modulating the abundance of specific host UPS components (Navarro et al., 2006).
Disruptions in the auxin signalling pathway also appear to affect arabidopsis resistance to TMV infection (Padmanabhan et al., 2005, 2006). IAA26 and IAA27, but not several other Aux/IAA family members, interact with the TMV replicase protein in yeast-two-hybrid assays (Padmanabhan et al., 2006), and this interaction was confirmed in vitro for IAA26 (Padmanabhan et al., 2005). Wild-type TMV can induce symptoms on arabidopsis, ecotype Shahdara, but the severity of specific phenotypes is reduced when the plants are inoculated with a mutant version of TMV (TMV-V1087I). This has a mutant replicase that spreads and accumulates normally within arabidopsis but shows diminished interaction with IAA26 in yeast-two-hybrid assays, suggesting that binding of the replicase to IAA26 is important for symptom development (Padmanabhan et al., 2005). Interestingly, co-transfection of IAA26:GFP and wild-type TMV results in a reduced percentage of cells with visible levels of fluorescence, suggesting that TMV could potentially destabilize IAA26 (Padmanabhan et al., 2005) and thus stimulate auxin signalling. In fact, a microarray analysis of TMV-infected plants shows that approx. 30 % of the transcriptionally altered genes lie downstream of auxin response elements (Golem and Culver, 2003; Padmanabhan et al., 2005). However, there is additional evidence that interactions with the replicase prompt fluorescently tagged forms of IAA26 and IAA27 to relocalize from the nucleus to the cytoplasm (Golem and Culver, 2003; Padmanabhan et al., 2005, 2006). This could also compromise their ability to perform their normal transcriptional regulatory roles in the nucleus, and could reduce the visibility of GFP- and DsRed-tagged versions of the proteins as they diffuse in the cytoplasm. So although there is evidence that TMV promotes movements of IAA26 and IAA27 within the cell, the question of whether TMV also induces their degradation during the infection process remains open. If this is the case, additional work will be required to ascertain if the well-characterized SCFTIR1/AFBx complex or other E3 ubiquitin ligases contribute to this proteolytic process.
Non-CRL RING proteins
Although many RING domain-containing proteins exist in plants, only a small subset has been shown to be transcriptionally up-regulated by biotic stress to date. Nevertheless, initial functional characterization of some of these putative E3 ligases suggests that they do play an important role in plant defence (reviewed in Zeng et al., 2006). For example, levels of ATL2 and ATL6 mRNAs rapidly rise in arabidopsis in response to chitin, a basal defence elicitor associated with fungal cell walls and insect exoskeletons. These genes encode two related RING E3 ligases (Salinas-Mondragon et al., 1999). The BRH1 RING protein, as mentioned above, also shows increased transcription following treatment with a chitin elicitor (Molnar et al., 2002). In a separate study, two forms of chitin spur mRNA accumulation of the arabidopsis At2g35000 gene (Ramonell et al., 2005), which encodes a RING domain-containing protein active in in vitro E3 ligase assays (Stone et al., 2005). Subsequent powdery mildew inoculation causes increased disease symptoms in three independent lines with a T-DNA insertion in At2g35000, demonstrating the importance of this gene in plant immune responses (Ramonell et al., 2005). The rice EL5 (elicitor-induced) RING E3 ligase is up-regulated by a different fungal elicitor. Functional analysis of this protein indicates that it possesses ubiquitylation activity in vitro when coupled with the OsUBC5a or OsUBC5b E2 conjugating enzymes. Interestingly, as described above, the latter E2 is up-regulated by the same elicitor, suggesting that these might function in the same specific response pathway, but their substrate is not known (Takai et al., 2002). The general bacterial flagellin peptide elicitor (flg22), known to stimulate basal defence responses (Felix et al., 1999), prompts greater than a 2·5-fold change in the levels of over 250 transcripts (out of a population of 8200 examined) in arabidopsis cell cultures and seedlings. Among those, ten RING finger putative E3 ligases are up-regulated, including RHA3b, RHA1b, RMA1 and ATL6 (Navarro et al., 2004).
RING domain-containing proteins have also surfaced in screens for gene-for-gene resistance-initiated transcriptional responses. For instance, the screen for Avr9-responsive transcripts that uncovered the F-box protein ACRE189 also identified the RING E3 ligase ACRE132. This protein is most closely related to the ATL2 protein of arabidopsis, implying functional conservation in fungal response pathways (Durrant et al., 2000).
Curiously, one protein with potential RING- and/or SCF-related function appears to affect plant resistance. Studies have also shown that SIP (Siah-Interacting Protein) from arabidopsis is transcriptionally induced by inoculation with P. syringae, as well as after wounding and hydrogen peroxide application (Kim et al., 2006). SIP appears to play a positive, though relatively minor, role in plant defence given that sip mutants show slightly increased susceptibility to this pathogen, whereas SIP overexpressers are somewhat more resistant to P. syringae. SIP is 21 % identical to SGT1b, but it is also 32 % identical to the human SIP protein. Human SIP functions in an atypical E3 ligase (Matsuzawa and Reed, 2001). It can bind to human Skp1, but it can also bind to the Siah-1 and Siah-2 RING-based E3 ligases (similar to SINAT5 in arabidopsis). In humans, beta-catenin can be degraded when it binds to the Ebi F-box protein. Ebi1 binds to Skp1, but then, rather than connecting to CUL1, links to the Siah E3 ubiquitin ligases through SIP. AtSIP does not interact with SINAT5 in yeast-two-hybrid assays, but no tests have yet been done to ascertain if it interacts with any of the four other arabidopsis SINAT family members or the ASK proteins (Kim et al., 2006). Therefore, the proteolytic function of arabidopsis SIP remains obscure, but its noted impact on plant defence leaves it well-poised to link further the UPS to plant resistance pathways.
U-box proteins
Recent results also reveal a prominent role for ARM-repeat-containing U-box E3 ligases in plant defence. In arabidopsis, 41 proteins contain these two domains (Mudgil et al., 2004) including PHOR1, implicated in GA signalling (see above). In these proteins, the U-box functions as the E2 interaction domain and the ARM repeat protein–protein interaction domain might contribute directly or indirectly to substrate recognition.
At least two U-box/ARM repeat E3 ligases were pulled out of the screen for ACRE genes (Durrant et al., 2000). ACRE276, found in tobacco and tomato, appears to be required for Cf4- and Cf9-stimulated hypersensitive response because after virus-induced gene silencing of ACRE276, tomatoes have increased susceptibility to C. fulvum leaf mould (Yang et al., 2006). Loss of this protein also compromises the plants' ability to generate the N-mediated hypersensitive response following treatment with the p50 elicitor of TMV. In this gene-for-gene interaction, the N TIR-NBS-LRR protein acts as a resistance factor against the Avr-like p50 elicitor from TMV. A GST:ACRE276 U-box protein does possess E3 ligase activity in vitro, as does a close homologue from arabidopsis, PUB17, and this activity seems to be required for the hypersensitive response based on experiments performed with mutant versions of the PUB17 protein expressed in tobacco (Yang et al., 2006).
Several other U-box/ARM-repeat-containing proteins are induced by elicitors in other plants and appear to participate in the plant defence response in both basal and R-mediated pathways. SPOTTED LEAF11 (Spl11) is induced in both a susceptible and a resistant cultivar of rice following inoculation with rice blight and may function in basal defence. It encodes a functional E3 ligase based on in vitro assays. As the plants with mutated spl11 exhibit a lesion mimic phenotype, it is hypothesized that SPL11 normally degrades a positive regulator of the hypersensitive response and programmed cell death (Zeng et al., 2004) (Fig. 2B).
In parsley (Petroselinum crispum) the Pep25 fungal peptide elicitor leads to rapid rises in the levels of the PcCMPG1/ELI17 (Cys-Met-Pro-Gly1/Elicitor-activated gene) transcript, which encodes a U-box/ARM protein. And when elements from the PcCMPG1 promoter are placed upstream of GUS and transformed into arabidopsis plants, GUS expression is increased at the site of infection when plants are inoculated using both fungal and bacterial general (flg22) and specific elicitors. Wounding alone, however, is insufficient to prompt GUS expression (Kirsch et al., 2001). Two arabidopsis homologues of this protein, AtCMPG1/PUB20 and AtCMPG2, display similarly rapid transcriptional induction by a fungal pathogen elicitor and by P. syringae (Heise et al., 2002). Further analysis of two homologues of PcCMPG1 from tobacco (NtCMPG1/ACRE74) and tomato (SlCMPG1) affirmed that these U-box/ARM proteins are transcriptionally up-regulated in response to a pathogen, and that NtCMPG1 possesses E3 ubiquitin ligase activity in vitro. However, unlike SPL11, the CMPG1 protein appears to function as a positive regulator of the hypersensitive response, as tomato and tobacco plants with reduced levels of CMPG1 display reduced hypersensitive responses following treatment with several elicitors (Gonzalez-Lamothe et al., 2006). Two additional U-box/ARM putative E3 ligases in arabidopsis, PUB12 and PUB5, are also induced by the flagellin (flg22) elicitor (Navarro et al., 2004).
Overall, these studies suggest that plants increase their transcription of numerous E3 components when challenged by pathogens, and highlight that among all of the various kinds of ubiquitin ligases, U-box/ARM E3 ligases are over-represented in screens for pathogen-responsive transcripts relative to their total number. In addition, these experiments support the idea that multiple pathogen perception systems converge on common downstream UPS elements, since, in many cases, both general elicitors and Avr-type specific elicitors up-regulate the same genes. For instance, a formal analysis identified homologues of the tomato Avr9-responsive (ACRE) genes in arabidopsis, and then determined that many of the homologues, such as PUB17, are also up-regulated in response to the general flagellin bacterial elicitor of basal defences (Navarro et al., 2004).
Potential substrates of the UPS involved in plant defence
There is ample evidence of E3 ligases responding to pathogen attack and contributing to plant defence, but what are their biological substrates that need to be degraded as part of the signalling process during plant/pathogen interactions? There are a few documented cases of plant defence proteins undergoing regulated proteolysis during pathogenic interactions. RPM1 specifically helps plants to defend themselves against P. syringae harbouring the avrRpm1 and avrB genes (Grant et al., 1995). This membrane-associated protein is one of a large class of R proteins that share predicted nucleotide binding sites (NBSs) and leucine-rich-repeats (LRRs). Rapid degradation of RPM1 coincident with the onset of the HR provided one of the first clues that proteolysis contributes to R-mediated gene-for-gene defence (Boyes et al., 1998). Loss of RPM1:Myc from leaf extracts only occurs in arabidopsis plants inoculated with P. syringae carrying the avrRpm1, avrB, avrRps4 or avrRpt2 genes, but not with comparable virulent strains. Importantly, several marker proteins do not show similarly rapid disappearance following bacterial inoculation, indicating that universal acceleration of protein degradation cannot explain RPM1 loss. The mode of RPM1 degradation remains unclear but the authors speculate that degradation of this specific R-protein in response to several different Avr proteins represents a general mechanism for limiting the spread of the HR (Boyes et al., 1998).
Recently, two proteins, RIN2 and RIN3, each with a RING domain and a ubiquitin binding domain called CUE were shown to interact with RPM1 (Kawasaki et al., 2005). GST-RIN2 and RIN3 fusion proteins possess E3 ubiquitin ligase activity in vitro. Analysis of the rin2 rin3 double mutant indicates that these two proteins contribute to pathogen-elicited RPM1-dependent ion leakage. It would be tempting to speculate that these enzymes catalyse RPM1 ubiquitylation and degradation coincident with the HR. But neither GST-tagged RIN2 nor RIN3 can ubiquitylate RPM1 in vitro, and there is no difference in pathogen-stimulated RPM1 disappearance in wild-type versus rin2 rin3 plants. RIN2 and RIN3 may require other proteins for in vitro activity and act redundantly in vivo with yet to be characterized E3s. Alternatively, they may act on a protein or proteins downstream in the signalling pathway (Kawasaki et al., 2005).
There is evidence for three additional proteins affecting RPM1 accumulation. rar1/pbs2/lra1 mutants were pulled out of multiple pathogenesis-related screens in barley and arabidopsis (Freialdenhoven et al., 1994; Shirasu et al., 1999; Warren et al., 1999; Tornero et al., 2002). By an unknown mechanism, RAR1 contributes to the accumulation of a subset of R proteins, including RPM1, as RPM1:Myc levels drop drastically in an rar1 mutant background (Tornero et al., 2002). Subsequent analysis revealed that RAR1 is required for defence responses downstream of several different R genes (Muskett et al., 2002), and not surprisingly has been shown to affect the stability of a number of R proteins including RPS5:HA in arabidopsis (Holt et al., 2005), MLA6:HA and MLA1:HA in barley, and Rx:HA from potato expressed in tobacco (Bieri et al., 2004).
Heat shock protein 90 (HSP90), a chaperonin required for several R-mediated defence pathways, also contributes to R-protein accumulation. For instance, viral-induced gene silencing of HSP90 in Nicotiana benthamiana reduces the levels of an Rx:HA fusion protein (Lu et al., 2003) and an hsp90·2 mutant possesses reduced levels of RPM1:Myc (Hubert et al., 2003). Intriguingly, in other organisms, HSP90 can contribute to both protein complex assembly and to ubiquitin-mediated degradation via the CHIP U-box E3 ubiquitin ligase (reviewed in Hohfeld et al., 2001).
Finally, the SGT1b protein can bind to both RAR1 and HSP90 (Azevedo et al., 2002; Takahashi et al., 2003) and SGT1b and SGT1a from arabidopsis and other plants function in several R-mediated pathways (Austin et al., 2002; Tor et al., 2002; Leister et al., 2005; Azevedo et al., 2006). As previously mentioned, SGT1b can associate with SCF E3 ligases (Azevedo et al., 2002; Y. Liu et al., 2002; Gray et al., 2003), and it appears to promote SCF-mediated degradation of E3 ligase substrates in budding yeast (Kitagawa et al., 1999) and in arabidopsis (Gray et al., 2003), potentially implicating an SCF in multiple R-gene-dependent pathways, but it remains unclear whether SGT1b enhances or suppresses substrate degradation in plant defence pathways. For instance, SGT1b appears to oppose RPS5:HA accumulation in arabidopsis plants (Holt et al., 2005) whereas a reduction in SGT1 in N. benthamiana causes Rx:HA levels to fall precipitously (Azevedo et al., 2006). Plants have a vested interest in tightly controlling the abundance of each R protein as there is evidence of dose-dependent resistance (Bieri et al., 2004) and increasing RPS5:HA levels correspond with increased speed of HR onset (Holt et al., 2005). The UPS could certainly contribute to this important process, but further exploration of this potential connection is warranted. On the one hand, treatment with proteasome inhibitors does not affect RPS5:HA stability in two different assays (Holt et al., 2005) and most experiments focus on total R protein accumulation without determining whether the actual rate of R protein degradation changes in response to pathogen attack or altered RAR1, HSP90 or SGT1 levels. But, on the other hand, both HSP90 and SGT1 provide tantalizing links to the UPS. And evidence that N-mediated gene-for-gene defence against TMV is compromised by suppression of SKP1, SGT1 or COP9 signalosome components (Y. Liu et al., 2002) gives additional impetus to a thorough investigation of the role of cullin-based E3 ligases in R-protein stability and signalling.
Another protein that associates with the RPM1 and RPS2 R proteins, RIN4, can also undergo regulated degradation during pathogen attack (M.G. Kim et al., 2005) and there is better experimental support for the involvement of the UPS in this process. The AvrRpt2 protein of the bacterial P. syringae pathogen functions as a cysteine protease capable of cleaving two distinct sites within membrane-bound RIN4 protein. One cleavage near the C-terminus leaves a small C-terminal anchor embedded in the plasma membrane, but releases the remainder of the RIN4 protein. As the N-terminal portion of the protein can only be observed in soluble fractions treated with proteasome inhibitors, it appears that the RIN4 released from the membrane is rapidly degraded in an uncharacterized proteasome-dependent manner (H.S. Kim et al., 2005). AvrRpt2-mediated cleavage at the second recognition site in RIN4 unmasks a tertiary destabilizing residue (asparagine) that can potentially be recognized by a specific UPS pathway called the N-end rule. Ubiquitylation via N-end rule (reviewed in Varshavsky, 2003) depends on the nature of the N-terminal residue, and certain amino acids at this position can be recognized directly by an E3 ligase (primary residues), recognized after addition of an arginyl group [secondary residues (aspartate and glutamate)] or recognized after removal of an amino group followed by addition of an arginyl group [tertiary residues (asparagine and glutamine)]. Presumably, presence of asparagine at the N-terminus of cleaved RIN4 leads to modification of the residue, recognition by an N-end rule E3 ligase, ubiquitylation and degradation of the RIN4 protein, given that replacing the asparagine with a residue not recognized by the N-end pathway (glycine) stabilizes an N-terminal RIN4-30 N(N11G):GFP reporter protein. Several RIN4-like proteins possess similar AvrRpt2 cleavage sites, suggesting this mechanism may not be restricted to RIN4 (Takemoto and Jones, 2005).
Degradation of the RIN4 protein has varying consequences for disease resistance depending on the suite of Avr and R proteins expressed by P. syringae and arabidopsis, respectively. RIN4 is required for defence against bacteria harbouring the AvrRpm1 or AvrB avirulence factors (Mackey et al., 2002). Thus, AvrRpt2-mediated loss of RIN4 blocks this defence mechanism. However, the RPS2 R protein ‘guards’ against pathogen-mediated loss of RIN4, so arabidopsis plants expressing RPS2 initiate an HR following RIN4 degradation (Axtell and Staskawicz, 2003; Mackey et al., 2003). RIN4 also plays a role in regulating basal defences so perturbations of RIN4 levels can potentially influence these basal reactions to a broad range of pathogens (M.G. Kim et al., 2005).
Although very few examples of ubiquitylated host proteins have been implicated in plant defence pathways to date, a number of experiments suggest that viral proteins may be substrates of the host UPS. A general linkage between ubiquitin and viral/host interactions was revealed in tobacco plants expressing a mutant variant of ubiquitin with lysine48 changed to arginine that exhibit spontaneous lesion development (Bachmair et al., 1990) and display several alterations in TMV susceptibility and symptom progression (Becker et al., 1993). Analysis of several plant virus particles demonstrates the existence of ubiquitin-conjugated proteins (Hazelwood and Zaitlin, 1990), although the length of the ubiquitin chains and their functional importance has not been determined. Treatment of virally infected tissue with proteasome inhibitors leads to the accumulation and stabilization of high-molecular-weight forms of the TMV movement protein, suggesting that this protein is normally subject to UPS-mediated degradation, as part of either the pathogenesis or the defence process (Reichel and Beachy, 2000). Furthermore, a 66-kDa protein from the Turnip Yellow Mosaic Virus (TYMV), believed to function as an RNA-dependent RNA polymerase (RdRp), can be phosphorylated at several serine and threonine residues within its PEST domain when expressed in insect cells (Hericourt et al., 2000). PEST domains appear to act as phospho-sensitive destabilization motifs (‘degrons’) in several eukaryotic substrates of the UPS (reviewed in Rechsteiner and Rogers, 1996), and further characterization of the 66-kDa RdRp protein indicates that it undergoes ubiquitylation in an insect expression system (Hericourt et al., 2000). It is possible that this RdRp is ubiquitylated in its natural plant hosts, but RdRp does not appear to be remarkably unstable when expressed in a proteolytically active rabbit reticulocyte system (Drugeon and Jupin, 2002). This could reflect a lack of proper modifying enzymes, or indicate that the protein is naturally degraded at a modest rate. However, under the same conditions, the 69-kDa movement protein of TYMV is degraded quite rapidly in an ATP- and proteasome-dependent manner. Furthermore, substantial evidence points to the accumulation of ubiquitylated forms of the 69-kDa movement protein in proteolytically compromised rabbit reticulocyte lysates (Drugeon and Jupin, 2002). To date, there is no conclusive evidence of whether these proteins are ubiquitylated in their hosts and, if so, what effect this has on TYMV pathogenicity.
In future characterizations of plant defence and the UPS, it will be interesting to see if there are any relationships between the set of UPS components shown to be induced by, or required for, plant responses to pathogens (e.g. the ARM/U-box E3 ligases) and the R-related proteins that are degraded in response to specific biotic challenges, or the viral proteins that may be ubiquitylated in plant cells, and to learn how all of these factors allow plants to mount integrated responses to an array of viral, bacterial, fungal and herbivore attacks.
Pathogenic usurpation of the host UPS
Given the demonstrated importance of the UPS for biotic defence and general plant growth and development, it makes sense that several plant pathogens hijack or interfere with components of the UPS to promote their own survival. For instance, the Polerovirus P0 protein possesses a minimal F-box motif that enables it to bind to the host ASK1/SKP1 and ASK2 proteins. This interaction allows the virus to suppress the plant's ability to defend itself via post-transcriptional silencing of viral RNAs presumably by promoting degradation of a component of the plant's RNA silencing machinery (Pazhouhandeh et al., 2006). The faba bean necrotic yellows virus (FBNYV) encodes a small protein, Clink, that can bind both to SKP1 and to the cell cycle protein pRB in vitro, in yeast and in planta (Aronson et al., 2000, 2002). Although mutations in the F-box portion of Clink do not interfere with replication of the FBNYV DNA, it is possible that Clink/SKP-mediated ubiquitylation of host or viral proteins could influence other aspects of FBNYV pathogenesis (Aronson et al., 2000). In addition, it was recently shown that Clink is not required for the induction of viral symptoms on Vicia faba (faba bean) plants in controlled experiments, but there does seem to be selective pressure to maintain this gene in the FBNYV genome given that the viral DNA from 98 % of the symptomatic plants tested retain the Clink gene. In addition, the authors postulate that Clink might be required for FBNYV infection of the wild relatives of the cultivated faba bean (TimchenKo et al., 2006) where its SKP-binding capabilities could possibly connect it to targeted protein degradation.
Another plant virus, Lettuce Mosaic Virus (LMV), influences the behaviour of the 20S proteasome (Ballut et al., 2005). Viral inoculation results in the formation of larger proteasome-containing complexes, as assayed by gel filtration. The viral protein HcPro can associate with purified 20S proteasomes and might be part of these proteasomal aggregates. Functionally, HcPro appears to have a slight stimulatory effect on the chymotrypsin- and trypsin-like enzymatic activities of the 20S proteasome in in vitro assays. Intriguingly, proteasomes purified from sunflower also possess endonuclease activity capable of cleaving TMV and LMV RNA (Ballut et al., 2003), and pre-incubation of purified 20S proteasomes with HcPro significantly inhibits degradation of a TMV RNA substrate. This points to a possible role for HcPro in ubiquitin-dependent and ubiquitin-independent proteasome-mediated processes in host/LMV interactions (Ballut et al., 2005).
In the realm of bacterial pathogens, the AvrPtoB protein of P. syringae (which does not have an endogenous UPS) is a ubiquitin ligase that can presumably work downstream of a host E1 and E2 enzyme to promote ubiquitylation of itself and/or unidentified host substrates. The level of E3 ligase activity exhibited by wild-type and mutant forms of AvrPtoB appears correlated with AvrPtoB's ability to suppress HR-based programmed cell death in susceptible tomato plants and N. benthamiana (Abramovitch et al., 2006; Janjusevic et al., 2006). Meanwhile, Agrobacterium tumefaciens introduces an F-box protein (VirF) into host cells and utilizes host components to form a functional SCF complex required for degradation of VirE2 and host VIP1 as these proteins must be eliminated to allow integration of the Agrobacterium's T-DNA into the host genome (Tzfira et al., 2004).
Nematodes may also co-opt components of the host UPS for their own advantage, as evidenced by the production of cDNAs for hexaubiquitin, a novel ubiquitin extension protein, a RING-H2 protein and an SKP1-like protein specifically within the dorsal esophageal gland cells of the nematode Heterodera glycines. These proteins bear a signal peptide to direct them for secretion into the host (Gao et al., 2003). An analysis of the secretion profile of the parasitic cyst nematode Heterodera schachtii led to the discovery of another member of the new class of ubiquitin extension proteins that is loaded into the host by the nematode (Tytgat et al., 2004). Plant-produced ubiquitin hydrolases most likely act on this protein to cleave ubiquitin and release the C-terminal portion to perform some sort of function that enhances nematode feeding.
In conclusion, pathogenic and symbiotic interactions of plants with their biosphere involve UPS components and often result in the promotion or inhibition of selective proteolysis. Similarly, as a plant reacts to alterations in its abiotic environment or focuses inward to respond to internal signals, the UPS plays a central role. Undoubtedly, future research will reveal the functions of the other UPS components that are currently only described by in silico predictions.
ACKNOWLEDGEMENTS
We thank Dr Sophia Stone for the ubiquitin pathway diagram, Sara Hotton, Mandy Hsia, Dr Jemma Jowett, Edward Kraft and Lucy Stewart for helpful comments on the manuscript, and Drs Ken Kaplan and Richard Michelmore for acronymic assistance. Research in the Callis lab is supported in part by grants from the National Science Foundation MCB-0519970 and IBN-0212659 and by the Paul K. and Ruth R. Stumpf Endowed Chair in Plant Biochemistry. K.D. was supported in part by National Institutes of Health Predoctoral Training Grant GM-0007377–27.
APPENDIX 1
Abbreviations used in the text
| Abbreviation* | Full name/description |
|---|---|
| 35S | Promoter from the cauliflower mosaic virus that makes a 35S RNA transcript |
| ABA | Abscisic acid |
| ABF | ABRE binding factor |
| ABI | Abscisic acid insensitive |
| ABRE | ABA response element |
| ACC | 1-amino-1-cyclopropane-carboxylic acid (ethylene precursor) |
| ACRE | Avr/Cf9 rapidly elicited |
| ACS | ACC synthase |
| AFB | Auxin signalling F-box protein |
| AFP | ABI5 binding protein |
| AIP | ABI3 interacting protein |
| AOS | Allene oxide synthase |
| AP | Apetala |
| APC | Anaphase promoting complex |
| ARF | Auxin response factor |
| ARIA | Arm protein repeat interacting with ABF2 |
| ARM | Armadillo, a protein interaction domain |
| ASK | Arabidopsis SKP1-like |
| At | Arabidopsis thaliana (arabidopsis) |
| ATL | Arabidopsis toxico para levadura (toxic to fungi) |
| Aux/IAA | Auxin/indole-3-acetic-acid |
| AuxRE | Auxin response element |
| Avr factor | Avirulence factor |
| AvrPtoB | Avirulence – Pseudomonas syringae pv. tomato |
| AvrRpm | Avirulence – resistance to Pseudomonas syringae pv. maculicola |
| avrRpt | Avirulence – resistance to Pseudomonas syringae pv. tomato |
| AXR | Auxin resistant |
| AXR3NT:GUS | Auxin resistant 3 N-terminus:beta-glucuronidase protein fusion |
| BAB | Bric-a-brac protein |
| BAK | BRI1 associated receptor kinase |
| BARD | BRCA1 associated RING domain |
| bes1-D | bri1-EMS-suppressor 1 dominant |
| BIN | Brassinosteroid insensitive |
| blastp | Basic local alignment search tool – protein |
| BRCA | Breast cancer (BRCA1 is an E3) |
| BRH | Brassinosteroid-responsive RING-H2 |
| BRI | Brassinosteroid insensitive |
| BSU | bri1 suppressor |
| BTB | Domain first found in the Drosophila proteins: broad complex, tramtrack and bric-a-brac |
| BY | Bright yellow |
| bzr1-1D | Brassinazole-resistant 1–1dominant |
| C. fulvum | Cladosporum fulvum |
| CAND | Cullin associated and neddylation-dissociated |
| Cdc | Cell-division cycle |
| CDD | COP10, DET1, DDB1 complex |
| CDPK | Calcium-dependent protein kinase |
| Cdt | Cdc10-dependent transcript 2 |
| C. elegans | Caenorhabditis elegans |
| Cf | Required for resistance to Cladosporum fulvum |
| CFP | Cyan fluorescent protein |
| CHIP | C-terminal HSP interacting protein |
| c-Jun | Gene for this protein cloned from avian sarcoma isolate #17; ju-nana is 17 in japanese |
| Clink | Cell cycle link |
| CMPG | Cysteine-methionine-proline-glycine (first 4 strictly conserved amino acids within domain) |
| COI | Coronitine insensitive |
| COP | Constitutive photomorphogenic |
| CRL | Cullin RING ligase |
| CSN | COP9 signalosome |
| Ctf | Chromosome transmission fidelity |
| CTR | Constitutive triple response |
| CUE | Ubiquitin-binding domain identified in yeast Cue1p |
| CUL | Cullin |
| DCAF | DDB1-cullin4-associated factor |
| DCN | Defective in cullin neddylation |
| DDB | Damaged DNA binding |
| DET | De-etiolated |
| DsRed | Discosoma sp. ‘red’ (fluorescent protein) |
| E1 | Enzyme 1 (same as UBA, ubiquitin activating enzyme) |
| E2 | Enzyme 2 (same as UBC, ubiquitin conjugating enzyme) |
| E2F | E2 promoter binding factor |
| E3 | Enzyme 3 (same as ubiquitin protein ligase) |
| EAR | ERF-associated amphiphilic repression |
| EBF | EIN3-binding F-box |
| Ebi | Shrimp (in Japanese) |
| ECR | E1 C-terminal related |
| EID | Empfindlicher im dunkelroten licht (sensitive to far-red light – German) |
| EIL | EIN3-like |
| EIN | Ethylene insensitive |
| EIR | Ethylene insensitive root |
| EL5 | Elicitor induced |
| ELI | Elicitor-activated gene |
| EOL | ETO-like |
| ERF | Ethylene response factor |
| ERS | Ethylene response sensor |
| ETO | Ethylene overproduction |
| ETR | Ethylene receptor |
| FBNYV | Faba bean necrotic yellow virus |
| FLAG | Synthetic epitope tag |
| flg22 | Flagellin (22 amino acids from N-terminal part of protein) |
| FUS | Fusca |
| GA | Gibberellic acid/gibberellins |
| GA2ox | GA 2-oxidase |
| GA3ox | GA 3-oxidase |
| GAI | GA (gibberellin) insensitive |
| GAMYB | Gibberellin-inducible Myb (identified from an oncogene in avian myeblastosis virus) |
| GFP | Green fluorescent protein |
| GID | Gibberellin insensitive dwarf |
| GMPOZ | GAMYB associated BTB/POZ protein |
| GSK | Glycogen synthase kinase |
| GST | Glutathione-S-transferase |
| GTPase | Guanosine triphosphatase |
| GUS | Beta-glucuronidase |
| HA | Peptide sequence from influenza haemagglutinin protein (epitope tag) |
| HAUSP | Herpes-associated ubiquitin-specific protease |
| HcPro | Helper component protease |
| HDAC | Histone deacetylase |
| HECT | Homologous to E6-AP COOH terminus |
| HLS | Hookless |
| HR | Hypersensitive response |
| HSL | Hormone-sensitive lipase |
| HSP90 | Heat shock protein 90 kDa |
| HSS | Hookless1 suppressor |
| Hv | Hordeum vulgare (barley) |
| HY | Long hypocotyl |
| IAA | Indole-3-acetic acid |
| INA | 2,6-dichloroisonicotinic acid (synthetic inducer of SAR) |
| ISR | Induced systemic resistance |
| JA | Jasmonic acid |
| JAI | Jasmonate insensitive |
| Keap | Kelch-like ECH-associated protein |
| LMV | Lettuce mosaic virus |
| LRA | Loss of recognition of avrRpm1 |
| LRR | Leucine-rich repeat |
| LUC | Photinus pyralis luciferase (firefly) |
| MAPK | Mitogen-activated protein kinase |
| MATH | Meprin and TRAF homology |
| MEI | Meiotic |
| MeJA | Methyl jasmonate |
| MEL | Maternal effect lethality |
| MG132 | Carboxybenzyl-leucyl-leucyl-leucinal (proteasome inhibitor) |
| Mla | Mildew resistance locus A |
| MPK | Mitogen-activated protein kinase |
| Myc | Epitope tag from c-Myc protein (originally identified from a chicken with myelocytomatosis) |
| 1-NAA | 1-naphthaleneacetic acid (synthetic auxin) |
| NAC | NAM, ATAF, and CUC transcription factors |
| NBS | Nucleotide binding site |
| NCBI | National Center for Biotechnology Information |
| NCED | Nine-cis-epoxycarotenoid dioxygenase |
| NEDD | Neural precursor cell expressed, developmentally down-regulated gene |
| NIM | Non-inducible immunity |
| NPB | Nuclear protein body |
| NPR | Non-expressor of pathogenesis-related genes |
| Nrf | NF-E2-related factor |
| Ns | Nicotiana sylvestris |
| Nt | Nicotiana tabacum (tobacco) |
| Os | Orzya sativa (rice) |
| P. syringae | Pseudomonas syringae |
| PAMP | Pathogen associated molecular pattern |
| PBS | avrPphB susceptible |
| Pc | Petroselinum crispum (parsley) |
| Plant defensin | |
| Pep25 | 25 amino acids from the 42-kDa Phytophthora sojae glycoprotein elicitor |
| PEST domain | Enriched in proline, glutamate, serine, threonine |
| PHOR | Photoperiod-responsive |
| Phy | Phytochrome |
| PI-II | Proteinase inhibitor-II |
| PIL | PIF3-like |
| PIN | Pin-formed |
| POZ | Pox virus and zinc finger |
| PR | Pathogenesis-related |
| pRB | Phosphorylated retinoblastoma tumour suppressor protein |
| PUB | Plant U-box |
| R gene/protein | Resistance gene/protein |
| RAC | ras-related C3 botulinum toxin substrate |
| RAR | Required for MlA12 resistance |
| RBX | RING-box protein, same as ROC1 and Hrt1p |
| RCE | RUB conjugating enzyme |
| Rcy | Recycling |
| RdRp | RNA-dependent RNA polymerase |
| RGA | Repressor of ga1-3 |
| RGL | RGA-like |
| RGS | Epitope tag with arginine-glycine-serine upstream of a polyhistidine tract |
| RHA | RING-H2 group A |
| RIN | RPM1-interacting protein |
| RING | Really interesting new gene protein domain |
| RMA | RING finger protein with membrane anchor |
| RNAi | RNA interference |
| ROC | Regulator of cullins |
| RPD | Reduced potassium dependency |
| RPM | Resistance to Pseudomonas syringae pv. maculicola |
| RPN | Regulatory particle non-ATPase |
| RPS | Resistance to Pseudomonas syringae pv. tomato |
| RUB | Related to ubiquitin |
| Rubisco | Ribulose 1,5-bisphosphate carboxylase/oxygenase |
| Rx | R protein conferring resistance to potato virus X |
| SA | Salicylic acid |
| SAI | Salicylic acid insensitive |
| SAR | Systemic acquired resistance |
| SCF | Skp1-Cullin1-F-box |
| SGT | Suppressor of G2 allele of skp1 |
| Siah | Seven in absentia homologue |
| SINA | Seven in absentia |
| SINAT | SINA of Arabidopsis thaliana |
| SIP | Siah-interacting protein |
| SIPK | Salicylic-acid induced protein kinase |
| SKP | S phase kinase-associated protein |
| Sl | Solanum lycopersicum (tomato) |
| SLN | Slender |
| SLR | Slender rice |
| SLY | Sleepy |
| SNE | Sneezy |
| SON | Suppressor of nim1-1 |
| Spd | S-phase delayed |
| Spl | Spotted leaf |
| TGA | Bind to DNA element with core sequence TGACG |
| TIR1 | Transport inhibitor response1 |
| TIR-NBS-LRR | Toll Interleukin1 Receptor–Nucleotide Binding Site–Leucine-Rich Repeat |
| TLP | TUBBY-like protein |
| TMV | Tobacco mosaic virus |
| ToMV | Tomato mosaic virus |
| TYMV | Turnip yellow mosaic virus |
| Ub | Ubiquitin |
| UBA | Ubiquitin activating enzyme |
| UBC | Ubiquitin conjugating enzyme |
| UbDHFR | Ubiquitin fused to the N-terminus of dihydrofolate reductase |
| UbKn | Ubiquitin lysine n, where n indicates specific lysine residue |
| U-box | UFD2-homology domain |
| UEV | Ubiquitin-conjugating E2 enzyme variant |
| UFO | Unusual floral organs |
| UPL | Ubiquitin protein ligase |
| UPS | Ubiquitin proteasome system |
| VIP | Vir-E2 interacting protein |
| VirE2 | Virulence E2 |
| VirF | Virulence F |
| WD40 | ∼40-amino-acid residue domain with tryptophan and aspartate residues in conserved positions |
| WDXR | Motif with the sequence tryptophan-aspartate-any amino acid-arginine. |
| Wnt | Wingless and int proteins |
| XBAT | XB3 orthologue 2 in Arabidopsis thaliana |
| XB3 | Rice Xanthomonas oryzae pv. oryzae resistance 21 binding protein |
* Numbers at the end of abbreviations have been removed when they do not convey functional information. All genes/proteins found in the text with the same abbreviations (but different numbers) have the same names.
APPENDIX 2
Diagrams of (A) a generic, and (B) specific Cullin RING ligases. The binding of the ubiquitin-charged E2 (ubiquitin conjugating enzyme) to the RING protein RBX is depicted only for the generic CRL. Many of the CRLs have alternate names (see Petroski and Deshaies, 2005). Question marks denote proteins with less well-characterized functions in CRLs. Recent experiments suggest that CRLs containing CUL1, CUL3 or CUL4a may homooligomerize in mammalian cells (Chew et al., 2007). Cullins associated with each class of CRL are listed below each diagram. The identity of putative DCAFs and other proteins participating in CUL4-based CRLs are also mentioned. The APC, which has a cullin-like subunit, is not shown. ECS: elongin BC, CUL2/5, SOCS/BC box; SOCS: suppressor of cytokine signalling.
[Chew E-H, Poobalasingama T, Hawkeya CJ, Thilo Hagen T. 2007. Characterization of cullin-based E3 ubiquitin ligases in intact mammalian cells — evidence for cullin dimerization. Cellular Signalling. In press, doi: 10.1016/j.cellsig.2006·12·002]

LITERATURE CITED
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biology. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB. Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proceedings of the National Academy of Science of the USA. 2006;103:2851–2856. doi: 10.1073/pnas.0507892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. Ethylene regulates arabidopsis development via the modulation of DELLA protein growth repressor function. The Plant Cell. 2003;15:2816–2825. doi: 10.1105/tpc.015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN. The ethylene signaling pathway. Science. 2004;306:1513–1515. doi: 10.1126/science.1104812. [DOI] [PubMed] [Google Scholar]
- Alonso-Peral MM, Candela H, del Pozo JC, Martinez-Laborda A, Ponce MR, Micol JL. The HVE/CAND1 gene is required for the early patterning of leaf venation in Arabidopsis. Development. 2006;133:3755–3766. doi: 10.1242/dev.02554. [DOI] [PubMed] [Google Scholar]
- Amador V, Monte E, Garcia-Martinez JL, Prat S. Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to. Drosophila armadillo. 2001;106:343–354. doi: 10.1016/s0092-8674(01)00445-7. Cell. [DOI] [PubMed] [Google Scholar]
- Andersen P, Kragelund BB, Olsen AN, Larsen FH, Chua NH, Poulsen FM, et al. Structure and biochemical function of a prototypical Arabidopsis U-box domain. Journal of Biological Chemistry. 2004;279:40053–40061. doi: 10.1074/jbc.M405057200. [DOI] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006 doi: 10.1038/nature05175. doi:10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. Fold prediction and evolutionary analysis of the POZ domain: structural and evolutionary relationship with the potassium channel tetramerization domain. Journal of Molecular Biology. 1999;285:1353–1361. doi: 10.1006/jmbi.1998.2394. [DOI] [PubMed] [Google Scholar]
- Aronson MN, Meyer AD, Gyorgyey J, Katul L, Vetten HJ, Gronenborn B, et al. Clink, a nanovirus-encoded protein, binds both pRB and SKP1. Journal of Virology. 2000;74:2967–2972. doi: 10.1128/jvi.74.7.2967-2972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson MN, Complainville A, Clerot D, Alcalde H, Katul L, Vetten HJ, et al. In planta protein–protein interactions assessed using a nanovirus-based replication and expression system. Plant Journal. 2002;31:767–775. doi: 10.1046/j.1365-313x.2002.01388.x. [DOI] [PubMed] [Google Scholar]
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JDG, Parker JE. Regulatory role of. SGT1 in early. 2002;295:2077–2080. doi: 10.1126/science.1067747. R gene-mediated plant defenses Science. [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;112:369–377. doi: 10.1016/s0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Azevedo C, Sadanandom A, Kitagawa K, Freiadldenhover A, Shirasu K, Schulze-Lefert P. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science. 2002;295:2073–2077. doi: 10.1126/science.1067554. [DOI] [PubMed] [Google Scholar]
- Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noel L, Sadanandom A, et al. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO Journal. 2006;25:2007–2016. doi: 10.1038/sj.emboj.7601084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboshina OV, Haas AL. Novel multiubiquitin chain linkages catalysed by the conjugating enzymes E2EPF and RAD6 are reconginzed by 26S proteasome subunit 5. Journal of Biological Chemistry. 1996;271:2823–2831. doi: 10.1074/jbc.271.5.2823. [DOI] [PubMed] [Google Scholar]
- Bachmair A, Becker F, Masterson RV, Schell J. Perturbation of the ubiquitin system causes leaf curling, vascular tissue alteration and necrotic lesions in a higher plant. EMBO Journal. 1990;9:4543–4549. doi: 10.1002/j.1460-2075.1990.tb07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Ballut L, Petit F, Mouzeyar S, Le Gall O, Candresse T, Schmid P, et al. Biochemical identification of proteasome-associated endonuclease activity in sunflower. Biochimica et Biophysica Acta. 2003;1645:30–39. doi: 10.1016/s1570-9639(02)00500-9. [DOI] [PubMed] [Google Scholar]
- Ballut L, Drucker M, Pugniere M, Cambon F, Blanc S, Roquet F, et al. HcPro, a multifunctional protein encoded by a plant RNA virus, targets the 20S proteasome and affects its enzymic activities. Journal of General Virology. 2005;86:2595–2603. doi: 10.1099/vir.0.81107-0. [DOI] [PubMed] [Google Scholar]
- Becker F, Buschfeld E, Schell J, Bachmair A. Altered response to viral infection by tobacco plants perturbed in ubiquitin system. Plant Journal. 1993;3:875–881. [Google Scholar]
- Bernhardt A, Lechner E, Hano P, Schade V, Dieterle M, Anders M, et al. CUL4 associates with DDB1 and DET1 and its downregulation affects diverse aspects of development in. Arabidopsis thaliana. Plant Journal. 2006;47:591–603. doi: 10.1111/j.1365-313X.2006.02810.x. [DOI] [PubMed] [Google Scholar]
- Bieri S, Mauch S, Shen QH, Peart J, Devoto A, Casais C, et al. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. The Plant Cell. 2004;16:3480–3495. doi: 10.1105/tpc.104.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C. Brassinosteroids and plant steroid signaling. The Plant Cell. 2002;14:S97–S110. doi: 10.1105/tpc.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biology. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, et al. The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes and Development. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Book AJ, Yang P, Scalf M, Smith LM, Vierstra RD. Tripeptidyl peptidase II. An oligomeric protease complex from Arabidopsis. Plant Physiology. 2005;138:1046–1057. doi: 10.1104/pp.104.057406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein G, Ganoth D, Hershko A. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proceedings of the National Academy of Science of the USA. 2006;103:11515–11520. doi: 10.1073/pnas.0603921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick M, Lochhead SR, Honda A, Palmer S, Callis J. Related to ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth, auxin signaling, and ethylene production in Arabidopsis. The Plant Cell. 2004;16:2418–2432. doi: 10.1105/tpc.104.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Nam J, Dangl JL. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proceedings of the National Academy of Science of the USA. 1998;95:15849–15854. doi: 10.1073/pnas.95.26.15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert WF, Delaure SL, De Bolle MFC, Cammue BPA. The role of ethylene in host–pathogen interactions. Annual Review of Phytopathology. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Broomfield S, Chow BL, Xiao W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proceedings of the National Academy of Science of the USA. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. Journal of Cell Science. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. The Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, et al. The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. The Plant Cell. 2003;15:2370–2382. doi: 10.1105/tpc.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS Protein. The Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau V, Tobias J, Bachmair A, Marriott D, Ecker D, Gonda D, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Chen H, Shen Y, Tang X, Yu L, Wang J, Guo L, et al. Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. The Plant Cell. 2006;18:1991–2004. doi: 10.1105/tpc.106.043224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-F, Etheridge N, Schaller G. Ethylene signal transduction. Annals of Botany. 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. AtCAND1, a HEAT-repeat protein that participates in auxin signaling in Arabidopsis. Plant Physiology. 2004;135:1020–1026. doi: 10.1104/pp.104.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HW, Zhang W, Gray WM. Arabidopsis ETA2, an apparent ortholog of the human cullin-interacting protein CAND1, is required for auxin responses mediated by the SCF(TIR1) ubiquitin ligase. The Plant Cell. 2004;16:1883–1897. doi: 10.1105/tpc.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Ryu C-M, Oh S-K, Kim RN, Park JM, Cho HS, et al. Suppression of pepper SGT1 and SKP1 causes severe retardation of plant growth and compromises basal resistance. Physiologia Plantarum. 2006;126:605–617. [Google Scholar]
- Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends in Cell Biology. 2004;14:103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Cope GA, Suh GSB, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, et al. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science. 2002;298:608–611. doi: 10.1126/science.1075901. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dahan J, Etienne P, Petitot AS, Houot V, Blein JP, Suty L. Cryptogein affects expression of alpha3, alpha6 and beta1 20S proteasome subunits encoding genes in tobacco. Journal of Experimental Botany. 2001;52:1947–1948. doi: 10.1093/jexbot/52.362.1947. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, editor. Plant hormones: biosynthesis, signal transduction, action! Boston, MA: Kluwer Academic Publishers; 2004. pp. 1–15. [Google Scholar]
- Deng XW, Caspar T, Quail PH. cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes and Development. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, et al. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell. 2003;15:2181–2191. doi: 10.1105/tpc.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto A, Turner JG. Jasmonate-regulated Arabidopsis stress signalling network. Physiologia Plantarum. 2005;123:161–172. [Google Scholar]
- Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, et al. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in. Arabidopsis. Plant Journal. 2002;32:457–466. doi: 10.1046/j.1365-313x.2002.01432.x. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005a;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F-box proteins. Developmental Cell. 2005b;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Dieterle M, Zhou YC, Schafer E, Funk M, Kretsch T. EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes and Development. 2001;15:939–944. doi: 10.1101/gad.197201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle M, Thomann A, Renou JP, Parmentier Y, Cognat V, Lemonnier G, et al. Molecular and functional characterization of Arabidopsis Cullin 3A. Plant Journal. 2005;41:386–399. doi: 10.1111/j.1365-313X.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- Dill A, Jung HS, Sun TP. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proceedings of the National Academy of Science of the USA. 2001;98:14162–14167. doi: 10.1073/pnas.251534098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A, Thomas SG, Hu J, Steber CM, Sun TP. The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. The Plant Cell. 2004;16:1392–1405. doi: 10.1105/tpc.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Wang L, Zhang Y, Zhang Y, Deng X, Xue Y. An auxin-inducible F-box protein CEGENDUO negatively regulates auxin-mediated lateral root formation in Arabidopsis. Plant Molecular Biology. 2006;60:599–615. doi: 10.1007/s11103-005-5257-5. [DOI] [PubMed] [Google Scholar]
- Dong X. NPR1, all things considered. Current Opinion in Plant Biology. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Doronkin S, Djagaeva I, Beckendorf SK. The COP9 signalosome promotes degradation of cyclin E during early. Drosophila oogenesis. 2003;4:699–710. doi: 10.1016/s1534-5807(03)00121-7. Developmental Cell. [DOI] [PubMed] [Google Scholar]
- Downes BP, Vierstra RD. Post-translational regulation in plants employing a diverse set of polypeptide tags. Biochemical Society Transactions. 2005;33:393–399. doi: 10.1042/BST0330393. [DOI] [PubMed] [Google Scholar]
- Downes BP, Stupar RM, Gingerich DJ, Vierstra RD. The HECT ubiquitin-protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant Journal. 2003;35:729–742. doi: 10.1046/j.1365-313x.2003.01844.x. [DOI] [PubMed] [Google Scholar]
- Drugeon G, Jupin I. Stability in vitro of the 69 K movement protein of turnip yellow mosaic virus is regulated by the ubiquitin-mediated proteasome pathway. Journal of General Virology. 2002;83:3187–3197. doi: 10.1099/0022-1317-83-12-3187. [DOI] [PubMed] [Google Scholar]
- Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, et al. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO Journal. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X. Systemic acquired resistance. Annual Review of Phytopathology. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- Durrant WE, Rowland O, Piedras P, Hammond-Kosack KE, Jones JD. cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. The Plant Cell. 2000;12:963–977. doi: 10.1105/tpc.12.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge N, Hall BP, Schaller GE. Progress report: ethylene signaling and responses. Planta. 2006;223:387–391. doi: 10.1007/s00425-005-0163-2. [DOI] [PubMed] [Google Scholar]
- Farras R, Ferrando A, Jasik J, Kleinow T, Okresz L, Tiburcio A, et al. SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO Journal. 2001;20:2742–2756. doi: 10.1093/emboj/20.11.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant Journal. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Feng S, Ma L, Wang X, Xie D, Dinesh-Kumar SP, Wei N, et al. The COP9 signalosome interacts physically with SCF COI1 and modulates jasmonate responses. The Plant Cell. 2003;15:1083–1094. doi: 10.1105/tpc.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Shen Y, Sullivan JA, Rubio V, Xiong Y, Sun TP, et al. Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathways controlled by ubiquitin/proteasome-mediated protein degradation. The Plant Cell. 2004;16:1870–1882. doi: 10.1105/tpc.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Parker JE. Interplay of signaling pathways in plant disease resistance. Trends in Genetics. 2000;16:449–455. doi: 10.1016/s0168-9525(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. The Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa P, Gusmaroli G, Serino G, Habashi J, Ma L, Shen Y, et al. Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes. in vivo. The Plant Cell. 2005;17:1180–1195. doi: 10.1105/tpc.105.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Sadis S, Monia BP, Boucher P, Ecker DJ, Crooke ST, et al. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Molecular and Cellular Biology. 1994;14:5501–5509. doi: 10.1128/mcb.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet CM, Sun T-p. A DELLAcate balance: the role of gibberellin in plant morphogenesis. Current Opinion in Plant Biology. 2005;8:77–85. doi: 10.1016/j.pbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Freialdenhoven A, Scherag B, Hollricher K, Collinge DB, Thordal-Christensen H, Schulze-Lefert P. Nar-1 and Nar-2, two loci required for Mla12-specified race-specific resistance to powdery mildew in barley. The Plant Cell. 1994;6:983–994. doi: 10.1105/tpc.6.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP. The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. The Plant Cell. 2004;16:1406–1418. doi: 10.1105/tpc.021386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop K, Tarayre S, Kelemen Z, Horvath G, Kevei Z, Nikovics K, et al. Arabidopsis anaphase-promoting complexes: multiple activators and wide range of substrates might keep APC perpetually busy. Cell Cycle. 2005;4:1084–1092. [PubMed] [Google Scholar]
- Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Molecular and Cellular Biology. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proceedings of the National Academy of Science of the USA. 2002;99:11519–11524. doi: 10.1073/pnas.162339999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, et al. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proceedings of the National Academy of Science of the USA. 2004;101:6803–6808. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J-M, Wiederkehr A, Seol JH, Haguenauer-Tsapis R, Deshaies RJ, Riezman H, et al. Skp1p and the F-Box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Molecular and Cellular Biology. 2001;21:3105–3117. doi: 10.1128/MCB.21.9.3105-3117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS. The parasitome of the phytonematode. Heterodera glycines. Molecular Plant–Microbe Interactions. 2003;16:720–726. doi: 10.1094/MPMI.2003.16.8.720. [DOI] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Gingerich DJ, Gagne JM, Salter DW, Hellmann H, Estelle M, Ma L, et al. Cullins 3a and 3b assemble with members of the Broad Complex/Tramtrack/Bric-a-Brac (BTB) protein family to form rssential ubiquitin-protein ligases (E3s) in Arabidopsis. Journal of Biological Chemistry. 2005;280:18810–18821. doi: 10.1074/jbc.M413247200. [DOI] [PubMed] [Google Scholar]
- Goldenberg SJ, Cascio TC, Shumway SD, Garbutt KC, Liu J, Xiong Y, et al. Structure of the Cand1-Cul1-Roc1 complex reveals regulatory mechanisms for the assembly of the multisubunit cullin-dependent ubiquitin ligases. Cell. 2004;119:517–528. doi: 10.1016/j.cell.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Golem S, Culver JN. Tobacco mosaic virus induced alterations in the gene expression profile of. Arabidopsis thaliana. Molecular Plant–Microbe Interactions. 2003;16:681–688. doi: 10.1094/MPMI.2003.16.8.681. [DOI] [PubMed] [Google Scholar]
- Gomez-Cadenas A, Zentella R, Walker-Simmons MK, Ho T-HD. Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. The Plant Cell. 2001;13:667–679. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Felix G, Boller T. A single locus determines sensitivity to bacterial flagellin in. Arabidopsis thaliana. Plant Journal. 1999;18:277–284. doi: 10.1046/j.1365-313x.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- Gomi K, Sasaki A, Itoh H, Ueguchi-Tanaka M, Ashikari M, Kitano H, et al. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant Journal. 2004;37:626–634. doi: 10.1111/j.1365-313x.2003.01990.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lamothe R, Tsitsigiannis DI, Ludwig AA, Panicot M, Shirasu K, Jones JD. The U-box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. The Plant Cell. 2006;18:1067–1083. doi: 10.1105/tpc.106.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, et al. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science. 1995;269:843–846. doi: 10.1126/science.7638602. [DOI] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, et al. Identification of an SCF ubiquitin-ligase complex required for auxin response in. Arabidopsis thaliana. Genes and Development. 1999;13:1678–1691. doi: 10.1101/gad.13.13.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- Gray WM, Hellmann H, Dharmasiri S, Estelle M. Role of the Arabidopsis RING-H2 protein RBX1 in RUB modification and SCF function. The Plant Cell. 2002;14:2137–2144. doi: 10.1105/tpc.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE. Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. The Plant Cell. 2003;15:1310–1319. doi: 10.1105/tpc.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, et al. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell. 2003;113:357–367. doi: 10.1016/s0092-8674(03)00316-7. [DOI] [PubMed] [Google Scholar]
- Groisman R, Kuraoka I, Chevallier O, Gaye N, Magnaldo T, Tanaka K, et al. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes and Development. 2006;20:1429–1434. doi: 10.1101/gad.378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for myb transactivation of a high-pl alpha-amylase gene promoter. The Plant Cell. 1995;7:1879–1891. doi: 10.1105/tpc.7.11.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Chandler PM, White RG, Llewellyn DJ, Jacobsen JV. Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression. Plant Physiology. 2002;129:191–200. doi: 10.1104/pp.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ, Ulmasov T, Hagen G. The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cellular and Molecular Life Sciences. 1998;54:619–627. doi: 10.1007/s000180050190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;116:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Han L, Mason M, Risseeuw EP, Crosby WL, Somers DE. Formation of an SCFZTL complex is required for proper regulation of circadian timing. Plant Journal. 2004;40:291–301. doi: 10.1111/j.1365-313X.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U-box proteins as a new family of ubiquitin-protein ligases. Journal of Biological Chemistry. 2001;276:33111–33120. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- Hatfield PM, Gosink MM, Carpenter TB, Vierstra RD. The ubiquitin-activating enzyme (E1) gene family in. Arabidopsis thaliana. Plant Journal. 1997;11:213–226. doi: 10.1046/j.1365-313x.1997.11020213.x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M. The armadillo family of structural proteins. International Review of Cytology. 1999;186:179–224. doi: 10.1016/s0074-7696(08)61054-2. [DOI] [PubMed] [Google Scholar]
- Hauck P, Thilmony R, He SY. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proceedings of the National Academy of Science of the USA. 2003;100:8577–8582. doi: 10.1073/pnas.1431173100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood D, Zaitlin M. Ubiquitinated conjugates are found in preparations of several plant viruses. Virology. 1990;177:352–356. doi: 10.1016/0042-6822(90)90490-i. [DOI] [PubMed] [Google Scholar]
- He JX, Gendron JM, Yang YL, Li JM, Wang ZY. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proceedings of the National Academy of Science of the USA. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise A, Lippok B, Kirsch C, Hahlbrock K. Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitor-response element of AtCMPG1. Proceedings of the National Academy of Science of the USA. 2002;99:9049–9054. doi: 10.1073/pnas.132277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, et al. Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligase in auxin regulation of embryogenesis. EMBO Journal. 2003;22:3314–3325. doi: 10.1093/emboj/cdg335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schafer E. Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiology. 2002;128:194–200. [PMC free article] [PubMed] [Google Scholar]
- Hericourt F, Blanc S, Redeker V, Jupin I. Evidence for phosphorylation and ubiquitinylation of the turnip yellow mosaic virus RNA-dependent RNA polymerase domain expressed in a baculovirus-insect cell system. Biochemical Journal. 2000;349:417–425. doi: 10.1042/0264-6021:3490417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Sebastia C, Hardin SC, Clouse SD, Kieber JJ, Huber SC. Identification of a new motif for CDPK phosphorylation. Archives of Biochemistry and Biophysics. 2004;428:81–91. doi: 10.1016/j.abb.2004.04.025. in vitro that suggests ACC synthase may be a CDPK substrate. [DOI] [PubMed] [Google Scholar]
- Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signaling. Current Opinion in Plant Biology. 2003;6:470–479. doi: 10.1016/s1369-5266(03)00090-6. [DOI] [PubMed] [Google Scholar]
- Hoecker U. Regulated proteolysis in light signaling. Current Opinion in Plant Biology. 2005;8:469–476. doi: 10.1016/j.pbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM. In vitro assembly and recognition of Lys-63 polyubiquitin chains. Journal of Biological Chemistry. 2001;276:27936–27943. doi: 10.1074/jbc.M103378200. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Reports. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt BF, 3rd, Belkhadir Y, Dangl JL. Antagonistic control of disease resistance protein stability in the plant immune system. Science. 2005;309:929–932. doi: 10.1126/science.1109977. [DOI] [PubMed] [Google Scholar]
- Hori T, Osaka F, Chiba T, Miyamoto C, Okabayashi K, Shimbara N, et al. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829–6834. doi: 10.1038/sj.onc.1203093. [DOI] [PubMed] [Google Scholar]
- Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nature Cell Biology. 2004;6:1003–1009. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, et al. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO Journal. 2003;22:5679–5689. doi: 10.1093/emboj/cdg547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata: V. microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiology. 2003;131:1877–1893. doi: 10.1104/pp.102.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Min KW, Tamura TA, Yoon JB. TIP120A associates with unneddylated cullin 1 and regulates its neddylation. FEBS Letters. 2003;541:102–108. doi: 10.1016/s0014-5793(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, et al. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene. GAI/RGA/RHT/D8. The Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293–297. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with Phototropin 1 in. Arabidopsis thaliana. The Plant Cell. 2004;16:887–896. doi: 10.1105/tpc.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Matsuoka M, Steber CM. A role for the ubiquitin-26S-proteasome pathway in gibberellin signaling. Trends in Plant Science. 2003;8:492–497. doi: 10.1016/j.tplants.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Jakobek JL, Smith JA, Lindgren PB. Suppression of bean defense responses by. Pseudomonas syringae. The Plant Cell. 1993;5:57–63. doi: 10.1105/tpc.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjusevic R, Abramovitch RB, Martin GB, Stebbins CE. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science. 2006;311:222–226. doi: 10.1126/science.1120131. [DOI] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Molecular Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, et al. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210:336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, et al. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes and Development. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KB, Hyman AA, Sorger PK. Regulating the yeast kinetochore by ubiquitin-dependent degradation and skp1p-mediated phosphorylation. Cell. 1997;91:491–500. doi: 10.1016/s0092-8674(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Nam J, Boyes DC, Holt BF, 3rd, Hubert DA, Wiig A, et al. A duplicated pair of Arabidopsis RING-finger E3 ligases contribute to the RPM1- and RPS2-mediated hypersensitive response. Plant Journal. 2005;44:258–270. doi: 10.1111/j.1365-313X.2005.02525.x. [DOI] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:283–307. [Google Scholar]
- Kepinski S, Leyser O. Auxin-induced SCFTIR1-Aux/IAA interaction involves stable modification of the SCFTIR1 complex. Proceedings of the National Academy of Science of the USA. 2004;101:12381–12386. doi: 10.1073/pnas.0402868101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Keshavarzi M, Soylu S, Brown I, Bonas U, Nicole M, Rossiter J, et al. Basal defenses induced in pepper by lipopolysaccharides are suppressed by Xanthomonas campestris pv. vesicatoria. Molecular Plant–Microbe Interactions. 2004;17:805–815. doi: 10.1094/MPMI.2004.17.7.805. [DOI] [PubMed] [Google Scholar]
- Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, et al. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. The Plant Cell. 2003;15:2707–2718. doi: 10.1105/tpc.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Delaney TP. Arabidopsis SON1 is an F-box protein that regulates a novel induced defense response independent of both salicylic acid and systemic acquired resistance. The Plant Cell. 2002;14:1469–1482. doi: 10.1105/tpc.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proceedings of the National Academy of Science of the USA. 2005;102:6496–6501. doi: 10.1073/pnas.0500792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, et al. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell. 2005;121:749–759. doi: 10.1016/j.cell.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi H-i, Ryu H-J, Park JH, Kim MD, Kim SY. ARIA, an arabidopsis Arm repeat protein interacting with a transcriptional regulator of abscisic acid-responsive gene expression, is a novel abscisic acid signaling component. Plant Physiology. 2004;136:3639–3648. doi: 10.1104/pp.104.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Yang SF. Turnover of 1-aminocyclopropane-1-carboxylic acid synthase protein in wounded tomato fruit tissue. Plant Physiology. 1992;100:1126–1131. doi: 10.1104/pp.100.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Ham BK, Paek KH, Park CM, Chua NH. An arabidopsis homologue of human Seven-in-Absentia-interacting protein is involved in pathogen resistance. Molecules and Cells. 2006;21:389–394. [PubMed] [Google Scholar]
- Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biology. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K. A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant Journal. 2001;26:217–227. doi: 10.1046/j.1365-313x.2001.01015.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Skowyra D, Elledge SJ, Harper LW, Hieter P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Molecular Cell. 1999;4:21–33. doi: 10.1016/s1097-2765(00)80184-7. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Koyama T, Ohme-Takagi M, Shinshi H, Sato F. Characterization of gene expression of NsERFs, transcription factors of basic PR genes from. Nicotiana sylvestris. Plant and Cell Physiology. 2000;41:817–824. doi: 10.1093/pcp/41.6.817. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiology. 2004;135:660–667. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyn PW, Fan W, Kovats SG, Lee JJ, Pulido JC, Wu Y, et al. Identification and characterization of the mouse obesity gene tubby: A member of a novel gene family. Cell. 1996;85:281–290. doi: 10.1016/s0092-8674(00)81104-6. [DOI] [PubMed] [Google Scholar]
- Ko J-H, Yang SH, Han K-H. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant Journal. 2006;47:343–355. doi: 10.1111/j.1365-313X.2006.02782.x. [DOI] [PubMed] [Google Scholar]
- Koyama T, Okada T, Kitajima S, Ohme-Takagi M, Shinshi H, Sato F. Isolation of tobacco ubiquitin-conjugating enzyme cDNA in a yeast two-hybrid system with tobacco ERF3 as bait and its characterization of specific interaction. Journal of Experimental Botany. 2003;54:1175–1181. doi: 10.1093/jxb/erg136. [DOI] [PubMed] [Google Scholar]
- Kraft E, Stone SL, Ma L, Su N, Gao Y, Lau OS, et al. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiology. 2005;139:1597–1611. doi: 10.1104/pp.105.067983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Takahashi N, Shimada H, Seki M, Shinozaki K, Matsui M. Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant and Cell Physiology. 2002;43:1073–1085. doi: 10.1093/pcp/pcf151. [DOI] [PubMed] [Google Scholar]
- Kurz T, Ozlu N, Rudolf F, O'Rourke SM, Luke B, Hofmann K, et al. The conserved protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature. 2005;435:1257–1261. doi: 10.1038/nature03662. [DOI] [PubMed] [Google Scholar]
- Lai CP, Lee CL, Chen PH, Wu SH, Yang CC, Shaw JF. Molecular analyses of the Arabidopsis TUBBY-like protein gene family. Plant Physiology. 2004;134:1586–1597. doi: 10.1104/pp.103.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD. A recessive mutation in the RUB1-conjugating enzyme, RCE1, reveals a requirement for RUB modification for control of ethylene biosynthesis and proper induction of basic chitinase and PDF1·2 in Arabidopsis. Plant Journal. 2004;38:626–638. doi: 10.1111/j.1365-313X.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- Lechner E, Xie D, Grava S, Pigaglio E, Planchais S, Murray JA, et al. The AtRbx1 protein is part of plant SCF complexes, and its down-regulation causes severe growth and developmental defects. Journal of Biological Chemistry. 2002;277:50069–50080. doi: 10.1074/jbc.M204254200. [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. Hookless1, an ethylene response gene, is required for differential cell elongation in the arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Leister RT, Dahlbeck D, Day B, Li Y, Chesnokova O, Staskawicz BJ. Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in. Nicotiana benthamiana. The Plant Cell. 2005;17:1268–1278. doi: 10.1105/tpc.104.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in arabidopsis. Developmental Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Li T, Chen X, Garbutt KC, Zhou P, Zheng N. Structure of DDB1 in complex with a paramyxovirus V protein: viral hijack of a propeller cluster in ubiquitin ligase. Cell. 2006;124:105–117. doi: 10.1016/j.cell.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Liu C, Powell KA, Mundt K, Wu L, Carr AM, Caspari T. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes and Development. 2003;17:1130–1140. doi: 10.1101/gad.1090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Poitelea M, Watson A, Yoshida S-h, Shimoda C, Holmberg C, et al. Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO Journal. 2005;24:3940–3951. doi: 10.1038/sj.emboj.7600854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ni W, Griffith ME, Huang Z, Chang C, Peng W, et al. The ASK1 and ASK2 genes are essential for arabidopsis early development. The Plant Cell. 2004;16:5–20. doi: 10.1105/tpc.017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Furukawa M, Matsumoto T, Xiong Y. NEDD8 modification of CUL1 dissociates p120CAND1, an inhibitor of CUL1-SKP1 binding and SCF ligases. Molecular Cell. 2002;10:1511–1518. doi: 10.1016/s1097-2765(02)00783-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. The Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Serino G, Deng XW, Dinesh-Kumar SP. Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. The Plant Cell. 2002;14:1483–1496. doi: 10.1105/tpc.002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Hannink M. CAND1-mediated substrate adaptor recycling is required for efficient repression of Nrf2 by Keap1. Molecular and Cellular Biology. 2006;26:1235–1244. doi: 10.1128/MCB.26.4.1235-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CM. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proceedings of the National Academy of Science of the USA. 2001;98:4782–4787. doi: 10.1073/pnas.081594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Kinoshita N, Chua N-H. AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes and Development. 2003;17:410–418. doi: 10.1101/gad.1055803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Current Opinion in Plant Biology. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. The Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Malcuit I, Moffett P, Ruiz MT, Peart J, Wu AJ, et al. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO Journal. 2003;22:5690–5699. doi: 10.1093/emboj/cdg546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke-Glaser S, Pintard L, Lu C, Mains PE, Peter M. The BTB protein MEL-26 promotes cytokinesis in C. elegans by a CUL-3-independent mechanism. Current Biology. 2005;15:1605–1615. doi: 10.1016/j.cub.2005.07.068. [DOI] [PubMed] [Google Scholar]
- Luo J, Shen G, Yan J, He C, Zhang H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant Journal. 2006;46:649–657. doi: 10.1111/j.1365-313X.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- Lyapina S, Cope G, Shevchenko A, Serino G, Tsuge T, Zhou C, et al. Promotion of NEDD8-CUL1 conjugate cleavage by COP9 signalosome. Science. 2001;292:1382–1385. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen K, Schaefer L, Menon S, Deng X-W, Miller JB, Wei N. Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E, and early embryonic death. Molecular and Cellular Biology. 2003;23:6790–6797. doi: 10.1128/MCB.23.19.6790-6797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Holt BF, 3rd, Wiig A, Dangl JL. RIN4 interacts with. Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. Cell. [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112:379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Macmillan CP, Blundell CA, King RW. Flowering of the grass Lolium perenne: effects of vernalization and long days on gibberellin biosynthesis and signaling. Plant Physiology. 2005;138:1794–1806. doi: 10.1104/pp.105.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden TL, Tatusov RL, Zhang J, Russell FD. Applications of network BLAST server. In: Doolittle RF, editor. Methods in enzymology. New York: Academic Press; 1996. pp. 131–141. [DOI] [PubMed] [Google Scholar]
- Magyar Z, De Veylder L, Atanassova A, Bako L, Inze D, Bogre L. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. The Plant Cell. 2005;17:2527–2541. doi: 10.1105/tpc.105.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, et al. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors. in vivo. Molecular and Cellular Biology. 2001;21:6782–6795. doi: 10.1128/MCB.21.20.6782-6795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Azuma K, Saijo M, Iemura S, Hioki Y, Natsume T, et al. DDB2, the xeroderma pigmentosum group E gene product, is directly ubiquitylated by Cullin 4A-based ubiquitin ligase complex. DNA Repair. 2005;4:537–545. doi: 10.1016/j.dnarep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S-i, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for Beta-Catenin degradation linked to p53 responses. Molecular Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Mazeyrat F, Mouzeyar S, Courbou I, Badaoui S, Roeckel-Drevet P, Tourvieille de Labrouhe D, et al. Accumulation of defense related transcripts in sunflower hypocotyls (Helianthus annuus L.) infected with Plasmopara halstedii. European Journal of Plant Pathology. 1999;105:333–340. [Google Scholar]
- McCall CM, Hu J, Xiong Y. Recruiting substrates to cullin 4-dependent ubiquitin ligases by DDB1. Cell Cycle. 2005;4:27–29. doi: 10.4161/cc.4.1.1396. [DOI] [PubMed] [Google Scholar]
- McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun TP, et al. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. The Plant Cell. 2003;15:1120–1130. doi: 10.1105/tpc.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaely P, Bennett V. The ANK repeat: a ubiquitous motif involved in macromolecular recognition. Trends in Cell Biology. 1992;2:127–129. doi: 10.1016/0962-8924(92)90084-z. [DOI] [PubMed] [Google Scholar]
- Min KW, Hwang JW, Lee JS, Park Y, Tamura TA, Yoon JB. TIP120A associates with cullins and modulates ubiquitin ligase activity. Journal of Biological Chemistry. 2003;278:15905–15910. doi: 10.1074/jbc.M213070200. [DOI] [PubMed] [Google Scholar]
- Molnar G, Bancos S, Nagy F, Szekeres M. Characterisation of BRH1, a brassinosteroid-responsive RING-H2 gene from Arabidopsis thaliana. Planta. 2002;215:127–133. doi: 10.1007/s00425-001-0723-z. [DOI] [PubMed] [Google Scholar]
- Monte E, Amador V, Russo E, Martínez-García J, Prat S. PHOR1: a U-Box GA signaling component with a role in proteasome degradation? Journal of Plant Growth Regulation. 2003;22:152–162. [Google Scholar]
- Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. The Plant Cell. 2004;16:3181–3195. doi: 10.1105/tpc.104.161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Garcia S, Vert G, Yin Y, Cano-Delgado A, Cheong H, Chory J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes and Development. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JR, Solomon E. BRCA1: BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Human Molecular Genetics. 2004;13:807–817. doi: 10.1093/hmg/ddh095. [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E. Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286:961–964. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- Mudgil Y, Shiu SH, Stone SL, Salt JN, Goring DR. A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiology. 2004;134:59–66. doi: 10.1104/pp.103.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskett PR, Kahn K, Austin MJ, Moisan LJ, Sadanandom A, Shirasu K, et al. Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defenses against multiple pathogens. The Plant Cell. 2002;14:979–992. doi: 10.1105/tpc.001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Shimada A, Takashi Y, Kim YC, Park SH, Ueguchi-Tanaka M, et al. Identification and characterization of Arabidopsis gibberellin receptors. Plant Journal. 2006;46:880–889. doi: 10.1111/j.1365-313X.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, et al. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiology. 2004;135:1113–1128. doi: 10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Ooka S, Sato K, Arima K, Okamoto J, Klevit RE, et al. Mass spectrometric and mutational analyses reveal lys-6-linked polyubiquitin chains catalysed by BRCA1-BARD1 ubiquitin ligase. Journal of Biological Chemistry. 2004;279:3916–3924. doi: 10.1074/jbc.M308540200. [DOI] [PubMed] [Google Scholar]
- Nodzon LA, Xu WH, Wang Y, Pi LY, Chakrabarty PK, Song WY. The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant Journal. 2004;40:996–1006. doi: 10.1111/j.1365-313X.2004.02266.x. [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Reviews. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant Journal. 2006;47:124–139. doi: 10.1111/j.1365-313X.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nature Structural Biology. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. The Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M, Hardtke C, Wei N, Deng X-W. Targeted destabilization of HY5 during light regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- Ozkan E, Yu H, Deisenhofer J. Mechanistic insight into the allosteric activation of a ubiquitin-conjugating enzyme by RING-type ubiquitin ligases. Proceedings of the National Academy of Science of the USA. 2005;102:18890–18895. doi: 10.1073/pnas.0509418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Friml J. Auxin signaling. Journal of Cell Science. 2006;119:1199–1202. doi: 10.1242/jcs.02910. [DOI] [PubMed] [Google Scholar]
- Padmanabhan MS, Goregaoker SP, Golem S, Shiferaw H, Culver JN. Interaction of the Tobacco mosaic virus replicase protein with the Aux/IAA protein PAP1/IAA26 is associated with disease development. Journal of Virology. 2005;79:2549–2558. doi: 10.1128/JVI.79.4.2549-2558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan MS, Shiferaw H, Culver JN. The Tobacco mosaic virus replicase protein disrupts the localization and function of interacting Aux/IAA proteins. Molecular Plant–Microbe Interactions. 2006;19:864–873. doi: 10.1094/MPMI-19-0864. [DOI] [PubMed] [Google Scholar]
- Parry G, Estelle M. Auxin receptors: a new role for F-box proteins. Current Opinion in Cell Biology. 2006;18:152–156. doi: 10.1016/j.ceb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Parthier B. Jasmonates: hormonal regulators or stress factors in leaf senescence? Journal of Plant Growth Regulation. 1990;9:57–63. [Google Scholar]
- Pazhouhandeh M, Dieterle M, Marrocco K, Lechner E, Berry B, Brault V, et al. F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proceedings of the National Academy of Science of the USA. 2006;103:1994–1999. doi: 10.1073/pnas.0510784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. The role of GA-mediated signalling in the control of seed germination. Current Opinion in Plant Biology. 2002;5:376–381. doi: 10.1016/s1369-5266(02)00279-0. [DOI] [PubMed] [Google Scholar]
- Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, et al. A proteomics approach to understanding protein ubiquitination. Nature Biotechnology. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nature Reviews Molecular Cell Biology. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochimica et Biophysica Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Pintard L, Kurz T, Glaser S, Willis JH, Peter M, Bowerman B. Neddylation deneddylation of CUL-3 is required to target MEI-1/Katanin for degradation at the meiosis-to-mitosis transition in. C. elegans. Current Biology. 2003a;13:911–921. doi: 10.1016/s0960-9822(03)00336-1. [DOI] [PubMed] [Google Scholar]
- Pintard L, Willis JH, Willems A, Johnson JL, Srayko M, Kurz T, et al. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature. 2003b;425:311–316. doi: 10.1038/nature01959. [DOI] [PubMed] [Google Scholar]
- Pointud J-C, Larsson J, Dastugue B, Couderc J-L. The BTB/POZ domain of the regulatory proteins Bric a brac 1 (BAB1) and Bric a brac 2 (BAB2) interacts with the novel Drosophila TAFII factor BIP2/dTAFII155. Developmental Biology. 2001;237:368–380. doi: 10.1006/dbio.2001.0358. [DOI] [PubMed] [Google Scholar]
- Pontier D, Privat I, Trifa Y, Zhou J-M, Klessig DF, Lam E. Differential regulation of TGA transcription factors by post-transcriptional control. Plant Journal. 2002;32:641–653. doi: 10.1046/j.1365-313x.2002.01461.x. [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Grava S, Koncz C, Genschik P. EIN3-dependent tegulation of plant ethylene hormone signaling by two arabidopsis F-Box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Estelle M. The Arabidopsis cullin AtCUL1 is modified by the ubiquitin-related protein RUB1. Proceedings of the National Academy of Science of the USA. 1999;96:15342–15347. doi: 10.1073/pnas.96.26.15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint M, Ito H, Zhang W, Gray WM. Characterization of a novel temperature-sensitive allele of the CUL1/AXR6 subunit of the SCF ubiquitin ligase. Plant Journal. 2005;43:371–383. doi: 10.1111/j.1365-313X.2005.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonell K, Berrocal-Lobo M, Koh S, Wan J, Edwards H, Stacey G, et al. Loss-of-function mutations in chitin responsive genes show increased susceptibility to the powdery mildew pathogen. Erysiphe cichoracearum. Plant Physiology. 2005;138:1027–1036. doi: 10.1104/pp.105.060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J, Zenser N, Leyser O, Callis J. Rapid degradation of Auxin/Indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. The Plant Cell. 2001;13:2349–2360. doi: 10.1105/tpc.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Naik C, Delacruz W, Laplaza JM, Tan S, Callis J, Fisher AJ. The Rub family of proteins: crystal structure of Arabidopsis RUB1 and expression of multiple RUBs in Arabidopsis. Journal of Biological Chemistry. 1998;273:34976–34982. doi: 10.1074/jbc.273.52.34976. [DOI] [PubMed] [Google Scholar]
- Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, et al. Nedd8 modification of Cul-1 activates SCF βTrCp-dependent ubiquitination of I kappa B alpha. Molecular and Cellular Biology. 2000;20:2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends in Biochemical Sciences. 1996;21:267–271. [PubMed] [Google Scholar]
- Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends in Plant Science. 2001;6:420–425. doi: 10.1016/s1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- Reichel C, Beachy RN. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. Journal of Virology. 2000;74:3330–3337. doi: 10.1128/jvi.74.7.3330-3337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, et al. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from. Arabidopsis. Plant Journal. 2003;34:753–767. doi: 10.1046/j.1365-313x.2003.01768.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Levchenko V, Hedrich R. ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant Journal. 2004;37:578–588. doi: 10.1111/j.1365-313x.2003.01985.x. [DOI] [PubMed] [Google Scholar]
- Rowland O, Ludwig AA, Merrick CJ, Baillieul F, Tracy FE, Durrant WE, et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. The Plant Cell. 2005;17:295–310. doi: 10.1105/tpc.104.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray W, Hobbie L, Turner J, Estelle M. The TIR protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes and Development. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell ID, Grancell AS, Sorger PK. The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex. The Journal of Cell Biology. 1999;145:933–950. doi: 10.1083/jcb.145.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- Salinas-Mondragon RE, Garciduenas-Pina C, Guzman P. Early elicitor induction in members of a novel multigene family coding for highly related RING-H2 proteins in. Arabidopsis thaliana. Plant Molecular Biology. 1999;40:579–590. doi: 10.1023/a:1006267201855. [DOI] [PubMed] [Google Scholar]
- Samach A, Klenz JE, Kohalmi SE, Risseeuw E, Haughn GW, Crosby WL. The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant Journal. 1999;20:433–445. doi: 10.1046/j.1365-313x.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- Sancho E, Vilá MR, Sánchez-Pulido L, Lozano JJ, Paciucci R, Nadal M, et al. Role of UEV-1, an inactive variant of the E2 ubiquitin-conjugating enzymes, in in vitro differentiation and cell cycle behavior of HT-29-M6 intestinal mucosecretory cells. Molecular and Cellular Biology. 1998;18:576–589. doi: 10.1128/mcb.18.1.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. Journal of Biological Chemistry. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development. 2006;133:251–261. doi: 10.1242/dev.02194. [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P, Bieri S. Plant sciences. Recognition at a distance. Science. 2005;308:506–508. doi: 10.1126/science.1111725. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Schwager K. Regulated proteolysis and plant development. Plant Cell Reports. 2004;23:353–364. doi: 10.1007/s00299-004-0858-z. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, et al. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science. 2001;292:1379–1382. doi: 10.1126/science.1059776. [DOI] [PubMed] [Google Scholar]
- Serrano M, Parra S, Alcaraz LD, Guzman P. The ATL gene family from Arabidopsis thaliana and Oryza sativa comprises a large number of putative ubiquitin ligases of the RING-H2 type. Journal of Molecular Evolution. 2006;62:434–445. doi: 10.1007/s00239-005-0038-y. [DOI] [PubMed] [Google Scholar]
- Shanklin J, Jabben M, Vierstra RD. Red light-induced formation of ubiquitin-phytochrome conjugates: Identification of possible intermediates of phytochrome degradation. Proceedings of the National Academy of Science of the USA. 1987;84:359–363. doi: 10.1073/pnas.84.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WH, Parmentier Y, Hellmann H, Lechner E, Dong AW, Masson J, et al. Null mutation of AtCUL1 causes arrest in early embryogenesis in Arabidopsis. Molecular Biology of the Cell. 2002;13:1916–1928. doi: 10.1091/mbc.E02-02-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by Phytochrome B and secondarily by Phytochrome A. Plant Physiology. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K, Lahaye T, Tan MW, Zhou F, Azevedo C, Schulze-Lefert P. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in. C. elegans. Cell. 1999;99:355–366. doi: 10.1016/s0092-8674(00)81522-6. [DOI] [PubMed] [Google Scholar]
- Sieberer T, Seifert GJ, Hauser MT, Grisafi P, Fink GR, Luschnig C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Current Biology. 2000;10:1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Jung HS, Dill A, Kawaide H, Kamiya Y, Sun TP. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. The Plant Cell. 2001;13:1555–1565. doi: 10.1105/TPC.010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annual Review of Plant Biology. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- Smalle J, Kurepa J, Yang P, Emborg TJ, Babiychuk E, Kushnir S, et al. The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. The Plant Cell. 2003;15:965–980. doi: 10.1105/tpc.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao QM, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. doi: 10.1016/s0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiology. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Ritchie S, Soule JD, McGinnis KM, Steber CM. Recessive-interfering mutations in the gibberellin signaling gene SLEEPY1 are rescued by overexpression of its homologue. SNEEZY. Proceedings of the National Academy of Science of the USA. 2004;101:12771–12776. doi: 10.1073/pnas.0404287101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annual Review of Plant Biology. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- Sundquist WI, Schubert HL, Kelly BN, Hill GC, Holton JM, Hill CP. Ubiquitin recognition by the human TSG101 protein. Molecular Cell. 2004;13:783–789. doi: 10.1016/s1097-2765(04)00129-7. [DOI] [PubMed] [Google Scholar]
- Swain SM, Singh DP. Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends in Plant Science. 2005;10:123–129. doi: 10.1016/j.tplants.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Casais C, Ichimura K, Shirasu K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proceedings of the National Academy of Science of the USA. 2003;100:11777–11782. doi: 10.1073/pnas.2033934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kuroda H, Kuromori T, Hirayama T, Seki M, Shinozaki K, et al. Expression and interaction analysis of Arabidopsis Skp1-related genes. Plant and Cell Physiology. 2004;45:83–91. doi: 10.1093/pcp/pch009. [DOI] [PubMed] [Google Scholar]
- Takai R, Matsuda N, Nakano A, Hasegawa K, Akimoto C, Shibuya N, et al. EL5, a rice N-acetylchitooligosaccharide elicitor-responsive RING-H2 finger protein, is a ubiquitin ligase which functions in vitro in co-operation with an elicitor-responsive ubiquitin-conjugating enzyme, OsUBC5b. Plant Journal. 2002;30:447–455. doi: 10.1046/j.1365-313x.2002.01299.x. [DOI] [PubMed] [Google Scholar]
- Takemoto D, Jones DA. Membrane release and destabilization of Arabidopsis RIN4 following cleavage by Pseudomonas syringae AvrRpt2. Molecular Plant–Microbe Interactions. 2005;18:1258–1268. doi: 10.1094/MPMI-18-1258. [DOI] [PubMed] [Google Scholar]
- Takizawa M, Goto A, Watanabe Y. The tobacco ubiquitin-activating enzymes NtE1A and NtE1B are induced by tobacco mosaic virus, wounding and stress hormones. Molecules and Cells. 2005;19:228–231. [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. The Plant Cell. 2002;14:2745–2760. doi: 10.1105/tpc.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Nibau C, Wu HM. RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. The Plant Cell. 2005;17:2369–2383. doi: 10.1105/tpc.105.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. Journal of Biological Chemistry. 2001;276:28051–28057. doi: 10.1074/jbc.M101543200. [DOI] [PubMed] [Google Scholar]
- Thomann A, Dieterle M, Genschik P. Plant CULLIN-based E3s: Phytohormones come first. FEBS Letters. 2005;579:3239–3245. doi: 10.1016/j.febslet.2005.02.068. [DOI] [PubMed] [Google Scholar]
- Thomas SG, Sun TP. Update on gibberellin signaling. A tale of the tall and the short. Plant Physiology. 2004;135:668–676. doi: 10.1104/pp.104.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H. Fresh insights into processes of nonhost resistance. Current Opinion in Plant Biology. 2003;6:351–357. doi: 10.1016/s1369-5266(03)00063-3. [DOI] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO Journal. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko T, Katul L, Aronson M, Vega-Arreguin JC, Ramirez BC, Vetten HJ, et al. Infectivity of nanovirus DNAs: induction of disease by cloned genome components of Faba bean necrotic yellows virus. Journal of General Virology. 2006;87:1735–1743. doi: 10.1099/vir.0.81753-0. [DOI] [PubMed] [Google Scholar]
- Tiryaki I, Staswick PE. An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant. axr1. Plant Physiology. 2002;130:887–894. doi: 10.1104/pp.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Hagen G, Guilfoyle T. Aux/IAA proteins are active repressors and their stability and activity are modulated by auxin. The Plant Cell. 2001;13:2809–2822. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle TJ. Aux/IAA proteins contain a potent transcriptional repression domain. The Plant Cell. 2004;16:533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tor M, Gordon P, Cuzick A, Eulgem T, Sinapidou E, Mert-Turk F, et al. Arabidopsis SGT1b is required for defense signaling conferred by Several downy mildew resistance genes. The Plant Cell. 2002;14:993–1003. doi: 10.1105/tpc.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL. RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. The Plant Cell. 2002;14:1005–1015. doi: 10.1105/tpc.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. The Plant Cell. 2002;14:S153–164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat T, Vanholme B, De Meutter J, Claeys M, Couvreur M, Vanhoutte I, et al. A new class of ubiquitin extension proteins secreted by the dorsal pharyngeal gland in plant parasitic cyst nematodes. Molecular Plant–Microbe Interactions. 2004;17:846–852. doi: 10.1094/MPMI.2004.17.8.846. [DOI] [PubMed] [Google Scholar]
- Tzfira T, Vaidya M, Citovsky V. Involvement of targeted proteolysis in plant genetic transformation by. Agrobacterium. Nature. 2004;431:87–92. doi: 10.1038/nature02857. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Biezen EA, Jones JDG. Plant disease-resistance proteins and the gene-for-gene concept. Trends in Biochemical Sciences. 1998;23:454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule and regulation of apoptosis. Nature Cell Biology. 2003;5:373–376. doi: 10.1038/ncb0503-373. [DOI] [PubMed] [Google Scholar]
- Vert G, Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annual Review of Cell and Developmental Biology. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proceedings of the National Academy of Science of the USA; 1998. pp. 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TA, Neal R, Merlo AO, Honma M, Hicks GR, Wolff K, et al. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-D or IAA in arabidopsis. Plant Physiology. 2006;142:542–552. doi: 10.1104/pp.106.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhai L, Xu J, Joo H-Y, Jackson S, Erdjument-Bromage H, et al. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Molecular Cell. 2006;22:383–394. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Wang KL, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Nakano T, Gendron J, He JX, Chen M, Vafeados D, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Developmental Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Wang Q, Chong K, Wang F, Wang L, Bai M, et al. The brassinosteroid signal transduction pathway. Cell Research. 2006;16:427–434. doi: 10.1038/sj.cr.7310054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Merritt PM, Holub E, Innes RW. Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics. 1999;152:401–412. doi: 10.1093/genetics/152.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Bernhardt A, Dieterle M, Hano P, Mutlu A, Estelle M, et al. Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiology. 2005;137:83–93. doi: 10.1104/pp.104.052654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Geyer RK, Toda T, Wolf DA. CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nature Cell Biology. 2005;7:387–391. doi: 10.1038/ncb1241. [DOI] [PubMed] [Google Scholar]
- Wen CK, Chang C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. The Plant Cell. 2002;14:87–100. doi: 10.1105/tpc.010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, et al. Human De-Etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science. 2004;303:1371–1374. doi: 10.1126/science.1093549. [DOI] [PubMed] [Google Scholar]
- Willems A, Schwab M, Tyers M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochimica et Biophysica Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Woo HR, Chung KM, Park JH, Oh SA, Ahn T, Hong SH, et al. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. The Plant Cell. 2001;13:1779–1790. doi: 10.1105/TPC.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodger FJ, Jacobsen JV, Gubler F. GMPOZ, a BTB/POZ domain nuclear protein, is a regulator of hormone responsive gene expression in barley aleurone. Plant and Cell Physiology. 2004;45:945–950. doi: 10.1093/pcp/pch100. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-T, Chan Y-R, Chien C-T. Protection of cullin-RING E3 ligases by CSN-UBP12. Trends in Cell Biology. 2006;16:362–369. doi: 10.1016/j.tcb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Wu K, Chen A, Pan ZQ. Conjugation of Nedd8 to CUL1 enhances the ability of the ROC1-CUL1 complex to promote ubiquitin polymerization. Journal of Biological Chemistry. 2000;275:32317–32324. doi: 10.1074/jbc.M004847200. [DOI] [PubMed] [Google Scholar]
- Xie Q, Frugis G, Colgan D, Chua NH. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes and Development. 2000;14:3024–3036. doi: 10.1101/gad.852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang SY, Weissman AM, Chua NH. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature. 2002;419:167–170. doi: 10.1038/nature00998. [DOI] [PubMed] [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, et al. The SCFCOI ubiquitin-ligase complexes are required for jasmonate response in arabidopsis. The Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M, et al. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425:316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun T-p. Phytochrome regulation and differential expression of gibberellin 3-beta-hydroxylase genes in germinating arabidopsis seeds. The Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y, Sullivan JA, Komatsu S, Gusmaroli G, Suzuki G, Yin J, et al. Arabidopsis COP10 forms a complex with DDB1 and DET1 in vivo and enhances the activity of ubiquitin conjugating enzymes. Genes and Development. 2004;18:2172–2181. doi: 10.1101/gad.1229504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–525. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- Yang CW, Gonzalez-Lamothe R, Ewan RA, Rowland O, Yoshioka H, Shenton M, et al. The E3 ubiquitin ligase activity of arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. The Plant Cell. 2006;18:1084–1098. doi: 10.1105/tpc.105.039198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Hu Y, Lodhi M, McCombie WR, Ma H. The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proceedings of the National Academy of Science of the USA. 1999;96:11416–11421. doi: 10.1073/pnas.96.20.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Menon S, Lykke-Andersen K, Tsuge T, Di X, Wang X, et al. The COP9 signalosome inhibits p27kip1 degradation and impedes G1-S phase progression via deneddylation of SCF Cul1. Current Biology. 2002;12:667–672. doi: 10.1016/s0960-9822(02)00791-1. [DOI] [PubMed] [Google Scholar]
- Yin YH, Wang ZY, Mora-Garcia S, Li JM, Yoshida S, Asami T, et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Yu Z-K, Gervais JLM, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proceedings of the National Academy of Sciences of the USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, et al. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. The Plant Cell. 2004;16:2795–2808. doi: 10.1105/tpc.104.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LR, Vega-Sanchez ME, Zhu T, Wang GL. Ubiquitination-mediated protein degradation and modification: an emerging theme in plant-microbe interactions. Cell Research. 2006;16:413–426. doi: 10.1038/sj.cr.7310053. [DOI] [PubMed] [Google Scholar]
- Zenser N, Ellsmore A, Leasure C, Callis J. Auxin modulates the degradation rate of Aux/IAA proteins. Proceedings of the National Academy of Science of the USA; 2001. pp. 11795–11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser N, Dreher KA, Edwards SR, Callis J. Acceleration of Aux/IAA proteolysis is specific for auxin and independent of AXR1. Plant Journal. 2003;35:285–294. doi: 10.1046/j.1365-313x.2003.01801.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Garreton V, Chua H-H. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes and Development. 2005;19:1535–1543. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Ni W, Feng B, Han T, Petrasek MG, et al. Members of the Arabidopsis-SKP1-like gene family exhibit a variety of expression patterns and may play diverse roles in arabidopsis. Plant Physiology. 2003;133:203–217. doi: 10.1104/pp.103.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DH, Yang M, Solava J, Ma H. The ASK1 gene regulates development and interacts with the UFO gene to control floral organ identity in Arabidopsis. Developmental Genetics. 1999;25:209–223. doi: 10.1002/(SICI)1520-6408(1999)25:3<209::AID-DVG4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zhao XC, Qu X, Mathews DE, Schaller GE. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiology. 2002;130:1983–1991. doi: 10.1104/pp.011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Yang X, Harrell JM, Ryzhikov S, Shim EH, Lykke-Andersen K, et al. CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Molecular Cell. 2002;10:1519–1526. doi: 10.1016/s1097-2765(02)00784-0. [DOI] [PubMed] [Google Scholar]
- Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
- Zhong GV, Burns JK. Profiling ethylene-regulated gene expression in Arabidopsis thaliana by microarray analysis. Plant Molecular Biology. 2003;53:117–131. doi: 10.1023/b:plan.0000009270.81977.ef. [DOI] [PubMed] [Google Scholar]
- Zhou C, Seibert V, Geyer R, Rhee E, Lyapina S, Cope G, et al. The fission yeast COP9/signalosome is involved in cullin modification by ubiquitin-related Ned8p. BioMed Central Biochemistry. 2001;2:7. doi: 10.1186/1471-2091-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhang L, Duan J, Miki B, Wu K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. The Plant Cell. 2005;17:1196–1204. doi: 10.1105/tpc.104.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Howley PM. Ubiquitination and degradation of the substrate recognition subunits of SCF ubiquitin-protein ligases. Molecular Cell. 1998;2:571–580. doi: 10.1016/s1097-2765(00)80156-2. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]