Abstract
Background and Aims
Species of the Nepenthaceae family are under-represented in studies of leaf traits and the consequent view of mineral nutrition and limitation in carnivorous plants. This study is aimed to complement existing data on leaf traits of carnivorous plants.
Methods
Physico-chemical properties, including construction costs (CC), of the assimilatory organs (leaf and pitcher) of a guild of lowland Nepenthes species inhabiting heath and/or peat swamp forests of Brunei, Northern Borneo were determined.
Key Results
Stoichiometry analyses indicate that Nepenthes species are nitrogen limited. Most traits vary appreciably across species, but greater variations exist between the assimilatory organs. Organ mass per unit area, dry matter tissue concentration (density), nitrogen (N), phosphorus (P), carbon, heat of combustion (Hc) and CC values were higher in the leaf relative to the pitcher, while organ thickness, potassium (K) and ash showed the opposite trend. Cross-species correlations indicate that joint rather than individual consideration of the leaf and the pitcher give better predictive relationships between variables, signalling tight coupling and functional interdependence of the two assimilatory organs. Across species, mass-based CC did not vary with N or P, but increases significantly with tissue density, carbon and Hc, and decreases with K and ash contents. Area-based CC gave the same trends (though weaker in strength) in addition to a significant positive correlation with tissue mass per unit area.
Conclusions
The lower CC value for the pitcher is in agreement with the concept of low marginal cost for carnivory relative to conventional autotrophy. The poor explanatory power of N, P or N : P ratio with CC suggests that factors other than production of expensive photosynthetic machinery (which calls for a high N input), including concentrations of lignin, wax/lipids or osmoregulatory ions like K+, may give a better explanation of the CC variation across Nepenthes species.
Key words: Botanical carnivory, leaf traits, leaf construction cost, mineral nutrition, Nepenthes
INTRODUCTION
Carnivorous plants (CPs) inhabit wet, sunny but nutrient-poor, acidic environments (Juniper et al., 1989; Albert et al., 1992; Ellison and Gotelli, 2002; Ellison and Farnworth, 2005; Ellison, 2006). Consequently, they have evolved strategies for dealing with shortages of soil minerals essential for growth, especially nitrogen (N), phosphorus (P) and potassium (K). In spite of their polyphyletic origin (Albert et al., 1992), all carnivorous plants share a convergent ability of their modified leaves or leaf parts to catch or trap prey, digest and absorb metabolites from the prey and utilize these metabolites for their growth and development (Adamec, 1997; Ellison et al., 2003; Ellison and Farnsworth, 2005). The nutrient properties and stoichiometry (the ratio between the contents of different nutrients, especially N, P and K) of these leaves have often been used to determine the mineral nutrition and limitation of carnivorous plants (e.g. Olde Venterink et al., 2003; Mendez and Karlsson, 2005; Wakefield et al., 2005). Adamec (1997) and many others (e.g. Schulze et al., 1997; Moran et al., 2001; Mendez and Karlsson, 2005) showed that most carnivorous plants are N limited as it is the primary nutrient gained from carnivory. Ellison (2006) from a comprehensive review of the literature on Dionaea, Drosera, Pinguicula, Sarracenia and Urticularia concluded that most carnivorous plants are either P or P + N limited. Members of the Nepenthaceae family, though limited in world-wide distribution but with a significant relative proportion of species amongst known carnivorous plants (approx. 10 %; Juniper et al., 1989), were under-represented in the data set of Ellison (2006), thus casting doubt on the generality of such an assertion.
Because carnivory is rare amongst plants (<0·2 % of angiosperm taxa), it is often assumed that the primary benefit of this form of assimilation should outweigh its production/maintenance and associated lower photosynthetic costs. Givinish et al. (1984) proposed a model to justify this assertion, but at the same time concluded that such adaptation makes carnivorous plants poorer competitors for water and light than non-carnivorous plants. Indeed, many studies have shown the benefits of carnivory via enhanced photosynthesis (e.g. Juniper et al., 1989; Ellison and Farnsworth, 2005; Wakefield et al., 2005), increased seed production and/or gain in whole plant biomass (e.g. Adamec, 1997; Ellison and Gotelli, 2002; Mendez and Karlsson, 2005). A corollary to this assertion is that for a carnivorous plant with a conspicuous flat, photosynthetic leaf base and modified parts (e.g. as in species of Nepenthaceae or Sarraceniaceae), the tissue construction cost (CC) of the two organs should differ in a predictable manner – an hypothesis that has not gained much, if any, attention in the ecological literature (but see Friday, 1992). We hypothesized that following the submission of Givinish et al. (1984) and many others (e.g. Friday, 1992; Ellison and Gotelli, 2001), irrespective of the taxonomic hierarchy considered, the CC of the modified leaf part of the carnivorous plant (e.g. the pitcher of Nepenthes) whose main function is to trap the supplier of the limiting nutrients (i.e. the prey) should be much lower than that of its conjoint receiving/photosynthetic leaf, or else evolution would not have favoured this extra pathway for nutrient uptake/assimilation. Additionally, because the modified leaf part is mainly involved in digestion of macromolecules, followed by absorption and transfer of the resulting nutrients to the conjoint leaves (or beyond it), nutrient levels, photosynthetic machineries and rates should be lower in the modified part (i.e. the pitcher) relative to the leaf. For example, essential elements, such as N and P that are the main constituents of photosystems I and II (especially Rubisco and ATPase) should be lower in the pitcher relative to the leaf. In contrast, it is hypothesized that mineral ions essential for osmo-regulatory and nutrient transfer processes (e.g. Ca2+, Na+, Cl−, K+), within the numerous large, multicellular glands lining the inner walls of the pitcher (Owen and Lennon, 1999) can be expected to be higher in the modified part (i.e. the pitcher) relative to the leaf. Indeed, data on leaf chemistry of carnivorous plant are scanty (but see compilation in Ellison, 2006). Very few studies have compared the leaf properties, including the cost of construction of the photosynthetic leaf part to that of its conjoint modified part (e.g. the leaf vs. its pitcher) (but see Friday, 1992; Ellison and Farnsworth, 2005).
Tissue CC is defined as the amount of glucose (+ minerals) required to synthesize 1 g of carbon skeleton (biomass) as well as the energy expended along the biosynthetic pathways leading to the construction of the tissue (Williams et al., 1987; Griffin, 1994; Poorter et al., 2006). Several physiological, biochemical and morphological traits, and nutrient levels in plants are related to the efficiency of resource utilization and growth (Reich et al., 2003; Wright et al., 2005), and ultimately to the tissue CC (Williams et al., 1989; Villar and Merino, 2001; Poorter et al., 2006). It is often hypothesized that CC should increase with increasing leaf mass per unit area (a unit of leaf economy), increasing content of expensive biochemical plant compounds such as protein (and hence N content), fibre, wax/lipid and lignin, and decrease or show less variation with inexpensive ones such as cellulose and inorganic ions (e.g. ash). For ash (total mineral ions), a negative trend will occur with CC because near a null cost is involved in their transportation in the xylem vessels, except for the root respiratory cost necessary to take them up (Poorter et al., 2006). Most of these hypothesized cause–effect trends have been documented (see Griffin et al., 1993; Villar and Merino, 2001; Osunkoya et al., 2004; Barthod and Epron, 2005; Poorter et al., 2006), although the pattern for protein and hence N has not been consistent as both positive (Griffin et al., 1993; Nagel and Griffin, 2001; Osunkoya et al., 2004) and null (Villar and Merino, 2001; Suarez, 2003; Barthod and Epron, 2005) correlations with CC have been observed. While there are an increasing numbers of studies on CC in relation to growth form (e.g. leaves of grass vs. erect shrubs vs. trees) and habitat (e.g. rainforest vs. Mediterranean vs. shrubland) (see Williams et al., 1989; Poorter and De Jong, 1999; Poorter et al., 2006), similar data sets for carnivorous plant are yet to be compiled.
Nepenthes are a unique group of carnivorous plant, the majority of which are evergreen, woody climbers or scrambling shrubs with shallow roots. Nepenthes distribution is mainly confined to the Madagascar–south-east Asia–northern Australia region. The centre of diversity appears to be in Borneo where 31 of the approx. 80 known species are endemic to the island (Clarke and Leen, 2004). Reported studies on members of this genus have focused on their distribution, pollination/breeding biology (Kato, 1993; Adam, 1998), captured prey identity, rates and mechanisms (e.g. Moran, 1996; Schulze et al., 1997; Moran et al., 1999, 2001; Bohn and Federle, 2004), pitcher microscopy and spectral quality (Moran and Moran, 1998; Moran et al., 1999; Owen and Lennon, 1999), pathway of nutrient exchange/uptake (Owen et al., 1999) and identification of trans-membrane transporters and mode of action within the pitcher (Schulze et al., 1999; An et al., 2001). In contrast, there is a dearth of comparative data on the physico-chemical properties of their assimilatory organs (leaves vs. pitchers). It is for this reason that the cross-species work reported here was embarked upon. Within such a genus, comparative study is very informative as it minimizes phylogenetic effects and allows rigorous testing of adaptive radiation (see Harvey and Pagel, 1991). Within the genus Nepenthes, although intraspecific leaf variation cannot be discounted, this is expected to be much lower than interspecific effects and therefore focus is on the latter. The following four questions were addressed. (1) Across species, what are the patterns of variation in the physical traits (e.g. mass per unit area, thickness, density) and nutrient contents (e.g. N, P and K) of the assimilatory organs? (2) Which is the more costly assimilatory structure to make – the leaf or the pitcher? (3) What are the links between the organ CC and its physico-chemical properties. (4) Are Nepenthes species similar in nutrient status and limitation to other carnivorous plants?
MATERIALS AND METHODS
Study species
The Nepenthes species used in this study are listed in Table 1. They occur sympatrically in sunny habitats of lowland heath (‘kerangas’) and/or peat-swamp as well as at the margins of secondary forests and open scrubland (‘padang’). Samplings were done in these habitats found in the Kuala Belait district of Brunei (4°34′N, 114°25′E). Two organs of assimilation are recognized in this carnivorous plant: (1) the main photosynthetic flat base (henceforth called the leaf); and (2) the modified non/limited-photosynthetic, jug-like leaf blade at the end of the leaf tendril/petiole (henceforth called the pitcher) – a passive trap that can catch/digest prey and absorb the breakdown products. The pitcher is a modified epiascidiate leaf blade, in which the adaxial surface curls around and fuses to form the inner wall of the pitcher tube (Albert et al., 1992; Owen and Lennon, 1999). Nepenthes plants produce two pitcher types, known as terrestrial (ovoid in shape) and aerial (cylindrical in shape) forms, which differ in their placement relative to the ground (see Clarke and Leen, 2004). Only the aerial pitchers were used in this study as a previous study indicated that elevation effect on the traits examined was minimal (Daud, 2006).
Table 1.
The Nepenthes species studied and the mean (± s.e.) morphometric trait values of their leaves and pitchers
| Species | Length (cm) |
Width (cm) |
Thickness (mm) |
Tissue mass per area (g cm−2) |
Tissue density (g cm−3) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Pitcher | Leaf | Pitcher† | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | |
| Nepenthes albomarginata | 19·66 ± 1·99 | 9·52 ± 1·09 | 3·40 ± 0·27 | 2·89 ± 0·51 | 0·21 ± 0·01 | 0·36 ± 0·01 | 0·013 ± 0·002 | 0·011 ± 0·000 | 0·567 ± 0·070 | 0·316 ± 0·020 |
| N. ampullaria | 19·55 ± 2·44 | 6·06 ± 1·33 | 5·94 ± 0·33 | 4·81 ± 0·62 | 0·26 ± 0·02 | 0·32 ± 0·01 | 0·011 ± 0·001 | 0·008 ± 0·001 | 0·407 ± 0·026 | 0·256 ± 0·020 |
| N. bicalcarata | 37·06 ± 1·72 | 12·47 ± 0·94 | 8·03 ± 0·24 | 4·61 ± 0·44 | 0·27 ± 0·01 | 0·37 ± 0·01 | 0·013 ± 0·001 | 0·016 ± 0·002 | 0·508 ± 0·054 | 0·455 ± 0·054 |
| N. gracilis | 11·62 ± 2·44 | 8·23 ± 1·33 | 2·27 ± 0·33 | 1·47 ± 0·62 | 0·17 ± 0·02 | 0·17 ± 0·01 | 0·011 ± 0·001 | 0·006 ± 0·000 | 0·654 ± 0·058 | 0·339 ± 0·034 |
| N. mirabilis | 22·92 ± 3·44 | 13·07 ± 1·89 | 5·48 ± 0·47 | 2·93 ± 0·88 | 0·18 ± 0·02 | 0·32 ± 0·02 | 0·009 ± 0·001 | 0·011 ± 0·001 | 0·492 ± 0·090 | 0·326 ± 0·017 |
| N. rafflesiana var. typical | 14·84 ± 2·44 | 14·22 ± 1·33 | 5·40 ± 0·33 | 4·64 ± 0·62 | 0·30 ± 0·02 | 0·29 ± 0·01 | 0·013 ± 0·000 | 0·010 ± 0·001 | 0·418 ± 0·036 | 0·346 ± 0·013 |
| N. rafflesiana var. elongata | 13·53 ± 1·80 | 23·64 ± 0·99 | 3·36 ± 0·25 | 5·43 ± 0·46 | 0·23 ± 0·01 | 0·22 ± 0·01 | 0·011 ± 0·001 | 0·007 ± 0·000 | 0·498 ± 0·011 | 0·314 ± 0·016 |
| N. rafflesiana var. gigantia | 24·03 ± 2·25 | 24·76 ± 1·24 | 6·33 ± 0·31 | 8·19 ± 0·58 | 0·27 ± 0·01 | 0·28 ± 0·01 | 0·014 ± 0·001 | 0·011 ± 0·000 | 0·546 ± 0·030 | 0·422 ± 0·023 |
| Mean | 22·58 ± 1·35 | 15·08 ± 0·94 | 5·29 ± 0·27 | 4·50 ± 0·30 | 0·24 ± 0·01 | 0·29 ± 0·01 | 0·012 ± 0·000 | 0·010 ± 0·001 | 0·518 ± 0·020 | 0·357 ± 0·014 |
| F-ratio amongst species within organs | 34·78*** | 18·31*** | 11·79*** | 48·76*** | 33·87*** | 9·90*** | 1·25 n.s. | 14·06*** | 2·42* | 3·19** |
| F-ratio between organs | 133·23*** | 9·62** | 126·10*** | 8·20*** | 49·63** | |||||
Data are based on leaves and pitchers from six sites per species with four measurements per site. *P < 0·05; **P < 0·02; ***P < 0·01; n.s., not significant.
†Indicates outer-wall peristome width.
Data for this comparative study came from five distinct species (Nepenthes albomarginata Lobb, N. ampullaria Jack, N. bicalcarata Hook. f., N. gracilis Korth, and N. mirabilis Druce) and three varieties of N. rafflesiana Jack (var. typical, var. elongata and var. gigantia). Currently there is uncertainty in the taxonomic delimitation of these three varieties of N. rafflesiana. However, gross differences between them, especially in their growth habit, size, morphology and spectral quality of their assimilatory organs, coupled with variation in reproductive biology, necessitated regarding them as three separate entities and hence ‘species’ (see also Phillip and Lamb, 1996; Moran, 1996; B. Di-Giusto, pers. obs.). Fuller descriptions, including morphology, growth habit and extent of distribution of these species can be found in Phillips and Lamb (1996) and Clarke and Leen (2004). No doubt significant variations exist in the morphometric traits of the leaf and pitcher of each of the Nepenthes species examined (see Table 1).
Collection and measurements
In July 2005, fresh, healthy and fully expanded sun-exposed leaves and pitchers were collected from mature plants of the study species from six peat-swamp/disturbed heath forests (populations) in the Kuala Belait district of Brunei, with a distance of at least 3 km between sites. At each site, leaves and their attached pitchers were collected under similar environmental conditions thus ensuring that available resources (light, soil water and nutrients) were essentially the same. The vine-like nature of Nepenthes species makes it difficult, at times, to distinguish individual plants [especially for N. gracilis as it grows as a scrambler among Gleichinia (a fern) bush]. Some also produce a low number of assimilatory organs per plant. Consequently, at every collection site and for each species, three to seven separate clusters (each approx. 3–5 m in radius) containing a reasonable number of individuals (usually four to six plants) were identified. It is from these areas that samples were collected (approx. 5000 cm2 per organ) from aerial branches, except for N. mirabilis and N. rafflesiana var. gigantia in which collection was confined only to the terrestrial branches, as their aerial pitchers and leaves were often vestigial or rare. For each species, the collections from different areas within a site were pooled and made into a composite, sealed in plastic bags, taken to the laboratory and kept in cold storage (4 °C) until processing, which normally occurred within 2 d.
The surface area of four leaves and pitchers (excluding the tendrils) from each composite sample (population) was determined with a portable area meter (model LI 300A LI-COR, Lincoln, NE, USA). Each pitcher was carefully cut lengthwise and laid flat to get an accurate measure of its area. Leaf size (maximum length and width), and pitcher maximum length and width of its peristome (the ridged, double-edged collar bordering the pitcher's mouth) were also determined (in centimetres). Thickness (in millimetres) was measured at three to five points per leaf (excluding major veins and midribs) and per pitcher (excluding the peristome and the lid), using a micrometer screw gauge. Materials were dried thereafter at 80 °C for 4 d to determine biomass. Tissue mass per unit area (TMA) was calculated as the ratio of leaf (or pitcher) dry weight to its corresponding area, and dry matter concentration (density) derived as TMA/leaf thickness. The remaining leaves and pitchers were washed with distilled water, followed by drying at 40 °C for several days. They were then ground into a fine powder using a ball mill. Samples for individual sites were then stored over silica gel in desiccators to maintain dryness prior to nutrient analyses.
Foliar and pitcher nutrient level and ash content
The following analyses were carried out per species. Total N was determined in triplicate on 1·0-g samples per site (replicate) by the micro-Kjeldahl method (AOAC, 1990). P and K were determined in triplicate with 1·0 g of sample per site by the vanadomolybdic spectrometric method and atomic absorption spectrometry, respectively (Ingle and Crouch, 1988). Carbon in two, 2–5-mg samples from three out of the six sites was obtained using an elemental analyser (Carlo-Erba, Singapore). Ash content (g g−1) was determined in triplicate by incinerating 1 g samples of the dried leaves in a muffle furnace at 500 °C until a white–grey residue remained (3–4 h).
Foliar and pitcher heat of combustion (Hc) and CC
For each species, the Hc was measured in triplicate per site using 1 g, with a Gallenkamp bomb calorimeter (model CRA-305, UK) calibrated against benzoic acid pellets of known energy value. Ash-free Hc was calculated by converting the Hc on a total dry weight to the corresponding ash-free weight. Leaf CC was calculated by a formula based on the growth efficiency of leaf tissue, Hc, ash and N content according to Williams et al. (1987):
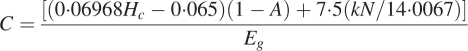 |
where C = construction cost (g glucose g−1), Hc = ash-free heat of combustion (kJ g−1), A = total ash content (g g−1), N = total Kjeldahl nitrogen (g g−1), k is the oxidation state of the N source (+5 for nitrate, or –3 for ammonium) and Eg is the growth efficiency (the fraction of the cost required to provide reductant that is not incorporated into the biomass; see Williams et al., 1987; Poorter and De Jong, 1999). The value of Eg used in this study was 0·87 (see Williams et al., 1987; Villar and Merino, 2001). In the calculation it was assumed that the N source was both nitrate and ammonium for Nepenthes. Although the principal source of N available to higher plants under most field conditions is nitrate (Villar and Merino, 2001), carnivorous plants are known to take up N as ammonium as well (Schulze et al., 1999). Hence CC is calculated using both k = + 5 and –3, and the average values are reported. To calculate CC per unit area (equivalent to grams glucose per square metre), the mass-based CC values for each individual site were multiplied by their corresponding TMA data.
Statistical analyses
In all, six sites and three readings per site generated 18 data points per organ for each species investigated, except for carbon where samples from only three sites were assayed, and hence nine data points were produced. The data were analysed using SPSS software (ver. 12·0). The data were confirmed for homogeneity of variances and hence there was no need for transformation prior to parametric analysis. Differences between sites (replicates), species and assimilatory organs for all the variables measured were tested using multi-factorial ANOVA. A preliminary analysis indicated that within species, site differences were not significant for the majority of the traits examined. Hence data for all sites were pooled. Where significant differences exist amongst species (P < 0·05), Bonferroni pair-wise comparison of means was applied. Using species means, correlations were carried out between all the variables measured. Regressions were also made to determine the relationship between leaf CC and other leaf variables. To examine if, overall, the pitcher traits were different from those of the leaf, and which of the traits were most influential across species, ordination was carried out using species mean data and principal component analysis.
RESULTS
Comparison among species
All attributes examined were significantly different among species, except for carbon content (Tables 1 and 2). Nepenthes bicalcarata and N. rafflesiana var. gigantia have the largest leaf and pitcher size, respectively (Table 1). Across species, thickness varied more in the leaves than in the pitchers, with N. bicalcarata and two varieties of N. rafflesiana (var. gigantia and var. typical) having the highest leaf thickness. For tissue mass per unit area (TMA) and density, cross-species variation was minimal in the leaves (F7,48 = 1·25, P = 0·14 and F7,48 = 2·42, P = 0·05, respectively) but well pronounced in the pitchers (F7,48 = 14·06, P = 0·0001 and F7,48 = 3·19, P = 0·007, respectively). In each species, mass per unit area was significantly higher in the leaf than in the pitcher, except for N. bicalcarata and N. mirabilis where the opposite trend occurred, but the difference was not statistically significant. The leaf of N. mirabilis exhibited the least mass per unit area amongst the species examined (0·009 g cm−2). Pitcher mass per unit area (PMA) and dry matter tissue concentration (density) were highest in N. bicalcarata (0·016 g cm−2 and 0·455 g cm−3, respectively). Lowest PMA was found in N. gracilis (0·006 g cm−2), but its leaf exhibited the highest density (0·65 g cm−3).
Table 2.
Mean (± s.e.) nutrient properties, heat of combustion (Hc) and construction costs (CC) of the assimilatory organs of Nepenthes species
| Species | N ( %) |
P ( %) |
K ( %) |
C ( %) |
Ash ( %) |
Hc (kJ g−1) |
CC mass (g glucose g−1) |
CC area (×1000) (g glucose cm−2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | Leaf | Pitcher | |
| Nepenthes albomarginata | 1·04 ± 0·04 | 0·76 ± 0·04 | 0·22 ± 0·01 | 0·18 ± 0·01 | 0·99 ± 0·07 | 1·21 ± 0·08 | 48·00 ± 0·78 | 44·60 ± 0·47 | 3·02 ± 0·12 | 4·10 ± 0·26 | 21·57 ± 0·27 | 19·86 ± 0·23 | 1·60 ± 0·04 | 1·46 ± 0·01 | 20·35 ± 5·79 | 17·10 ± 0·71 |
| N. ampullaria | 0·74 ± 0·05 | 0·52 ± 0·04 | 0·15 ± 0·02 | 0·13 ± 0·01 | 1·22 ± 0·09 | 1·58 ± 0·09 | 46·28 ± 1·36 | 43·97 ± 0·81 | 3·64 ± 0·36 | 6·19 ± 1·30 | 20·57 ± 0·33 | 19·18 ± 0·28 | 1·51 ± 0·01 | 1·37 ± 0·03 | 16·91 ± 2·23 | 11·95 ± 1·00 |
| N. bicalcarata | 0·62 ± 0·03 | 0·50 ± 0·03 | 0·15 ± 0·01 | 0·15 ± 0·01 | 0·93 ± 0·06 | 1·22 ± 0·07 | 48·00 ± 0·68 | 45·43 ± 0·40 | 2·92 ± 0·23 | 4·31 ± 0·19 | 20·78 ± 0·23 | 19·28 ± 0·20 | 1·54 ± 0·01 | 1·44 ± 0·01 | 20·31 ± 1·76 | 23·76 ± 1·62 |
| N. gracilis | 0·86 ± 0·05 | 0·75 ± 0·04 | 0·20 ± 0·02 | 0·20 ± 0·01 | 1·22 ± 0·09 | 1·68 ± 0·09 | 46·91 ± 0·96 | 45·64 ± 0·57 | 3·73 ± 0·24 | 4·88 ± 0·29 | 20·97 ± 0·33 | 19·12 ± 0·28 | 1·52 ± 0·02 | 1·47 ± 0·02 | 15·48 ± 0·76 | 9·57 ± 0·38 |
| N. mirabilis | 0·96 ± 0·07 | 1·15 ± 0·06 | 0·22 ± 0·02 | 0·23 ± 0·02 | 1·41 ± 0·13 | 1·81 ± 0·13 | 46·33 ± 0·96 | 44·57 ± 0·57 | 4·10 ± 0·32 | 6·82 ± 1·56 | 20·10 ± 0·46 | 19·08 ± 0·40 | 1·46 ± 0·02 | 1·35 ± 0·04 | 12·92 ± 3·07 | 16·03 ± 1·26 |
| N. rafflesiana var. typical | 0·83 ± 0·05 | 0·82 ± 0·04 | 0·26 ± 0·02 | 0·27 ± 0·01 | 1·18 ± 0·09 | 1·79 ± 0·09 | – | – | 4·83 ± 0·31 | 8·63 ± 1·65 | 17·87 ± 0·33 | 16·41 ± 0·28 | 1·35 ± 0·04 | 1·19 ± 0·02 | 18·57 ± 0·84 | 12·61 ± 0·58 |
| N. rafflesiana var. elongata | 0·80 ± 0·03 | 0·66 ± 0·03 | 0·21 ± 0·01 | 0·20 ± 0·01 | 0·99 ± 0·07 | 1·56 ± 0·07 | 47·67 ± 0·68 | 44·00 ± 0·40 | 4·51 ± 0·38 | 7·79 ± 0·99 | 20·47 ± 0·24 | 18·66 ± 0·21 | 1·49 ± 0·01 | 1·29 ± 0·03 | 16·41 ± 0·66 | 9·38 ± 0·52 |
| N. rafflesiana var. gigantia | 0·64 ± 0·04 | 0·49 ± 0·04 | 0·14 ± 0·01 | 0·15 ± 0·01 | 0·97 ± 0·08 | 1·43 ± 0·09 | 46·24 ± 0·96 | 42·73 ± 0·57 | 3·74 ± 0·23 | 5·35 ± 0·35 | 20·50 ± 0·30 | 17·98 ± 0·26 | 1·50 ± 0·01 | 1·29 ± 0·04 | 23·17 ± 1·40 | 16·08 ± 0·73 |
| Mean | 0·81 ± 0·02 | 0·68 ± 0·00 | 0·19 ± 0·01 | 0·19 ± 0·01 | 1·08 ± 0·03 | 1·50 ± 0·04 | 47·18 ± 0·03 | 44·35 ± 0·26 | 3·78 ± 0·38 | 5·80 ± 0·35 | 20·50 ± 0·16 | 18·78 ± 0·15 | 1·52 ± 0·01 | 1·37 ± 0·01 | 18·70 ± 1·15 | 15·23 ± 1·80 |
| F-ratio amongst species within organs. | 13·76*** | 23·95*** | 9·58*** | 12·11*** | 3·29*** | 7·36*** | 2·46 n.s. | 1·04 n.s. | 3·74** | 4·31*** | 12·28*** | 16·43*** | 3·45** | 7·71*** | 0·91 n.s. | 24·20*** |
| F-ratio between organs | 41·51*** | 1·78 n.s. | 85·75*** | 47·12*** | 35·35*** | 132·31*** | 99·13** | 11·08** | ||||||||
Data are based on leaves and pitchers from six sites per species with at least three readings per site, except for carbon (three sites).
*P < 0·05; **P < 0·02; ***P < 0·01; n.s., not significant.
The N and P contents, although generally low (<1 % and 0·25 %, respectively), were higher in N. albomarginata, N. gracilis, N. mirabilis and N. rafflesiana var. typical than the rest of the species (Table 2). Leaves and pitchers of N. mirabilis had the highest K values (1·4 % and 1·81 %, respectively). The mean values for carbon content in the leaves (range 46–48 %) or pitchers (range 42–46 %) did not differ significantly among the eight species (Table 2). Ash content was highest in the leaf (4·83 %) and pitcher (8·63 %) of N. rafflesiana var. typical and lowest in that of N. bicalcarata (2·92 % and 4·31 %, respectively) and N. albomarginata (3·02 % and 4·10 %, respectively). Heat of combustion (Hc) differed significantly among the species (Table 2) and was of the following order: N. rafflesiana var. typical < N. rafflesiana var. gigantia ≤ N. rafflesiana var. elongata ≤ N. mirabilis < N. ampullaria ≤ N. bicalcarata ≤ N. gracilis < N. albomarginata. Mass-based CC and area-based CC also differed significantly among the species, though variation was minimal (8·86 %) in the latter compared with the former (43·1 %) (Table 2). Mass-based CC was highest in the leaf (1·60 g glucose g−1) and pitcher (1·46 g glucose g−1) of N. albomarginata, and least in the leaf (1·35 g glucose g−1) and pitcher (1·19 g glucose g−1) of N. rafflesiana var. typical. Leaves and pitchers of N. albomarginata, N. bicalcarata and N. rafflesiana var. gigantia had the highest area-based CC values.
Comparison between organs
There were major differences between the assimilatory organs of each of the Nepenthes species examined (Tables 1 and 2). The effect of organ type was much more pronounced than the effects due to species differences as judged by the F-ratio values of the factorial ANOVA (Tables 1 and 2). Organ thickness was higher in the pitcher, but the leaf exhibited greater tissue content as it has higher TMA and density values (Table 1). Overall, the N and carbon contents were higher in the leaves (0·8 % and 47·2 %, respectively) than in the pitchers (0·68 % and 44·4 %, respectively), while P content (0·19 %) did not differ significantly between the two organs (Table 2). On the other hand, the pitchers contain significantly higher K (1·50 %) and ash content (0·06 g g−1 or 6 %) than the leaves (1·08 % and 0·04 g g−1 or 4 %, respectively; Table 2). Hc, mass-based CC and area-based CC were also significantly higher in the leaves than in pitchers, with mean values of 20·50 kJ g−1, 1·52 g glucose g−1 and 0·019 g glucose m−2, respectively for the leaves, compared with 18·78 kJ g−1, 1·37 g glucose g−1 and 0·015 g glucose m−2, respectively for the pitchers.
Cross-species correlations in attributes of the assimilatory organs
The correlations across species among the 11 measured leaf and pitcher attributes are given in Tables 3 and 4, and their relationships with mass-based CC are shown in Fig. 1. Significant trends (P < 0·05) that are common in both the leaf and the pitcher are as follows: positive relationships exist between (a) N and P, (b) tissue mass per unit area (TMA) and area-based CC, and (c) Hc and mass-based CC; negative relationships exist between (d) Hc and ash content, and (e) CC mass and ash content (Table 3). In the pitcher but not in the leaf, tissue thickness is directly and significantly related to its TMA, which in turn is also positively correlated with tissue density (Table 3). At the individual organ level, the mass-based CC trend with K was not statistically significant for either the leaf or the pitcher, while the area-based CC trend with K was significant for leaves (r = –0·73, P = 0·04), but not for the pitchers (r = –0·62, P = 0·10; Table 3). Lastly, K increased significantly with decreased carbon content in the leaves, but not in the pitchers, of Nepenthes species.
Table 3.
Matrix of correlation coefficients (r) for leaf (lower left section of the matrix) and pitcher (right upper section of the matrix), physical properties, nutrient composition, mass-based and area-based construction costs across eight Nepenthes species in nutrient-poor ‘kerangas’ and peat swamp forests habitats in Brunei, Northern Borneo
| Trait | Thickness | TMA | Density | N | P | K | C | Ash | Hc | CCmass | CCarea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness | – | 0·80* | 0·18 | 0·02 | −0·24 | −0·05 | 0·01 | −0·25 | 2·21 | 0·25 | 0·79* |
| TMA | 0·62‡ | – | 0·72* | −0·18 | −0·2 | −0·55 | 0·07 | −0·37 | 0·06 | 0·16 | 0·97** |
| Density | −0·68‡ | 0·14 | – | −0·29 | −0·06 | −0·31 | 0·05 | −0·36 | −0·18 | −0·03 | 0·70* |
| N | −0·62‡ | −0·56 | 0·22 | – | 0·73* | 0·48 | 0·22 | 0·31 | −0·01 | 0·13 | −0·14 |
| P | −0·18 | −0·26 | −0·02 | 0·76* | – | 0·56 | 0·24 | 0·61‡ | −0·58 | −0·65‡ | −0·29 |
| K | −0·34 | −0·69‡ | −0·18 | 0·30 | 0·23 | – | −0·25 | 0·73* | −0·59 | −0·67‡ | −0·62‡ |
| C | 0·09 | 0·29 | 0·23 | 0·12 | 0·32 | − 0·76* | – | −0·42 | 0·71‡ | 0·69‡ | 0·16 |
| Ash | 0·26 | −0·36 | 0·36 | −0·04 | 0·43 | 0·39 | −0·47 | – | − 0·73* | − 0·86** | −0·54 |
| Hc | −0·62‡ | −0·16 | 0·61 | 0·14 | −0·43 | −0·33 | 0·58 | − 0·77* | – | 0·97** | 0·26 |
| CCmass | −0·45 | −0·10 | 0·44 | 0·05 | −0·52 | −0·44 | 0·62 | − 0·83** | 0·96** | – | 0·37 |
| CCarea | 0·55 | 0·87** | 0·09 | −0·40 | −0·37 | − 0·73* | 0·22 | −0·28 | 0·09 | 0·22 | – |
n = 8 for the leaf and pitcher, respectively, except for correlations with carbon (C) where data are not available for N. rafflesiana var. typical and hence n = 7. Significant trends are highlighted in bold. ‡P < 0·10; *P < 0·05; **P < 0·02.
Table 4.
Matrix of correlation coefficients (r) between pairs of physico-chemical attributes using species mean data of the leaf and pitcher as two independent data points for each of the bivariate relationships
| Trait | Thickness | TMA | Density | N | P | K | C | Ash | Hc | CCmass | CCarea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness | – | 0·48† | − 0·49* | −0·30 | −0·22 | 0·09 | −0·43 | 0·23 | −0·38 | −0·31 | 0·37 |
| TMA | – | 0·50* | −0·16 | −0·17 | − 0·59** | 0·29 | −0·43† | 0·18 | 0·26 | 0·94*** | |
| Density | – | 0·19 | 0·03 | − 0·69** | 0·72* | − 0·67* | 0·62** | 0·61** | 0·55* | ||
| N | – | 0·72** | 0·06 | 0·34 | 0·03 | 0·21 | 0·14 | 0·09 | |||
| P | – | 0·23 | 0·26 | 0·34 | −0·35 | −0·41 | −0·26 | ||||
| K | – | − 0·78** | 0·82** | − 0·72** | − 0·78** | − 0·71** | |||||
| C | – | − 0·75* | 0·91*** | 0·90** | 0·39 | ||||||
| Ash | – | − 0·82** | − 0·89** | − 0·60* | |||||||
| Hc | – | 0·98*** | 0·39 | ||||||||
| CCmass | – | 0·48‡ | |||||||||
| CCarea | – |
n = 16, except for correlation with C where data are not available for N. rafflesiana var. typical and hence n = 14.
Significant trends are highlighted in bold. †P < 0·10; *P < 0·05; **P < 0·02; ***P < 0·01.
Fig. 1.
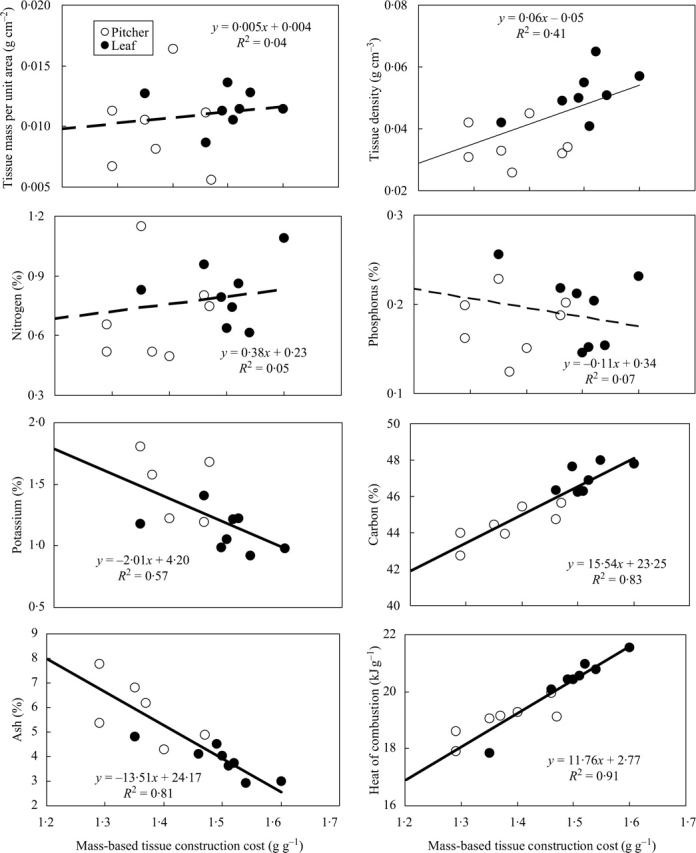
Relationships between mass-based construction costs (g glucose g−1) and physico-chemical properties of the leaf (closed circles) and the pitcher (open cycles) of eight Nepenthes species from Brunei, Borneo. Each point is the mean values from six sites, except for carbon where data are from three sites only. Continuous lines indicate significant correlation across species and assimilatory organs at P ≤ 0·05; dashed lines indicate non-significant trends of P > 0·05. For significance of trends within each organ type, see Table 3.
Note that a greater number of the bivariate relationships were significant (P < 0·05) when leaf and pitcher data are used as two independent data points (23 out of 55 pair-wise comparisons; Table 4 and Fig. 1) than when using either the leaf (7 out of 55) or the pitcher data alone (10 out of 55 pair-wise comparisons) (Table 3). Thus overall, trends can be summarized as follows: of the three physical traits examined, variation in tissue concentration (density) was the most influential on the chemical properties – being positively related to carbon, Hc, mass- and area-based CC, and negatively associated with K and total ash (Table 4). N increased with P but neither was correlated with mass-based CC or with area-based CC (Fig. 1). It appears that Hc increases significantly with increasing organ carbon content (r = 0·91) and tissue density (r = 0·62), and decreases with increasing K (r = –0·72) and ash contents (r = –0·82). As tissue mass per unit area and carbon content decreased, K increased significantly. There was a strong positive correlation between ash content and K. Also, mass-based CC correlated more strongly with most of the measured variables than did area-based CC (Fig. 1 and Table 4). Mass-based CC was significantly correlated with tissue density (r = 0·61), K (r = –0·78), carbon (r = 0·90), ash (r = –0·89) and Hc (r = 0·98) (Fig. 1). Area-based CC showed the same trends (though weaker in strength, especially with carbon, ash and Hc), in addition to a highly significant positive correlation with tissue mass per unit area (Table 4).
Ordination of species
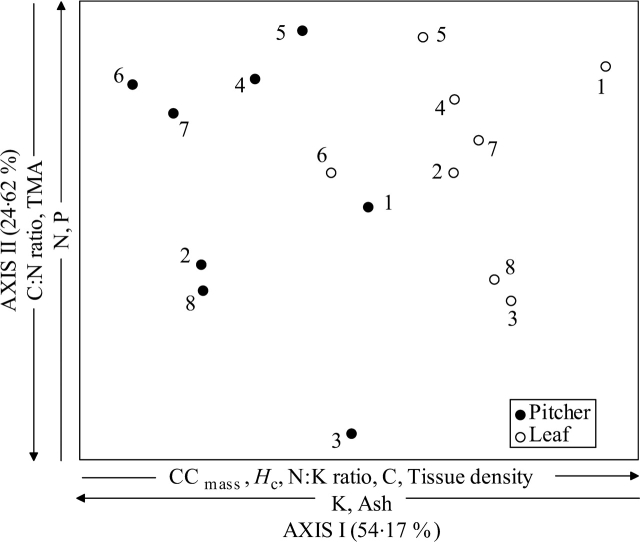
Figure 2 shows the result of a principal component analysis of the eight lowland Nepenthes species based on 11 direct attributes (see Tables 1 and 2) and three nutrient ratio values (C:N, N:P and N:K) of their assimilatory organs. Overall, the first two principal components explained 79 % of the total variation in the data. Axis I of the ordination accounted for 54 % of the total variation, and principally driven, in decreasing order, by mass-based CC, Hc, K, N:K ratio, carbon, tissue concentration (density) and ash content. The second axis explained 25 % of the variation with the minerals (C:N ratio, N and P) and a physical trait (TMA) being the main drivers. P and TMA again loaded moderately on axis III, but this axis explained a much lower fraction of the total variation (13 %) and is considered non-influential (data not shown). Overall, the leaves and the pitchers of the Nepenthes species emerged separately in two distinctive groups. Leaves were grouped mainly on the right-hand and upper side of the ordination plot while the pitchers tended to be grouped on the left-hand side of the ordination. Note also that the pitcher of N. bicalcarata appeared far removed and hence different physico-chemically from the other sympatric species.
Fig. 2.
Ordination on axes I and II of eight Nepenthes species based on 11 primary properties and three derived nutrient ratios (N : P, C : N and N : K) of their leaves and pitchers (see Tables 1 and 2) and using a principal component analysis. Variables that load significantly are indicated, as well as the proportion of variation captured by each axis. Organ CC mass, heat of combustion, N : K ratio, carbon and tissue concentration (density) were strongly positively correlated with Axis I (r = + 0·91, + 0·89, +0·85, +0·81 and + 78, respectively), while K and ash contents gave significant negative trends with this axis (–0·86 and –0·74, respectively). On Axis II, N and P (with r = + 0·83 and + 0·74, respectively), and C : N ratio and TMA (with r = –0·84 and –0·62, respectively) were the main drivers. 1, Nepenthes albomarginata; 2, N. ampullaria; 3, N. bicalcarata; 4, N. gracilis; 5, N. mirabilis; 6, N. rafflesiana var. typical; 7, N. rafflesiana var. elongata, 8, N. rafflesiana var. gigantia.
Nutrient stoichiometry of Nepenthes species
Tissue stoichiometry relationships amongst different nutrients (especially tri-partite links between N, P and K) are often used to determine if their relative concentrations limit plant growth (see Olde Venterink et al., 2003; Mendez and Karlsson, 2005; Ellison, 2006). N limitation is implied by N < 20 mg g−1, N : P < 14 mg mg−1, and N : K < 2·1, whereas P or P + N limitations is implied by P < 1 mg g−1, N : P >16 and K : P > 3·4. Co-limitation of N and P is implied when the concentrations of N and P are individually limiting and when 14 ≤ N : P ≤ 16. Based on these criteria, the N content in the leaves and the pitchers of Nepenthes is well below 1 % (10 mg g−1), while P and K are around 0·2 % (2 mg g−1) and 1·2 % (12 mg g−1), respectively (Table 2). These imply a N : P ratio of 5 and a N : K ratio of 0·83 and suggests that most Nepenthes species are N limited.
DISCUSSION
Nutrient contents of Nepenthes compared with those of other plants
Overall, the average leaf concentrations of N (approx. 0·81 %) and total ash [approx. 0·038 g g−1 (3·8 %)] in the Nepenthes species studied are significantly lower than those in the leaves of non-carnivourus plants co-habiting with them [e.g. species of Melastoma, Ploiarium, Alphitonia, Commersonia and Symplocos; average = 1·16%, 0·06 g g−1 (6 %), respectively; see Osunkoya et al. (2004)]. This observation occurs, irrespective of the plant growth form of the non-carnivorous plants used in the contrast. However, the average levels of leaf P (0·19 %) and K (1·08 %) found in the Nepenthes species are on a par with those of non-carnivorous plants listed above (0·12 %, 1·05 %, respectively) and elsewhere (see Larcher, 2003; Wright et al., 2005; Ellison, 2006). P and K values of the Nepenthes leaves and pitchers are also in the same range as other carnivorous plants around the globe (see Adamec, 1997; Ellison and Gotelli, 2001, 2002; Ellison, 2006). The comparatively low N level, backed by the tri-partite stoichiometry analytical results is a further boost to the idea that Nepenthes species are N (but not P or K) limited, and have thus evolved the pitcher to assist principally in their uptake of N. It is known that prey captured in the pitcher contributes 10–90 % of the N budget of Nepenthes plants (see Moran and Moran, 1998; Ellison and Gotelli, 2001). However, the present finding is slightly different from those of Ellison (2006) who suggested that most carnivorous plants are P or P + N limited. Scrutiny of Ellison (2006) data set showed that species of Nepenthes were conspicuously missing, except for N. rafflesiana.
Construction costs in relation to organ type and physical structure
For each of the eight species, it was found that CC of the pitcher was much less than that of its leaf (approx. 10–20 % less; Table 2). The presence of expensive photosynthetic machinery and compounds such as enzymes (Rubisco) in leaves coupled with higher tissue mass per unit area (except for N. bicalcarata) and density, and possibly thicker cuticle and epidermal layer to reduce water loss (implying high lipid content and presence of lignified cell walls) no doubt contributes to this trend (see Villar and Merino, 2001; Osunkoya et al., 2004; Barthod and Epron, 2005; Poorter et al., 2006). In contrast, the pitchers of Nepenthes need not contain much of the expensive photosynthetic machinery as they have evolved mainly to capture, kill and digest prey, followed by transfer of the inorganic ions to other sites where they are needed. In summary, the low cost of the cup relative to the leaf as found in this study verified one of the present hypotheses and lends support to the assertion that carnivory in plants, such as in Nepenthes and Sarracenia, may have evolved because the marginal benefits especially in terms of additional nutrients obtained from prey captured (and hence increased whole-plant photosynthetic capacity) far exceeds its marginal construction and maintenance cost (see Ellison and Farnsworth, 2005; Ellison, 2006).
Area-based estimates of CC are useful for economic interpretations and for relating CC to processes driven by light interception, including CO2 diffusion to the plant surface (Griffin, 1994). The leaves of N. albomarginata, N. bicalcarata, N. rafflesiana var. gigantia and N. rafflesiana var. typical and the pitcher of N. bicalcarata have the highest TMA (Table 1) due to their thicker leaf laminas and higher tissue density or both (Westoby et al., 2002; Larcher, 2003), and can be expected to have, comparatively, higher CC of all the eight species tested. Plants with high TMA contain more cell layers per unit area or an accumulation of non-structural carbohydrates like soluble sugars and starch (Griffin et al., 1993; Poorter et al., 2006), more cell-wall components, sclerenchyma tissue, lignin and other secondary compounds which are energetically costly to make (Osunkoya et al., 2004; Poorter et al., 2006). Consequently the CC would increase on an area basis with TMA but may not necessarily do so on a mass (weight) basis as shown in Tables 3 and 4. A similar strong and positive relationship between TMA and area-based CC have been reported in various ecosystems and plants (e.g. Williams et al., 1989; Nagel and Griffin, 2001; Suarez, 2003; Osunkoya et al., 2004; Barthod and Epron, 2005).
Minerals, Hc and CC
As in many other studies (e.g. Wright et al., 2005), there was a direct relationship between N and P contents (Tables 3 and 4). However, in contrast to expectation, N and P concentrations did not contribute to the significant changes observed in the interspecific variation in CC (Fig. 1) and have comparatively limited roles in the differentiation of the assimilatory organs of the Nepenthes species investigated (Fig. 2). Possible factors contributing to this apparent discrepancy include the following: (a) NH3 is often the main source of N for plants in nutrient-poor soils and costs less to incorporate than NO3 (Williams et al., 1987; Griffin, 1994); (b) N and P are often in short supply both in the assimilatory organs and in the soils of heath and peat-swamp forests inhabited by Nepenthes species, and may thus require evolution of a similar strategy across species within a genus to take them up; and (c) a co-ordination rather than a correlation effect may be operating between N (or P) and CC. Westoby et al. (2002), Reich et al. (2003) and Wright et al. (2005) argued that some functional ecological traits are substantially independent of each other (as seen here for N and CC), expressing different aspects of a plant's ecology and may thus not have strong causal, unidirectional relationships. Rather, such traits may exhibit a co-ordination (reciprocal) effect in which trait values function more successfully as a combination rather than because one trait drives another mechanically or physiologically.
It is noteworthy that K content is significantly higher in the pitcher than in the leaf for each of the eight species studied (Table 2 and Fig. 1) – a pattern that did not emerge for other elements examined. Perhaps most Nepenthes species, like another carnivorous plant group – the Sarracenia species – preferentially take up more K ions from the prey relative to other ions (see Karlsson, 1988). It will be informative to test this hypothesis, and work is underway with regards to this. Alternatively, it could be a reflection of greater osmoregulatory activities linked with transfer of ions and nutrients in the pitcher walls relative to the leaf lamina, but data in this area are lacking (see Owen and Lennon, 1999; Schulze et al., 1999; An et al., 2001). K is usually not considered as part of the leaf economic spectrum (see Reich et al., 2003; Wright et al., 2005). Yet in this study it is the only mineral ion of all the ones examined that tends to have a significant relationship (negative though) with the physical properties and CC of the assimilatory organs of the Nepenthes species. The negative trend of K with CC (Fig. 1) may be because most of the K is not used in many structural materials, but rather remains in the cell sap as dissolved ions (Larcher, 2003).
Carbon content was high (42–48 %) as in other plants (Poorter and De Jong, 1999), but there was no significant difference within the leaves or pitchers of the different Nepenthes examined (Table 2). If additional carbon from degraded amino acid (taken in via the pitcher, see Schulze et al., 1999) and CO2 uptake (fixed by the leaves) is synthesized into expensive secondary compounds (e.g. lignin) and remain in the leaf, or in the upper part of the pitcher as wax, then the CC should increase with increasing carbon content, as found in this study. If fixed carbon accumulates as non-structural carbohydrates, or is exported for growth of new tissues, there may be no net effect on the per gram CC of assimilatory organs (see also Griffin et al., 1993; Griffin, 1994).
Plant ash is composed of minerals – largely N, P, K, Ca, Mg, Na, S and Si – as well as oxides and nitrate formed during combustion. Overall, the pitchers of Nepenthes have higher ash content (mean = 0·06 g g−1) than the leaves (mean = 0·04 g g−1) (Table 2) but these values are within the range reported for trees and shrubs inhabiting the same area (Osunkoya et al., 2004). Most of the inorganic elements are derived from digestion and absorption of prey trapped inside the pitchers, and this could account for the higher ash content in the pitchers compared with the leaves. It is interesting to note that across species, total ash correlates significantly with K, suggesting that K may be the main constituent of the ash and/or that K plays a significant role in nutrient transfer and thus mineral nutrition of Nepenthes plants, as discussed earlier.
Leaf energy content (Hc = 18 – 22 kJ g−1) was in the range reported for many herbaceous plants and trees, including those in a similar habitat to the Nepenthes investigated (see Osunkoya et al., 2004, and references therein). There was a strong positive and significant correlation between Hc and mass-based CC, suggesting that Hc is a reliable estimate of the synthetic cost of assimilatory organs (see also Griffin, 1994; Suarez, 2003; Osunkoya et al., 2004).
CONCLUSIONS
Data on leaf traits of Nepenthaceae, an Old World enigmatic group of species, are conspicuously lacking in the literature and hence also from the view of mineral nutrition and limitations of carnivorous plants. The present study adds to the existing data set on carnivorous plants, and at the same time challenges some of the prevailing paradigms. For example, from previous studies the general consensus is that many terrestrial carnivorous plants preferentially extract N and P over K, Ca or Mg (Karlsson, 1988; Adamec, 1997), and that most are either P or P + N limited (Ellison, 2006). The present study indicates that this may not be all inclusive as Nepenthes species appeared to be K enriched and N limited. It will be interesting to follow the results of this general survey of N limitation in Nepenthes as reported in this paper with the species' response to inorganic ions and prey addition to the soil and the pitcher itself.
Ordination has shown that the leaf and its pitcher are individually unique in their biophysical and chemical properties (Fig. 2). However, because a higher number of cross-species bivariate relationships were significant when both organs were considered jointly rather than separately (Tables 3 and 4), it is evident that the two organs are functionally tightly coupled and inter-dependent with the tendril acting as a bridge for ‘super two-way’ conduit for the exchange of materials. Studies on the movement patterns of ions and macromolecules between the two organs are scarce, but deserve more attention (see also Owen et al., 1999; An et al., 2001; Ellison and Farnsworth, 2005).
CC varied appreciably across species, but by far the greatest difference was detected between the pitcher and the leaf. The lower CC value for the pitcher is in agreement with the concept of low marginal cost for carnivory relative to conventional autotrophy. However, a weak link was detected between CC and N or P, but a strong one with K, although N serves as one of the direct input variables to the derivation of CC. It suggests that for Nepenthes species, factors other than the production of photosynthetic machinery (which calls for a high N input), including accumulation of compounds to provide greater resistance against organ deformation (e.g. lignin) or to reduce water loss/increase prey-capture (wax/lipids), may better explain CC values. This is currently being investigated. The decrease in CC with increasing K suggests that this ion has a large role to play in the mineral nutrition of Nepenthes plants, perhaps as a major player in osmo-regulation and nutrient transfer, especially in the pitcher.
ACKNOWLEDGEMENTS
We thank the UBD Chemistry department for providing facilities for carbon and heat of combustion determination, and the Director of Agriculture for granting access to the plant and soil nutrient analytical laboratory, Brunei Agriculture Research Centre, Brunei where N, P and K analyses were carried out. Thanks also to David Marshall and Jan Beck for thoughtful discussion on carnivorous plants.
LITERATURE CITED
- Adam JH. Reproductive biology of Bornean Nepenthes (Nepenthaceae) species. Journal of Tropical Forest Science. 1998;10:456–471. [Google Scholar]
- Adamec L. Mineral nutrition of carnivorous plants – a review. Botanical Reviews. 1997;63:273–299. [Google Scholar]
- Albert VA, Williams SE, Chase MW. Carnivorous plants: phylogeny and structural evolution. Science. 1992;257:1491–1495. doi: 10.1126/science.1523408. [DOI] [PubMed] [Google Scholar]
- An C-II, Fukisaki E-I, Kobayashi A. Plasma membrane H+-ATPases are expressed in pitchers of the carnivorous plant. Nepenthes alata Blanco. Planta. 2001;212:547–555. doi: 10.1007/s004250000455. [DOI] [PubMed] [Google Scholar]
- Helrich K, editor. AOAC. Official methods of analysis. 15th edn. Vol. 1. Arlington, VA: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Barthod S, Epron D. Variations of construction cost associated to leaf area renewal in saplings of two co-occurring temperate tree species Acer platanoides L. and Fraxinus excelsior L.) along a light gradient. Annals of Forest Science. 2005;62:545–551. [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences of the USA; 2004. pp. 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C, Leen C. Pitcher plants of Sarawak. Sabah, Malaysia: Natural History Publications Borneo Sdn. Bhd; 2004. [Google Scholar]
- Daud SD. Variation in properties and construction costs of leaf and pitcher: patterns within and between eight Nepenthes species of Brunei. BSc Thesis. Brunei: Universiti Brunei Darussalam; 2006. [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:1–8. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Farnsworth EJ. The cost of carnivory for Darlingtonia californica (Sarraceniaceae): evidence from relationships among leaf traits. American Journal of Botany. 2005;92:1085–1093. doi: 10.3732/ajb.92.7.1085. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution. 2001;16:623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant. Sarracenia purpurea. Proceedings of the National Academy of Sciences of the USA; 2002. pp. 4409–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ, Brewer JS, Cochran-Stafira DL, Kneitel JM, Miller TE, et al. The evolutionary ecology of carnivorous plants. Advances in Ecological Research. 2003;33:1–74. [Google Scholar]
- Friday LE. Measuring investment in carnivory: seasonal and individual variation in trap numbers and biomass in Utricularia vulgaris L. New Phytologist. 1992;121:439–445. doi: 10.1111/j.1469-8137.1992.tb02944.x. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the Bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Griffin KL. Calorimetric estimates of construction cost and their use in ecological studies. Functional Ecology. 1994;8:551–562. [Google Scholar]
- Griffin KL, Thomas RB, Strain BR. Effects of nitrogen supply and elevated carbon dioxide on construction cost in leaves of Pinus taeda L. seedlings. Oecologia. 1993;95:575–580. doi: 10.1007/BF00317443. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Ingle Jr, JD, Crouch SR. Spectrochemical analysis. Englewood, NJ: Prentice Hall; 1988. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Karlsson PS. Seasonal patterns of nitrogen, phosphorus and potassium utilization by three Pinguicula species. Functional Ecology. 1988;2:203–209. [Google Scholar]
- Kato M. Floral biology of Nepenthes gracilis (Nepenthaceae) in Sumatra. American Journal of Botany. 1993;80:924–927. [Google Scholar]
- Larcher W. Physiological plant ecology: ecophysiology and stress physiology of functional groups. 4th edn. Berlin: Springer-Verlag; 2003. [Google Scholar]
- Mendez M, Karlsson PS. Nutrient stoichiometry in Pinguicula vulgaris: nutrient availability, plant size, and reproductive status. Ecology. 2005;86:982–991. [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Moran AJ. Foliar reflectance and vector analysis reveal stress in prey-captured pitcher plants. Nepenthes rafflesiana. International Journal of Plant Sciences. 1988;159:996–1001. [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implication for prey capture. Annals of Botany. 1999;85:521–528. [Google Scholar]
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata: evidence from stable isotope analysis. Annals of Botany. 2001;88:307–331. [Google Scholar]
- Nagel JM, Griffin KL. Construction cost and invasive potential: comparing Lythrum salicaria (Lythraceae) with co-occurring native species along pond banks. American Journal of Botany. 2001;88:2252–2258. [PubMed] [Google Scholar]
- Olde Venterink H, Wassen MJ, Verkroost AWM, De Ruiter PC. Species richness-productivity patterns differ between N-, P- and K-limited wetlands. Ecology. 2003;84:2191–2199. [Google Scholar]
- Osunkoya OO, Bujang D, Moksin H, Wimmer FL, Holige TM. Leaf properties and the construction cost of common, co-occurring plant species of disturbed heath forest in Borneo. Australian Journal of Botany. 2004;52:499–507. [Google Scholar]
- Owen TPJ, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Owen TPJ, Lennon KA, Santo MJ, Anderson AN. Pathways of nutrient transport in the pitchers of the carnivorous plant. Nepenthes alata. Annals of Botany. 1999;84:459–466. [Google Scholar]
- Phillipps A, Lamb A. Pitcher-plants of Borneo. Sabah, Malaysia: Natural History Publications Borneo Sdn. Bhd; 1996. [Google Scholar]
- Poorter H, De Jong R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist. 1999;143:163–176. [Google Scholar]
- Poorter H, Pepin S, Rijkers T, de Jong Y, Evans JR, Körner C. Construction costs, chemical composition and payback time of high- and low-irradiance leaves. Journal of Experimental Botany. 2006;57:355–371. doi: 10.1093/jxb/erj002. [DOI] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craines JM, Oleksyn J, Westoby M, et al. The evolution of plant functional variation: traits, spectra and strategies. International Journal of Plant Science. 2003;164:S143–164. [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants. Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Schulze W, Frommer WB, Ward JM. Transporters of ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant. Nepenthes. The Plant Journal. 1999;17:637–646. doi: 10.1046/j.1365-313x.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Suarez N. Leaf longevity, construction, and maintenance costs of three mangrove species under field conditions. Photosynthetica. 2003;41:373–381. [Google Scholar]
- Villar R, Merino J. Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytologist. 2001;151:213–226. doi: 10.1046/j.1469-8137.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- Wakefield AE, Gotelli NJ, Wittman SE, Ellison AM. Prey addition alters nutrient stoichiometry of the carnivorous plant. Sarracenia purpurea. Ecology. 2005;86:1737–1743. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Williams K, Percival F, Merino J, Mooney HA. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant, Cell and Environment. 1987;10:725–734. [Google Scholar]
- Williams K, Field CB, Mooney HA. Relationships amongst leaf construction cost, leaf longevity and light environment in rainforest plants of the genus. Piper. American Naturalist. 1989;133:198–211. [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, et al. Assessing the generality of global leaf trait relationships. New Phytologist. 2005;166:485–496. doi: 10.1111/j.1469-8137.2005.01349.x. [DOI] [PubMed] [Google Scholar]