Abstract
Background and Aims
Etiolation symptoms and the greening process are usually studied on dark-germinated seedlings and this raises the question – can these results be generalized for plants growing under field conditions? This work examines various aspects of the plastid differentiation under the covering of the achene wall, which often remains attached to the cotyledons of sunflower (Helianthus annuus) seedlings grown under light.
Methods
Cotyledons of 7- to 10-d-old sunflower seedlings grown in the dark and on light were examined. The partially covered cotyledons were sectioned into light-exposed, covered and transition zones. Pigment contents, 77 K fluorescence spectroscopy, electron microscopy and fluorescence imaging, along with fluorescence kinetic methods, were used.
Key Results
The light-exposed zone of the partially covered cotyledons was similar to cotyledons developed without achene covering. However, some of the plastids had prolamellar bodies among the granal thylakoid membranes; despite this no protochlorophyllide was detected. The fully covered, yellowish sections contained protochlorophyllide forms emitting at 633 and 655 nm and well-developed prolamellar bodies, similar to those of etiolated cotyledons. In addition, reduced amounts of chlorophyll a, chlorophyll b and stacked thylakoid membrane pairs were found in this region. The transitional sections showed a mixture of the characteristics of the covered and exposed sections. Various, but significantly different values of the photosynthetic activity parameters were found in each sector of the partially covered cotyledons.
Conclusions
The partial covering of the achene wall shades the cotyledon tissues effectively, enough to provoke the appearance of etiolation phenomena, i.e. the permanent presence of flash-photoactive protochlorophyllide complexes and prolamellar bodies (with or without protochlorophyllide), which proves that these phenomena may appear under natural illumination conditions.
Key words: Cotyledon, etio-chloroplast, etioplast, etiolation, Helianthus annuus, photosynthetic activity, protochlorophyllide, prolamellar body, sunflower
INTRODUCTION
Dark-grown seedlings are often used when studying the effect of light on plastid differentiation and the greening process. Leaves of etiolated angiosperm seedlings contain etioplasts instead of chloroplasts. In the absence of light, the tissues synthesize low amounts of the chlorophyll (Chl) precursors: protochlorophyllide (Pchlide) and its esterified form protochlorophyll (Pchl; see Table 1 for list of abbreviations). The majority of the etioplast inner membranes build up highly regular, paracrystalline prolamellar bodies (PLBs) from which lamellar prothylakoids (PTs) stretch out (Gunning, 1965, 2001). The hexagonal PLB structure is thought to be strongly associated with the presence of the membrane-bound, NADPH: protochlorophyllide oxidoreductase (POR) enzyme (EC 1·3·1·33) (Selstam and Widell-Wigge, 1993; Selstam, 1998). POR is the only light-activated enzyme of Chl biosynthesis, and it plays a key role in the regulation of the whole process (Beale, 1999; Masuda and Takamiya, 2004). Subpopulations of Pchlide or Pchl feature distinct spectral forms, reflecting various microenvironments and molecular interactions. When it is not possible to judge whether the studied pigment is Pchl or Pchlide, the abbreviation ‘Pchl(ide)’ is used. In the 77 K fluorescence emission spectra of dark-grown leaves three main emission bands have been identified (Böddi et al., 1992). The band at 631–633 nm represents monomeric Pchlide molecules localized mainly in the PTs and probably not bound to POR (Böddi et al., 1989; Kis-Petik et al., 1999). The emission bands at 644 nm and 655 nm correspond to POR-bound pigment molecules. NADPH: protochlorophyllide oxidoreductase units are ternary complexes of the protein NADPH and Pchlide; these units aggregate into dimers (Wiktorsson et al., 1993; Ouazzani-Chahdi et al., 1998) and oligomers (Böddi et al., 1989; Wiktorsson et al., 1993; Ouazzani-Chahdi et al., 1998) having the emission maxima at 644 nm and 655 nm, respectively. The Pchlide forms are usually studied in leaves of dark-germinated seedlings because in such samples they are present in much higher amounts than in light-grown plant tissues. Consequently, their absorption and fluorescence bands are not overlapped by those of chlorophylls (Amirjani and Sundqvist, 2004). However, the 3- to 8-d continuous darkness used for growing the experimental material for these studies is unusual in nature. On the other hand, some symptoms of etiolation were observed under low light intensities in greening leaves in case of red light (Treffry, 1973) or white light (Argyroudi-Akoyunoglou et al., 1976). Chloroplasts having grana and PLBs simultaneously – named etio-chloroplasts – have been found in young cells at the base of maize leaves grown under natural light intensities (Whatley, 1977; Rascio et al., 1980).
Table 1.
List of abbreviations
| Chl | Chlorophyll |
| Chlide | Chlorophyllide |
| ETR | Rate of electron transport |
| Fm | Maximal fluorescence |
| F0 | Minimal fluorescence |
| Fv | Variable fluorescence |
| LHCII | Light-harvesting complex II |
| NPQ | Non-photochemical quenching |
| Pchl | Protochlorophyll |
| Pchlide | Protochlorophyllide |
| PAR | Photosynthetically active radiation |
| POR | NADPH: protochlorophyllide oxidoreductase (EC 1·3·1·33) |
| PLB | Prolamellar body |
| PSI | Photosystem I |
| PSII | Photosystem II |
| PT | Prothylakoid |
Leaf primordia developing in the inner regions of organs masked by outer leaves or scales have both etioplasts and etio-chloroplasts and both Pchl and Pchlide under natural conditions (Solymosi et al., 2004, 2006; Solymosi and Böddi, 2006). Protochlorophyllide accumulation was found in the innermost leaves of white cabbage heads (Solymosi et al., 2004), horse chestnut buds and the buds of several other plant species (Solymosi and Böddi, 2006; Solymosi et al., 2006).
Although several plant organs have chlorenchyma tissues, the majority of studies are aimed at the greening process and the chlorophyll biosynthesis of leaves and fewer data are available on non-leaf organs. Reports on Pchlide forms and their phototransformation, or plastid development in non-leaf organs include stems (Böddi et al., 1994, 1996; Skribanek et al., 2000) and cotyledons (i.e. clementine: Casadoro and Rascio, 1987; bean: Schoefs et al., 1994; spinach: Schoefs et al., 2000a; mustard: Jabben and Mohr, 1975; arabidopsis: Sperling et al., 1997, 1998; Oosawa et al., 2000; Cucumis sativus: Kuroda et al., 1996).
In this paper, the presence of Pchlide and the formation etio-chloroplasts is described in the epigeal cotyledons of sunflower (Helianthus annuus) seedlings. To characterize the photosynthetic apparatus in these cotyledons, fluorescence imaging and fluorescence kinetic parameters were also measured.
MATERIALS AND METHODS
Plant material
Cotyledons of sunflower varieties with completely black (Helianthus annuus ‘ES Lolita’ and ‘Swingy’) and with black and white striped pericarps (Helianthus annuus ‘Iregi szürke csíkos’) were examined. Seedlings were grown on wet filter paper in Petri dishes and in other experiments in sandy (loose) or clayey (compact) soils at room temperature for 7 d under shaded natural light (20 µmol s−1 m−2, 12/12 h day/night cycles). In parallel, seedlings were grown in the same conditions but in the dark.
The samples were irradiated with a Chinon-8000 (Yokohama, Japan) photoflash apparatus; the energy output was 160 J/2 ms. Samples were cooled to 77 K 10 s after irradiation. The size of the seedlings varied between 5 cm and 8 cm, the etiolated seedlings were 7–13 cm tall. The pericarp of the achene often covered certain regions of the cotyledons of these seedlings. The cotyledons were collected and the pericarp was carefully removed in dim green light, which was previously tested not to cause phototransformation.
Light intensity measurements
The light intensity was measured with a LI-189 type, Li-Cor photometer (Li-Cor Inc., Lincoln, NE, USA).
Transmission and absorption spectroscopy
Transmission spectra were recorded with a Shimadzu UV-2101 PC (Shimadzu Corp., Kyoto, Japan) spectrophotometer, in the 400- to 800-nm region. The optical slits were 0·5 nm. The data frequency was 1 nm. Pieces of pericarps were fixed on the surface of a cuvette equipped with a black mask leaving a 2 × 15 mm window. The light beam of the spectrophotometer projected on the cuvette was completely covered. The absorption spectra of the pigment extractions were recorded with the same spectrophotometer in 1-cm cuvettes.
Fluorescence spectroscopy
To analyse the native arrangement of chlorophyllous pigments, low-temperature (77 K) fluorescence spectra were recorded with a Fluoromax-3 (Jobin Yvon-Horiba, Paris, France) spectrofluorimeter. The excitation wavelength was 440 nm. The excitation and emission slits were set to 2 nm and 5 nm, respectively. The integration time was 0·1 s. The spectra were analysed with the SPSERV V3·14 program (© C. Bagyinka, Institute of Biophysics, Biological Research Center of the Hungarian Academy of Sciences, Szeged, Hungary). Baseline correction and a combination of three-point and five-point linear smoothing were carried out. The spectra were corrected for the wavelength-dependent variations of the detector's response.
Pigment extraction and pigment content measurements
Cotyledons from etiolated and from light-grown seedlings were used for the pigment extraction. The light-exposed sections of the partially covered cotyledons were dissected in dim green light then the pericarp-covered sections were collected separately. The samples were smashed in mortars and the pigments were extracted with 80 % (v/v) acetone at room temperature. The water content of the cotyledons was 16·8 % (m/m), which influenced the final acetone concentration of the pigment extract only with 0·1 % (v/v). For this reason the extractions and the calculations were done with 80 % (v/v) acetone according to Porra et al. (1989).
To determine the Pchl(ide) contents, absorption spectra and fluorescence emission spectra with 430 nm excitation were also measured. Using a dilution series of Pchl solution [prepared from pumpkin (Cucurbita pepo L. ‘stájer’) seed coat], a calibration curve was measured to calculate the relation between the fluorescence intensity and the absorbance values. The molar extinction coefficient for Pchlide (30400 dm3 mol−1 cm−1) was used for the calculation according to Brouers and Michel-Wolwertz (1983). The Chl a and Chl b contents were calculated using equations from Porra et al. (1989).
Electron microscopy
Cotyledons were fixed in 2·5 % glutaraldehyde for 3 h in the dark and post-fixed in 1 % OsO4 for 2 h. The fixatives were buffered with 70 mm Na-K phosphate (pH 7·2). The samples were rinsed in the same buffer after fixation steps. After dehydration in an alcohol series, the samples were embedded in Durcupan ACM resin (Fluka Chemie AG, Buchs, Switzerland).
Ultrathin sections (70 nm) were cut with a Reichert Jung Ultracut E microtome (Reichert-Jung AG., Vienna, Austria). The sections were stained with 5 % uranyl acetate dissolved in methanol for 5 min and treated with Reynolds' lead citrate solution for 5 min.
The sections were investigated with a Hitachi 7100 TEM (transmission electron microscope, Hitachi, Tokyo, Japan) at 75-kV accelerating voltage.
Fluorescence induction
Cotyledons were examined with PAM-imaging. Photosynthetic activity of the cotyledons was studied by measuring variable chlorophyll fluorescence. In order to detect heterogeneity in fluorescence emission, fluorescence images were taken either with a FluorCam imaging fluorometer (Photon Systems Instruments, Brno, Czech Republic) or with an imaging-PAM M-Series chlorophyll fluorescence system, mini-head (Heinz Walz GmbH., Effeltrich, Germany). With both instruments, samples were dark-adapted for 15 min prior to the onset of actinic light to obtain a measure of minimal (F0) and maximal (Fm) fluorescence in darkness, the latter by applying a saturating flash (8000 µmol m−2 s−1). The maximum quantum efficiency of photosystem II (PSII) was calculated as Fv/Fm = (Fm − F0)/Fm (Björkman and Demmig, 1987). After measuring Fv/Fm, photochemistry was driven by constant low intensity (25 µmol m−2 s−1) actinic light and photosynthesis was probed by a saturating flash every 15 s for 1·5 min, by measuring chlorophyll fluorescence parameters as described below. In another set of experiments, a light response curve was measured after determining Fv/Fm, by keeping the sample at various, increasing photosynthetically active radiation (PAR) levels. Each level of actinic light was maintained for 60 s, and then the fluorescence was measured before (F′) and after (Fm′) the saturating flash. The following parameters were calculated, following the nomenclature and equations summarized by Rosenqvist and van Kooten (2003): the quantum yield of PSII photochemistry in light [ϕPSII = (Fm′ − F′)/Fm′], the Stern–Volmer non-photochemical quenching [NPQ = (Fm − Fm′)/Fm′] and the rate of electron transport (ETR = ϕPSII × PAR × 0·5 × 0·853) as μmol m−2 s−1 electrons transported. When calculating ETR, 0·5 was used as the approximate fraction of absorbed light directed to PSII and 0·853 as mean light absorption (Björkman and Demmig, 1987). It is important to note that these two constants are based on optical properties of green leaves, and are probably different (lower) for cotyledons at early greening stages. Consequently, electron transport rates in greening tissues studied in this work can only be regarded as an estimation.
RESULTS AND DISCUSSION
The behaviour of the pericarps during germination and their spectral properties
Depending on the growth conditions, the pericarp remained tightly attached to the cotyledons even of 7-d-old seedlings. Of the seedlings, around 70 % on wet filter paper, 43 % in sandy soil and 33 % in clayey soil bore the pericarp (Fig. 1A). The covered regions of the cotyledons were pale yellow while the light-exposed sections were dark-green (Fig. 1B). This indicated that the shading effect of the achene was so strong that the Chl biosynthesis was inhibited in the covered regions. To quantify this shading effect, pericarp pieces were collected and their transmittance was measured with a light meter (i.e. the sensor of a light meter was firmly covered by the achene walls). These measurements showed that only 1 % of the external light was transmitted; no significant differences were found between achene varieties with completely black or black and white striped pericarps. To study the spectral properties of these samples, the transmission spectra were recorded. No specific signals were found in the spectra, the transmittance value was only 1–5 % between 400 and 800 nm (data not shown). Similar properties of Lactuca and Taraxacum achene pericarps were reported earlier (Widell and Vogelmann, 1985, 1988).
Fig. 1.
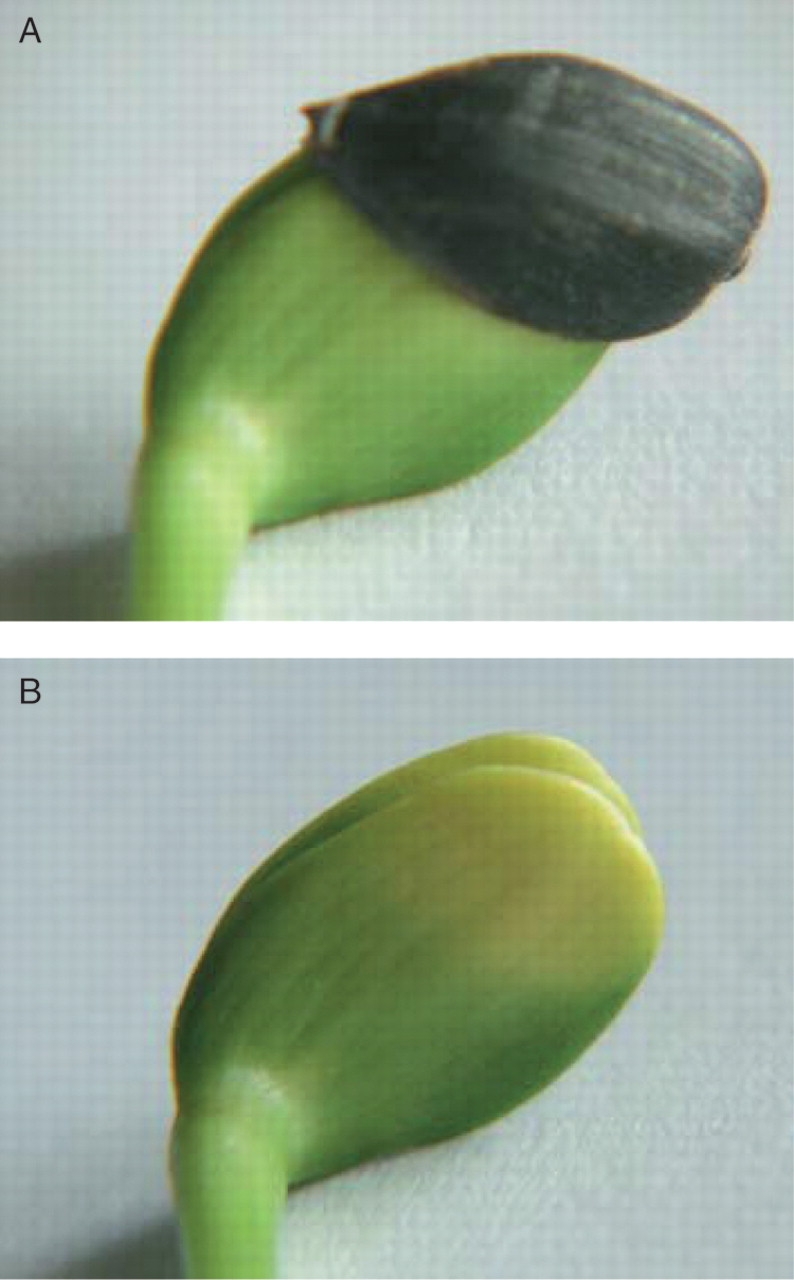
(A) The achene wall often covers the cotyledons of young (7 d old in the figure) sunflower (Helianthus annuus) seedlings. (B) After removing the achene wall, the yellowish, pale colour of the covered cotyledon part can be seen.
Pigment contents of the etiolated, covered and light-exposed regions of light-grown cotyledons
The sunflower cotyledons were suitable for studying the changes of the pigment contents because their tissues differentiated to chlorenchyma tissues similar to those of true leaves in a certain period of the seedling development. The cotyledons in dry fruits had a more or less homogenous tissue structure, which transformed in 3–4 d into a dorsiventral leaf structure with palisade and spongy cells on the adaxial and abaxial sides, respectively. This arrangement was stable for 3–4 weeks, and then the cotyledons showed senescence symptoms, they gradually dried out and fell off. The photosynthetic activity of cotyledons can play an essential role in the early development of seedlings when the primary leaves differentiate slowly.
To characterize the chlorenchyma tissues in cotyledons or cotyledon regions developed under various light conditions, the chlorophyllous pigments were extracted and the pigment contents were determined (Table 2). The 7-d-old etiolated cotyledons contained only Pchl(ide) the amount of which was around 6·8 µg g−1 f · wt, a value similar to those of etiolated true leaves (Akoyunoglou and Siegelman, 1967; Gassmann, 1973) and of etiolated leaves exposed to low intensities of red light (0·05 mW cm−2) (Treffry, 1973).
Table 2.
Pigment contents of various regions of 7-d-old sunflower (Helianthus annuus) cotyledons grown in the light (20 µmol s−1 m−2, at 12/12 h day/night cycles) and of dark-grown cotyledons
| Chlorophyll a (μg g−1 f · wt) | Chlorophyll b (μg g−1 f · wt) | Chl a : Chl b ratio | Protochlorophyll(ide) μg g−1 f · wt | |
|---|---|---|---|---|
| Region exposed to light- | 477·4 ± 30·7 | 205·3 ± 21·6 | 2·3 | – |
| Covered region | 65·7 ± 6·3 | 20·1 ± 3·5 | 3·3 | 2·1 ± 0·4 |
| Etiolated cotyledon | – | – | – | 6·8 ± 0·5 |
The pigment contents were calculated from absorption and fluorescence measurements (n = 6).
The pigment content and the Chl a : Chl b ratio of the light-grown cotyledons (Table 2) were similar to those found for 2- to 3-week-old light-grown sunflower leaves and cotyledons (Fambrini et al., 2004) and in young cotyledons emerging from the soil (La Rocca et al., 1996) as well as in light-grown clementine cotyledons developing at 120 µmol s−1 m−2 for 7 d (Casadoro and Rascio, 1987). The pericarp covered regions of the cotyledons of light-grown seedlings contained 2·10 ± 1·08, 65·67 ± 16·17 and 20·08 ± 9·05 µg g−1 fresh mass Pchl(ide), Chl a and Chl b, respectively (Table 2). Similar pigment compositions and contents are characteristic for innermost leaf primordia in breaking buds (Solymosi and Böddi, 2006; Solymosi et al., 2006) and for intermediary leaf layers of cabbage heads (Solymosi et al., 2004). The Chl a : Chl b ratio was higher in the covered than in the light-exposed regions of the cotyledons (Table 2). A high Chl a : Chl b ratio is characteristic of the early steps of greening of etiolated leaves (Treffry, 1973; Schoefs et al., 1998). However, in sunflower cotyledons studied in this work, this can be an effect of continuous low light illumination, which was reported to retard the synthesis of Chl b to a much larger extent than that of Chl a (Bennett et al., 1987).
Native arrangements of the pigments in the cotyledons
To examine the native arrangements of the pigments, 77 K fluorescence emission spectra of various sections of partially covered cotyledons and those of cotyledons developed without pericarp covering were measured and compared.
To observe transitional stages between the covered and light-exposed regions of the cotyledons, the cotyledons of partially covered achenes were divided into 2-mm-wide slices, cut parallel to the edge of the pericarp, and their fluorescence spectra were measured separately.
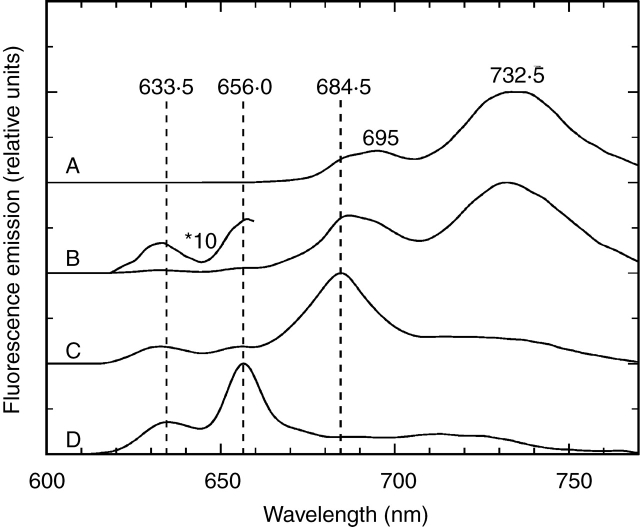
The emission spectra of the light-exposed sections had bands at 685, 695 and 730–740 nm (Fig. 2, curve A); these spectra were similar to those of any young green leaves and indicated the presence of all Chl–protein complexes of photosystems I and II (PSI and PSII, respectively) (Nielsen et al., 1979). These spectra were identical to those of cotyledons of seedlings germinated without a pericarp covering.
Fig. 2.
Fluorescence emission spectra (77 K) of 7-d-old sunflower (Helianthus annuus) cotyledons grown in the light (curves A–C) or in the dark (curve D). Spectra of light-exposed (A) and achene wall-covered regions of the cotyledons (B, C). (B) Sample from the sector next to the light-exposed region; (C) sample from the fully covered cotyledon tip. The light-grown seedlings developed at 20 µmol s−1m−2 photon flux density with 12/12 h day/night cycles. The spectra are normalized at their maxima and shifted along the y axis. Excitation wavelength = 440 nm.
The sections containing tissues close to the edge of the pericarp (i.e. the transient section) showed emission bands of low intensities at 631–633 and 655–657 nm, corresponding to Pchl or Pchlide forms (Fig. 2, curve B). Besides these emission bands, maxima were found at 686, 692 and 732 nm, indicating the parallel occurrence of Chl–protein complexes of green leaves (Fig. 2, curve B). Fluorescence emission bands at 631–633 and 655–657 nm were also found in the spectra of fully covered regions but the relative amplitudes of these bands were higher than those in the spectra of transient sections.
There was a significant difference in the region of emission bands of Chl–protein complexes, the fully covered sections had an emission band at 685 nm and the amplitude of the fluorescence around 730–740 nm was very low; it had a great variability but often no band was found in this region (Fig. 2, curve C). These spectra were similar to fluorescence measured from the innermost leaves of buds of several plant species, e.g. horse chestnut (Aesculus hippocastanum), flowering ash (Fraxinus ornus), common walnut (Juglans regia), tree of heaven (Ailanthus altissima) (Solymosi and Böddi, 2006; Solymosi et al., 2006), in intermediary leaf layers of white cabbage (Brassica oleracea) (Solymosi et al., 2004), in re-etiolated plant material (Minkov et al., 1988; Amirjani and Sundqvist, 2004) or in etiolated and low-light illuminated leaves at early stages of greening (Franck and Strzałka, 1992; Franck et al., 1993; Schoefs et al., 2000b). In sunflower cotyledons, however, the pigment biosynthesis was activated at the start of the germination at the stage when the pericarp was totally closed. Consequently, the Chl biosynthesis was arrested at Pchlide similarly to tissues grown under artificial etiolation. In the later phase of the germination, when the pericarp opened, Chl biosynthesis was completed in the light-exposed regions. In the covered regions, however, the light intensity was not enough for the full photoconversion of Pchlide to chlorophyllide (Chlide). To prove that the lack of light and not the photo-inactivity was the reason for the presence of Pchlide forms in the covered regions, the achene wall was removed from the covered part of the achene and the cotyledons were illuminated with a light flash. Full phototransformation of the 655-nm Pchlide form was found under these conditions (Fig. 3A). The spectra of these illuminated samples had an emission band at 633 nm, indicating that a part of Pchl(ide) was not flash-photoactive, like forms with similar emission bands found in other etiolated tissues [633-nm Pchlide form in etiolated wheat leaves (Kahn et al., 1968); 629 and 636 nm Pchlide forms in etiolated pea epicotyl (Böddi et al., 1994; 1996)].
Fig. 3.
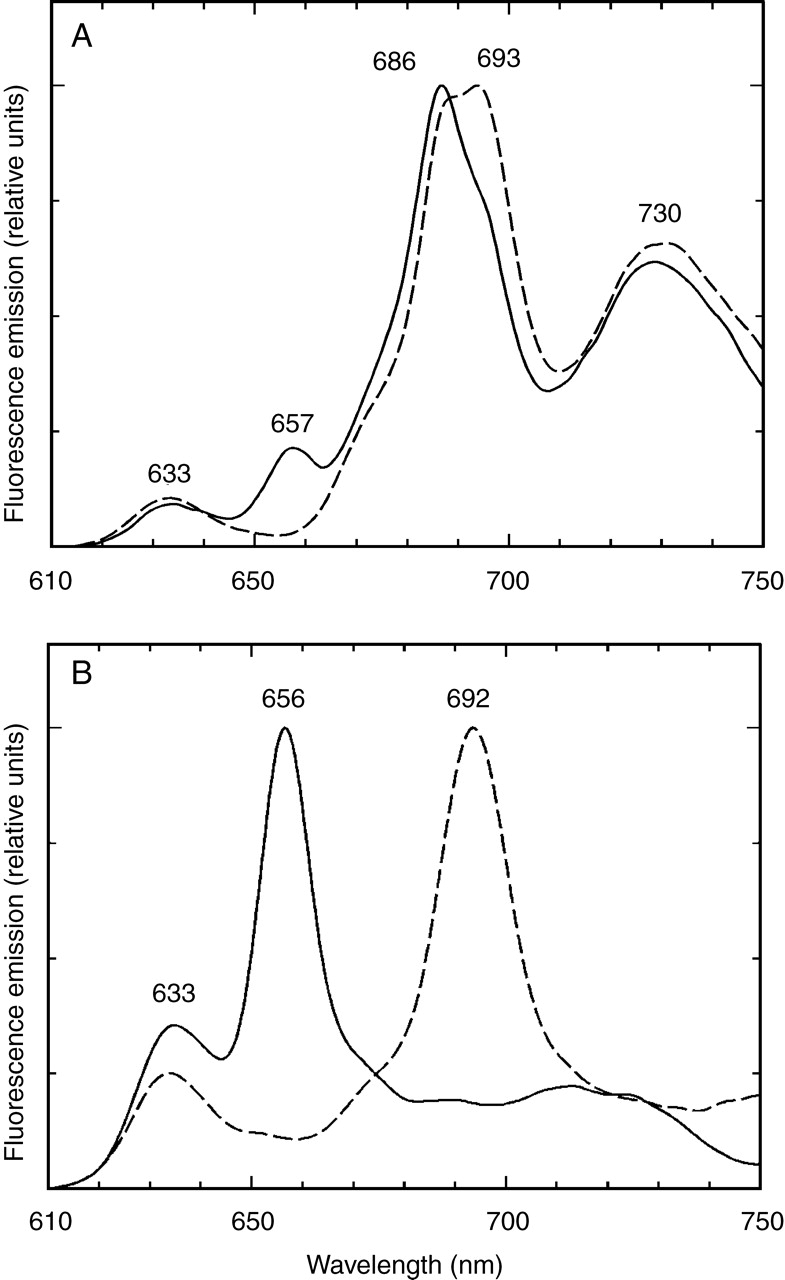
The effect of irradiation on completely covered regions of 7-d-old, light-grown sunflower (Helianthus annuus) cotyledons (A) and on the cotyledons of 7-d-old etiolated sunflower seedlings (B). The 77 K fluorescence emission spectra are normalized at their maxima. Continuous line: samples cooled to 77 K before irradiation, broken line: samples irradiated with a light flash (160 J 2 ms−1) and cooled to 77 K 10 s later. Excitation wavelength = 440 nm.
The pigment forms and their ratios found in the covered regions of the light-grown cotyledons were compared with those of cotyledons of dark-germinated seedlings. The emission spectra of the dark-grown cotyledons resembled those of true leaves of most etiolated seedlings featuring bands at 632–634 and 655–657 nm (Fig. 2, curve D). At 440 nm excitation, the amplitude ratio of these forms was 1 : 3 as often found in spectra of etiolated leaves (Böddi et al., 1992; Amirjani and Sundqvist, 2004). The emission spectra measured with the 460-nm excitation had a band at 655–657 nm (not shown). Illumination with a light flash resulted in the disappearance of the 655-nm band and the parallel appearance of a band at 692–694 nm indicating the formation of Chlide (Fig. 3B). Emission bands with low amplitudes were found in the 635- to 650-nm region, indicating the presence of flash-inactive Pchlide forms also in the dark-grown cotyledons. NADP +–Pchlide–POR forms are also likely to contribute to the emission at 635–650 nm (Franck et al., 1999). Therefore, it can be assumed that the same spectral Pchlide forms are present in the covered regions of light-grown cotyledons and in the cotyledons of etiolated seedlings (Figs 2, curves C and D, and 3A, B).
Plastid differentiation
Electron microscopic investigations were carried out to observe the effect of light on plastid differentiation by comparing dark- and light-grown and light-grown but covered cotyledons. Fully developed chloroplasts like in any green leaf were found in cotyledons of light-grown seedlings when the cotyledons were exposed to the light from very early developmental stages. The diameter of the plastids in the sunflower cotyledons was 3·6 µm on average (it varied between 1·9 and 6·1 µm). The characteristic features of the thylakoids were their dense lumen having thus an ‘inverse contrast’ (Fig. 4). Similarly, ‘inverse contrast’ or lightly staining membranes were described earlier in sunflower leaves (Casadoro and Rascio, 1978), sunflower cotyledons and in several other plant species (Casadoro and Rascio, 1979; Rascio et al., 1979; Keresztes and Sárvári, 2001). This feature is explained by the presence of phenolic compounds in the membranes (Keresztes and Sárvári, 2001).
Fig. 4.
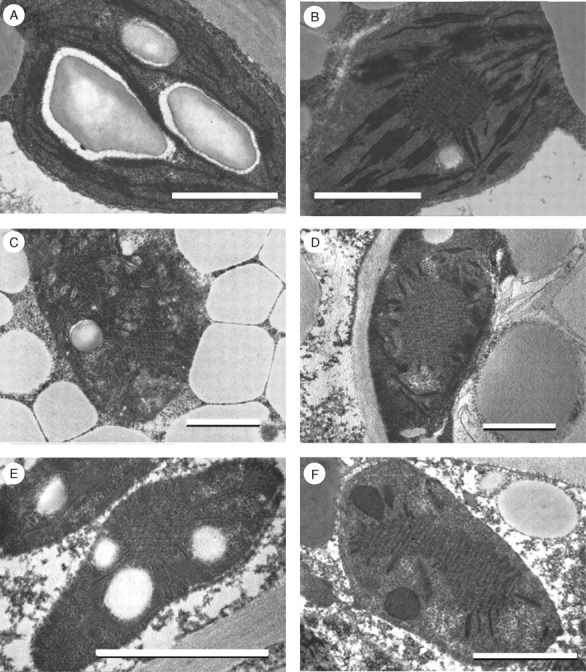
Electron micrographs of plastids from 7-d-old sunflower (Helianthus annuus) cotyledons grown in the light (A–D) or in the dark (E and F). The light-grown cotyledons developed at 20 µmol s−1 m−2 photon flux density with 12/12 h day/night cycles. (A) Regular chloroplast from the light-exposed region of the cotyledon. (B) Etio-chloroplast from the light-exposed region: prolamellar bodies (PLBs), grana and stroma thylakoids, (C and D) Etio-chloroplast from the covered region: PLBs and low grana consisting of two, swollen, stacking membranes present. (E) Etioplast from the mesophyll cell of a dark-grown cotyledon. (F) Etioplast from the epidermis of dark-grown cotyledons: PLBs and prothylakoids present. Scale bar = 1 µm.
The light-exposed regions of the partially covered cotyledons had fully developed chloroplasts (Fig. 4A) and, interestingly, a significant number of etio-chloroplasts with grana and interconnected, regular PLBs (Fig. 4B). Plastids containing grana and stroma thylakoids with and without PLBs were often found even within the same cell. In the case of the 88 plastid sections studied, PLBs were present in 61 sections, which means that typical etio-chloroplasts profiles can be found in 69 % of the plastids. Moreover, in most plastids two or three small PLBs could be observed. Similar, etio-chloroplasts were described in the cotyledons of the hypogeal seeds of clementine developing under continuous irradiation (at 120 and 240 µmol m−2 s−1) as well as in the dark (Casadoro and Rascio, 1987). Treffry (1973) found etio-chloroplasts in seedlings irradiated with low light intensities without the simultaneous accumulation of Pchlide. The presence of PLBs is especially interesting if we consider that the fluorescence spectra measured from the same, light-exposed regions did not indicate the presence of any of the Pchlide forms (Fig. 2, curve A).
The fully covered regions of the cotyledons contained etio-chloroplasts with PLBs as well as developing thylakoids (Fig. 4C). The mean size of the plastids was 3·4 µm (it varied between 2·1 and 5·4 µm); they were smaller than those in light-exposed regions. Often the stroma of the plastids was unusually dense; it was difficult to distinguish the inner membrane structures (Fig. 4C, D). The plastids contained stacked membrane regions but no true grana were found; the thylakoid membranes appeared as swollen membrane vesicles of developing grana, composed of a few (only two or three) stacking membranes. The PLBs in these plastids were larger (2 µm on average) than those in the light-exposed regions. From the 81 plastid profiles studied, PLBs were found in 55 plastid sections (67 %), a similar value to that found in the light-exposed region.
The absence of developed grana was probably caused and is in agreement with the low amounts of Chl a and Chl b pigments detected. Chlorophyll b accumulation is essential for LHCII formation, therefore granum development is hindered without significant amounts of Chl b, as it was shown in corn leaves developed under low (1–10 µmol s−1 m−2) PAR (Ryberg et al., 1980; Bennett et al., 1987). The presence of PLBs in the etio-chloroplasts is associated with the accumulation of POR (Bennett et al., 1987; Sperling et al., 1997, 1998; Selstam, 1998). POR plays an important role against photooxidative damage (Sperling et al., 1997, 1998), which can easily proceed in leaf primordia of buds of trees (Solymosi et al., 2006), low-light-grown maize (Rascio et al., 1980; Bennett et al., 1987) and in cotyledons of hypogeal seeds of clementine (Casadoro and Rascio, 1987) and of germinating sunflower (Walles, 1966, 1972; Casadoro and Rascio, 1979; Rascio et al., 1979; La Rocca et al., 1996; Lebkuecher et al., 1999) when they are exposed to natural light intensities. The macrodomain of POR usually contains surplus NADPH (Ryberg and Sundqvist, 1988) that prevents photooxidation. The excitation energy therefore is used mainly for Pchlide photoreduction rather than for photooxidation.
The etiolated cotyledons had etioplasts with PLBs and single PT lamellae (Fig. 4E–F). These etioplasts were small (2·6 µm on average; varied between 1·4 µm and 6·7 µm). However, the stroma of most plastids was electron-dense and the membranes had ‘inverse contrast’ (see above). Similar plastids were characteristic for both the adaxial and abaxial epidermis, for the mesophyll areas and also for the vascular tissues of these leaves. The plastids in the epidermis cells had better contrast; an etioplast from an epidermis cell is shown in Fig. 4F. The PLBs and PTs were similar to those found in etioplasts of dark-grown sunflower cotyledons (Walles, 1972; Rascio and Casadoro, 1979; Rascio et al., 1979) and etiolated leaves of other species (Gunning, 1965, 2001; Henningsen and Boynton, 1969).
Photosynthetic activity
Considering differences in pigment contents, organizations of pigment–protein complexes and plastid ultrastructures between uncovered and pericarp-covered regions of the cotyledons, differences were also expected in their photosynthetic activities. To study this, photosynthetic parameters of pericarp-covered and light-exposed tissues were compared, using variable chlorophyll fluorescence imaging. Figure 5 shows that the whole cotyledon had variable cholorophyll fluorescence – even the formally covered area was photosynthetically active. Minimal fluorescence of the dark-adapted cotyledon (F0) was not significantly different in covered and exposed areas, indicating that the number of excited chlorophyll molecules that did not initiate either photochemistry or energy conversion to heat, was the same. In the developing, partly covered areas such chlorophylls could be either free (Marder and Raskin, 1993; Myśliwa-Kurdziel et al., 1997) or embedded in the light-harvesting complexes but energetically uncoupled to the reaction centres. Maximal intensity of PSII, characterized by Fv/Fm, was about 30 % less in the covered area than in the exposed (Fig. 5A). The F0 and Fv/Fm values of the light-exposed regions were similar to those observed in light-grown sunflower leaves (Fambrini et al., 2004). The F0 and Fv/Fm values of the covered regions resemble those obtained during the greening of 6-d-old etiolated sunflower cotyledons illuminated with continuous white light (100 µmol m−2 s−1) for 6 h (Lebkuecher et al., 1999). However, it should be noted that Pchlide phototransformation is triggered by the light pulses used during the measurements, therefore the fluorescence of the newly formed Chlide might slightly influence the actual values of these parameters.
Fig. 5.
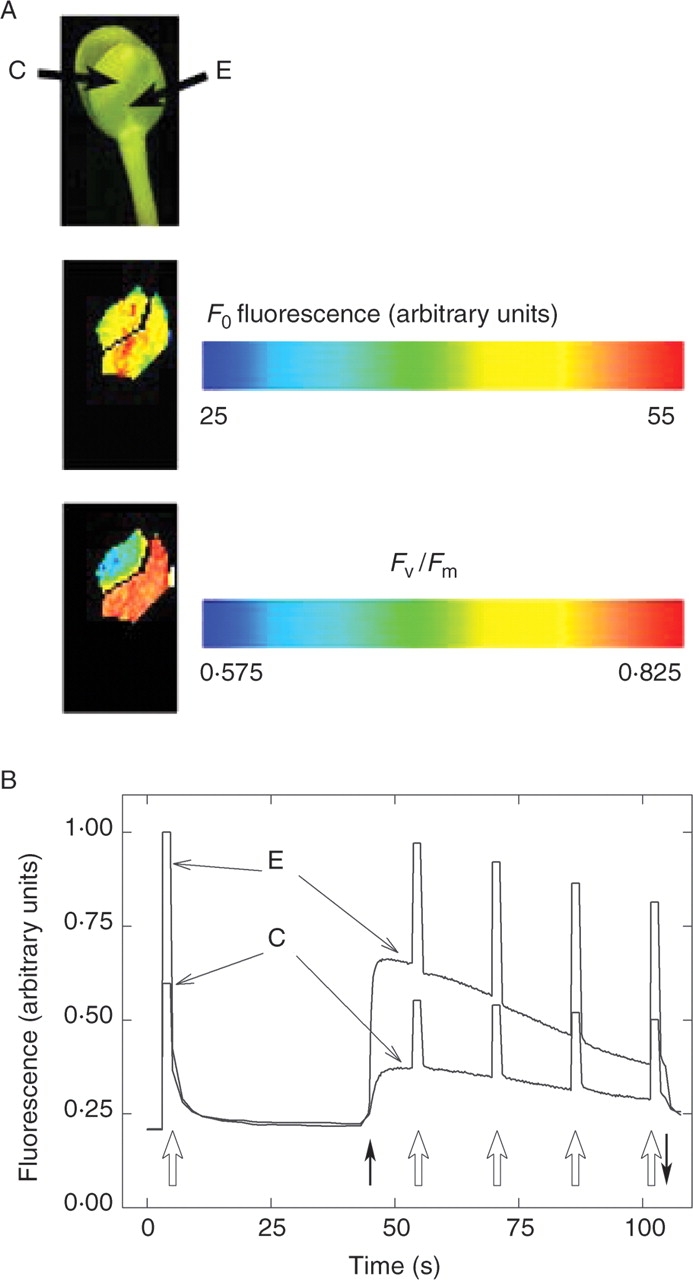
The whole sunflower cotyledon, even the formally covered area was photosynthetically active. (A) Division of leaf areas into formerly pericarp covered (C) and uncovered, exposed (E) ones for the photosynthesis measurements in Figs 6–7, and an illustration of differences between chlorophyll fluorescence parameters of these two areas. F0 fluorescence intensities and Fv/Fm ratios were colour-coded as indicated. (B) Typical traces of the induction of photosynthesis in these two areas. Open upward arrows indicated saturating pulses (8000 µmol m−2 s−1), closed arrows show on- and offsets of actinic illumination (25 µmol m−2 s−1).
Fluorescence images in Fig. 5A show that these parameters were not uniform, even in similar cotyledon areas. For further analysis, parameters were averaged from the formerly covered (C) and exposed (E) areas of several samples, as illustrated with one cotyledon in Fig. 5A. Induction of photosynthesis upon exposure to actinic light followed different kinetics in covered (C) and exposed (E) areas: fluorescence was generally lower in the former area of the cotyledon (Fig. 5B). Quenching analysis performed under a series of saturating pulses showed that the light-induced dissipation of energy in non-photochemical pathways was initially faster in the formerly covered than in the exposed areas. However, this difference disappeared after about 30 s, and the two regions gave similar NPQ values afterwards (Fig. 6A, B). At low PAR intensities the light saturation curves of NPQ (Fig. 6C) showed no significant difference between the light-exposed and covered areas. These NPQ values were similar to those found in light-grown sunflower leaves irradiated with 25 µmol m−2 s−1 (Fambrini et al., 2004). At higher PAR intensities, the light response of NPQ was markedly different in the two areas. Covered regions, which developed under lower light, were less able to develop NPQ than the exposed regions (Fig. 6C). Plants are able to prevent photodegradation by increasing the NPQ values and in, this way, dissipating the excitation energy mostly in the form of thermal energy, instead of inducing photochemical reactions (Holt et al., 2004). The results show that at the onset of illumination the covered region is more effective in energy dissipation than the light-exposed area (Fig. 6A, B). This might protect the developing photosynthetic apparatus from the harmful effects of the high natural light intensity when the pericarp falls down. However, at increasing PAR intensities, that the light-exposed areas are able to increase the NPQ values to a higher extent than the covered region (Fig. 6C) may be explained by the less-developed photosynthetic apparatus.
Fig. 6.
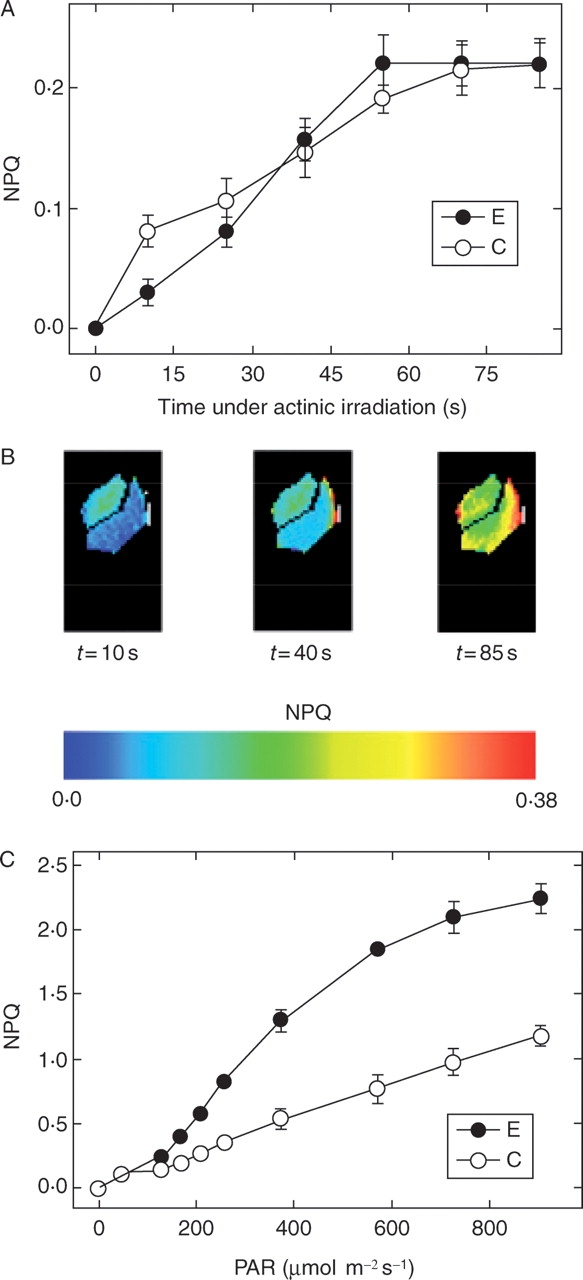
(A) Development of non-photochemical quenching in formerly pericarp-covered (C) and uncovered, exposed (E) areas of dark-adapted sunflower cotyledons upon illuminating with 100–120 µmol m−2 s−1 PAR. Data were collected and averaged from respective areas of different cotyledons. Typical fluorescence images are shown in (B) using colour-coding of NPQ. (C) Light saturation curves of NPQ measured at the two areas studied in response to increasing PAR. All data in (A) and (C) are mean values of four independent measurements, error bars represent s.d.
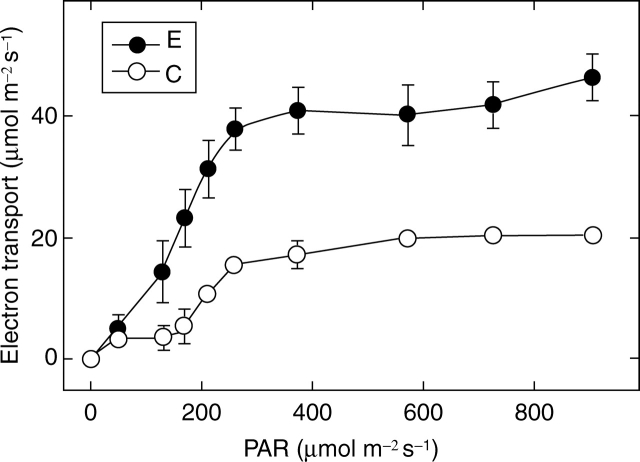
Photosynthetic electron transport was also different in the two areas. In the formerly covered regions not only the maximal yield of PSII (Fig. 5A) but also quantum yields under illumination were lower than in the areas grown exposed to light (Fig. 7). The light-saturated electron transport was approximately double in the latter than in the former areas (Fig. 7). These results clearly indicate that the photosynthetic electron transport chain has developed also in the covered region; however, its efficiency is much lower than that of the light-exposed area. This may give a photoprotection for these tissues. The formation of the photosynthetic apparatus can be stimulated by light-piping, i.e. low intensity light can reach the covered regions even through non-exposed tissues. This may induce some Pchlide phototransformation and phytochrome activation of rapid development of the photosynthetic activity (Smith, 2000; Quail, 2002).
Fig. 7.
Light response curves of photosynthetic electron transport measured from formerly pericarp-covered (C) and uncovered, exposed (E) areas of sunflower cotyledons. All data are mean values of four independent measurements; error bars represent s.d.
CONCLUSIONS
The epigeal sunflower cotyledons develop chlorenchyma tissues organized similarly to those of any dorsiventral true leaves, when exposed to light. These cotyledons are normally green and live about 28 d after germination and are thus suitable for studying the formation of their photosynthetic apparatus. However, because the achene fruit wall may remain attached, partly shading the cotyledons, pigment and plastid development can proceed in these areas in vivo, first after the removal of the pericarp. The very low light intensity reaching the shaded areas allows the formation of Pchlide and PLBs characteristic of etiolated cotyledons, but Chl–protein complexes of PSI and PSII, capable of photosynthetic electron transport are also formed to some extent. In this way, the sudden exposure to PAR caused by the subsequent fall of the achene wall, drives the Pchlide–Chlide photoreduction and development of photosynthetic activity rather than causing photooxidation and bleaching. These results prove that the etio-chloroplasts with Pchlide forms and PLBs previously studied in dark-grown laboratory plants can also develop under natural conditions.
ACKNOWLEDGEMENTS
We thank Katalin M. Gergely and Csilla Jónás for their assistance in the electron microscope sample preparation. This work has been supported by the Hungarian Scientific Research Fund (OTKA T038003).
LITERATURE CITED
- Akoyunoglou GA, Siegelman HW. Protochlorophyllide resynthesis in dark-grown bean leaves. Plant Physiology. 1967;43:66–68. doi: 10.1104/pp.43.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirjani MR, Sundqvist C. Regeneration of protochlorophyllide in green and greening leaves of plants with varying proportions of protochlorophyllide forms in darkness. Physiologia Plantarum. 2004;121:377–390. [Google Scholar]
- Argyroudi-Akoyunoglou JH, Kondylaki S, Akoyunoglou G. Growth of grana from ‘primary’ thylakoids in Phaesolus vulgaris. Plant and Cell Physiology. 1976;17:939–954. [Google Scholar]
- Beale SI. Enzymes of chlorophyll biosynthesis. Photosynthesis Research. 1999;60:43–73. [Google Scholar]
- Bennett J, Schwender JR, Shaw EK, Tempel N, Ledbetter M, Williams RS. Failure of corn leaves to acclimate to low irradiance: role of protochlorophyllide reductase in regulating levels of five chlorophyll-binding proteins. Biochimica et Biophysica Acta. 1987;892:118–129. [Google Scholar]
- Björkman O, Demmig B. Photon yields of O2 evolution and chlorophyll fluorescence characteristics at 77-K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Böddi B, Lindsten A, Ryberg M, Sundqvist C. On the aggregational states of protochlorophyllide and its protein complexes in wheat etioplasts. Physiologia Plantarum. 1989;76:135–143. [Google Scholar]
- Böddi B, Ryberg M, Sundqvist C. Identification of four universal protochlorophyllide forms in dark-grown leaves by analyses of the 77 K fluorescence emission spectra. Journal of Photochemistry and Photobiology B: Biology. 1992;12:389–401. [Google Scholar]
- Böddi B, McEwen B, Ryberg M, Sundqvist C. Protochlorophyllide forms in non-greening epicotyls of dark-grown pea (Pisum sativum) Physiologia Plantarum. 1994;92:706–713. [Google Scholar]
- Böddi B, Evertsson I, Ryberg M, Sundqvist C. Protochlorophyllide transformations and chlorophyll accumulation in epicotyls of pea (Pisum sativum) Physiologia Plantarum. 1996;96:706–713. [Google Scholar]
- Brouers M, Michel-Wolwertz MR. Estimation of protochlorophyll(ide) contents in plant extracts; re-evaluation of the molar absorption coefficient of protochlorophyll(ide) Photosynthesis Research. 1983;4:265–270. doi: 10.1007/BF00052130. [DOI] [PubMed] [Google Scholar]
- Casadoro G, Rascio N. Thylakoid membranes in sunflower and in other plants. Journal of Ultrastructure Research. 1978;65:30–35. doi: 10.1016/s0022-5320(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Casadoro G, Rascio N. Plastid ultrastructural features in the various tissues of sunflower leaves. Cytobios. 1979;24:157–166. [PubMed] [Google Scholar]
- Casadoro G, Rascio N. Cotyledonal chloroplasts in the hypogeal seeds of clementine. Planta. 1987;170:300–307. doi: 10.1007/BF00395020. [DOI] [PubMed] [Google Scholar]
- Fambrini M, Castagna A, Dalla Vecchia F, Degl'Innocenti E, Ranieri A, Vernieri P, et al. Characterization of a pigment-deficient mutant of sunflower (Helianthus annuus L.) with abnormal chloroplast biogenesis, reduced PS II activity and low endogenous level of abscisic acid. Plant Science. 2004;167:79–89. [Google Scholar]
- Franck F, Strzałka K. Detection of the photoactive protochlorophyllide-protein complex in the light during the greening of barley. FEBS Letters. 1992;309:73–77. doi: 10.1016/0014-5793(92)80742-y. [DOI] [PubMed] [Google Scholar]
- Franck F, Barthélémy X, Strzałka K. Spectroscopic characterization of protochlorophyllide photoreduction in the greening leaf. Photosynthetica. 1993;29:185–194. [Google Scholar]
- Franck F, Bereza B, Böddi B. Protochlorophyllide–NADP+ and protochlorophyllide-NADPH complexes and their regeneration after flash illumination in leaves and etioplast membranes of dark-grown wheat. Photosynthesis Research. 1999;59:53–61. [Google Scholar]
- Gassmann ML. A reversible conversion of phototransformable protochlorophyll(ide)650 to photoinactive protochlorophyll(ide)633 by hydrogen sulfide in etiolated bean leaves. Plant Physiology. 1973;51:139–145. doi: 10.1104/pp.51.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES. The greening process in plastids. 1. The structure of the prolamellar body. Protoplasma. 1965;60:111–130. [Google Scholar]
- Gunning BES. Membrane geometry of ‘open’ prolamellar bodies. Protoplasma. 2001;215:4–15. doi: 10.1007/BF01280299. [DOI] [PubMed] [Google Scholar]
- Henningsen KW, Boynton JE. Macromolecular physiology of plastids. VII. The effect of brief illumination on plastids of dark-grown barley leaves. Journal of Cell Science. 1969;5:757–793. doi: 10.1242/jcs.5.3.757. [DOI] [PubMed] [Google Scholar]
- Holt NE, Fleming GR, Niyogi KK. Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry. 2004;43:8281–8289. doi: 10.1021/bi0494020. [DOI] [PubMed] [Google Scholar]
- Jabben M, Mohr H. Stimulation of the Shibata shift by phytochrome in the cotyledons of the mustard seedling Sinapis alba L. Photochemistry and Photobiology. 1975;22:55–58. doi: 10.1111/j.1751-1097.1975.tb06721.x. [DOI] [PubMed] [Google Scholar]
- Kahn A. Developmental physiology of bean leaf plastids. III Tube transformation and protochlorophyll(ide) photoconversion by a flash irradiation. Plant Physiology. 1968;43:1781–1785. doi: 10.1104/pp.43.11.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes Á, Sárvári É. Investigations into the ‘inverse contrast’ of chloroplast thylakoids. Acta Botanica Croatica. 2001;60:253–265. [Google Scholar]
- Kis-Petik K, Böddi B, Kaposi AD, Fidy J. Protochlorophyllide forms and energy transfer in dark-grown wheat leaves Studies by conventional and laser excited fluorescence spectroscopy between 10 K–100 K. Photosynthesis Research. 1999;60:87–98. [Google Scholar]
- Kuroda H, Masuda T, Ohta H, Shioi Y, Takamiya K. Effects of light, developmental age and phytohormones on the expression of the gene encoding NADPH-protochlorophyllide oxidoreductase in Cucumis sativus. Plant Physiology and Biochemistry. 1996;34:17–22. [Google Scholar]
- La Rocca N, Barbato R, Casadoro G, Rascio N. Early degradation of photosynthetic membranes in carob and sunflower cotyledons. Physiologia Plantarum. 1996;96:513–518. [Google Scholar]
- Lebkuecher JG, Haldeman KA, Harris CE, Holz SL, Joudah SA, Minton DA. Development of photosystem-II activity during irradiance of etiolated Helianthus (Asteraceae) seedlings. American Journal of Botany. 1999;86:1087–1092. [PubMed] [Google Scholar]
- Marder JB, Raskin VI. The assembly of chlorophyll into pigment–protein complexes. Photosynthetica. 1993;28:243–248. [Google Scholar]
- Masuda T, Takamiya K. Novel insights into the enzymology, regulation and physiological functions of light-dependent protochlorophyllide oxidoreductase in angiosperms. Photosynthesis Research. 2004;81:1–29. doi: 10.1023/B:PRES.0000028392.80354.7c. [DOI] [PubMed] [Google Scholar]
- Minkov IN, Ryberg M, Sundqvist C. Properties of reformed prolamellar bodies from illuminated and redarkened etiolated wheat plants. Physiologia Plantarum. 1988;72:725–732. [Google Scholar]
- Myśliwa-Kurdziel B, Barthélemy X, Strzałka K, Franck F. The early stages of photosystem II assembly monitored by measurements of fluorescence lifetime, fluorescence induction and isoelectric focusing of chlorophyll-proteins in barley etiochloroplasts. Plant and Cell Physiology. 1997;38:1187–1196. [Google Scholar]
- Nielsen NC, Smillie RM, Henningsen KW, Von Wettstein D. Composition and function of thylakoid membranes from grana-rich and grana-deficient chloroplast mutants of barley. Plant Physiology. 1979;63:174–182. doi: 10.1104/pp.63.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa N, Masuda T, Awai K, Fusada N, Shimada H, Ohto H, Takamiya K. Identification and light-induced expression of a novel gene of NADPH:protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Letters. 2000;474:113–136. doi: 10.1016/s0014-5793(00)01568-4. [DOI] [PubMed] [Google Scholar]
- Ouazzani-Chahdi MA, Schoefs B, Franck F. Isolation and characterisation of photoactive complexes of NADPH:protochlorophyllide oxidoreductase from wheat. Planta. 1998;206:673–680. [Google Scholar]
- Porra RJ, Thompson WA, Kriedermann PE. Determination of accurate extinction coefficients and simultaneous equation for assaying chlorophyll-a and b extracted with different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Quail PH. Phytochrome photosensory signalling networks. Nature Reviews Molecular Cell Biology. 2002;3:85–93. doi: 10.1038/nrm728. [DOI] [PubMed] [Google Scholar]
- Rascio N, Casadoro G. Sunflower etioplast membranes. Journal of Ultrastructure Research. 1979;68:325–327. doi: 10.1016/s0022-5320(79)90164-3. [DOI] [PubMed] [Google Scholar]
- Rascio N, Casadoro G, di Chio L. Etioplast–chloroplast transformation in sunflower cotyledons. Protoplasma. 1979;100:45–52. [Google Scholar]
- Rascio N, Mariani Colombo P, Orsenigo M. The ultrastructural development of plastids in leaves of maize plants exposed to continuous illumination. Protoplasma. 1980;102:131–139. [Google Scholar]
- Rosenqvist E, van Kooten O. Chlorophyll fluorescence: a general description and nomenclature. In: DeEll JR, Toivonen PMA, editors. Practical applications of chlorophyll fluorescence in plant biology. Boston: Kluwer; 2003. pp. 32–77. [Google Scholar]
- Ryberg H, Axelsson L, Widell K-O, Virgin HI. Chlorophyll b accumulation and grana formation in low intensities of red light. Physiologia Plantarum. 1980;49:431–436. [Google Scholar]
- Ryberg M, Sundqvist C. The regular ultrastructure of isolated prolamellar bodies depends on the presence of membrane-bound NADPH-protochlorophyllide oxidoreductase. Physiologia Plantarum. 1988;73:218–226. [Google Scholar]
- Schoefs B, Garnir HP, Bertrand M. Comparison of the photoreduction of protochlorophyllide to chlorophyllide in leaves and cotyledons from dark-grown bean as a function of age. Photosynthesis Research. 1994;41:405–417. doi: 10.1007/BF02183043. [DOI] [PubMed] [Google Scholar]
- Schoefs B, Bertrand M, Lemoine Y. Changes in the photosynthetic pigments in bean leaves during the first photoperiod of greening and the subsequent dark-phase: comparison between old (10-d-old) leaves and young (2-d-old) leaves. Photosynthesis Research. 1998;57:203–213. [Google Scholar]
- Schoefs B, Bertrand M, Funk C. Photoactive protochlorophyllide regeneration in cotyledons and leaves from higher plants. Photochemistry and Photobiology. 2000a;72:660–668. doi: 10.1562/0031-8655(2000)072<0660:pprica>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schoefs B, Bertrand M, Franck F. Spectroscopic properties of protochlorophyllide analyzed in situ in the course of etiolation and in illuminated leaves. Photochemistry and Photobiology. 2000b;72:85–93. doi: 10.1562/0031-8655(2000)072<0085:spopai>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Selstam E. Development of thylakoid membranes with respect to lipids. In: Siegentahler P-A, Murata N, editors. Lipids in photosynthesis: structure, function and genetics. Dordrecht: Kluwer; 1998. pp. 209–224. [Google Scholar]
- Selstam E, Widell-Wigge A. Chloroplast lipids and the assembly of membranes. In: Sundqvist C, Ryberg M, editors. Pigment–protein complexes in plastids: synthesis and assembly. San Diego, CA: Academic Press; 1993. pp. 241–277. [Google Scholar]
- Skribanek A, Apatini D, Inaoka M, Böddi B. Protochlorophyllide and chlorophyll forms in dark-grown stems and stem-related organs. Journal of Photochemistry and Photobiology B: Biology. 2000;55:172–177. doi: 10.1016/s1011-1344(00)00044-0. [DOI] [PubMed] [Google Scholar]
- Smith H. Phytochromes and light signal perception by plants — an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- Solymosi K, Böddi B. Optical properties of bud scales and protochlorophyll(ide) forms in leaf primordia of closed and opened buds. Tree Physiology. 2006;26:1075–1085. doi: 10.1093/treephys/26.8.1075. [DOI] [PubMed] [Google Scholar]
- Solymosi K, Martinez K, Kristóf Z, Sundqvist C, Böddi B. Plastid differentiation and chlorophyll biosynthesis in different leaf layers of white cabbage (Brassica oleracea cv. capitata) Physiologia Plantarum. 2004;121:520–529. [Google Scholar]
- Solymosi K, Bóka K, Böddi B. Transient etiolation: protochlorophyll(ide) and chlorophyll forms in differentiating plastids of closed and breaking leaf buds of horse chestnut (Aesculus hippocastanum) Tree Physiology. 2006;26:1087–1096. doi: 10.1093/treephys/26.8.1087. [DOI] [PubMed] [Google Scholar]
- Sperling U, van Cleve B, Frick G, Apel K, Armstrong GA. Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. The Plant Journal. 1997;12:649–658. doi: 10.1046/j.1365-313x.1997.00649.x. [DOI] [PubMed] [Google Scholar]
- Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA. Etioplast differentation in Arabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. The Plant Cell. 1998;10:283–296. doi: 10.1105/tpc.10.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffry T. Chloroplast development in etiolated peas: reformation of prolamellar bodies in red light without accumulation of protochlorophyllide. Journal of Experimental Botany. 1973;24:185–195. [Google Scholar]
- Walles B. Plastid structures of carotenoid-deficient mutants of sunflower (Helianthus annuus L.). II. The yellow mutant. Hereditas. 1966;56:131–136. [Google Scholar]
- Walles B. An electron microscope study on photodestruction of plastid ribosomes in β-carotene-deficient mutants of Helianthus annuus L. Protoplasma. 1972;75:215–227. [Google Scholar]
- Whatley JM. Variations in the basic pathway of chloroplast development. New Phytologist. 1977;78:407–420. [Google Scholar]
- Widell K-O, Vogelmann TC. Optical properties of Lactuca and Taraxacum seed and fruit coats: their role as light filters. Physiologia Plantarum. 1985;64:34–40. [Google Scholar]
- Widell K-O, Vogelmann TC. Fiber optic studies of light gradients and spectral regime within Lactuca sativa achenes. Physiologia Plantarum. 1988;72:706–712. [Google Scholar]
- Wiktorsson B, Engdahl S, Zhong LB, Böddi B, Ryberg M, Sundqvist C. The effect of cross-linking of the subunits of NADPH:protochlorophyllide oxidoreductase on the aggregational state of protochlorophyllide. Photosynthetica. 1993;29:205–218. [Google Scholar]