Abstract
Background and Aims
Lichens can be both nitrogen- (N) and phosphorous- (P) limited and thus may be susceptible to nutrient enrichment. Nutrient enrichment with N and P may have differing impacts on the lichen structure because of different physiological responses of fungal and algal partners to these nutrients. The hypothesis was tested that the differential responses of lichen symbionts to enhanced availability of N and P is reflected in the lichen thallus structure and the wall-to-wall interface between the algal and fungal cells.
Methods
Lichen cushions of Cladonia stellaris were treated with one P and two N concentrations alone and in combination that yielded total depositions of approx. 300 (moderate) and 1000 (high) mg N m−2 and 100 (high) mg P m−2 over an experiment lasting 14 weeks. The effects of N and P inputs on the relative volumes of fungal and algal cell in the medullary tissue and on the thallus structure were studied using light microscopy. The interface between algal and fungal cell walls was examined using transmission electron microscopy.
Key Results
The influence of excess P on the lichen thallus structure was stronger than that of additional N. Addition of P reduced the N : P ratio in podetia, the proportion of the medullary layer volume occupied by the algal cells, the thallus volume occupied by the internal lumen, and the algal cell-wall area covered by fungal hyphae.
Conclusions
Ecologically realistic changes in the availability of key macronutrients can alter the growth of symbionts. Reduction in the proportion of photobiont cells indicates that the application of P either stimulates fungal hyphal growth in the medullary tissue or impairs the cell division of the algal cells. The results suggest that both the N and P availability and thallus N : P ratio affect the growth rates of lichen symbionts.
Key words: Lichen, N, P, nutrient status; algal cell; fungal cell; thallus structure; Cladina stellaris
INTRODUCTION
Several field and laboratory studies have indicated that the growth of large foliose and fruticose lichens may be limited by nitrogen (N; Crittenden et al., 1994 and references therein; Kurina and Vitousek, 1999; Palmqvist and Dahlman, 2006). However, little is known about the effects of nutrient deficiency on lichen structure. The results of fertilization experiments (Kauppi, 1980; Roy-Arcand et al., 1989) suggest that the impact of enhanced N on lichen growth may be biased towards the growth of the photobiont rather than the mycobiont.
Increases in photobiont populations and a concomitant disruption of the symbiosis, followed by thallus disintegration in extreme cases, have long been known to be a response to ecologically unrealistic N concentrations (e.g. Smith and Griffiths, 1969). Over-growth of the algal cells has been reported in highly N-enriched Cladina lichens (e.g. Kauppi, 1980). However, the impact of moderate or ecologically realistic changes in the N input on lichen anatomy and especially on the symbiotic interaction between photobiont and mycobiont are still poorly understood.
The growth of lichens, especially N-fixing lichens, may also be limited by P (Crittenden et al., 1994). However, to our knowledge, there are no experimental data available regarding the effect of P-limitation on lichen growth. Hyvärinen and Crittenden (2000) have shown with Cladina portentosa that P recycles from the senescing parts of thalli to the growing apices. They interpreted these findings as a source–sink relationship within the thallus and an adaptation of lichen to P-limited sites. In fungi, P translocation is suggested to be symplastic, based on cytoplasmic streaming through multiperforate septa (Wetmore, 1973; Hyvärinen and Crittenden, 2000). N has also been shown to resorb from senescent basal tissue and to be recycled within the thallus (Kytöviita, 1993; Ellis et al., 2005). Both key macronutrients must be actively translocated from the older parts to promote the growth of apices. Thus alterations in the availability of N and P may also result in physiological changes in the lichen thallus.
Dahlman et al. (2003) measured the concentrations of chlorophyll a, ergosterol and chitin in Hypogymnia physodes and Platismatia glauca subjected to N-rich fertilization for several years. They concluded that the relative investment of N in the lichen thallus was radically changed in favour of the photobiont and suggested that increased photosynthetic capacity may be an adaptation to alleviate the carbon cost of high-N tissues. These indirect measurements of the relative investment of N into the different symbionts show that the physiological changes in the photobiont and mycobiont may be coupled with the general nutrient regime. Whether these changes are also reflected in the lichen thallus structure remains to be shown.
The aim of this study was to investigate the effect of moderately enhanced N or P availability, alone and in combination, on the growth of alga and fungus, and on the podetium structure in Cladina stellaris. Each cell of the mature photobiont is usually enveloped by hyphae (Nash, 1996). We hypothesized that the proportion of the algal cell-wall area covered by fungal hyphae might vary if the growth rate of lichen partners either increase or decrease. It was expected that the combined N and P treatments would not necessarily bias the growth of symbionts as both the alga and fungus might benefit from the moderate nutrient enhancement.
MATERIALS AND METHODS
Study species
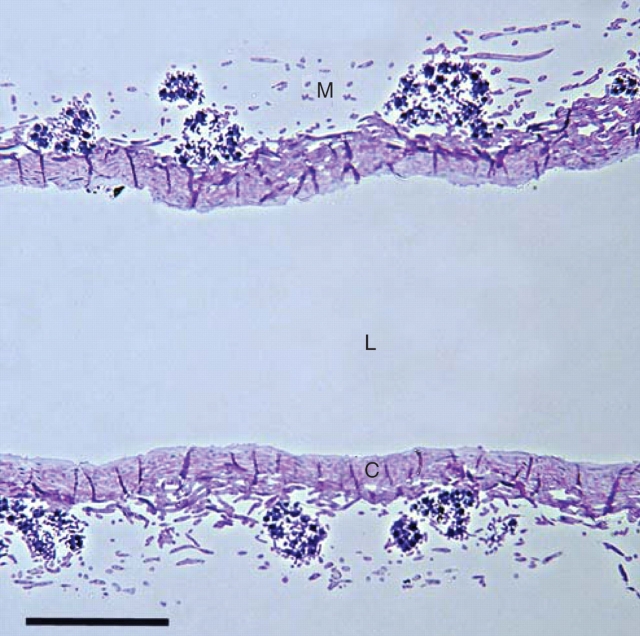
Cladina stellaris (Opiz) Brodo is a mat-forming fruticose lichen that dominates field layers of the oligotrophic and well-drained environments of boreal and arctic zones (Longton, 1988; Ahti and Oksanen, 1990). Cladina stellaris grows vegetatively by producing new growth at the top of each podetium, thus lengthening the internode formed in the previous year. The thallus structure of C. stellaris and other Cladina spp. differs from many other fruticose lichens in that it lacks an outer cortex. The outer layer of C. stellaris consists of algal cells and loosely packed hyphal tissue resembling the medulla (Figs 1 and 2). Below the upper layer is the inner cortex in which fungal cells are tightly packed. The core of podetium, which is empty, is called here an internal lumen.
Fig. 1.
Longitudinal semi-thin section of the thallus of C. stellaris, showing the medulla (M) with algal cells, the inner cortex (C) and the internal lumen (L). Scale bar = 100 µm.
Fig. 2.
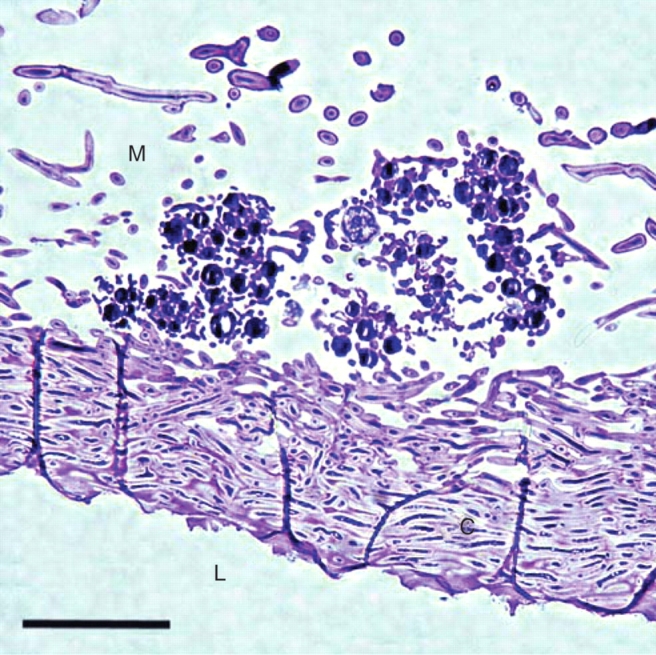
Longitudinal semi-thin section of the thallus of C. stellaris. The medulla (M), the inner cortex (C) and the internal lumen (L). Scale bar = 50 µm.
Experimental design and fertilization treatment
Intact 20 × 26 cm cushions of Cladina stellaris together with 5-cm depth of topsoil were transported from the island of Hailuoto (65°01′N, 24°47′E) in northern Finland to the experimental field of the University of Oulu (approx. 25 km east from the site of collection). Six blocks, each of which contained seven 20 × 26 cm quadrats separated by 30–40 cm buffer zones, were set up randomly in the field roughly 30 m apart from each other, with lichen cushions originating from the same place forming one block. Lichen cushions and the underlying soil were kept in plastic boxes with drainage holes in the bottom and embedded within soil.
The following treatments were applied in factorial combinations to lichen cushions within each block: P (one concentration), N (in two concentrations), and distilled water that represented the ‘control’ level of the nitrogen and phosphorus treatments (subsequently referred to as P: control and high; and N: control, moderate and high). In addition, a dry control (DC) that was not treated with any solution was included in each block in order to test whether the addition of water alone influenced thallus structure.
Nitrogen was applied as NH4NO3 in concentrations of 0·16 and 0·48 mm for the ‘moderate’ and ‘high’ N treatments, respectively, and P as Na2PO4.H2O at a concentration of 0·043 mm for the ‘high’ P treatment. Applications were made three times a week, and the pH of solutions ranged from 5·8 to 6·2. The concentrations were selected to yield total depositions of approx. 100 (high) mg P m−2, and 300 (moderate) and 1000 (high) mg N m−2 over 14 weeks that the experiment was carried out. The level of N deposition in the ‘high’ treatment was roughly the same magnitude as in industrialized areas in Britain where Cladina lichens are still found (see Hyvärinen and Crittenden, 1998b and references therein). In the combined N and P treatments the salts were prepared in the same solution to keep the spraying volume the same for all treatments.
The background deposition of total N during the experiment was 48·5 mg N m−2 and 51·9 mg N m−2 as collected in two rain gauges located 0·7 km south-east and 2·4 km south-west from the study site. The background deposition of P was not measured at those sites since it is generally considered to be negligible. The study site was surrounded by forests and hence protected from potential short-distance deposition of P-rich particulates (e.g. from agriculture), which are generally regarded to be the main form of P deposition (e.g. Newman, 1995).
All the quadrats except DC were sprayed with 100 mL of solution three times a week during the study period from 28 May to 3 September, 1998. Spraying took place only during natural rainfall events or early in the morning when the lichens were naturally moist after the morning dew. Precipitation in the area was 241·2 mm during the exposure period (Helminen et al., 1998), and the spray irrigation increased the amount of water by approx. 31 % in the experimental plots. A 2-week period without irrigation preceded the harvest in order to allow the surface deposits to be assimilated or washed away by rainwater.
N : P ratio
Biomass N : P ratio was calculated as a quotient of [N] and [P] at each application. The concentrations of N and P in the apices of C. stellaris were determined as described by Hyvärinen et al. (2003).
Microscopy
Microscopic examination was conducted on five C. stellaris thalli chosen randomly from each treatment in six blocks. The fourth internode from the top of each podetium was fixed in 2·5 % glutaraldehyde in phosphate buffer (pH 7), and post-fixed in 1 % buffered OsO4 (Holopainen, 1982). Samples were dehydrated in ethanol and embedded in Ladd's LX-112 resin. Semi-thin sections were cut with an Ultratome III (LKB-Producter AB, Sweden) and stained with toluidine blue. A longitudinal section through the centre of each internode was photographed (TMAX ASA 100) with a NIKON Microphot-FXA light microscope (Nikon Coorporation, Japan) at × 4 magnification.
The proportions of the lumen, inner cortex (i.e. a central cylinder of Cladina; Nash, 1996) and medulla within the secondary thallus were analysed using point frequency analysis as described in Tarhanen et al. (1997). A 10 × 15 grid with a random point within each grid cell was placed on the photograph and numbers of points hitting the target were counted. The longitudinal section size of the internode was estimated by multiplying the area of the grid cell by the number of counted points. The relative volumes of medulla, cortex and internal lumen within each thallus internode were derived from the surface area measurements on longitudinal sections. The volume density of algal cells (%) was estimated by calculating the proportion of the medullary layer volume occupied by the algal cells. The medullary layer volume consisted of the algal and fungal cells, and the interhyphal air spaces.
Ultrathin sections of the lichens were cut with an Ultracut E ultramicrotome (Reichert-Jung AG, Austria) and stained with uranyl acetate and lead citrate. The samples were examined with a JEM 12000 EX electron microscope (JEOL Ltd, Japan), and images of contacts between algal and fungal symbionts were acquired with a digital camera. The length of interface between algal and fungal cells was measured on the cell-wall surface (i.e. perimeter) of cross-sectional algal cell using NIH-Image software for Windows (Scion Corporation, Maryland, USA). The wall-to-wall interface between symbionts was expressed as a percentage of the algal cell perimeter (Lfungal wall : Lalgal cell perimeter). In addition, size and maximum and minimum diameters of the algal cells were analysed. The shape of cells was determined by comparing the ratio of maximum and minimum diameters. The mean number of algal cells examined per treatment was 369·6 (range, 286–485).
Statistical analysis
The impact of N and P application on the thallus structure in C. stellaris was tested by factorial ANOVA with randomized blocks using the statistical package SPSS (SPSS Inc, Chicago, IL, USA). The model (N + P + block) + (N × P) was fitted to the data. Whenever the influence of ‘block’ was statistically insignificant the term was dropped from the final model.
RESULTS
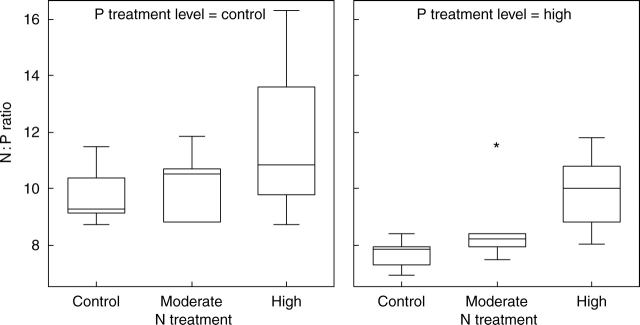
In control lichens, the average thallus N : P ratio was 10 : 1 (±1·0, s.d.) and increased with increasing N supply (F2,25 = 4·58, P < 0·05; Fig. 3). The addition of P reduced this ratio by 15–20 % in all the N treatments (F1,25 = 10·66, P < 0·01).
Fig. 3.
Box-plot showing N : P ratio in the apices of thalli of C. stellaris at the end of the experiment. The line within the box indicates the median value of the data values. The ends of the vertical lines indicate the minimum and maximum data values. Asterisk = extremes; circle = outliers.
The blocks in the experimental design did not have any effect on the structural characteristics of the thallus that were measured, and the term was therefore dropped from the models given in Tables 1, 2 and 3.
Table 1.
Factorial ANOVA for the volumes (%) of medulla, inner cortex and internal lumen in the thallus of C. stellaris
| Source of variation | d.f. | Sum of squares | Mean squares | F | P | |
|---|---|---|---|---|---|---|
| Medulla | N treatment | 2 | 0·02 | 0·01 | 2·26 | 0·118 |
| P treatment | 1 | 0·04 | 0·04 | 8·24 | 0·007 | |
| N × P | 2 | 0·02 | 0·00 | 1·75 | 0·189 | |
| Residuals | 36 | 0·16 | ||||
| Inner cortex | N treatment | 2 | 0·00 | 0·00 | 0·36 | 0·699 |
| P treatment | 1 | 0·00 | 0·00 | 0·79 | 0·380 | |
| N × P | 2 | 0·00 | 0·00 | 0·25 | 0·783 | |
| Residuals | 36 | 0·07 | 0·00 | |||
| Internal lumen | N treatment | 2 | 0·01 | 0·00 | 1·97 | 0·154 |
| P treatment | 1 | 0·06 | 0·06 | 18·57 | <0·001 | |
| N × P | 2 | 0·02 | 0·00 | 3·13 | 0·056 | |
| Residuals | 36 | 0·11 | 0·00 |
Table 2.
Factorial ANOVA for the algae volume (%) in the medulla layer of C. stellaris
| Source of variation | d.f. | Sum of squares | Mean squares | F | P |
|---|---|---|---|---|---|
| N treatment | 2 | 0·00 | 0·00 | 0·14 | 0·892 |
| P treatment | 1 | 0·09 | 0·00 | 25·96 | <0·001 |
| N × P | 2 | 0·00 | 0·00 | 0·12 | 0·883 |
| Residuals | 36 | 0·12 | 0·00 |
Table 3.
Factorial ANOVA for the wall-to-wall interface between algal and fungal cells (%) in the thallus of C. stellaris
| Source of variation | d.f. | Sum of squares | Mean squares | F | P |
|---|---|---|---|---|---|
| N treatment | 2 | 0·00 | 0·00 | 0·01 | 0·991 |
| P treatment | 1 | 0·02 | 0·02 | 9·48 | 0·004 |
| N × P | 2 | 0·00 | 0·00 | 0·21 | 0·812 |
| Residuals | 36 | 0·07 | 0·00 |
The main effect of P was observed as a significant change in the proportions of thallus volume occupied by the lumen and medulla (Table 1). The high P treatment increased the proportion of thallus volume occupied by the medullary tissue and reduced the volume density of the internal lumen (Fig. 4). In contrast to the P treatment, addition of N, particularly in the moderate N treatment, reduced the volume density of the medullary tissue and increased that of the lumen (Table 1 and Fig. 4). The lumen volume density was correlated negatively with [P] and positively with the thallus N : P ratio (Table 4). Both [N] and [P] (% of thallus dry mass) were positively correlated with the thallus volume occupied by the inner cortex (Table 4). However, the main effect of N and P treatments on the volume density of inner cortex was not statistically significant (Fig. 4 and Table 1). The total longitudinal section area of internode was positively correlated with the internal lumen volume density (%; r = 0·351, P < 0·05, n = 42). The total longitudinal section area of the thallus internode was not significantly affected by the different nutrient treatments applied (data not shown).
Fig. 4.
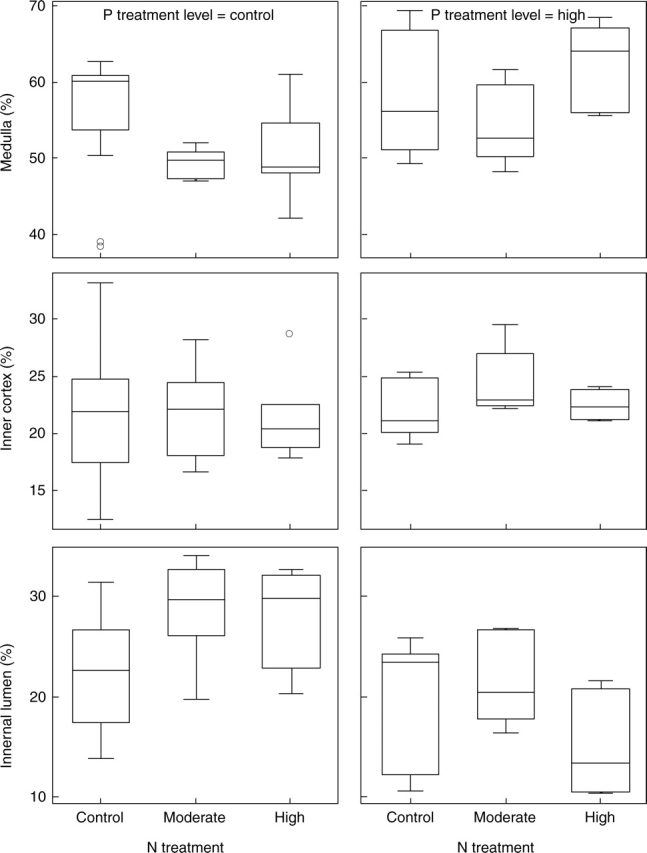
Box-plot showing the volumes (% of thallus volume) of medulla, inner cortex and internal lumen in C. stellaris after N and P treatments. The line in the box indicates the median value of the data values. The ends of the vertical lines indicate the minimum and maximum data values. Asterisk = extremes; circle = outliers.
Table 4.
Correlation matrix of thallus morphological characteristics, nitrogen and phosphorus concentrations, and N : P ratio in C. stellaris
| [N] (% of thallus d. wt) | [P] (% of thallus d. wt) | N : P ratio | |
|---|---|---|---|
| n | 32 | 33 | 31 |
| [P] (% of thallus d. wt) | 0·640*** | ||
| N : P ratio | −0·094 | −0·789*** | |
| Medulla (% of thallus internode) | −0·036 | 0·170 | −0·211 |
| Fungal volume (% of medulla) | 0·024 | 0·305° | −0·348º |
| Algal volume (% of medulla) | 0·066 | −0·173 | 0·180 |
| Inner cortex (% of thallus internode) | 0·376* | 0·369* | −0·261 |
| Lumen (% of thallus internode) | −0·154 | −0·409* | 0·360* |
| Wall-to-wall interface between symbionts (%) | −0·229 | −0·170 | 0·102 |
°P < 0·10, *P < 0·05, **P < 0·01, ***P < 0·001·
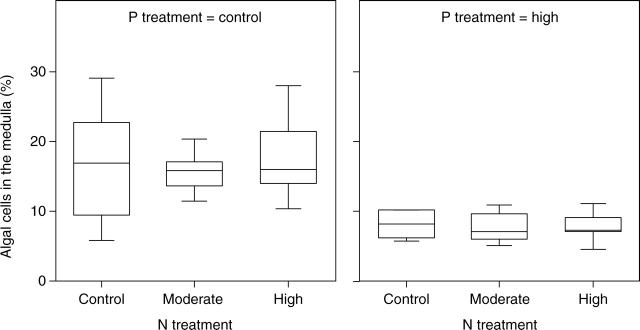
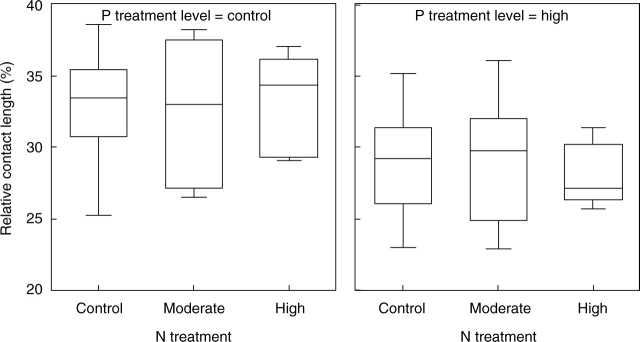
The proportion of medullary layer volume occupied by the algal cells (% of medulla) was significantly affected by P (Table 2). The high P treatment reduced the volume density of the algal cells (Fig. 5) which varied from 5 to 29 % in the low P treatment, and from 5 to 11 % in the high P treatment. Addition of P also significantly reduced the wall-to-wall interface between algal and fungal cells (Table 3 and Fig. 6). The volume density of the algal cells and wall-to-wall contact between symbionts were not significantly affected by the N supply (Table 2), nor were there significant changes in the size and shape of algal cells in any of the fertilization treatments (data not shown).
Fig. 5.
Box-plot of relative algal cell volume (% of medullary layer volume) after N and P treatments. The line in the box indicates the median value of the data values. The ends of the vertical lines indicate the minimum and maximum data values.
Fig. 6.
Box-plot showing the relative cover of fungal hyphae on the algal cell wall (% algal cell perimeter) after N and P treatments. The line in the box indicates the median value of the data values. The ends of the vertical lines indicate the minimum and maximum data values.
DISCUSSION
The present study revealed that moderately enhanced availability of the macronutrients N and P affects the structure of podetia in C. stellaris. Enhancements of N and P, when given independently, caused changes in the thallus volumes occupied by the internal lumen and the medulla, in both high P and moderate N treatments. Moreover, it appears that the influence of P fertilization on the lichen thallus structure was stronger than that for N fertilization. Given the fact that both N and P treatments moderately increased the total concentrations of those elements in the thalli (Hyvärinen et al., 2003), it can be speculated that thallus anatomy may change due to the nutrient content of the lichen rather than nutrient availability.
The N uptake efficiency varies depending on the N economy of the lichen as well as the mode of uptake of N into the thallus. In general, when N is received in ionic form in low concentrations, either from natural rainfall (Hyvärinen and Crittenden, 1998b) or applied solutions (Crittenden, 1996), the estimated uptake efficiency of non-N-fixing lichens has ranged between 90–100 %. The uptake efficiency of NO3− and NH4+ may be considerably lower in N-fixing lichens. For example, Dahlman et al. (2002) estimated that only 2–27 % of N supplied as aqueous NH4+ or NO3− solution to Peltigera aphthosa and Nephroma arcticum could be taken up by the lichen thalli.
There are few estimates of P uptake efficiency in lichens. Farrar (1976) demonstrated that Hypogymnia physodes can rapidly absorb P from bathing solutions containing PO43− in concentrations typical of rainfall (Newman, 1995). In addition, Hyvärinen and Crittenden (1998a) measured PO43− uptake efficiency of Cladina portentosa in a spray chamber (lysimeter). In their experiment, the lichen mat was able to scavenge approx. 80 % of P supplied at a concentration of 30 µg P L−1 and at a rate of approx. 100 mL h−1. In our study the P concentration of the solution applied was considerably higher (0·043 mm, i.e. 1·33 mg P L−1) and therefore it is difficult to estimate how much of the P would have been immediately absorbed. Nevertheless, the increase in P concentration was statistically significant (Hyvärinen et al., 2003), confirming that a great proportion of the P was scavenged by C. stellaris.
One problem commonly faced with studies involving fertilization of lichens is the adverse effects of ammonium ions. Toxicity of ammonium ions to ATP formation in chloroplasts and mitochondria is well known in the higher plants (e.g. Salisbury and Ross, 1992). Because ion uptake occurs over the entire thallus surface of lichens, it may be assumed that they could be more susceptible to harmful effects of ions than vascular plants. However, such toxic effects of NH4+ usually occur only at concentrations beyond the levels used in our study. For example, Gaio-Oliveira et al. (2004) studied the effect of ammonium ion addition on Xanthoria parietina. They concluded that ammonium ions resulted in toxic effects on the mycobiont and photobiont at different threshold concentrations (0·34 and 0·69 m NH4+, respectively), the latter being more tolerant to ammonium ions. In terms of N, these concentrations are approx. 350–700 times higher than the highest value used in the present study (including background deposition; see Methods).
It should be noted that the relative growth rate of lichens in terms of dry mass was not significantly promoted by addition of N and P (Hyvärinen et al., 2003). Given a weak positive relationship between the total internode longitudinal sectional area and the thallus volume occupied by the lumen, the lumen volume density gain may indicate an increase in radial growth and lichen area (i.e. cover) expansion. Data on lichens with green algal symbionts such as C. stellaris are lacking, but it has been recently shown with nitrogen-fixing lichens (i.e. Nephroma arcticum and Peltigera aphthosa) that lichen expansion is limited by thallus N status (Sundberg et al., 2001; Dahlman et al., 2002), while lichen weight gain was mainly affected by irradiance and carbon assimilation, and not by N supply (Sundberg et al., 2001).
The low volume density of the algal cells (% of medulla) together with the increased thallus volume occupied by medullary tissue after the high P treatment might indicate an increase in fungal hyphal growth in relation to the number of algal cells. The reduced wall-to-wall interface between the algal and fungal cells also seems to point to enhanced fungal growth. Studies on ectomycorrhizal associations have shown that additional P application to the hyphal compartment stimulates external fungal hyphal growth, simultaneously improving the nutrient status of host plant (Jentschke et al., 2001). In this study, P enrichment may have resulted in nutrient imbalances in the lichen that led to enhanced hyphal production in the medullary layer. The reduced proportion of thallus volume occupied by algal cells might also be explained either by the lack of growth stimulation or impaired algal cell division due to excess P. Data for lichen are lacking, but Martínez-Abaigar et al. (2002) found in a laboratory study that high tissue P concentration in liverwort (Jungermannia exsertifolia) was capable of reducing net photosynthesis and increasing the proportion of carotenoids to chlorophylls, indicating P toxicity in the bryophyte.
It has been shown previously that P addition increases the concentration of usnic acid in C. stellaris but that the increase was less evident in moderate N + P treatments (Hyvärinen et al., 2003). The enhanced usnic acid level might be associated with increased fungal hyphal growth in the medulla because usnic acid mainly occurs on the surface of fungal hyphae. The P-induced elevation in the usnic acid concentration may also explain the low medullary layer volume occupied by the algal cells. Bačkor et al. (1998) demonstrated that usnic acid inhibited the growth of the photobiont Trebouxia irregularis isolated from Cladina mitis. By increasing hyphal growth and secondary metabolite production, the fungal partner probably inhibits the cell division of the algal partner.
Knowledge is still limited about the habitat factors or environmental conditions that affect the symbiotic interaction between algae and fungi in relation to lichen growth. Sun and Friedmann (2005) found a positive relationship between alga-to-fungus ratio and habitat summer temperature in Cladina rangiferina. They suggested that regulation of the ratio of producer (alga) to consumer (fungus) directly contributes to adaptation to a wide range of thermal regimes and to the distribution of lichens. The differential responses of fungal and algal growth to N and P fertilization observed in the present study suggest that the tissue nutrient content, and particularly the nutrient balance, affect resource allocation in the lichen thallus. In aquatic ecosystems (Sridhar and Bärlocher, 2000; Fong et al., 2004), as well as in isolated lichen symbionts (Crittenden et al., 1994), the growth of free-living algal and fungal cells have been shown to be N- and P-limited. In the lichen symbiosis, the fungal partner primarily determines thallus form and the structural characters of tissue layers, but the algal partner may also influence it (Nash, 1996). The balance between N and P supply, as reflected in N : P ratios in plant biomass, has been used mainly to assess whether N or P is more limiting for biomass production. Variation in [N] is often more important in determining the N : P ratios of bryophytes or lichens (Güsewell, 2004). In this short-term experiment, addition of P clearly reduced the N : P ratios. The negative effect of P on the algal partner suggests that algal growth was N-limited even though the N concentration of the thalli increased.
ACKNOWLEDGEMENTS
We are grateful to Dr P. D. Crittenden and two anonymous reviewers for constructive comments on the manuscript. We are also indebted to the staff of the Botanical Gardens of the University of Oulu for their help in setting up the field experiment. The study was supported by the Academy of Finland (projects 40951 and 43004).
LITERATURE CITED
- Ahti T, Oksanen J. Epigeic lichen communities of taiga and tundra regions. Vegetatio. 1990;86:39–70. [Google Scholar]
- Bačkor M, Hudak J, Repcak M, Ziegler W, Bačkorova M. The influence of pH and lichen metabolites (vulpinic acid and (+) usnic acid) on the growth of the lichen photobiont. Trebouxia irregularis. Lichenologist. 1998;30:577–582. [Google Scholar]
- Crittenden PD. The effect of oxygen deprivation on inorganic nitrogen uptake in an Antarctic macrolichen. Lichenologist. 1996;28:347–354. [Google Scholar]
- Crittenden PD, Kałucka I, Oliver E. Does nitrogen supply limit the growth of lichens? Cryptogamic Botany. 1994;4:143–155. [Google Scholar]
- Dahlman L, Näsholm T, Palmqvist K. Growth, nitrogen uptake, and resource allocation in the two tripartite lichens Nephroma arcticum and Peltigera aphthosa during nitrogen stress. New Phytologist. 2002;153:307–315. [Google Scholar]
- Dahlman L, Persson J, Näsholm T, Palmqvist K. Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta. 2003;217:41–48. doi: 10.1007/s00425-003-0977-8. [DOI] [PubMed] [Google Scholar]
- Ellis CJ, Crittenden PD, Scrimgeour CM, Ashcroft CJ. Translocation of 15N indicates nitrogen recycling in the mat-forming lichen. Cladonia portentosa. New Phytologist. 2005;168:423–434. doi: 10.1111/j.1469-8137.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- Farrar JF. The uptake and metabolism of phosphate by the lichen Hypogymnia physodes. New Phytologist. 1976;77:127–134. [Google Scholar]
- Fong P, Fong JJ, Fong CR. Growth, nutrient storage, and release of dissolved organic nitrogen by Enteromorpha intestinalis in response to pulses of nitrogen and phosphorus. Aquatic Botany. 2004;78:83–95. [Google Scholar]
- Gaio-Oliveira G, Dahlman L, Plamqvist K, Máguas C. Ammonium uptake in the nitrophytic lichen Xanthoria parietina and its effects on vitality and balance between symbionts. Lichenologist. 2004;36:75–86. [Google Scholar]
- Güsewell S. N : P ratios in terrestrial plants: variation and functional significance. New Phytologist. 2004;164:243–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- Helminen J, Nordlund A, Karlsson P. Ilmastokatsaus 12/98. Finnish Meteorological Institute; 1998. [Google Scholar]
- Holopainen T. Summer versus winter condition of the ultrastructure of the epiphytic lichens Bryoria capillaris and Hypogymnia physodes in central Finland. Annales Botanici Fennici. 1982;19:39–52. [Google Scholar]
- Hyvärinen M, Crittenden PD. Phosphate uptake in. Cladonia portentosa. Lichenologist. 1998a;30:297–301. [Google Scholar]
- Hyvärinen M, Crittenden PD. Relationships between atmospheric nitrogen inputs and the vertical nitrogen and phosphorus concentration gradients in the lichen. Cladonia portentosa. New Phytologist. 1998b;140:519–530. doi: 10.1111/j.1469-8137.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Hyvärinen M, Crittenden PD. 33P translocation in the thallus of the mat-forming lichen. Cladonia portentosa. New Phytologist. 2000;145:281–288. [Google Scholar]
- Hyvärinen M, Walter B, Koopmann R. Impact of fertilisation on phenol content and growth rate of Cladina stellaris: a test of the carbon-nutrient balance hypothesis. Oecologia. 2003;134:176–181. doi: 10.1007/s00442-002-1105-3. [DOI] [PubMed] [Google Scholar]
- Jentschke G, Brandes B, Kuhn AJ, Schröder WH, Godbold DL. Interdependence of phosphorus,nitrogen, potassium and magnesium translocation by the ectomycorrhizal fungus. Paxillus involutus. New Phytologist. 2001;149:327–337. doi: 10.1046/j.1469-8137.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- Kauppi M. The influence of nitrogen-rich pollution components on lichens. Acta Universitatis Ouluensis. 1980;A101(9):1–25. [Google Scholar]
- Kurina LM, Vitousek PM. Controls over the accumulation and decline of a nitrogen-fixing lichen, Stereocaulon vulcani, on young Hawaiian lava flows. Journal of Ecology. 1999;87:784–799. [Google Scholar]
- Kytöviita M-M. Effects of acid rain on growth and nutrient relations in mat-forming lichens. UK: University of Nottingham; 1993. PhD thesis UK. [Google Scholar]
- Longton RE. The biology of polar bryophytes and lichens. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Martínez-Abaigar J, Nunez-Olivera E, Beaucourt N. Short-term physiological responses of the aquatic liverwort Jungermannia excertifolia subsp. cordifolia to KH2PO4 and anoxia. The Bryologist. 2002;105:86–95. [Google Scholar]
- Nash TH., III . Lichen biology. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Newman EI. Phosphorus inputs to terrestrial ecosystems. Journal of Ecology. 1995;83:713–726. [Google Scholar]
- Palmqvist K, Dahlman L. Responses of the green algal foliose lichen Platismatia glauca to increased nitrogen supply. New Phytologist. 2006;171:343–356. doi: 10.1111/j.1469-8137.2006.01754.x. [DOI] [PubMed] [Google Scholar]
- Roy-Arcand L, Delisle CE, Briére FG. Effects of simulated acid precipitation on the metabolic activity of Cladina stellaris. Canadian Journal of Botany. 1989;67:1796–1802. [Google Scholar]
- Salisbury FB, Ross CW. Plant physiology. Belmont: Wadsworth; 1992. (CA). [Google Scholar]
- Smith EC, Griffiths H. The occurrence of the chloroplast pyrenoid is correlated with the activity of a CO2 concentrating mechanism and carbon isotope discrimination in lichens and bryophytes. Planta. 1969;198:6–16. [Google Scholar]
- Sridhar KR, Bärlocher F. Initial colonization, nutrient supply, and fungal activity on leaves decaying in streams. Applied and Environmental Microbiology. 2000;66:1114–1119. doi: 10.1128/aem.66.3.1114-1119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Näsholm T, Palmqvist K. The effect of nitrogen on growth and key thallus components in the two tripartite lichens, Nephroma arcticum and Peltigera aphthosa. Plant, Cell and Environment. 2001;24:517–527. [Google Scholar]
- Sun HJ, Friedmann EI. Communities adjust their temperature optima by shifting producer-to-consumer ratio, shown in lichens as models: II: experimental verification. Microbial Ecology. 2005;49:528–535. doi: 10.1007/s00248-005-3679-x. [DOI] [PubMed] [Google Scholar]
- Tarhanen S, Holopainen T, Oksanen J. Ultrastructural changes and electrolyte leakage from ozone fumigated epiphytic lichens. Annals of Botany. 1997;80:611–621. [Google Scholar]
- Wetmore CM. Multiperforate septa in lichens. New Phytologist. 1973;72:535–538. [Google Scholar]