Abstract
Background and Aims
Intra-specific variation in nectar chemistry under natural conditions has been only rarely explored, yet it is an essential aspect of our understanding of how pollinator-mediated selection might act on nectar traits. This paper examines intra-specific variation in nectar sugar composition in field and glasshouse plants of the bumblebee-pollinated perennial herbs Aquilegia vulgaris subsp. vulgaris and Aquilegia pyrenaica subsp. cazorlensis (Ranunculaceae). The aims of the study are to assess the generality of extreme intra-plant variation in nectar sugar composition recently reported for other species in the field, and gaining insight on the possible mechanisms involved.
Methods
The proportions of glucose, fructose and sucrose in single-nectary nectar samples collected from field and glasshouse plants were determined using high performance liquid chromatography. A hierarchical variance partition was used to dissect total variance into components due to variation among plants, flowers within plants, and nectaries within flowers.
Key Results
Nectar of the two species was mostly sucrose-dominated, but composition varied widely in the field, ranging from sucrose-only to fructose-dominated. Most intra-specific variance was due to differences among nectaries of the same flower, and flowers of the same plant. The high intra-plant variation in sugar composition exhibited by field plants vanished in the glasshouse, where nectar composition emerged as a remarkably constant feature across plants, flowers and nectaries.
Conclusions
In addition to corroborating the results of previous studies documenting extreme intra-plant variation in nectar sugar composition in the field, this study suggests that such variation may ultimately be caused by biotic factors operating on the nectar in the field but not in the glasshouse. Pollinator visitation and pollinator-borne yeasts are suggested as likely causal agents.
Key words: Abiotic environment, Aquilegia pyrenaica subsp. cazorlensis, Aquilegia vulgaris subsp. vulgaris, biotic factors, field conditions, glasshouse, Iberian Peninsula, inter- and intra-specific variation, nectar-sugar composition, nectary, variance components
INTRODUCTION
Nectar represents a key link between insect-pollinated plants and their pollinators (Simpson and Neff, 1983). The frequency and duration of pollinator visits to nectariferous flowers depend on nectar production rate (Biernaskie et al., 2002; Shafir et al., 2003; Nicolson and Nepi, 2005) and chemical composition, including the type and relative amounts of sugars, amino acids and lipids (Baker and Baker, 1983a, 1986; Bernardello et al., 1999). Specifically, variation in nectar sugars, which are the dominant constituents of most nectars, has been thoroughly investigated in relation to pollinator assemblages in angiosperms (e.g. Baker and Baker 1983b; Baker et al., 1998; Galetto and Bernardello, 2003; Dupont et al., 2004; Jürgens, 2004; among many others). The vast majority of studies on nectar sugar composition have traditionally focused on comparisons at the species level or above (for a recent review and historical background, see Herrera et al., 2006). A handful of studies, however, have revealed that nectar chemistry, including sugar proportions, may differ among individuals, populations, cultivars or subspecies of the same species (Baker and Baker, 1983b; Severson and Erickson, 1984; Freeman et al., 1985; Reid et al., 1985; Freeman and Wilken, 1987; Gottsberger et al., 1989; Lanza et al., 1995; Witt et al., 1999; Roldán-Serrano and Guerra-Sanz, 2004). Furthermore, intra-plant variation in nectar sugar composition may also be extensive (Freeman and Wilken, 1987; Davis et al., 1998; Langenberger and Davis, 2002; Herrera et al., 2006), but this level of intra-specific variation has been investigated even less often than variation among individuals or populations.
A detailed knowledge of the proportions of total intra-specific variance in nectar composition due to variation among individuals and to smaller-scale variation occurring within plants under natural conditions (i.e. among flowers and among separate nectaries within a flower) is crucial to our understanding of how pollinator-mediated selection might act on nectar traits. Only one previous investigation seems to have dissected intra-specific variation in nectar chemistry occurring in nature from this latter perspective. Herrera et al. (2006) described variation in nectar sugar composition in a population of the perennial herb Helleborus foetidus (Ranunculaceae) in south-eastern Spain, and dissected it into components due to variation among plants, flowers of the same plant, and nectaries of the same flower. That study revealed that population-wide variance was mainly accounted for by variation among flowers of the same plant (56 % of total) and nectaries of the same flower (30 %) and only minimally by differences among plants (14 %). Such a high variance in nectar sugar composition at the among- and within-plant levels occurring under natural conditions may reflect intrinsic plant features, external factors, or some combination of these. Nevertheless, no attempt was made by Herrera et al. (2006) to evaluate the possible causes of intra-plant nectar variation observed in the field.
In this paper, a sampling and analytical approach similar to that of Herrera et al. (2006) is adopted to dissect intra-specific variation in nectar sugar composition in the insect-pollinated perennial herbs Aquilegia vulgaris subsp. vulgaris and Aquilegia pyrenaica subsp. cazorlensis (Ranunculaceae), using plants growing in the field and in the glasshouse. Each Aquilegia flower bears five independent, separate nectaries. Using individual nectaries as the sampling units for nectar composition analyses, variation in nectar sugar composition is quantified at the among- and within-plant levels. By obtaining separate nectar samples from different nectaries of the same flower, it is possible to extend the partition of within-plant variance in nectar composition down to the within-flower level. In addition, a comparison of the patterns of intra-specific variation in nectar sugar composition exhibited by plants, growing under natural conditions and in a controlled glasshouse environment, is undertaken. That comparison may help to suggest possible causes of observed variation in the field. If intrinsic plant features were ultimately shaping patterns of natural variation in nectar sugar composition, by comparing them with samples obtained from plants growing under homogeneous glasshouse conditions and excluded from pollinators, a similar pattern of variation should be obtained. On the contrary, if some external factor was involved in the natural variation in nectar sugar composition, significant differences in nectar composition between field and glasshouse samples would be expected. As far as is known, only Freeman and Wilken (1987) in their study on Ipomopsis longiflora (Polemoniaceae) have compared intra-plant variation in nectar sugar composition under field and glasshouse conditions.
The objective of this paper is 2-fold: (1) to assess whether the extreme intra-plant variation in nectar sugar composition described by Herrera et al. (2006) for Helleborus foetidus is a more general phenomenon occurring also in other Ranunculaceae species; and (2) by concurrently examining patterns of variation under field and glasshouse conditions, to obtain some insight on the possible mechanisms underlying the extensive variation in nectar sugar composition exhibited by plants in the field.
MATERIALS AND METHODS
Study species
Aquilegia pyrenaica subsp. cazorlensis (Heywood) Galiano and Rivas-Martínez (A. p. cazorlensis hereafter) and A. vulgaris subsp. vulgaris L. (A. vulgaris hereafter) are perennial herbs of the Ranunculaceae family. A. vulgaris is widely distributed throughout Eurasian mountain forests, and it sometimes occurs in open woodlands and meadows at or around sea level. In the area studied, A. vulgaris grows along stream margins or poorly drained open meadows around springs at 900–1700 m a.s.l. A. p. cazorlensis is a narrow endemic restricted to a few populations in the Sierras de Cazorla and El Pozo in the Spanish province of Jaén, occurring at 1200–1950 m a.s.l. A. p. cazorlensis grows in rifts of limestone outcrops and on sandy soils in shady, damp sites at cliff bases. The flowering periods of the two species differ, with A. vulgaris flowering from May to early June, and A. p. cazorlensis from June to early July. Plants produce one or a few inflorescences, each bearing 1–13 (A. vulgaris) and 1–8 (A. p. cazorlensis) showy flowers ranging from pale blue to purple. Flowers consist of five petaloid sepals, five petals elongated into nectar-producing spurs, 45–60 (A. vulgaris) or 40–55 (A. p. cazorlensis) stamens, and 5–10 (A. vulgaris) or 4–6 (A. p. cazorlensis) carpels. Flower lifespan in the field is slightly shorter in A. vulgaris (4–6 d) than in A. p. cazorlensis (6–8 d). Flowers of both species are protandrous, spending approx. 1–2 d in male phase (anthers releasing pollen), approx. 3–4 d in hermaphrodite phase (stigmas visible while anthers still shedding pollen), and approx. 1–2 d in female phase (stigmas receptive, anthers no longer releasing pollen) before petals wither. The main pollinators for both species are bumblebees (Bombus pascuorum, B. pratorum, B. terrestris). Further details on these species may be found in Medrano et al. (2006).
Study sites and field methods
The field part of this study was carried out during May–July 2005 on two populations of A. p. cazorlensis and A. vulgaris located at the Barranco del Escalón and Barranco del Guadalentín, respectively, in the Parque Natural Sierras de Cazorla-Segura-Las Villas, Jaén province, south-eastern Spain. Glasshouse populations of A. p. cazorlensis and A. vulgaris were established during 2004–2006 from seeds collected from wild plants growing at Barranco del Guadalentín (A. vulgaris) and near Barranco del Escalón (A. p. cazorlensis). Glasshouse plants were cultivated in flowerpots with similar soil mixture (75 % peat moss, 25 % vermiculite), watering (three times per week), light (natural daylight), temperature (approx. 27 ºC) and air humidity (approx. 46 %). Insect pollinators and herbivores were excluded from glasshouse plants for the whole study.
Samples of nectar were collected from field plants during the flowering peak of each population on typical spring days, i.e. sunny days with approx. 28 ºC ambient temperature, approx. 40 % air humidity. Eight plants bearing inflorescences were randomly selected in each population, and three recently open flowers were chosen in each plant for nectar sampling. To ensure consistency in posterior nectar comparisons only male-phase flowers (1–2 d old) were chosen. In these flowers, pollinator access to nectaries was precluded by placing small cotton plugs at spur entrances. Plugs were kept in place for 24 h, and nectar was collected at the end of the exclusion period. Two spurs were randomly selected per flower, split longitudinally, and the nectar present was immediately absorbed onto 10 × 2 mm wicks paper (Whatman 3MM), separately for each nectary. Wicks were individually placed into small clean envelopes, stored in plastic bags containing silica gel, and kept at room temperature. The same sampling protocol was used when sampling nectar from the flowers of the glasshouse plants, with the exception that in this case nectar was sampled from three spurs per flower and two male-phase flowers per plant. In the peak flowering period, eight plants of A. p. cazorlensis were sampled for nectar in the glasshouse. Unfortunately, only one plant of A. vulgaris survived to maturity and flowered in the glasshouse.
Nectar analysis
Nectar-containing wicks were individually placed into 2-mL Eppendorf tubes, and 500 μL of high performance liquid chromatography (HPLC)-grade water were added to each one. Wicks were kept soaking during 24 h at 4 ºC. Two microlitres were taken from each tube and placed into a new Eppendorf tube containing 198 µL of HPLC-grade water. Then each tube-nectar solution was measured independently twice. For each measurement, 5 µL of solution was filtered through a 0·4-μm polyvinylidenedifluoride filter (Análisis Vínicos SL, Tomelloso, Spain) and injected into a Dionex DX 500 HPLC system (Dionex, Sunnyvale, CA, USA).
The HPLC system was equipped with an eluent degas module, a GP 40 gradient pump, a guard column CarboPac PA10 (4×50 mm) and an analytical column CarboPac PA10 (4×250 mm), as well as an ED 40 electrochemical detector for pulsed amperometric detection in integrated amperometric mode, with the normal preloaded wave form for sugar detection (Dionex, 1994). The output range of the detector was set to 100 nC. The column was eluted (flow rate 1 mL min–1) isocratically with 40 mm NaOH (50 % solution obtained from J. T. Baker, Deventer, The Netherlands) and kept at 24 ºC during analysis. Retention times were calibrated daily for d-glucose, d-fructose and sucrose (Sigma-Aldrich, Madrid, Spain) by injecting 10 µL of a calibration mixture containing 5·5 mg L−1, 13·75 mg L−1 and 13·75 mg L−1 of these sugars, respectively. The proportions of the three different sugars (glucose, fructose, sucrose) in each sample analysed were estimated by integrating the area under the chromatogram peaks. Only sucrose, glucose and fructose appeared in all samples.
Statistical analyses
Data were analysed using SAS statistical software (SAS Institute, Cary, NC, USA). Differences between species and between growing conditions (field and glasshouse) in the percentage of each sugar in the nectar were analysed with a fully nested hierarchical ANOVA as implemented in the MIXED procedure with the restricted maximum likelihood method (REML) (Littell et al., 1996). The two replicate measurements obtained per nectar sample allowed for estimation of measurement error and for assessing the statistical significance of the within-flower, among-nectary component of variation. Variance components in the percentages of glucose, fructose and sucrose in nectar were calculated separately for each species and growing condition using the VARCOMP procedure and the REML method. A hierarchical variance partition was used to dissect total variance into components due to variation among plants, flowers within plant, and nectaries within flower. Statistical significance was estimated with the RAMDOM statement of the GLM procedure, which produces an unbiased F-test for each hierarchical level. The among-plant component could not be estimated for A. vulgaris in the glasshouse, but components due to variation among flowers and among nectaries within flower were estimated for the single plant available.
RESULTS
Summary statistics for the variation among individual nectaries in nectar sugar proportions were based on a single set of values for sugar proportions for each nectary, obtained by averaging the two replicate measurements (Table 1). On average, nectar samples obtained from field plants were sucrose-dominated, both in A. p. cazorlensis and A. vulgaris. Nevertheless, there were significant interspecific differences in percentage sucrose (t9 = 7·4, P = 0·02, n = 94), glucose (t92 = 5·1, P = 0·04, n = 94) and fructose (t92 = 7·1, P = 0·02, n = 94). Under field conditions, the nectar of A. p. cazorlensis contained proportionally more sucrose, on average, and was lower in glucose and fructose than the nectar of A. vulgaris (Table 1).
Table 1.
Summary statistics for the relative amounts of individual sugars in single-nectary nectar samples of the two Aquilegia species under field and glasshouse conditions
| Aquilegia p. cazorlensis | Aquilegia vulgaris | |||
|---|---|---|---|---|
| Sugar | Field | Glasshouse | Field | Glasshouse |
| Glucose (%) | ||||
| Mean ± s.d. | 2·4 ± 2·7 | 1 ± 0·5 | 3·6 ± 2·4 | 1·1 ± 0·5 |
| Range | 0–13·5 | 0·4–2·9 | 0–9·5 | 0·4–2·5 |
| Interquartile range | 0·7–3·1 | 0·6–1·3 | 1·9–5·4 | 0·7–1·3 |
| Fructose (%) | ||||
| Mean ± s.d. | 10·9 ± 15·4 | 0·6 ± 0·6 | 20·5 ± 19·3 | 1·1 ± 0·4 |
| Range | 0–60·6 | 0–2·3 | 0–78·5 | 0·3–3 |
| Interquartile range | 1·3–10·9 | 0·2–0·7 | 6·6–27·1 | 0·6–1·4 |
| Sucrose (%) | ||||
| Mean ± s.d. | 86·6 ± 17·1 | 98·4 ± 0·86 | 75·9 ± 21·2 | 97·9 ± 0·9 |
| Range | 35·3–100 | 94·9–99·6 | 11·9–100 | 94·5–99·3 |
| Interquartile range | 84·1–97·9 | 98·1–98·9 | 69·4–91·7 | 97·4–98 |
Aquilegia p. cazorlensis: field, n = 48 nectaries from 24 flowers and 8 plants; glasshouse, n = 48 nectaries from 16 flowers and 8 plants. A. vulgaris: field, n = 46 nectaries from 24 flowers and 8 plants; glasshouse n = 39 nectaries from 13 flowers and 1 plant.
Within species, mean nectar sugar composition differed significantly between field and glasshouse plants (Table 1). In A. p. cazorlensis, the nectar of glasshouse-grown plants contained significantly more sucrose (t88 = 9·6, P = 0·009, n = 90) and less fructose (t88 = 9·9, P = 0·008, n = 90) and glucose (t88 = 4·7, P = 0·05, n = 90) than the nectar of field plants. A similar pattern was shown by A. vulgaris, although the glasshouse data were obtained from a single plant. On average, nectar from glasshouse samples contained much more sucrose (t83 = 7·12, P = 0·0001, n = 85), slightly less glucose (t83 = 3·28, P = 0·002, n = 85) and considerably less fructose (t83 = 6·68, P = 0·0001, n = 85) than the nectar from field plants.
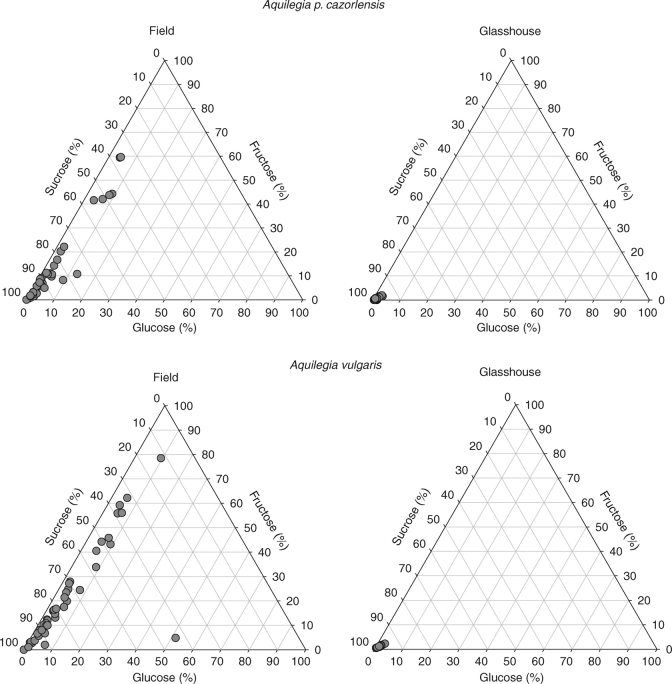
Mean figures describe the central tendency in the ‘population’ of nectaries sampled. There was, however, extreme variation around these figures in both species, as revealed by the broad ranges and interquartile ranges of the relative amounts of different sugars in single-nectary samples (Table 1). Intra-specific variation is also illustrated in Fig. 1. Within each species, the nectar varied from pure or nearly pure sucrose solutions in some nectaries to fructose-dominated mixtures in others (Table 1 and Fig. 1). Glucose was in all cases a minor nectar component. Hierarchical variance partitions for individual sugars showed similar sources of variation in nectar composition for A. p. cazorlensis and A. vulgaris (Table 2). In general, the two main sources of variance in relative amounts of glucose, fructose and sucrose in nectar were the variation occurring among flowers within plants, and among nectaries within individual flowers. The among-plant component of variation in nectar sugar composition did not differ significantly from zero for any individual sugar in either A. p. cazorlensis or A. vulgaris (Table 2), thus reinforcing the conclusion that variation within plants was by far the main source of variation in nectar sugar composition in the two species.
Fig. 1.
Ternary diagrams illustrating intra-specific variation in sugar nectar composition in field- and glasshouse-grown plants of two Aquilegia species. Each point depicts the proportional sugar composition of the nectar from a single nectary. The distance of a point from a side of the triangle is proportional to the relative importance of that sugar in the sample.
Table 2.
Hierarchical variance partition of the main nectar sugars of Aquilegia
| Aquilegia p. cazorlensis | Aquilegia vulgaris | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field | Glasshouse | Field | Glasshouse | |||||||||
| Significance | Significance | Significance | Significance | |||||||||
| F | P | Variance | F | P | Variance | F | P | Variance | F | P | Variance | |
| Glucose (%) | ||||||||||||
| Plant | 1·7 | 0·20 | 19·0 | 2·9 | 0·11 | 64·7 | 0·3 | 0·92 | 0 | — | — | — |
| Flower within plant | 9·6 | <0·0001 | 78·0 | 2·0 | 0·08 | 27·0 | 26 | <0·0001 | 97·5 | 3·0 | 0·01 | 43·2 |
| Nectary within flower | 2·7 | 0·07 | 2·9 | 0·3 | 0·73 | 8·3 | 3·7 | 0·03 | 2·5 | 11·2 | <0·0001 | 56·8 |
| Fructose (%) | ||||||||||||
| Plant | 1·1 | 0·41 | 3·9 | 3·1 | 0·10 | 52·3 | 0·4 | 0·92 | 0 | — | — | — |
| Flower within plant | 15·6 | <0·0001 | 81·1 | 2·0 | 0·09 | 22·9 | 13·2 | <0·0001 | 84·7 | 4·8 | 0·0005 | 58·5 |
| Nectary within flower | 15·1 | <0·0001 | 15·0 | 0·5 | 0·60 | 24·8 | 5·2 | 0·01 | 15·3 | 10·5 | <0·0001 | 41·5 |
| Sucrose (%) | ||||||||||||
| Plant | 1·2 | 0·36 | 6·4 | 3·2 | 0·09 | 55·5 | 0·3 | 0·94 | 0 | — | — | — |
| Flower within plant | 16·3 | <0·0001 | 81·6 | 2·1 | 0·07 | 23·1 | 14·9 | <0·0001 | 86·1 | 3·0 | 0·03 | 50·7 |
| Nectary within flower | 11·8 | <0·0001 | 12·1 | 0·5 | 0·60 | 21·4 | 5·4 | 0·01 | 13·9 | 12·5 | <0·0001 | 49·3 |
The percentages of variance into the hierarchical context of analysis, together with their statistical significances, both field and glasshouse conditions, are shown for the two species.
Patterns of intra-specific variance in sugar proportions differed markedly between growing conditions in the two species. Glasshouse plants exhibited considerably smaller variation in nectar sugar composition than field ones, as revealed by the much narrower ranges and interquartile ranges of the former group (Table 1) and the contrasting spread of points in Fig. 1. Hierarchical patterns of variance differed also between field and glasshouse plants. In glasshouse-grown A. p. cazorlensis, the variance components of glucose, fructose and sucrose proportions did not differ significantly from zero at any hierarchical level (plant, flower within plant, nectary within flower). In contrast, in field plants the among-flower and among-nectary variance components for glucose (78 %), fructose (81 %) and sucrose (82 %) were large and statistically significant (Table 2). In the single glasshouse-grown plant of A. vulgaris, variance in nectar sugar composition was nearly equally accounted for by variation among flowers and nectaries within flowers (Table 2).
DISCUSSION
Flowers of A. p. cazorlensis and A. vulgaris are very similar morphologically and, despite the contrasting habitats of the two species, are visited and pollinated by the same pollinators, almost exclusively bumblebees (Medrano et al., 2006). The similarity in sugar composition of the nectar of the two species revealed by the present study is therefore consistent with expectations relating pollinator type and nectar composition. In spite of variation in nectar sugar composition, nectaries of both species tended to produce sucrose-dominated nectar, which has been associated with flowers pollinated by hummingbirds or long-tongued insects (Baker, 1975; Freeman et al., 1984). Furthermore, the finding that the nectar of the two species was predominantly made up of sucrose corroborates Percival's report (Percival, 1961) for the nectar of A. vulgaris in England, and is also consistent with her pioneering suggestions that sucrose-dominated nectars tend to be associated with long-tubed flowers having protected nectars and with bumblebee pollination. The nectar of A. p. cazorlensis and A. vulgaris differed significantly in the proportions of major sugars, with that of A. p. cazorlensis being higher in sucrose and lower in fructose than that of A. vulgaris. Given the relatively small magnitude of these interspecific differences, particularly under glasshouse conditions, it is unclear whether they are biologically relevant from the viewpoint of the interaction of plants with pollinators.
The two most significant results of this investigation are (1) nectar sugar composition of field-collected nectar samples, each corresponding to the production of one elemental secretory structure (i.e. individual nectaries), varied widely in the two species studied, with most of the variation taking place at the restricted within-plant scale; (2) such variability virtually vanished in plants grown in the glasshouse, where nectar sugar composition emerged as a remarkably constant feature across plants (only in the case of A. p. cazorlensis), flowers and nectaries of a given species. These two aspects will be discussed in turn below.
The magnitude of intra-plant variation in nectar sugar composition reported here for field-grown plants of A. p. cazorlensis and A. vulgaris is similar or even greater than that ordinarily found in interspecific comparisons (e.g. Baker and Baker, 1983a). This finding contrasts with the notion of intra-specific constancy prevailing in most recent literature on nectar sugar composition (for a review, see Herrera et al., 2006), and corroborates the results of Herrera et al. (2006) for Helleborus foetidus. In this latter species, as in the two Aquilegia species studied here, variation in nectar composition among individual plants was quantitatively negligible, yet extensive variation occurred at a very fine-grained spatial scale due to broad differences between flowers of the same plant and nectaries of the same flower. In both investigations, disclosing this dominant source of intraspecific variation was possible because nectar was sampled using a sampling unit (the individual nectary) that was commensurate with the spatial scale at which variation takes place. The importance of the variation among flowers of the same plant, and among nectaries of the same flower, would have remained undetected had pooled samples been analysed from different nectaries and flowers, a common practice in nectar composition studies. From a methodological viewpoint, therefore, extensive intra-plant variability in nectar sugar composition calls for more elaborate sampling designs to capture that source of variance, particularly for species with individual nectaries in the same flower, as discussed by Herrera et al. (2006). Additionally, the broad intra-plant variation in nectar sugar composition exhibited in the field by the two Aquilegia species could have important effects on the foraging patterns of insect pollinators and thus, presumably, on the selective pressures exerted by them on that floral trait (Herrera et al., 2006).
An important result of this study is the demonstration that plants of the same species and geographical provenance, grown in the field and under controlled homogeneous conditions, differ in intra-specific variance in nectar sugar composition. In sharp contrast with the extensive variability exhibited by plants growing in the field, glasshouse-grown plants were characterized by remarkable intra-specific constancy in nectar sugar composition. Glasshouse plants originated from seeds collected at the same (A. vulgaris) or geographically very close (A. p. cazorlensis) populations where the field study was conducted; hence it is unlikely that the observed contrast in the magnitude of intra-specific variation was due to different genetic backgrounds. Extrinsic abiotic or biotic factors seem therefore the most likely candidates to account for observed patterns.
Among abiotic factors, differences in ambient temperature have been shown to induce changes in the nectar sugar profiles of some species (Freeman and Head, 1990). Differences in the thermal regime experienced by wild and glasshouse plants could therefore contribute to partly explain their differences in average nectar sugar composition. It is extremely unlikely, however, that differences in the small-scale spatial patterning of microclimatic variables experienced by wild and glasshouse plants could account for the marked difference between these two groups in the within-plant variability of nectar sugar composition. This explanation would require that small-scale differences in microclimate (light, temperature, humidity) experienced by the different flowers of the same plant, and the different nectaries of the same flower, are larger and, particularly, more constant over time in the field than in the glasshouse, so as to result in greater small-scale variation in sugar profile. This seems implausible, particularly as it applies to differences among nectaries of the same flower that are only a few millimetres apart.
Differences between field and glasshouse plants in nectar sugar variability may be also due to differential exposure to biotic agents such as nectar removal by pollinators (Galleto and Bernardello, 1992, 1993), addition of enzymes by pollinator hypopharingeal secretions (Vonderohe, 1994), and specifically pollinator-borne microorganisms (Gilliam et al., 1983; Eisikowitch et al., 1990; Davis, 1997). In the field, recently open flowers that almost certainly had already received some visit by bumblebees were sampled. In the glasshouse, in contrast, plants were excluded from pollinators, and sampled flowers had not been previously visited by any insect. Therefore, it is hypothesized that differences between field and glasshouse plants in the variability in the nectar sugar profile are the consequence of contrasting histories of prior visitation by pollinators leading to differential patterns of nectar contamination by some pollinator-borne microorganisms. Floral nectar may harbour highly diverse yeast communities for which pollinators represent major vehicles for dispersal among flowers (Brysch-Herzberg, 2004, and references therein). Furthermore, yeasts will ordinarily modify the original sugar profile by adding enzymes which hydrolyse the disaccharide sucrose into the monosaccharides glucose and fructose (Barnett, 1997). We suggest that glasshouse flowers, which were all similar in not having received any insect visit, consistently exhibited an unmodified, fairly constant nectar sugar profile inherent to the species. Field flowers and nectaries, in contrast, should be highly heterogeneous with regard to prior pollinator visitation and associated yeast contamination history. According to this hypothesis, therefore, the large intra-specific variance in nectar sugar composition of the two Aquilegia species in the field would reflect the strong stochastic component underlying differences between flowers and nectaries in prior pollinator visitation, frequency of yeast contamination, composition of yeast communities and metabolic characteristics of component yeast species.
The preceding hypothesis cannot be tested with the data available, but some indirect evidence is consistent with it. Microbiological studies by Brysch-Herzberg (2004) have revealed that individuals of bumblebee species involved in pollinating Aquilegia in the present study area (Bombus terrestris and B. pascuorum) ordinarily harbour dense and taxonomically diverse yeast populations on their bodies and proboscides, so they could easily act as vectors of yeast contamination of nectar as they visit flowers. Furthermore, the unequal proportions of glucose and fructose in nectar from field but not glasshouse plants probably provide an indication of the metabolic activity of yeasts. In addition to hydrolysing sucrose into glucose and fructose, yeasts often metabolize preferentially one of these monosaccharides, eventually leading to non-stoichiometric proportions among them departing from the 1:1 ratio that would be expected from simple sucrose hydrolysis (Vonderohe, 1994; Barnett, 1997). The very unequal, non-stoichiometric proportions of glucose and fructose found in nectar of field plants of Aquilegia in this study, and also in H. foetidus nectar by Herrera et al. (2006), may thus be interpreted as a chemical ‘signature’ of prior yeast activity on nectar. Finally, budding and vegetative yeast cells have been found on the tongue of several B. terrestris and B. pratorum individuals collected in the study area while they were visiting H. foetidus flowers (A. Canto, unpubl. res.). Experimental studies are needed to corroborate the hypothesis that variable yeast contamination is responsible for extensive intra-plant variation in nectar sugar composition in wild populations of bumblebee-pollinated plants.
ACKNOWLEDGEMENTS
A.C. and M.C.C. were supported by post-doctoral grants from the Spanish Ministerio de Educación y Ciencia. M.M. was supported by a post-doctoral contract from the I3P program of the Consejo Superior de Investigaciones Científicas – Fondo Social Europeo. Permission to work in the Sierra de Cazorla was granted by the Consejería de Medio Ambiente, Junta de Andalucía, who also provided invaluable facilities. We are greatly indebted to the staff of the San Jerónimo nursery, Red de Viveros de la Consejería de Medio Ambiente, for invaluable assistance in growing and maintaining the plants. This work was partly supported by grant BOS2003-03979-C02-1 from the Ministerio de Educación y Ciencia to C.M.H.
LITERATURE CITED
- Baker HG. Sugar concentrations in nectars from hummingbird flowers. Biotropica. 1975;7:37–41. [Google Scholar]
- Baker HG, Baker I. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York, NY: Van Nostrand Reinhold; 1983a. pp. 117–141. [Google Scholar]
- Baker HG, Baker I. A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T, editors. The biology of nectaries. New York, NY: Columbia University Press; 1983b. pp. 126–152. [Google Scholar]
- Baker HG, Baker I. The occurrence and significance of amino acids in floral nectar. Plant Systematics and Evolution. 1986;151:175–186. [Google Scholar]
- Baker HG, Baker I, Hodges SA. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica. 1998;30:559–586. [Google Scholar]
- Barnett JA. Sugar utilization by Saccharomyces cerevisiae. In: Zimmerman FK, Entian KD, editors. Yeast sugar metabolism. Lancaster: Technomic Publishing Company; 1997. pp. 34–43. [Google Scholar]
- Bernardello G, Galetto L, Forcone A. Floral nectar chemical composition of some species from Patagonia II. Biochemical Systematics and Ecology. 1999;27:779–790. [Google Scholar]
- Biernaskie JM, Cartar RV, Hurly TA. Risk-averse inflorescence departure in hummingbirds and bumble bees: could plants benefit from variable nectar volumes? Oikos. 2002;98:98–104. [Google Scholar]
- Brysch-Herzberg M. Ecology of yeasts in plant–bumblebee mutualism in Central Europe. FEMS Microbiology Ecology. 2004;50:87–100. doi: 10.1016/j.femsec.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Davis AR. Influence of floral visitation on nectar-sugar composition and nectary surface changes in Eucalyptus. Apidologie. 1997;28:27–42. [Google Scholar]
- Davis AR, Pylatuik JD, Paradis JC, Low NH. Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta. 1998;205:305–318. doi: 10.1007/s004250050325. [DOI] [PubMed] [Google Scholar]
- Dionex . Optimal setting for pulsed amperometric detection of carbohydrates using Dionex pulsed electrochemical and amperometric detectors. Sunnyvale: Dionex Corp., USA: 1994. Technical Note No. 21. [Google Scholar]
- Dupont YL, Hansen DM, Rasmussen JT, Olesen JM. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Functional Ecology. 2004;18:670–676. [Google Scholar]
- Eisikowitch D, Kevan PG, Lachance MA. The nectar-inhabiting yeast and their effect on pollen germination in common milkweed, Asclepias syriaca L. Israel Journal of Botany. 1990;39:217–225. [Google Scholar]
- Freeman CE, Head KC. Temperature and sucrose composition of floral nectars in Ipomopsis longiflora under field conditions. Southwestern Naturalist. 1990;35:423–426. [Google Scholar]
- Freeman CE, Wilken DH. Variation in nectar sugar composition at the intraplant level in Ipomopsis longiflora (Polemoniaceae) American Journal of Botany. 1987;74:1681–1689. [Google Scholar]
- Freeman CE, Reid WH, Becvar JE, Scogin R. Similarity and apparent convergence in the nectar-sugar composition of some hummingbird-pollinated flowers. Botanical Gazette. 1984;145:132–135. [Google Scholar]
- Freeman CE, Reid WH, Worthington RD. Patterns of floral nectar-sugar composition of Ipomopsis longiflora (Polemoniaceae) near the contact zone of its subspecies longiflora and australis. American Journal of Botany. 1985;72:1662–1667. [Google Scholar]
- Galetto L, Bernardello G. Nectar secretion pattern and removal effects in six Argentinean Pitcairnioideae (Bromeliaceae) Botanica Acta. 1992;105:292–299. [Google Scholar]
- Galetto L, Bernardello G. Nectar secretion pattern and removal effects in three species of Solanaceae. Canadian Journal of Botany. 1993;71:1394–1398. [Google Scholar]
- Galetto L, Bernardello G. Nectar sugar composition in angiosperms from Chaco and Patagonia (Argentina): do animal visitors matter? Plant Systematics and Evolution. 2003;238:69–86. [Google Scholar]
- Gilliam M, Moffett JO, Kauffeld NM. Examination of floral nectar of citrus, cotton, and Arizona desert plants for microbes. Apidologie. 1983;14:299–302. [Google Scholar]
- Gottsberger G, Arnold T, Linskens HF. Intraspecific variation in the amino acid content of floral nectar. Botanica Acta. 1989;102:141–144. [Google Scholar]
- Herrera CM, Pérez R, Alonso C. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. American Journal of Botany. 2006;93:575–581. doi: 10.3732/ajb.93.4.575. [DOI] [PubMed] [Google Scholar]
- Jürgens A. Nectar sugar composition and floral scent compounds of diurnal and nocturnal Conophytum species (Aizoaceae) South African Journal of Botany. 2004;70:191–205. [Google Scholar]
- Langenberger MW, Davis AR. Temporal changes in floral nectar production, reabsorption, and composition associated with dichogamy in annual caraway (Carum carvi; Apiaceae) American Journal of Botany. 2002;89:1588–1598. doi: 10.3732/ajb.89.10.1588. [DOI] [PubMed] [Google Scholar]
- Lanza J, Smith GC, Sack S, Cash A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia. 1995;102:113–119. doi: 10.1007/BF00333318. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Medrano M, Castellanos MC, Herrera CM. Comparative floral and vegetative differentiation between two European Aquilegia taxa along a narrow contact zone. Plant Systematics and Evolution. 2006;262:209–224.. [Google Scholar]
- Nicolson SW, Nepi M. Dilute nectar in dry atmospheres: nectar secretion patterns in Aloe castanea (Asphodelaceae) International Journal of Plant Sciences. 2005;166:227–233. [Google Scholar]
- Percival MS. Types of nectar in angiosperms. New Phytologist. 1961;60:235–281. [Google Scholar]
- Reid WH, Freeman CE, Becvar JE. Nectar sugar variability in two species of Agave (Agavaceae) Southwestern Naturalist. 1985;30:443–445. [Google Scholar]
- Roldán-Serrano AS, Guerra-Sanz JM. Dynamics and sugar composition of sweet pepper (Capsicum annuum L.) nectar. Journal of Horticultural Science and Biotechnology. 2004;79:717–722. [Google Scholar]
- Severson DW, Erickson EH. Quantitative and qualitative variation in floral nectar of soybean cultivars in southeastern Missouri. Environmental Entomology. 1984;13:1091–1096. [Google Scholar]
- Shafir S, Bechar A, Weber EU. Cognition-mediated coevolution: context-dependent evaluations and sensitivity of pollinators to variability in nectar rewards. Plant Systematics and Evolution. 2003;238:195–209. [Google Scholar]
- Simpson BB, Neff JL. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York, NY: Van Nostrand Reinhold; 1983. pp. 142–159. [Google Scholar]
- Vonderohe W. Unifloral honeys: chemical conversion and pollen reduction. Grana. 1994;33:292–294. [Google Scholar]
- Witt T, Jürgens A, Geyer R, Gottsberger G. Nectar dynamics and sugar composition in flowers of Silene and Saponaria species (Caryophyllaceae) Plant Biology. 1999;1:334–345. [Google Scholar]