Abstract
Background and Aims
Flexistyly is a sexual dimorphism where there are two morphs that differ in the temporal expression of sexual function and also involve reciprocal movement of the stigmatic surface through a vertical axis during the flowering period. The adaptive significance of flexistyly has been interpreted as a floral mechanism for outcrossing, but it may also function to reduce sexual interference in which styles and stigmas impede the pollen export. Here these two explanations of flexistyly were tested in Alpinia blepharocalyx through a hand-pollination experiment.
Methods
Hand-pollinations were performed in two temporal morphs and consisted of two sequential pollination treatments, namely self-pollination in the morning and inter-morph pollination in the afternoon (treatment 1) or conversely inter-morph pollination in the morning and self-pollination in the afternoon (treatment 2), and two simultaneous self- and inter-morph cross-pollination treatments either in the morning (treatment 3) or in the afternoon (treatment 4). Seed paternity was then determined to assess relative success of self- versus cross-pollen using allozyme markers.
Key Results
In the sequential pollination treatments, whether the stigmas of recipients are receptive in the morning is crucial to the success of the pollen deposited. When the cataflexistylous (protandrous) morph served as pollen recipient, early-arriving pollen in the morning can sire only a very small proportion (<15%) of seeds because the stigmas were then unreceptive. However, when the anaflexistylous (protogynous) morph served as pollen recipient, early pollen did gain a large competitive advantage over the late pollen, particularly when cross-pollen arrived first. Simultaneous self- and inter-morph cross-pollination indicated that outcross-pollen is more competitive than self-pollen on receptive stigmas.
Conclusions
Differential maturing of male and female organs in Alpinia blepharocalyx is sufficient for selfing avoidance, obviating the need for style movements. Instead, the upward style curvature of the cataflexistylous morph in the morning and the anaflexistylous morph in the afternoon most likely represents a means of reducing interference with pollen export.
Key words: Alpinia, flexistyly, heterodichogamy, pollen competition, self-pollination, sexual interference
INTRODUCTION
Hermaphroditism, the presence of female and male sex organs within one flower, is the predominant sexual condition in flowering plants. The prevalence of this sex system reflects its advantage of economizing on the resources allocated to pollinator attraction and/or the potential reproductive assurance in the absence of mates or pollinators (Charnov, 1982; Lloyd, 1987). However, when female and male sex organs are expressed simultaneously and housed together within a flower, hermaphroditism can cause intra-floral self-fertilization and subsequent inbreeding depression. Therefore, inbreeding avoidance has been widely accepted as a major driving force shaping floral traits and the evolution of plant mating systems (Charlesworth and Charlesworth, 1987; Barrett, 2003). Another disadvantage of hermaphroditism, i.e. the potential for physical interference between male and female floral functions, had been largely ignored until Lennart van der Pijl first clearly pointed it out in 1978 (van der Pijl, 1978). Sexual interference in hermaphroditic animal-pollinated plants stems from functional conflicts between pollen dispersal and receipt during pollination and mating, which may result in gamete wastage and reduce mating opportunities (Barrett, 2002a). Reproductive losses resulting from sexual interference may not involve the genetic costs of inbreeding depression. Lloyd and Webb (1986) proposed that sexual interference is one of the most important selective forces driving the evolution of floral traits. Segregation of sex organs either in space (herkogamy) or in time (dichogamy) has been viewed as an adaptation that avoids interference and increases reproductive success (Lloyd and Webb, 1986; Webb and Lloyd, 1986; Bertin and Newman, 1993). The interference hypothesis has been examined for a limited number of species (Kohn and Barrett, 1992; Griffin et al., 2000; Harder et al., 2000; Fetscher, 2001; Cesaro et al., 2004; Routley and Husband, 2006).
Now documented in 24 species of the Zingiberaceae (Kress et al., 2005), flexistyly is a novel floral strategy, unique and ‘active’ floral dimorphism achieved by both changing the position of the style and separating the maturation of male and female organs at different times (Cui et al., 1995; Li et al., 2001a; Li et al., 2002; Zhang et al., 2003; Takano et al., 2005). Flexistylous populations are composed of equal frequencies of two style morphs of hermaphrodites that differ in the direction of movement that styles undergo during flowering. Styles of flowers that disperse pollen in the morning are curved upwards, so that stigmas are spatially separated from anthers and cannot contact pollinators; at this time stigmas are still unreceptive (Zhang et al., 2003). At noon, after the male function is complete, styles grow downwards into a position where stigmas can contact pollinators (Fig. 1A). Patterns of style growth in the reciprocal morph are reversed, with stigmas receiving cross-pollen in the morning and upward style curvature occurring in the afternoon (Fig. 1B). The upward style curvature in both morphs is unlikely to be an anti-selfing mechanism, since the stigmas when held in an upward position either are unreceptive (cataflexistylous morph) or have already received cross-pollen (anaflexistylous morph). The occurrence of 1:1 morph ratios in natural populations of flexistylous species (Li et al., 2001a) suggests that a single diallelic locus governs the two morphs with anaflexistylous morph dominant to cataflexistylous morph (Renner, 2001; Barrett, 2002b; Zhang and Li, 2002).
Fig. 1.
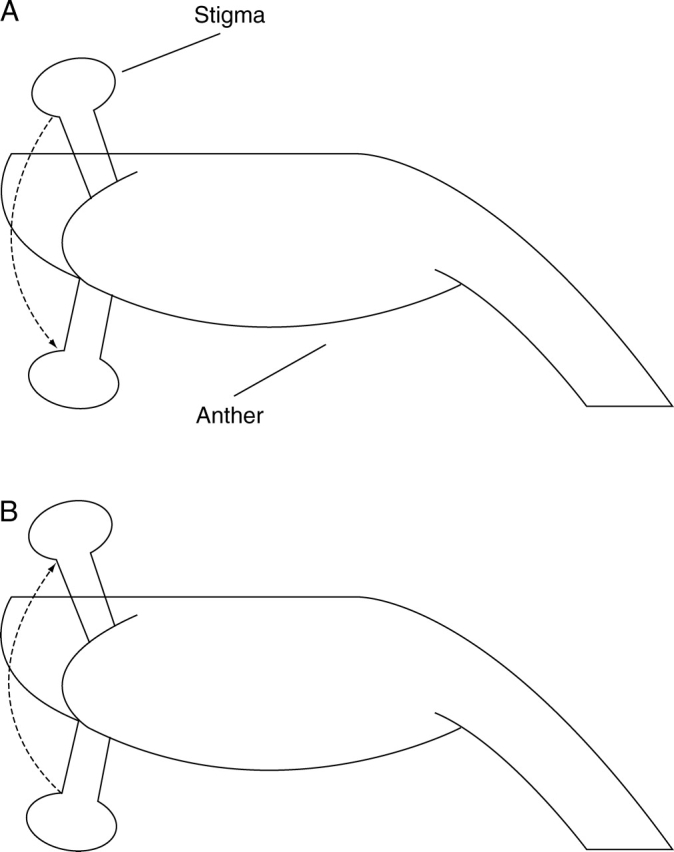
A sketch of style movement in Alpinia blepharocalyx. Anthers are held in the same position throughout the 1-d flowering period, but shed pollen only when styles in the upward position. (A) Cataflexistylous (protandrous) style; (B) anaflexistylous (protogynous) style. The arrow with a dotted line indicates the direction of style movement.
Dichogamy and herkogamy have been regarded by some (e.g. Richards, 1997; Li et al. 2001a, b), as mechanisms that function solely to promote outcrossing. Flexistyly involves both heterodichogamy and herkogamy, and this redundancy seems unnecessary if flexistyly is only to promote outcrossing. Furthermore, heterodichogamy in the absence of style movement could not alleviate physical interference between anther and stigma function, resulting in losses of pollen dispersal. The aim of this study was to demonstrate that heterodichogamy in a flexistylous plant, Alpinia blepharocalyx, is sufficient for avoiding self-fertilization; thus, herkogamy via style movement most likely functions to reduce conflict between the male and female functions. To assess the role of heterodichogamy in avoiding self-fertilization, the siring success of self- vs. cross-pollen applied sequentially or simultaneously to stigmas of A. blepharocalyx was compared. If style movement functions to avoid self-fertilization, the anaflexistylous styles with their stigmas pollinated by outcross-pollen from the other morphs in the morning would have no need to curve up at noon, unless the self-pollen deposited on the stigmas in the afternoon is competitively superior to the outcross-pollen, which will prove to be untrue through the experiments conducted in this study.
MATERIALS AND METHODS
Study organism and sites
Alpinia blepharocalyx K. Schum. is a self-compatible, clonal, flexistylous perennial herb, usually 1–3 m tall (Zhang et al., 2003). The inflorescences are terminal on leafy shoots and 20–30 cm long. The flower has a very special structure, a conspicuous three-lobed labellum produced by the fusion of two staminodes, which are flesh-coloured and red with a yellow centre. The labellum forms a tube, the free part of which is expanded and forms a landing platform for pollinators. Only one fertile stamen with two anthers develops and the style extends through the anthers. Bracteoles are green and elliptic, dry and brittle, and fall off at anthesis. During blooming each inflorescence produces two to ten open flowers per day, and each flower lasts 1 d. Flowering occurs from March to late April and capsules ripen by August–September.
All hand-pollination experiments were carried out in a monsoon evergreen broad-leaved forest in the Caiyanghe nature reserve of Simao, Yunnan province, southwestern China (22°30′N, 101°22′E; 1200 m a.s.l.). Here, the plants of A. blepharocalyx are distributed in the evergreen broad-leaved forest dominated by Betula alnoides and Alnus nepalensis along several valleys (Zhang et al., 2003). The outcrossing rates of cataflexistylous and anaflexistylous morph in this population were estimated to be 0·615 ± 0·115 and 0·751 ± 0·097, respectively (Sun, 2006). Experimental manipulations were conducted in March–April 2004.
Methods
To evaluate the effect of heterodichogamy on self-fertilization in this flexistylous plant, hand-pollination treatments were carried out in two temporal morphs, using a pair of pollen donors consisting of self-pollen and cross-pollen from the other morph. The plants used as pollen donors or recipients were chosen on the basis of the dissimilarity of the alcohol dehydrogenase (ADH) locus, such that anaflexistylous plants were homozygous for allele A and cataflexistylous plants were homozygous for allele B. Progeny expressing genotype AB must have resulted from cross-pollination between two morphs.
Two types of hand-pollination treatments were executed on two temporal morphs: sequential pollinations and mixed-donor pollinations. In sequential pollination, pollen donors were chosen in the following order: (1) self-pollination in the morning plus inter-morph cross-pollination in the afternoon; and (2) inter-morph cross-pollination in the morning plus self-pollination in the afternoon. Mixed-donor pollination was carried out using each of the mixed pair of pollen donors consisted of an equal mass of pollen from the same number of flowers of two pollen donors in the following treatments: (1) self- and inter-morph cross-pollen applied simultaneously in the morning; and (2) self- and inter-morph cross-pollen applied simultaneously in the afternoon.
Flowers on recipient plants were bagged prior to anthesis and re-bagged after hand-pollination to exclude pollinators. Hand-pollinations were performed in the morning (1030 h to1130 h) and afternoon (1600 h to 1700 h), respectively. When anaflexistylous flowers were used as pollen donors in the morning when the pollen sacs had not dehisced, the indehiscent anthers were opened and pollen collected. In sequential hand-pollination treatments, the entire stigmatic surface with the first pollen application was covered, so that early pollen could gain an advantage due to better access to the stigmatic surface. An approximately equal mass of pollen from the self- vs. cross-donors was mixed in the vial during the applications.
The siring success of each type of donor could be determined by scoring seed from pollination treatments. Seeds were assayed for the enzyme system ADH (EC 1·1·1·1) by using vertical slab polyacrylamide gel electrophoresis. Allozymes were extracted by using a Tris–HCL–PVP buffer (pH 7·5) and kept at −20°C until assayed on 7 % polyacrylamide gel. A continuous Tris–glycine buffer system (pH 8·3) was used. The treatment of self-pollination in the morning and inter-morph pollination in the afternoon on anaflexistylous flowers produced 18 fruits and all seeds from these fruits were scored electrophoretically; the other treatments produced over 30 fruits and 20 fruits were randomly selected from each treatment and all seeds assessed from these fruits (Table 1). The null hypothesis of equal proportion of the seeds sired by self- vs. cross-donors was assessed by using a chi-square test of the goodness-of-fit. The two-way ANOVA was performed to assess differences in the proportion of selfed seeds and the number of seeds per fruit among the treatments using SPSS 13·0.
Table 1.
Summary of hand-pollination treatments, numbers of recipients used in each treatment, fruit set, the number of seeds per fruit, and total number of progeny scored
| Treatment | Morphs as recipients | Total no. of flowers | Fruit set | No. of seeds per fruit (n)* | Total no. of progeny scored |
|---|---|---|---|---|---|
| (1) Self-pollination in AM + cross-pollination in PM | Cata-M | 199 | 0·59 | 24·2 ± 0·90 (80) | 494 |
| Ana-M | 29 | 0·62 | 18·33 ± 1·58 (18) | 330 | |
| (2) Cross-pollination in AM + self-pollination in PM | Cata-M | 107 | 0·64 | 27·33 ± 1·49 (40) | 502 |
| Ana-M | 31 | 1·00 | 25·25 ± 0·94 (24) | 503 | |
| (3) Self- and cross-pollen applied simultaneously in AM | Cata-M | 145 | 0·48 | 20·2 ± 1·14 (42) | 363 |
| Ana-M | 42 | 0·81 | 19·6 ± 0·87 (20) | 392 | |
| (4) Self- and cross-pollen applied simultaneously in PM | Cata-M | 186 | 0·55 | 27·81 ± 0·96 (44) | 526 |
| Ana-M | 73 | 0·44 | 20·53 ± 1·72 (30) | 429 |
* n = number of fruits inspected.
RESULTS
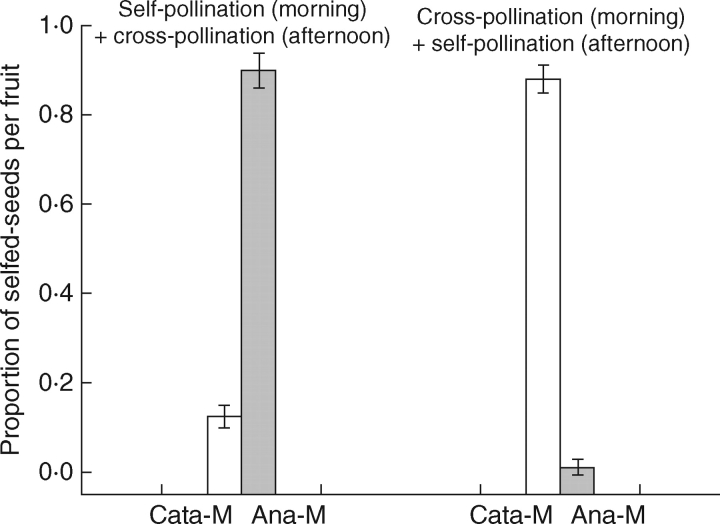
The proportion of selfed seeds per fruit shows significant variation among treatments (F = 7·05, d.f. = 3, P < 0·001) and between morphs (F = 10·93, d.f. = 1, P < 0·001), and also a significant morph × treatment interaction (F = 200·81, d.f. = 3, P < 0·001), indicating that the response to hand-pollination treatments was not the same in the two morphs. The proportion of seeds sired by self- versus outcross-pollen donors is subject to the position of the stigmas of recipients at the time of hand-pollinations. In the treatments of cataflexistylous flowers serving as recipients, when the stigma is curved downward to the anther and receptive in the afternoon, late donors sired significantly more seeds than early donors, with the mean proportions (±s.e.) of seeds sired by early-deposited pollen per fruit being 0·11 ± 0·025 (selfing) and 0·11 ± 0·032 (outcrossing), respectively (Fig. 2). In cataflexistylous flowers, the fact that early-deposited pollen in the morning did not have a competitive advantage may be due to temporal patterns of stigma receptivity in A. blepharocalyx: the stigmas are simply unreceptive when still curved up in the morning (Zhang et al., 2003). In the treatments where anaflexistylous flowers served as recipients and the stigmas are receptive in the morning, early donors sired significantly more seeds than late donors. In these cases, the mean proportions of seeds sired by the early-arriving pollen were 0·90 ± 0·039 (selfing) and 0·99 ± 0·006 (outcrossing), respectively (Fig. 2).
Fig. 2.
The mean proportion of seeds per fruit sired by self-pollen (mean ± s.e.) following sequential pollinations in the cataflexistylous morphs (Cata-M) and anaflexistylous morphs (Ana-M) of Alpinia blepharocalyx. Chi-squared tests were used to compare observed values with the expected ratio of 1:1 if siring success of self- and outcross donors is equal, and a significance level of P < 0·001 was found for all cases.
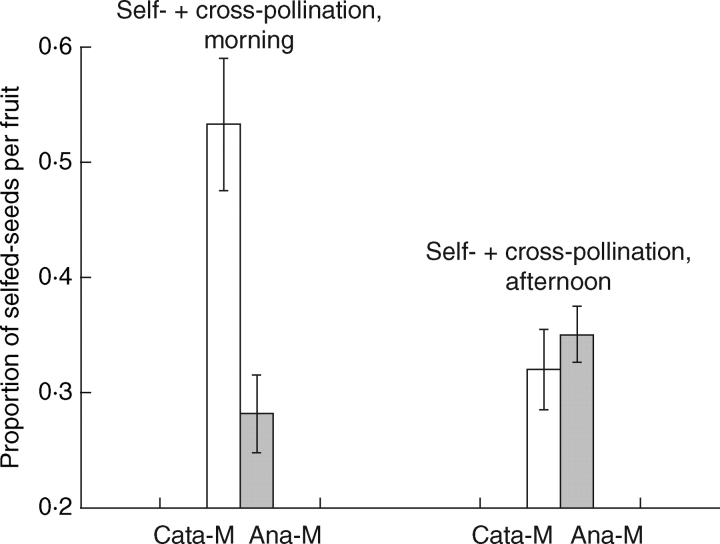
In contrast, the results of mixed-donor pollinations indicated that when the stigmas of recipients are at the position able to contact pollinators (and receptive), viz. cataflexistylous stigmas in the afternoon and anaflexistylous stigmas in the morning and afternoon, inter-morph pollen donors sired significantly more seeds than self-pollen donors (Fig. 3). But in the treatment of simultaneous pollinations with cataflexistylous stigmas above the anthers in the morning, self-pollen donors sired more seeds than inter-morph cross-pollen donors (P = 0·0588; Fig. 3). This result may be due to variation in pollen maturity between self- and inter-morph pollen when stigmas become receptive gradually. Pooling data from all pollination treatments showed that inter-morph cross-pollen is more competitive than self-pollen under simultaneous pollinations (P < 0·001).
Fig. 3.
The mean proportion of seeds per fruit sired by self-pollen (mean ± s.e.) following simultaneous pollinations in the cataflexistylous morph (Cata-M) and anaflexistylous morph (Ana-M) of Alpinia blepharocalyx. Chi-square tests were used to compare observed values with the expected ratio of 1:1 if siring success of self- and outcross donors is equal. A significance level of P < 0·001 was found for all treatments except for mixed pollination in the morning for Cata-M (P = 0·0588).
Moreover, when the cataflexistylous morphs served as recipients, the proportions of seeds sired by self- or inter-morph cross-pollen donors applied in the morning from sequential pollinations (0·11 and 0·10, respectively) are significantly smaller than those by the same donors in mixed pollinations applied in the morning (0·53 and 0·47, respectively). This further indicated that early-arriving pollen in the morning could not gain a competitive advantage over late-arriving pollen in the afternoon on the stigmas of the cataflexistylous morph.
The number of seeds produced per fruit shows significant variation among treatments (F = 8·33, d.f. = 3, P < 0·001) and between morphs (F = 16·45, d.f. = 1, P < 0·001). In both morphs prior self-pollination resulted in fewer seeds per fruit than prior cross-pollination (Table 1), suggesting that early inbreeding depression might occur.
DISCUSSION
Flexistyly is not only an example of heterodichogamy (Renner, 2001), but it is also an example of herkogamy via style movement. Flexistyly results both in a reduction in within- and among-flower (geitonogamous) self-pollination within an individual and a reduction in cross-pollination among individuals of the same morph (Li et al., 2001a; Zhang et al., 2003), but apparently does not come to a full stop with self-fertilization (Sun, 2006). Selfing could arise from incomplete pollen removal due to insufficient pollinators (Zhang et al., 2003) and/or pollen carryover, both of which are, however, unrelated to style movement. The present study illustrates that the heterodichogamy component of flexistyly in A. blepharocalyx can be sufficient to avoid self-pollination, and to explain style movement it is necessary to resort to an alternative explanation: reduction of sexual interference.
The function of dichogamy depends on the rates of pollen deposition and removal, which, in turn, depend on pollinator visitation rates and foraging behaviour (Griffin et al., 2000). In flexistylous Alpinia species, the visit frequencies of the pollinators show a bimodal pattern during the day, in a good match with the floral behaviour of the flexistylous plants (Li et al., 2001b; Takano et al., 2005). In the cataflexistylous morph, anthers dehisce before stigmas become receptive in the morning (Zhang et al., 2003), and the late-deposited pollen in the afternoon showed significant competitive advantage, regardless of prior pollination in the morning (Fig. 2). In this case, early-arriving self-pollen may have difficulty in adhering to unreceptive stigmas and thus seems not to derive an advantage from early arrival. Stigmas of cataflexistylous flowers become receptive after they are located at the position of pollination. Long delays between self-pollination and pollen germination (Spira et al., 1996) or the environment of the stigmatic surface may negate any advantage of early-arriving pollen. If cataflexistylous styles were not curved up in the morning but were kept in a downward position during the 1-d flowering period, self-fertilization would still be unlikely due to the timing of stigma receptivity and the weaker competitive ability of self-pollen (Fig. 2).
In the anaflexistylous morph, where stigmas are receptive in the morning before anthers dehisce in the afternoon, protogyny provides opportunities for the receipt of outcross-pollen before self-pollen is shed. This synchronous protogyny is very effective in reducing self-fertilization even though anaflexistylous styles are kept in a downward position and are unable to curve up in the afternoon (Fig. 2). Actually, not to curve up in the afternoon may even benefit the plant because it can assure reproduction in the case of insufficient outcross-pollen or pollinators in the morning, although in A. blepharocalyx strong selection prevents selfed offspring from reaching reproductive maturity as a result of inbreeding depression, which was evaluated by using allozyme markers to measure changes in the inbreeding coefficient from parents to offspring (S. Sun, unpubl. res.). Taken together, heterodichogamy primarily protects self-compatible, flexistylous plants from the harmful effects of self pollination, while reciprocal style movements primarily reduce interference between male and female function.
The experimental results from simultaneous mixed-donor hand-pollination treatments showed that when the stigmas are receptive, cross-pollen is more competitive than self-pollen (Fig. 3), further reducing the need for avoiding self-pollination. Barrett et al. (2000) proposed that the function of all stylar polymorphisms in plants is to increase the precision of cross-pollination and reduce lost mating opportunities associated with self-interference. If both stigmas and anthers in flexistylous plants are housed in approximately the same position within a flower to facilitate pollen removal and deposition, stigmas below anthers may obstruct pollen export and be pollinated by self-pollen, resulting in pollen discounting. Flexistyly is effective both to avoid self-fertilization and to resolve sexual conflict between male and female function. It reduces gamete wastage and increases mating opportunities through more effective pollen dispersal. Nevertheless it is also noted that prior self-pollination in our experiments results in both smaller fruit set and significantly fewer seeds per fruit for both morphs (Table 1). Thus it cannot be ruled out that style movement also functions to enhance female reproductive success.
Future manipulative studies on style movement are needed to demonstrate its functional significance in pollen–stigma interference. For instance, styles of anaflexistylous flowers may be manipulated not to curve up in the afternoon. If the male fitness of manipulated flowers is reduced in comparison with intact flowers, then this would constitute strong direct evidence for pollen–stigma interference.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (30430160) and the Doctoral Program of Higher Education (20030027021). We are grateful to Pan-Yu Ren, Xiao-Bao Deng and Min Liu for their help with the field experiments. Dr John Pannell and two anonymous referees made very useful comments on the earlier versions of this manuscript.
LITERATURE CITED
- Barrett SCH. Sexual interference of the floral kind. Heredity. 2002a;88:154–159. doi: 10.1038/sj.hdy.6800020. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Review Genetics. 2002b;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany. 2000;85(Suppl. A):253–265. [Google Scholar]
- Bertin RI, Newman CM. Dichogamy in angiosperms. Botanical Review. 1993;59:112–152. [Google Scholar]
- Cesaro AC, Barrett SCH, Murice S, Vaissiere BE, Thompson JD. An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. Journal of Evolutionary Biology. 2004;17:1367–1376. doi: 10.1111/j.1420-9101.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Charnov EL. The theory of sex allocation. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Cui XL, Wei RC, Huang RF. A preliminary study on the genetic system of Amomum tsao-ko. Journal of Yunnan Univerisity (Natural Science) 1995;17:290–297. [Google Scholar]
- Fetscher AE. Resolution of male–female conflict in an hermaphroditic flower. Proceedings of the Royal Society of London. Series B. Biological Sciences. 2001;268:525–529. doi: 10.1098/rspb.2000.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SR, Mavraganis K, Eckert CG. Experimental analysis of protogyny in Aquilegia canadensis (Ranunculaceae) American Journal of Botany. 2000;87:1246–1256. [PubMed] [Google Scholar]
- Harder LD, Barrett SCH, Cole WW. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society of London. Series B. Biological Sciences. 2000;267:315–320. doi: 10.1098/rspb.2000.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn JR, Barrett SCH. Floral manipulations reveal the cause of male fitness variation in experimental population of Eichhoria paniculata (Pontederiaceae) Functional Ecology. 1992;6:590–595. [Google Scholar]
- Kress WJ, Liu AZ, Newman M, Li QJ. The molecular phylogeny of Alpinia (Zingiberaceae): a complex and polyphyletic genus of gingers. American Journal of Botany. 2005;92:167–178. doi: 10.3732/ajb.92.1.167. [DOI] [PubMed] [Google Scholar]
- Li QJ, Kress WJ, Xu ZF, Mia YM, Zhang L, Deng XB, Gao JY. Mating system and stigmatic behaviour during flowering of Alpinia kwangsiensis (Zingiberaceae) Plant Systematics and Evolution. 2002;232:123–132. [Google Scholar]
- Li QJ, Xu ZF, Kress WJ, Xia YM, Zhang L, Deng XB, et al. Flexible style that encourages outcrossing. Nature. 2001a;410:432–432. doi: 10.1038/35068635. [DOI] [PubMed] [Google Scholar]
- Li QJ, Xu ZF, Xia YM, Zhang L, Deng XB, Gao JY. Study on the flexistyly pollination mechanism in Alpinia plants (Zingiberaceae) Acta Botanica Sinica. 2001b;43:364–369. [Google Scholar]
- Lloyd DG. Allocations to pollen, seeds, and pollination mechanisms in self-fertilizaing plants. Functional Ecology. 1987;1:83–89. [Google Scholar]
- Lloyd DG, Webb CJ. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany. 1986;24:135–162. [Google Scholar]
- van der Pijl L. Reproductive intergration and sexual disharmony in floral function. In: Richards AJ, editor. The pollination of flowers by insects. London: Academic Press; 1978. pp. 79–88. Linnean Society Symposium Series 6. [Google Scholar]
- Renner SS. How common is heterodichogamy? Trends in Ecology and Evolution. 2001;16:595–597. [Google Scholar]
- Richards AJ. Plant beeding systems. 2nd edn. New York: Chapman and Hall; 1997. [Google Scholar]
- Routley MB, Husband BC. Sexual interference within flowers of Chamerion augustifolium. Evolutionary Ecology. 2006;20:331–343. [Google Scholar]
- Spira TP, Snow AA, Puterbaugh MN. The timing and effectiveness of sequential pollination in Hibiscus moscheutos. Oecologia. 1996;105:230–235. doi: 10.1007/BF00328551. [DOI] [PubMed] [Google Scholar]
- Sun S. Beijing: Beijing Normal University; 2006. Study on reproductive ecology of Alpinia blepharocalyx (Zingiberaceae) and adaptive significance of flexistyly. PhD Thesis. [Google Scholar]
- Takano A, Gisil J, Yusoff M, Tachi T. Floral and pollinator behaviour of flexistylous Bornean ginger, Alpinia nieuwenhuizii (Zingiberaceae) Plant Systematics and Evolution. 2005;252:167–173. [Google Scholar]
- Webb CJ, Lloyd DG. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkgamy. New Zealand Journal of Botany. 1986;24:163–178. [Google Scholar]
- Zhang L, Li QJ. Flexistyly and its evolutionary-ecological significance. Acta Phytoecologica Sinica. 2002;26:385–390. [Google Scholar]
- Zhang L, Li QJ, Deng XB, Ren PY, Gao JY. Reproductive biology of Alpinia blepharocalyx (Zingiberaceae): another example of flexistyly. Plant Systematics and Evolution. 2003;241:67–76. [Google Scholar]