Abstract
Background and Aims
There is considerable confusion in the literature concerning impermeability of seeds with ‘hard’ seed coats, because the ability to take up (imbibe) water has not been tested in most of them. Seeds of Opuntia tomentosa were reported recently to have a water-impermeable seed coat sensu lato (i.e. physical dormancy), in combination with physiological dormancy. However, physical dormancy is not known to occur in Cactaceae. Therefore, the aim of this study was to determine if seeds of O. tomentosa are water-permeable or water-impermeable, i.e. if they have physical dormancy.
Methods
The micromorphology of the seed coat and associated structures were characterized by SEM and light microscopy. Permeability of the seed-covering layers was assessed by an increase in mass of seeds on a wet substrate and by dye-tracking and uptake of tritiated water by intact versus scarified seeds.
Key Results
A germination valve and a water channel are formed in the hilum–micropyle region during dehydration and ageing in seeds of O. tomentosa. The funicular envelope undoubtedly plays a role in germination of Opuntia seeds via restriction of water uptake and mechanical resistance to expansion of the embryo. However, seeds do not exhibit any of three features characteristic of those with physical dormancy. Thus, they do not have a water-impermeable layer(s) of palisade cells (macrosclereids) or a water gap sensu stricto and they imbibe water without the seed coat being disrupted.
Conclusions
Although dormancy in seeds of this species can be broken by scarification, they have physiological dormancy only. Further, based on information in the literature, it is concluded that it is unlikely that any species of Opuntia has physical dormancy. This is the first integrative study of the anatomy, dynamics of water uptake and dormancy in seeds of Cactaceae subfamily Opuntioideae.
Key words: México Valley; Opuntia tomentosa; physical dormancy, physiological dormancy; seed anatomy; seed germination; water uptake by seeds
INTRODUCTION
Seeds with a water-impermeable seed or fruit coat have physical dormancy (PY; sensu Baskin and Baskin, 2004), and such seeds are known to occur in 16 families of angiosperms but no gymnosperms (Baskin et al., 2000, 2006; Baskin and Baskin, 2003). In all cases, PY is associated with a water-impermeable layer(s) of palisade cells (macrosclereids) (Baskin et al., 2000). However, considerable confusion occurs in the literature concerning seeds with ‘hard’ seed coats. These seeds have a seed (or fruit) coat that is hard when pinched with one's fingers, but they may or may not be water-permeable, depending on the species. The only way to determine if seeds with hard (to-the-touch) coats are water-permeable is to conduct imbibition studies. If seeds fail to imbibe water, they have PY. To add to the confusion, mechanical or acid scarification of water-permeable, hard (to-the-touch) seeds may promote germination. Low growth potential (or ‘push power’) of the embryo is the major reason why imbibed seeds with fully developed embryos fail to germinate; these seeds have physiological dormancy (PD). If the coat of such seeds is scarified, mechanical restriction is decreased, and the embryo is able to expand (germinate). However, when physiological dormancy is broken in intact seeds, the embryo gains sufficient growth potential to overcome the restraint of the seed coat (Baskin and Baskin, 2004).
Seeds of Opuntia species have hard (to-the-touch) seed covers, and pressures of 440·83–44·35 daN may be required to break them (Aguilar, 2003). The hardness of the Opuntia seed covers is related to the presence of a funicular envelope, which completely encloses the seed (Archibald, 1939). Further, physiological dormancy is present in seeds of many cacti (Baskin and Baskin, 1998; C. C. Baskin and J. M. Baskin, unpubl. res.), including those of Opuntia spp., e.g. O. macrorhiza (Timmons, 1942), O. compressa (Baskin and Baskin, 1977), O. tomentosa (Olvera-Carrillo et al., 2003), O. rastrera (Mandujano et al., 1997, 2005) and O. stricta (Reinhardt et al., 1999). Exposing seeds of O. macrorhiza (Timmons, 1942) and of O. compressa (Baskin and Baskin, 1977) to outdoor winter temperatures in Kansas and Kentucky, respectively, was effective in breaking dormancy. Various dormancy-breaking treatments, including scarification with sulfuric acid (Archibald, 1939; Wiggins and Focht, 1967; Baskin and Baskin, 1977; Potter et al., 1984; Olvera-Carrillo et al., 2003; Mandujano et al., 2005), mechanical scarification (Johnson, 1918; Archibald, 1939; Hamilton, 1970a, b; Pilcher, 1970; Baskin and Baskin, 1977; Pendley, 2001; Mandujano et al., 2005), after-ripening (Mandujano et al., 1997, 2005; Reinhardt et al., 1999; Pendley, 2001), cold stratification (Timmons, 1942; Baskin and Baskin, 1977; Olvera-Carrillo et al., 2003), leaching (Wiggins and Focht, 1967; Pilcher, 1970; Hamilton, 1970a; Pendley, 2001), passage through the digestive tract of animals (Timmons, 1942; Potter et al., 1984; Mandujano et al., 1997; Gimeno and Vila, 2002), soaking in hot water (Reinhardt et al., 1999), and some combinations of these treatments have been reported to enhance germination of Opuntia seeds. However, these treatments do not always promote germination and, in some cases, treated seeds have germinated to lower percentages than those in the control (e.g. Wiggins and Focht, 1967; Baskin and Baskin, 1977; Mandujano et al., 2005). Gibberellic acid (GA3) alone seems to be only moderately effective in promoting germination of seeds of Opuntia spp. However, it induces a high percentage of germination in combination with other treatments such as rinsing/washing (Pendley, 2001) or chemical or mechanical scarification (Sánchez-Venegas, 1997).
In a recent paper, Olvera-Carrillo et al. (2003) reported PY in seeds of Opuntia tomentosa Salm-Dyck (Cactaceae), but this class of dormancy is not (otherwise) known to occur in the Cactaceae (Baskin et al., 2000; Baskin and Baskin, 2003). Further, seeds of O. tomentosa required gibberellin and acid scarification to germinate, but scarification time for full germination varied from 5 to 90 min, depending on seed batch (Olvera-Carrillo et al., 2003). However, after 15–18 months of dry storage, seeds only germinated following scarification, and GA3 (1000 and 2000 ppm) reduced germination.
Although Olvera-Carrillo et al. (2003) showed that scarification significantly increased germination of O. tomentosa seeds, they did not compare water uptake (imbibition) of scarified versus non-scarified seeds. Further, it was observed that non-scarified seeds of O. tomentosa did not germinate, and they did not appear to swell when placed on a wet substrate. Thus, the purpose of the present study was to address questions concerning water uptake in the hard (to-the-touch) seeds of O. tomentosa. In particular, the anatomy of the seed cover layers was investigated in relation to imbibition and germination, with the primary objectives being to determine if the seed cover layers are water-impermeable or water-permeable, i.e. whether or not seeds have PY, and the pathway of water entry into the seed.
MATERIALS AND METHODS
Seed collection
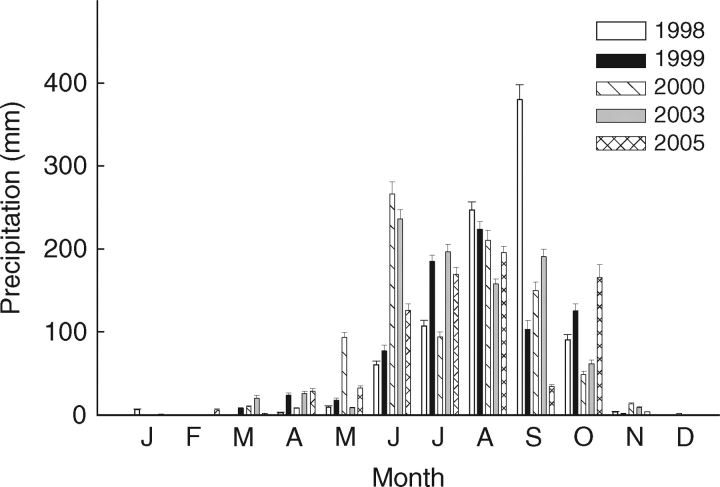
Opuntia tomentosa (cactus pear) occurs in the Central Plateau and the south-west of Mexico (Guzman et al., 2003), where it grows in open xerophilous shrubland vegetation developed on a lava field (Rzedowski, 1994; Scheinvar, 1982). Ripe fruits were collected from August to November in 1998, 1999, 2000, 2003 and 2005 from more than 12 adult plants growing at the El Pedregal de San Angel Reserve (Mexico, City, 19°19′N, 99°11′W, 2240 m a.s.l.). After dormancy break, seeds from these collections had high viability (77 ± 11·4 %, A. Orozco-Segovia et al., unpubl. res.). Mean annual rainfall at the reserve is 803 mm, 93 % of which occurs from May to October, and the mean annual temperature is 15·5 °C (Barradas et al., 1999). Precipitation during the collection years is shown in Fig. 1. Seeds were removed from fruits and stored dry in paper bags at room temperature (23–25 °C, 20–50 % relative humidity) until used in the study; some treatments were limited by seed availability.
Fig. 1.
Amount and monthly distribution of precipitation during the seed collection years.
Seed micromorphology and anatomy
The primary purpose of this part of the study was to characterize the micromorphology of the seed coat and the funicular envelope in relation to water uptake. Longitudinal cuts in seeds collected in 1998 and in 2003 were made in 2004 with a tissue chopper (Sorvall, TC-2; Smith & Farquhar, Newtown, CT, USA). The samples were mounted on aluminium stubs using carbon double tape and coated with approx. 200 nm of gold with a sputter coater (Desk II; Denton Vacuum Inc., Norristown, NJ, USA). Finally, seeds were observed with a scanning electron microscope (JSM-5310LV; Electron Optics Div., Medford, MA, USA).
In 2004, non-scarified seeds and seeds scarified for 45 or 90 min (1998 and 2003 collections) in concentrated sulfuric acid (see below) were immersed for 12, 24, 48, 72, 96, 120 and 144 h in methylene blue (1 % in water; Sigma-Aldrich Química S. A. de C. V., México) or for 96 h in neutral red stain (1 % in water, Sigma-Aldrich Química S. A. de C. V., México). Then, the seeds were cut longitudinally and observed with a photomicroscope (Olympus Provis AX-70; Olympus, Tokyo, Japan). Additionally, longitudinal cuts in seed covers (seed coat and funicular envelope), stained with red oil ‘O’ to determine if lipids were present, and funicular cells, disassociated in HNO3, were examined under the photomicroscope. To identify tannins, longitudinal cuts were treated with an aqueous solution containing 5 % formalin and 10 % Fe2SO4 for 48 h (Johansen, 1940).
Seed treatments: general procedures
In all treatments, seeds were incubated in a growth chamber at 24 °C under 20-W cool white fluorescent and 25-W incandescent lamps. The photoperiod was 12 h d−1, and photon flux density (PFD, 400–700 nm) was 33 µmol m−2 s−1. PFD was determined using a portable radiometer (LI 185 B; LI-COR Inc., Lincoln, NE, USA). Seeds, seed covers and embryos were weighed with an electronic balance with a readability of 10−5 g. All scarification treatments were done with concentrated sulfuric acid (details later), after which seeds were washed vigorously with tap water for 5 min and then air dried under laboratory conditions.
Burial treatment
Olvera-Carrillo (2001) demonstrated that after burial O. tomentosa seeds germinated to high percentages. Thus, to determine if changes in water uptake occur during burial, seeds collected in 1998 were buried in soil at a depth of 5 cm immediately after collection in November 1998 at the El Pedregal de San Angel Reserve. The seeds were buried from the beginning of the dry season, when they are naturally dispersed, to the beginning of the rainy season, when they could germinate. Seeds were placed inside a 25 × 25 cm double layer nylon bag, which in turn was placed in a 30 × 30 cm plastic mesh bag and buried 5 cm deep. Seeds were exhumed 7 months later, at the beginning of the following rainy season, washed with tap water, sterilized with a 0·2 % fungicide solution [Captan 50 (cis-N-[(trichloromethyl)thio]-4-ciclohexene-1,2-dicarboxymide); AGM, México], air dried and stored in paper bags in the laboratory at room temperature until used. Water content of exhumed seeds was determined after 2 months and after 6 years of storage in the laboratory.
Water content of seeds and mass of seed covers
In 2004, 20 seeds each collected in 1998, 1999, 2000, 2003 and 2005 were weighed and then cut longitudinally. The embryo was dissected out and weighed separately from the seed covers. The contribution of seed covers to seed mass was calculated as a percentage of seed mass: (mass of seed covers/total seed mass)×100. Embryos from seeds in the four collections were dried at 80 °C for 24 h and reweighed. Seed water content was calculated on a dry mass basis [(fresh mass – dry mass)/dry mass]×100. Water content of seeds collected in 2003 was also determined immediately after they were removed from the fruits. Osmotic potential (Ψπ) and pH of the juicy pulp in fruits from this collection were determined with an automatic osmometer (Osmette A; Precision Systems, Inc., Sudbury, MA, USA) and pH meter (HI 9219; Hanna Instruments, MI, USA), respectively; mOsm were converted to MPa.
Uptake of tritiated water
To measure water uptake by the embryo, seeds were imbibed in tritiated water. After 6 years of dry storage in the laboratory, water uptake into the embryonic tissue was determined in seeds collected in 1998 using tritiated water (500 µCi) diluted in 30 mL of tap water. Ten non-scarified seeds and ten seeds scarified for 90 min (see Olvera-Carrillo et al., 2003) were imbibed in 1·5 mL of a solution containing 25 µCi of tritiated water for 72 h inside Eppendorf tubes at room temperature. Then, the seeds were washed with 10 mL of deionized water, rinsed with tap water for 3 min, immediately frozen in liquid nitrogen and stored at −80 °C until the amount of tritium in the tissue was determined.
To determine tritium levels in the embryonic tissue, embryos were removed from the seeds, put into a 1·5-mL Eppendorf tube and ground into a fine powder, which was homogenized in 100 µL of distilled water. After centrifugation, 80 µL of this suspension were placed into a scintillation vial with 5 mL of scintillation liquid (ACS-II Scintillation Cocktail; Amersham Corp., Arlington Heights, IL, USA). Counts per minute (cpm) were determined in a Beckman Scintillation Counter (Model LS6000IC; Beckman Instruments Inc., Fullerton, CA, USA). Additionally, cpm were measured in empty vials (control 1) and in vials containing embryos of seeds which had imbibed deionized water (control 2). Finally, to determine if freezing/thawing affected water uptake by the embryo, some seeds were placed in water for a few seconds, frozen in liquid nitrogen and hydration (or not) observed in the thawed embryo.
Seed hydration
In 2004, seeds collected in 1998 were weighed, and four replicates of ten seeds per treatment were subsequently scarified in concentrated sulfuric acid for 0 (control), 1, 5, 30, 45, 60 or 120 min. After washing in tap water, seeds were air-dried for 48 h and then weighed (initial mass) and incubated on agar. Subsequently, individual seeds were weighed at short intervals during the first day and then daily for 9 d, i.e. final mass on day 10. Each time seeds were taken from the Petri dish, they were blotted with a paper towel, immediately weighed and returned to the Petri dish. Hydration of non-scarified seeds collected in 1999 and in 2000 and a sample of exhumed seeds (collected in 1998) were evaluated at the same time in the same way. Thus, water uptake was calculated as [(final mass – initial mass)/initial mass]×100.
Water uptake by embryo
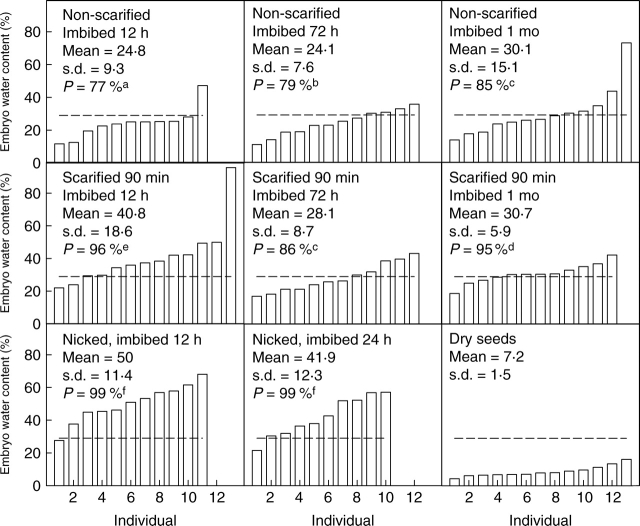
In 2004, embryo water content was determined for seeds collected in 1998 that had received various treatments: (a) dry-stored, non-scarified seeds that were not allowed to imbibe, (b) non-scarified seeds allowed to imbibe for 72 h, (c) non-scarified seeds allowed to imbibe for 1 month, (d) seeds acid scarified for 90 min and allowed to imbibe for 72 h, and (e) exhumed, non-scarified seeds allowed to imbibe for 72 h. Also in 2004, embryo water content of seeds collected in 2003 was determined for non-scarified seeds that were not allowed to imbibe and for scarified (90 min) and non-scarified seeds following 12 h, 72 h or 1 month of imbibition. In other seeds collected in 2003, the funiculus was nicked with clippers at the hypocotyl end and in the dorsal area, and seeds were imbibed for 12 or 24 h. In all experiments, there were 15 seeds per treatment. After imbibition, pieces of each embryo, as extracted, were weighed immediately, dried for 24 h at 80 °C and immediately weighed again. Water content also was determined for embryos in dry seeds and for those in seeds just beginning to germinate (incipient germination). During imbibition, seeds were placed in Petri dishes and nearly covered with water. At the end of treatments, the number of embryos evaluated varied slightly due to difficulties during embryo extraction. Water content of the embryos was calculated on a dry mass basis.
Effect of germination valves on water uptake by embryos inside seeds
The valve (see Fig. 2F) is a segment of the seed coat/funicular envelope that is dislodged during germination. The purpose of this portion of the study was to determine if water uptake increased when the valve was removed from the seeds and/or when a similar opening was artificially made in the seed in the valve area. Using a needle, the valve was removed from dry-stored, non-scarified seeds collected in 1998 and from seeds collected in 1998 that were exhumed after burial in soil in the field for 7 months. Also, seeds collected in 2003 in which the valve was not preformed were used to evaluate water uptake through an artificial opening; i.e. in 2004 an opening was made in seeds collected in 2003 using a rotary tool (DREMEL, Multipro 3956-02, Mexico) equipped with a jewelry borer (0·8 mm diameter) in the same position and with a size similar to that of the valve present on seeds collected in 1998; an opening was not made in control seeds. Subsequently, seeds with and without an open valve or an artificial opening were allowed to imbibe for 72 h on 1 % agar containing 0 or 1000 ppm GA3 (based in Olvera-Carrillo et al., 2003), and then pieces of the embryos were dissected out of the seeds, weighed, dried at 80 °C for 24 h, and reweighed. All the fifteen seeds used per treatment were always sown in the same position with the area corresponding to the valve in contact with the agar surface. Embryo water content also was determined simultaneously for dry-stored seeds from all the seed batches used. Embryo water content was calculated on a dry mass basis.
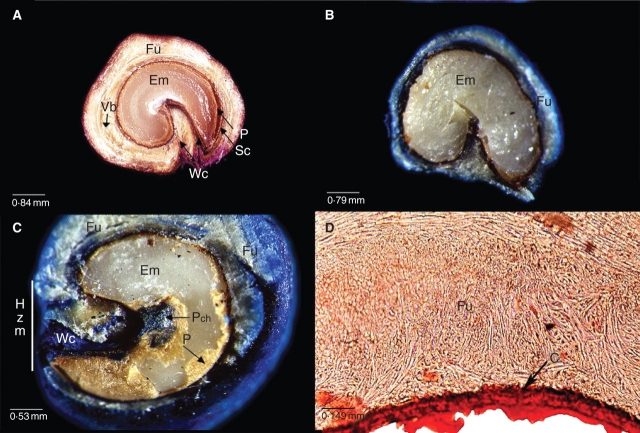
Fig. 2.
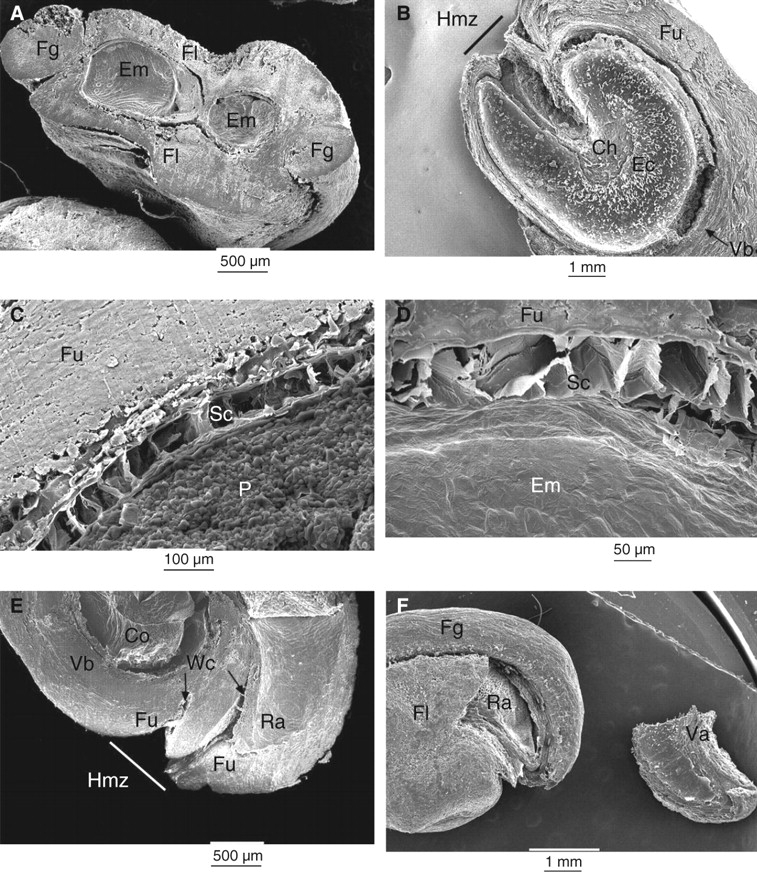
Scanning electron micrographs of (A) transverse and (B–E) longitudinal sections of O. tomentosa seeds. (B) Empty cavity in funicular envelope previously occupied by embryo. The seed coat remains can be seen attached to funiculus inside the embryo cavity. (C, D) Seed coat. (E) Hilum–micropylar zone showing water channels. (F) External morphology of seed and valve (shown on the right) detached from a dry seed. Ch, Position of the chalazal zone; Co, cotyledon; Ec, embryo cavity; Em, embryo; Fg, funicular girdle; Fl, funicular flanks; Fu, funiculus; Hmz, hilum–micropylar zone; P, perisperm; Ra, radicle; Sc, seed coat; Va, valve; Vb, vascular bundle; Wc, water channel.
Effect of burial and of seed age on opening of germination valve
Immediately after collection, a sample of seeds collected in 2003 was buried 5 cm deep in soil in the Pedregal from November 2003 to June 2004, at which time they were exhumed (mean, minimum and maximum soil temperatures during the period of burial were 21·7, 10·6 and 41·5 °C, respectively). Pressure was applied with a needle to the valve area of 20 seeds to detach the valve. Also, 20 dry, laboratory-stored seeds collected in 2003 (27 months old) or 2005 (3 months old) were exposed to 80°, 0° and −20 °C for 24 and 48 h in February 2006. The first temperature simulates the thermal time (cumulative temperature) during heat shocks produced by fires, which are common in the reserve, and it is fairly close to the high daily summer temperatures recorded on rocks in the reserve (>60 °C). The second temperature (0 °C) simulates winter temperatures at 2240 m a.s.l. (Olvera-Carrillo, 2001; Vivar-Evans et al., 2006). The third temperature (−20 °C) is commonly used for storage of orthodox seeds. Then, for each of the three samples of seeds collected in 2003 and in 2005, as well as for those from both collections that were not exposed to these temperatures (controls), the funiculus area corresponding to the valve was pushed with a needle in an attempt to detach it from the seed.
Analysis
Percentages of final seed water uptake were normalized (Shapiro–Wilcox test) with a Box–Cox transformation (Crawley, 1993). Homogeneity of variances was confirmed by Bartlett's test and then data were compared by ANOVA. Multiple comparisons were done with Tukey's test. Seed water content and scintillation counts were assessed statistically with the Kruskal–Wallis test and visually compared with box-and-whiskers plots (Tukey, 1977; Statgraphics, ver. 5·0, Statistical Graphics Co., Rockville, MD, USA). The probability that embryos reach the minimal water content for germination was calculated by logistic regression analysis using JMP ver. 4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Seed micromorphology and anatomy
In O. tomentosa, the ovule is campylotropous, and the distal funiculus fully encircles the seed forming a funicular envelope (Figs 2A, B and 3A). Funiculus trajectory begins and ends in the hilum–micropylar region, and its lateral extensions cover the entire seed surface (Figs 2B, E and 3A). The part of the funiculus closest to the testa (Fig. 2A–F) consists primarily of lignified, thick-walled sclereids and fibres that form a hard cover. The red oil ‘O’ stain indicated the presence of abundant lipids in the funicular envelope and seed coat and the presence of a thick cuticle (Fig. 3D). Outside this lignified layer, the parenchymatous funiculus cells form a soft, juicy, sweet tissue that could easily be separated manually from the seed. Seed coat cells (Fig. 2C, D) contained a large quantity of tannins, as shown by staining positive with Fe2SO4 (figure not shown).
Fig. 3.
Longitudinal sections of O. tomentosa seeds previously immersed for 144 h in 1 % solutions of (A) neutral red stain or (B and C) methylene blue. (B) Seeds were scarified for 120 min and then immersed for 144 h in the methylene blue solution. (D) Longitudinal cut stained with red oil ‘O’. C, Cuticle and seed coat; Em, embryo; Fu, funiculus; Hmz, hilum–micropylar zone; P, perisperm; Pch, perisperm covering the chalaza; Sc, seed coat; Vb, vascular bundle; Wc, water channel.
The proximal part of the funiculus is the junction of the placenta and the seed. The distal part of the long funiculus forms the hard funicular envelope and the juicy tissue. Therefore, in the mature seed that has separated from the fruit, the proximal part of the funiculus has been lost, but the hard distal portion remains, covering the seed. Two thin, weak areas are formed in the internal face of the nonvascularized funicular flanks. Longitudinal cuts show the thin areas located at both sides of the hilar region. With dehydration and ageing, these thin areas become cracks that are channels for uptake of water (Figs 2E and 3A). In old seeds (1998), the tissue between the two channels is degraded, thus forming one wide channel (Figs 2B and 3C). Twenty-seven months after collection of the 2003 seeds, water channels were not evident in all seeds, and scarification and staining did not reveal them (Fig. 3B).
In median cuts of previously acid-scarified seeds, neutral red stain entered the seeds by water channels situated in the hilar region (Fig. 3A), and methylene blue also penetrated the seed in this region up to the chalaza (Fig. 3C). In older seeds (1998), the funiculus was also stained (e.g. Fig. 3C); nevertheless, methylene blue reached the embryo only by the water channels. Scarification did not modify the route by which methylene blue penetrated into the seed (Fig. 3B). During imbibition in water, the part of the embryo in contact with the chalaza imbibed first, while in most seeds the radicular and cotyledonary ends remained dry. This could be observed when the embryo segments were compressed with a dissecting needle; thus, the first segment was wet and looked soft and brilliant and the latter two dry, firm and opaque.
During germination, the radicle pushed the seed covers, tearing the valve situated in the funicular envelope and with the adjacent seed coat attached to it (Fig. 2F). The valve was situated between two weak lines, in only one seed face. One weak line was situated near one of the water channels and the other just between the funicular girdle (dorsal part of the funiculus; sensu Stuppy, 2002) and the flanks (Fig. 2F). The valve had a scalene-like triangular form and could become totally detached from the seed (collected in 1998; Fig. 2F) or remain attached to it by one side. In old dry seeds (1998), the valve could be totally detached by pushing on it with a needle, whereas in young (2003, 2005) seeds pushing with a needle did not dislodge the valve.
Water content of seeds and mass of seed covers
The contribution of seed covers to seed mass in collections from the dry years 1998, 1999, 2000 and 2005 (precipitation concentrated in the second half of the rainy season) was significantly greater than that for seeds collected in the rainy year 2003 [precipitation distributed throughout the rainy season (Fig. 1), H = 17·18, P = 0·001; Fig. 4]. In the 2003 and 2005 collections, contribution of the seed covers to seed mass had a wide frequency distribution, while in the 1998, 1999 and 2000 collections it was skewed to higher percentages.
Fig. 4.
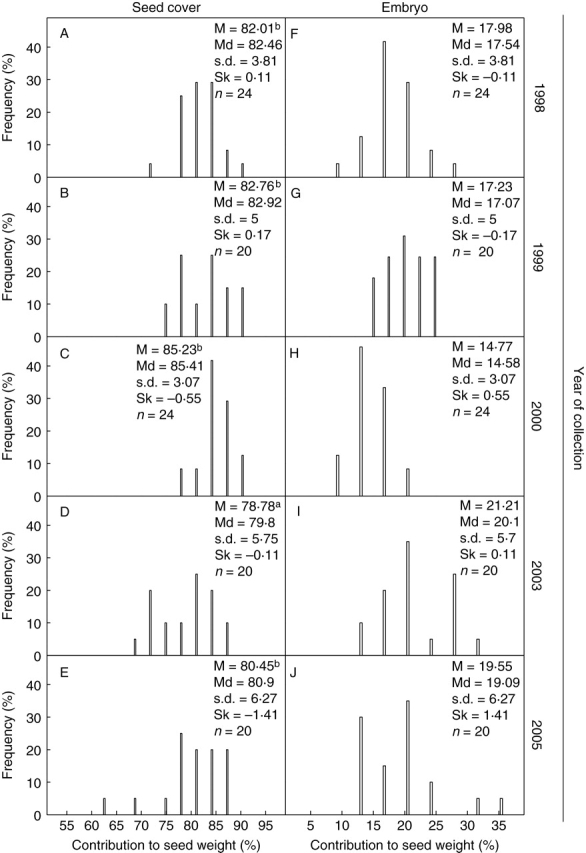
Percentage frequency distribution of mass of seed covers (funiculus and seed coat) and of embryos of O. tomentosa seeds collected in different years. Mean (M), median (Md), standard deviation (s.d.) and skewness (Sk). Letters indicate significant differences.
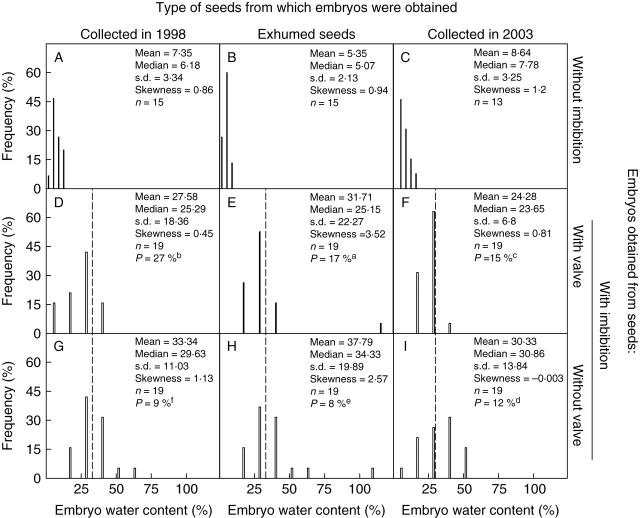
The pulp of the fruit (2003 collection), which is composed mainly of soft, juicy funicular tissue, was acidic (pH = 4) and had a low osmotic potential (−3·54 MPa). Water content of seeds immediately after they were extracted from the juicy fruit was relatively high (20·28 ± 7·8 %) in relation to the seed water content after 2 (14·21 ± 8·97) and 5 (8·64 ± 3·25) weeks of dry storage at room temperatures. Water content of the seeds collected in 2003 and stored for 5 weeks did not differ significantly from that of seeds collected in 1998 (7·35 ± 3·34), 1999 (7·57 ± 3·53), 2000 (6·36 ± 0·86) or 2005 (7·7 ± 0·8) that had been stored dry at room temperature. Water content was 5·35 ± 2·13 for exhumed seeds following 2 months of air drying and for those after 6 years of dry storage in the laboratory. Differences in seed water content among the samples were not significant (H = 11·44, P = 0·07).
Uptake of tritiated water
Embryos of non-scarified seeds had the highest and controls 2 and 1 the lowest cpm (H = 17·4, P = 0·004). The cpm in control 1 did not differ significantly from that of seeds scarified for 90 min. cpm did not differ significantly among the scarified seeds. Tritiated water was easily washed out of the scarified seeds during the brief washing with running water at the end of the imbibition period in tritiated water. Water on the surface of seeds immersed in liquid nitrogen entered the seeds after thawing; therefore, the embryos looked fully hydrated.
Seed hydration
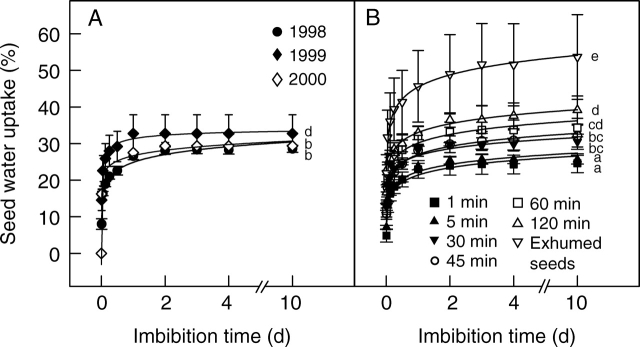
Imbibition curves for non-scarified and for scarified seeds were similar (Fig. 5). However, water uptake by exhumed seeds was significantly higher than that of any of the other seeds. In all cases, about 80 % of the water uptake occurred during the first 3 h of imbibition, and after 72 h the hydration curves were nearly asymptotic. Nevertheless, a minimal amount of water continued to be taken up for an additional 3–7 d, mainly by exhumed seeds. In seeds collected in 1998, final water uptake was significantly higher in seeds scarified for 30–120 min than in those scarified for 1 or 5 min (W = 0·98, P = 0·78; Barlett's test = 1·44, P = 0·39; F9,38 = 20·42, P = 0·00001). Non-scarified seeds took up significantly more water than those scarified for 1 or 5 min. Only non-scarified seeds collected in 1999 took up water to a similar percentage as those scarified for 60 and 120 min. Water uptake in non-scarified seeds was primarily due to hydration of druses and mucilage, which were corroded by sulfuric acid in scarified seeds.
Fig. 5.
Percentage of water uptake by O. tomentosa seeds on agar surface. (A) Non-scarified seeds collected in 1998, 1999 and 2000. (B) Seeds collected in 1998 and scarified for 1, 5, 30, 45, 60 or 120 min, and non-scarified seeds exhumed after a 7-month period of burial in the field. After collection or exhumation, all seeds were stored in the laboratory (23–25°C, 20–50% RH) inside paper bags until used. Bars indicate s.d. Letters indicate significant differences.
Water uptake by the embryo
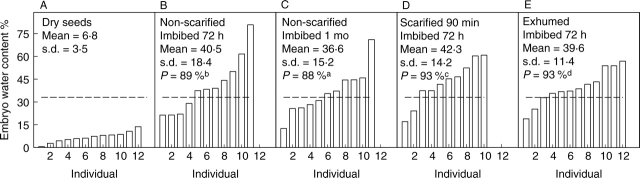
Water content of incipiently germinating embryos, determined when the radicle was visible beneath the valve, varied widely for seeds collected in 1998 (76·05 ± 46·5, mean ± s.d.) and in 2003 (66·61 ± 43·4). Most embryos from all treatments took up water; however, not all of them reached the minimal water content of incipiently germinating embryos (33 % and 29 % for collections made in 1998 and 2003, respectively). For seeds collected in 1998, the probability of reaching the minimal water content for germination was high, but it varied among treatments. The relatively lowest probabilities were for non-scarified seeds (89 %; F1,87 = 43, P = 0·0001; Fig. 6B, C); a few embryos did not reach this percentage. Embryos from exhumed and from scarified seeds allowed to imbibe for 72 h had the highest probabilities of reaching the minimal water content for germination (94 %; F1,87 = 78·18, P = 0·0001; Fig. 6D, E). Seeds collected in 2003 had a similar pattern. Non-scarified seeds had the lowest probability of reaching the minimal water content for germination (77 %; F2,187 = 366·7, P = 0·0001; Fig. 7A), and embryos from nicked seeds had the highest probability (99 %) of reaching this level of hydration. The non-scarified seeds collected in 2003 had lower probabilities than seeds collected in 1998. Most of the embryos from exhumed (Fig. 6E), nicked (Fig. 7G, H) and scarified (Fig. 7D–F) seeds reached the minimal water content for germination after 12 h of imbibition. Nonetheless, embryos from seeds scarified or nicked and allowed to imbibe for >12 h had a lower water content than embryos from the same treatments allowed to imbibe for 12 h.
Fig. 6.
Water content (dry mass basis) of embryos isolated from (A) dry seeds of O. tomentosa (collected in 1998) and from those that were (B, C) non-scarified, (D) acid-scarified or (E) field-stored (exhumed seeds) and incubated nearly covered by water in Petri dishes. Dashed lines indicate minimal water content required for germination in the seed population. P = probability of reaching the minimal water content required for germination. Lower-case letters indicate significant differences.
Fig. 7.
Water content (dry mass basis) of embryos isolated from (A) seeds of O. tomentosa (collected in 2003) that were (A–C) non-scarified, (D–F) acid-scarified or (G, H) nicked and incubated nearly covered by water in Petri dishes. (I) Dry seeds. Dashed lines indicate minimal water content required for germination in the seed population. P = probability of reaching the minimal water content required for germination. Lower-case letters indicate significant differences.
Effect of germination valves on water uptake by embryos inside seeds
Not all seeds had taken up the same amount of water after 72 h of imbibition, and in all treatments there was significant variation among individuals and treatments (Fig. 8). The probability that seeds sown on the wet agar surface did not reach the maximal water content for germination after 72 h was significantly higher for seeds with a valve (19 %) than for those without a valve (10 %; F1,295 = 481·6, P = 0·0001). Dry-stored seeds collected in 1998 had significantly higher probabilities of not reaching this minimal water content (16·6 %) than exhumed seeds collected in 1998 (13 %) or of those collected in 2003 (14 %, F2,295 = 10·51, P = 0·0001). In only a relatively few embryos was water content in the seeds with or without a valve lower than the minimal water content for germination (Fig. 8D–F and G–I). GA3 did not modify water uptake (data not shown).
Fig. 8.
Percentage frequency distribution of water content (dry mass basis) of embryos isolated from (A–C) dry seeds of O. tomentosa and of those with (D–F) and without (G–I) a valve imbibed on agar for 72 h. Dashed lines indicate the minimal water content required for each group of seeds to germinate. Exhumed seeds were collected in 1998. P = probability that seeds do not reach the minimal water content required for germination. Lower-case letters indicate significant differences.
Effect of burial and of seed age on opening of germination valve
In all exhumed seeds, the valve was manually removed without any temperature treatment. For all seeds collected in 2003 and stored in the three temperature treatments, it was possible to remove the valve in 50 ± 0 % and 91·66 ± 2·89 % of them after 24 and 48 h, respectively, with no variation among treatments. On the other hand, in laboratory stored seeds collected in 2003 that were not given these three temperature treatments, and in all seeds collected in 2005, this was not possible because the lines of weakness that delimit the valve apparently had not formed. Therefore, the area corresponding to the valve was firmly attached to the funicular cover.
DISCUSSION
Anatomical and functional evidence showed that the seed covers of O. tomentosa are permeable to water. Nevertheless, the hard funicular envelope acts as a partial barrier to water diffusion into the seed and to radicle protrusion. Changes occurring in the funicular cover during ageing and seed dehydration play an important role in water uptake dynamics and germination of the seed. Scanning electron micrographs of O. tomentosa seeds (Fig. 2) showed that the seed coat is covered by a hard funicular envelope (Ganong, 1898; Bregman and Bouman, 1983; Bregman, 1988; Stuppy, 2002). However, neither the seed coat nor the funicular layer had a palisade layer(s) of macrosclereids (also see figs 44–52 in Maheshwari and Chopra, 1955) characteristic of seeds (or fruits) with physical dormancy (Baskin et al., 2000). Both scarified and non-scarified (nonburied) seeds imbibed some water, with about 80 % of the water uptake occurring within 3 h (Fig. 5). Thus, it is concluded that seeds of O. tomentosa do not have physical dormancy. Lack of water-impermeability in nontreated seeds of Opuntia in the present study is supported by observations on seeds of several other Opuntia species. Wiggins and Focht (1967) reported that seeds of Opuntia echios var. gigantea imbibed water without treatment, and Freeman (1969) stated that untreated seeds of O. basilaris imbibed water quickly. Deno (1993, pp. 182–183) stated, ‘It has been reported that cactus have impervious seed coats. Extensive experiments on O. phaecantha showed that at least for this species impervious seed coats are not the problem [i.e. for lack of germination]’(also see Deno, 1994). Although Reyes-Agüero et al. (2006) said that, ‘The Opuntia seed of several species presents dormancy associated with tegument impermeability …’, the present results and those of Wiggins and Focht (1967), Freeman (1969) and Deno (1993, 1994) do not support this conclusion. However, although seeds of O. tomentosa do not have physical dormancy, the covering layers of the embryo play an important role in the dynamics of the water movement into and out of the seeds, which delay germination and thus promote formation of a soil seed bank (A. Orozco-Segovia et al., unpubl. res.; this study).
As in other Opuntia species (Sawaya et al., 1983; Pimienta and Engleman, 1985), freshly matured seeds of O. tomentosa are immersed in a juicy tissue with a low osmotic potential, which nevertheless is insufficient to dry the seeds. Thus, seeds are dispersed with a relatively high water content, and the final stages of drying occur after dispersal. One of the consequences of drying could be the formation of a channel in the hilar region (Fig. 2F), which is important in the water relations of the seed. Longitudinal sections of seeds that had been exposed to methylene blue showed that water enters the seed only through this channel. Further, the pathway of dye penetration was not modified by 90 min of acid scarification, possibly due to the cutin and tannins in the seed cover. However, there was much variation in formation of the water channel, which resulted in water uptake varying widely among seed collections and among individuals within a seed lot. This heterogeneity of the seed covers probably can be attributed to maternal effects (Fig. 4; Aitken, 1939; Marbach and Mayer, 1974; Argel and Humpreys, 1983; Clua and Gimenez, 2003). For example, high amount of water applied by irrigation during seed development increased seed mass in Opuntia ficus-indica (Mulas and D'hallewin, 1997).
In addition to opening a water channel, which has not been reported by previous authors, germination of O. tomentosa seeds was also preceded by the formation of lines of weakness that delimit the valve during seed drying and ageing (Fig. 2E). Thus, the steps in germination are: (a) opening of the water channel; (b) a little water enters the water channel; (c) the embryo takes up some water; (d) the slightly swollen embryo pushes against the preformed valve; (e) the lines around the edge of the valve become externally visible; (f) following more uptake of water by the embryo the valve opens; (g) imbibition of water by the embryo is completed; and (h) the radicle emerges. Each step leading to germination of O. tomentosa seeds can occur at different times, depending on climate (precipitation and temperature) and on microsite characteristics, such as those in the heterogeneous lava-derived soil in the ‘El Pedregal of San Angel’ (Vivar-Evans et al., 2006) and in other microsites within the geographical range of the species; therefore, germination can vary widely in time and space.
Bregman and Bouman (1983) described the micropylar area, which corresponds to the position of the valve in the present study, in seeds of (the inoperculate) Opuntioideae as the weakest region in the funicular envelope and thus the place where the radicle emerges. This type of germination morphology is characteristic of Opuntia, and the valve could play a role similar to an operculum. However, according to Werker (1997) germination in Opuntioideae is delayed by the hard funiculus, and seeds do not have an operculum. In contrast, seeds of other Cactaceae such as Echinocactus and Cereus have an operculum but no funicular envelope. In seeds with a hard funiculus, valve formation occurs during seed drying and ageing, or simultaneously with formation of the water channel, and imbibition can occur via the water channel before valve delimitation. Thus, the valve is not a specialized water-restricting structure (‘water gap’) characteristic of water-impermeable (i.e. physically dormant) seeds (Baskin, 2003).
Drying conditions can determine funicular envelope characteristics and the time required for water channel and valve formation. After exhumation, the valve was completely delimited in 100 % of the seeds collected in 2003; thus, it was removed easily with a needle or expelled during germination (personal observations). However, it was not possible to remove the valve with a needle in non-scarified seeds from the 2003 and 2005 collections stored dry in the laboratory for 27 and 3 months, respectively. In contrast, the valve could be removed easily from seeds collected in 1998 following 6 years of dry storage in the laboratory, suggesting that under laboratory conditions dehydration of the funicular envelope could be a slow, but effective, way of weakening the union between the lignified cells. Heating and freezing for 24 and 48 h also promoted weakening of these cells, suggesting that under field conditions valve formation could occur in a relatively brief period of time.
The water content in individual incipiently germinating embryos of O. tomentosa varied widely; therefore, it was difficult to determine the critical water content for germination. Nevertheless, based on the minimal water content of embryos in which radicle protrusion had just begun, some embryos did not take up water, some took up an insufficient amount to germinate and many others a sufficient amount to germinate. The changes in water uptake by non-scarified seeds were insufficient to assess seed water uptake, due to the fact that water uptake by non-scarified seed collected in the different years was higher than that in seeds scarified for a few minutes. This was related to the water retained by the druses and mucilage present on the seed surface; nevertheless, embryo water uptake confirms that water entered the seeds.
In embryos from all collections (excepting exhumed seeds) that took up at least a minimal amount of water, the cotyledon and radicle ends remained dry, while the chalazal region imbibed, suggesting that the funicular envelope also represents a restriction to increase in embryo volume and thus to water uptake. This restriction may be related to the lack of external evidence for water uptake and to lack of germination in seeds of Opuntia tomentosa and several other Opuntia species, which did not swell when in contact with a moist substrate (Hamilton, 1970a; Beltran, 1984). Further, seed envelopes of O. tomentosa seeds produced in 1998 were more resistant to water uptake than those of seeds produced in 2004, perhaps due to the climatic conditions produced by ‘El niño’ in 1998, when precipitation occurred mainly in the second half of the rainy season, and temperatures in spring–summer were higher than those in other years.
Although seeds of O. tomentosa do not have physical dormancy, seed hydration can be an important factor limiting germination. Extended periods of drying, e.g. 6 years in dry storage, promoted opening of the water channel, but extended periods of incubation in the soil may be the major factor in opening the water channel in nature. For example, the valve could be pushed off of all seeds of O. tomentosa buried in the field from November to June. In other studies on O. tomentosa, non-scarified seeds incubated on a moist substrate for up to 120 d germinated to only about 20 % (Olvera-Carrillo et al., 2003), but lack of germination does not prove that the water channel was closed. In fact, based on rates of imbibition of non-scarified seeds in the study (Fig. 5), it appears that seeds do imbibe partially when incubated on a moist substrate. However, imbibition may not result in immediate germination. Decreases in embryo water content after 72 h of imbibition suggest that during partial imbibition cell division precedes germination, as has been reported for seeds imbibed under restricted water availability (Gray et al., 1990). The seed hydration study using tritiated water showed that cpm was higher for embryos in non-scarified seeds than for those in scarified seeds. This could occur because the cover layers (funiculus/seed coat) of scarified seeds were more permeable than those of non-scarified seeds. In which case, more of the tritiated water was rinsed from scarified than from non-scarified seeds. Scarification made the funicular envelope more permeable and weaker, thus favouring uptake of water and embryo expansion, as occurred in exhumed seeds.
If seeds of O. tomentosa and other Opuntia species imbibe water before physiological dormancy is broken, the embryo may not have enough growth potential (‘push power’) to open the valve. On the other hand, if seeds imbibe water after physiological dormancy has been partly (e.g. O. tomentosa seeds that were buried from November to June) or completely broken, the embryo has enough growth potential to loosen the valve, thereby facilitating further water uptake and thus emergence of the radicle (Fig. 5B). Archibald (1939) reported that seeds of O. aurantiaca from which ‘the micropylar end of the funicle’ had been removed germinated to 92 % (versus 0 % in the control). Interestingly, he concluded that, ‘The well-developed embryo and the structure of the seed coat [including funiculus] indicated that it was probably mechanical resistance to the expansion of the embryo, which caused the prolonged dormancy [i.e. physiological dormancy] which has been found to exist by preliminary germination tests.’ In the case of O. tomentosa embryos with low growth potential, it is easy to understand why scarification of seeds would reduce the mechanical restriction on the embryo and promote germination (Olvera-Carrillo et al., 2003). Thus, it appears that a non-dormant embryo, as well as opening the water channel and valve delimitation and its eventual expulsion from the seed, are required for germination of O. tomentosa seeds, along with, of course, appropriate temperature, moisture and light conditions. All these germination requirements can be adaptive advantages that enhance dispersal of germination in space and time in heterogeneous and changing environments, such as lava fields and deserts. Compared with laboratory-stored seeds, exhumed seeds showed a reduction in water uptake heterogeneity due to funicular envelope weakness, which included valve formation. Nevertheless, the dynamics and frequency distribution of water uptake by embryos in all of the seed samples and treatments confirmed the lack of physical dormancy and the role of the funicular envelope in the restriction of water uptake and for radicle protrusion.
ACKNOWLEDGEMENTS
The authors thank Dr E. Mark Engleman for comments on the manuscript, Ms Alejandro Martínez Mena and Biol. José Antonio Hernández for taking the microphotomicrographs, Dr Silvia Espinosa and biologist Yolanda Ornelas for scanning electron microscopy and Dr Marcela Aguilar, Ms Ricardo Wong and Biol. Esther Zúñiga Sánchez for technical support (CONACyT grant 47859Q).
LITERATURE CITED
- Aitken Y. The problem of hard seeds in subterranean clover. Proceedings of the Royal Society of Victoria. 1939;51:187–213. [Google Scholar]
- Aguilar A. Caracterización de la semillas de 403 variantes de nopal (Opuntia spp.) y sus implicaciones agroindustriales. México: Universidad Autónoma de San Luis Potosí; 2003. Bachelor Thesis. [Google Scholar]
- Archibald EEA. The development of the ovule and seeds of jointed cactus (Opuntia aurantiaca) South African Journal of Science. 1939;36:195–211. [Google Scholar]
- Argel PJ, Humphreys LR. Environmental effects on seed development and hardseedness in Stylosanthes hamata cv. Verano. II. Moisture and illuminance. Australian Journal of Agricultural Research. 1983;34:271–277. [Google Scholar]
- Barradas VL, Tejeda-Martínez A, Jáuregui E. Energy balance measurements in a suburban vegetated area in Mexico City. Atmospheric Environment. 1999;33:4109–4113. [Google Scholar]
- Baskin CC. Breaking physical dormancy in seeds-focusing on the lens. New Phytologist. 2003;158:229–232. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. 1st edn. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. Seed and seedling ecology of Opuntia compressa in Tennessee cedar glades. Journal of the Tennessee Academy of Science. 1977;52:118–122. [Google Scholar]
- Baskin JM, Baskin CC. Classification, biogeography, and phylogenetic relationships of seed dormancy. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. London: Royal Botanical Gardens, Kew; 2003. pp. 517–544. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Beltran M. Evaluación del potencial germinativo en el laboratorio de cinco especies de Opuntia de los estados de San Luis Potosí y Zacatecas. Morelia, Michoacán, México: Universidad Michoacana de San Nicolás de Hidalgo; 1984. Bachelor Thesis. [Google Scholar]
- Bregman R. Forms of seed dispersal in Cactaceae. Acta Botanica Neerlandica. 1988;37:395–402. [Google Scholar]
- Bregman R, Bowman F. Seed germination in Cactaceae. Botanical Journal of the Linnean Society. 1983;86:357–374. [Google Scholar]
- Clua AA, Gimenez DO. Environmental factors during seed development of narrow-leaved birds's-foot-trefoil (Lotus tenuis) influences subsequent dormancy and germination. Grass and Forage Science. 2003;58:333–338. [Google Scholar]
- Crawley MJ. GLIM for ecologist. 1st edn. Oxford: Blackwell Scientific Publications; 1993. [Google Scholar]
- Deno NC. Seed germination theory and practice. 1st edn. PA, USA: Published by the author, State College; 1993. [Google Scholar]
- Deno NC. The critical role of gibberellins in germination and survival of certain cacti. Cactus and Succulent Journal (USA) 1994;66:28–30. [Google Scholar]
- Freeman TP. The development of Opuntia basilaris. I. Embryo, root, and transition zone. American Journal of Botany. 1969;56:1067–1074. [Google Scholar]
- Ganong WF. Upon polyembryony and its morphology in Opuntia vulgaris. Botanical Gazette. 1898;25:221–228. +plate XVI. [Google Scholar]
- Gimeno I, Vila M. Recruitment of two Opuntia species invading abandoned olive groves. Acta Oecologia. 2002;23:239–246. [Google Scholar]
- Gray D, Steckel JRA, Hands LJ. Responses of vegetable seeds to controlled hydration. Annals of Botany. 1990;66:227–235. [Google Scholar]
- Guzmán U, Arias S, Dávila P. Catalogo de cactáceas mexicanas. 1st edn. México, D. F, México: Universidad Nacional Autónoma de México and CONABIO; 2003. [Google Scholar]
- Hamilton MW. Seedling development of Opuntia bradtiana (Cactaceae) American Journal of Botany. 1970a;57:599–603. [Google Scholar]
- Hamilton MW. The comparative morphology of three cylindropuntias. American Journal of Botany. 1970b;57:1255–1263. [Google Scholar]
- Johansen DA. Plant microtechnique. 1st edn. New York, NY: McGraw-Hill; 1940. [Google Scholar]
- Johnson DS. The fruits of Opunita fulgida: a study of perennation and proliferation in fruits of certain Cactaceae. 1st edn. Carnegie Institution of Washington; 1918. Publication No. 269. [Google Scholar]
- Maheshwari P, Chopra RN. The structure and development of the ovule and seed of Opuntia dillenii Haw. Phytomorphology. 1955;5:112–122. [Google Scholar]
- Mandujano MC, Golubov J, Montaña C. Dormancy and endozoochorous dispersal of Opuntia rastrera seeds in the northern Chihuahuan desert. Journal of Arid Environments. 1997;36:259–266. [Google Scholar]
- Mandujano MC, Montaña C, Rojas-Aréchiga M. Breaking seed dormancy in Opuntia rastrera from the Chihuahuan desert. Journal of Arid Environments. 2005;62:15–21. [Google Scholar]
- Marbach I, Mayer AM. Permeability of seed coats to water as related to drying conditions and metabolism of phenolics. Plant Physiology. 1974;54:817–820. doi: 10.1104/pp.54.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulas M, D'hallewin G. Fruit quality of four cactus pear (Opuntia ficus-indica Mill.) cultivars as influenced by irrigation. Acta Horticulturae. 1997;438:97–103. [Google Scholar]
- Olvera-Carrillo Y. Estudio ecofisiológico de la germinación, sobrevivencia y crecimiento de Opuntia tomentosa S. D. en la Reserva del Pedregal de San Angel. México, México: Universidad Nacional Autónoma de; 2001. Bachelor Thesis, Facultad de Ciencias. [Google Scholar]
- Olvera-Carrillo Y, Márquez-Guzmán J, Barradas VL, Sánchez-Coronado ME, Orozco-Segovia A. Germination of the hard seeds of Opuntia tomentosa S. D., a cactus from the Mexico Valley. Journal of Arid Environments. 2003;55:29–42. [Google Scholar]
- Pendley GK. Seed germination experiments in Opuntia (Cactaceae) of the northern Chihuahuan desert. Haseltonia. Yearbook of the Cactus and Succulent Society of America. 2001;8:42–50. [Google Scholar]
- Pilcher BL. Germination of seeds of four species of Opuntia. Cactus and Succulent Journal (USA) 1970;42:281–282. [Google Scholar]
- Pimienta BE, Engleman EM. Desarrollo de la pulpa y proporción, en volumen, de los componentes del lóbulo maduro en tuna (Opuntia ficus-indica [L.] Miller) Agrociencia. 1985;62:51–56. [Google Scholar]
- Potter RL, Petersen JL, Ueckert DN. Germination responses of Opuntia spp. to temperature, scarification, and other seed treatments. Weed Science. 1984;32:106–110. [Google Scholar]
- Reinhardt CF, Rossouw L, Thatcher L, Lotter WD. Seed germination of Opuntia stricta: implications for management strategies in the Kruger National Park. South African Journal of Botany. 1999;65:295–298. [Google Scholar]
- Reyes-Agüero JA, Aguirre RJR, Valiente-Banuet A. Reproductive biology of Opuntia: a review. Journal of Arid Environments. 2006;64:549–585. [Google Scholar]
- Rzedowski J. Vegetación del Pedregal de San Angel. In: Rojo A, editor. Reserva ecológica del Pedregal de San Ángel Ecología, historia natural y manejo. México, D. F, México: Universidad Nacional Autónoma de México; 1994. pp. 9–65. [Google Scholar]
- Sánchez-Venegas G. Germinación, viabilidad y características distintivas de la semilla de Opuntia joconostle Weber, forma cuaresmeño. Cactáceas y Suculentas Mexicanas. 1997;42:16–21. [Google Scholar]
- Sawaya WN, Khatchadourian HA, Safi WM, Al-Muhammad HM. Chemical characterization of prickly pear pulp, Opuntia ficus-indica and the manufacturing of prickly pear jam. Journal of Food Technology. 1983;18:183–193. [Google Scholar]
- Scheinvar L. La familia de las cactáceas en el Valle de México. México, D. F., México: Universidad Nacional Autónoma de México; 1982. PhD Thesis. [Google Scholar]
- Stuppy W. Seed characters and the generic classification of the Opuntioideae (Cactaceae) In: Hunt D, Taylor NP, editors. Succulent plant research. Vol 6. London: Royal Botanical Gardens, Kew; 2002. pp. 25–58. [Google Scholar]
- Timmons FL. The dissemination of prickly pear seed by jack rabbits. Journal of the American Society of Agronomy. 1942;34:513–520. [Google Scholar]
- Tukey JW. Exploratory data analysis. 1st edn. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Vivar-Evans S, Barradas VL, Sánchez-Coronado ME, Gamboa A, Orozco-Segovia A. Seed germination of wild Dahlia coccinea (Asteraceae) in a spatially heterogeneous fire-prone habitat. Acta Oecologica. 2006;29:187–195. [Google Scholar]
- Werker L. Seed anatomy. Encyclopedia of plant anatomy. 1st edn. Berlin: Gebrúder Borntraeger; 1997. [Google Scholar]
- Wiggins IL, Focht DW. Seeds and seedlings of Opuntia echios var. gigantea Dawson. Cactus and Succulent Journal (USA) 1967;39:26–30. [Google Scholar]