Abstract
Background and Aims
Plant aerial development is well known to be affected by day length in terms of the timing and developmental stage of floral transition. Arabidopsis thaliana is a ‘long day’ plant in which the time to flower is delayed by short days and leaf number is increased. The aim of the work presented here was to determine the effects of different day lengths on individual leaf area expansion. The effect of flower emergence per se on the regulation of leaf expansion was also tested in this study.
Methods
Care was taken to ensure that day length was the only source of micro-meteorological variation. The dynamics of individual leaf expansion were analysed in Ler and Col-0 plants grown under five day lengths in five independent experiments. Responses at cellular level were analysed in Ler plants grown under various day lengths and treatments to alter the onset of flowering.
Key Results
When the same leaf position was compared, the final leaf area and both the relative and absolute rates of leaf expansion were decreased by short days, whereas the duration of leaf expansion was increased. Epidermal cell number and cell area were also altered by day-length treatments and some of these responses could be mimicked by manipulating the date of flowering.
Conclusions
Both the dynamics and cellular bases of leaf development are altered by differences in day length even when visible phenotypes are absent. To some extent, cell area and its response to day length are controlled by whole plant control mechanisms associated with the onset of flowering.
Key words: Arabidopsis thaliana, day length, leaf expansion rate, duration of expansion, cell division, flowering
INTRODUCTION
Generally, studies of the effect of day length concentrate upon photoperiod sensing and the control of floral transition (Onouchi and Coupland, 1998; Carré, 2001; Yanovsky and Kay, 2002). There has been little detailed characterization of the effects of day length on individual leaf expansion per se. Previous investigations of this type have generally been done on plants grown under field conditions in which the precise effects of photoperiod on development have been difficult to elucidate because different day lengths occurred with changes in leaf temperature and/or cumulative daily light supply (e.g. Slafer et al., 1994; Bulter et al., 2002). In a few studies during the 1960s, the part played by daily light supply and its two components, day length and light intensity, in influencing leaf development was investigated in plant culture chambers (Arney, 1956; Newton, 1963). It was reported that the amount of radiation per day had a far greater influence on individual leaf area than either of its components per se (Newton, 1963). Nevertheless, significant effects of day length on individual leaf expansion have been reported. For example, final leaf size was increased by short-day treatments in Cucumis sativus (Newton, 1963) or by long-day treatments in strawberry (Arney, 1956) and temperate grasses (Hay, 1990).
Arabidopsis thaliana is a ‘long day’ plant in which the time to flower is delayed by short days and consequently leaf number is increased. In this species, the effect of day length on floral transition has been studied in many accessions, mutants and transgenic lines (Lempe et al., 2005). Physiological and molecular events associated with these responses have been described (Corbesier and Coupland, 2005). Some genes that regulate sensitivity to day length also have a role in the regulation of leaf development at the cellular level (Wang et al., 2003), suggesting that both processes are somehow related. This work aims to investigate the effect of different day length conditions on individual leaf area and on the dynamics and cellular bases of leaf expansion in this species. Leaf expansion and cell division are thought to depend upon the number of leaves growing at a given time (Wilson, 1966) and on the presence or absence of the vegetative shoot apical meristem (Ashby, 1948), which both are affected by day length. Therefore, the impact of whole-plant control mechanisms associated with leaf expansion was also investigated by manipulating the timing of floral transition and examining its effect on leaf development.
MATERIALS AND METHODS
Plant culture conditions
Seeds of A. thaliana (L.) Heynh., ecotypes Landsberg erecta (Ler) and Columbia-0 (Col-0), (Nottingham Arabidopsis Stock Centre) were grown in growth chambers for five experiments. Light in the growth chambers was provided by a bank of cool-white fluorescent tubes and sodium lamps. Air temperature and relative humidity were measured by sensors at 20-s intervals (HMP35A Vaisala Oy, Helsinki, Finland). Leaf temperature was measured with copper-constantan thermocouples touching the lower side of the lamina after leaf emergence. All measurements of temperature, PPFD (photosynthetic photon flux density) and relative humidity were averaged and stored every 600 s by a datalogger (Campbell Scientific, LTD- CR10 Wiring Panel, Shepshed, Leics., UK). Vapour pressure deficit (VPD) was calculated and monitored continuously during the experiments and kept below 0·8 kPa. Air temperature was maintained between 18·5 and 20 °C and air humidity was maintained to regulate the desired VPD in all treatments. Data concerning the environmental conditions of plant culture are given in Table 1.
Table 1.
Mean environmental conditions during expts 1–5
| Expt | Ecotype | Day length (h) | Daily incident PPFD (mol m2 d−1) | Instantaneous incident PPFD (μmol m−2 s−1) | Relative humidity (%) | Air temp. (°C) | Leaf temp. (°C) |
|---|---|---|---|---|---|---|---|
| 1 | Ler | 12 | 12·1 | 279 | 73·1 | 19·3 | 19·2 |
| 1 | Ler | 16 | 11·8 | 205 | 67·4 | 19·8 | 19·4 |
| 2 | Col-0 | 12 | 7·6 | 177 | 72·5 | 18·9 | 20·5 |
| 3 | Col-0 | 16 | 7·7 | 134 | 80·9 | 18·5 | 20·3 |
| 4 | Ler | 10 | 9·3 | 257 | 81·9 | 18·7 | 19·6 |
| 4 | Ler | 10 h→20 h | 9·3→9·2 | 257→128 | 78·7 | 18·7 | 19·6 |
| 4 | Ler | 20 | 9·2 | 128 | 75·5 | 18·8 | 19·5 |
| 5 | Ler | 14 | 9·3 | 184 | 76·4 | 19·8 | 19·7 |
Seeds were stored at 4 °C before sowing. In expts 1, 4 and 5, five seeds were sown in 250 cylindrical pots (53 mm in diameter and 88 mm in height) containing a 50 : 50 mixture (v/v) of loamy soil and organic compost. In expts 2 and 3, 80 seeds were sown in eight large plastic containers (0·5 m wide, 0·5 m long and 0·15 m deep). To avoid population density effects, young seedlings were thinned to one plant per pot 10 d after plant germination in expts 1, 4 and 5 and to 40 plants per container in expts 2 and 3. In each experiment, the substrate was maintained at 80 % of field capacity (corresponding to a soil water content of 0·50 g g−1 of dry soil) by weighing the pots or container once a day and watering them with a modified one-tenth strength Hoagland's solution with additional micronutrients (Hoagland and Arnon, 1950).
During expt 1, Ler plants were grown under either a 12- or a 16-h day length and the daily PPFD was approx. 12 mol m2 d−1 which, on average, corresponded to 279 and 205 µmol m2 s−1, respectively. During expt 2, Col-0 plants were cultured under a 12-h day length, whereas during expt 3 they were grown under a 16-h day length. Daily PPFD during these two experiments was of 7·6 and 7·7 mol m2 d−1, respectively which, on average, corresponded to 134 and 177 µmol m2 s−1, respectively. During expt 4, Ler plants were grown under either a 10- or a 20-h day length with a daily PPFD of 9·2–9·3 mol m2 d−1. This corresponded to an instantaneous incident PPFD of 128 and 257 µmol m−2 s−1 for plants grown under a 20- or a 10-h day length. An additional treatment was imposed during expt 4 when leaf 10 was initiated half the plants grown under the 10-h day length were transferred to the 20-h day length to accelerate the onset of flowering. During expt 5, Ler plants were grown under a 14-h day length and when the flower bud emerged it was removed from half of the plants being cultured.
In all experiments, neutral shading was used to alter the light quantity in the chambers without affecting its spectrum as measured using a LI-1800 spectroradiometer (LI-COR, Lincoln, NB, USA; data not shown).
Growth analysis
Number of leaves initiated
Five plants were harvested at intervals of 1–3 d. Plants were dissected in a drop of water using a microscope (Leica stereomicroscope, wild F8Z; Wetzlar, Germany) at a magnification of × 160. The number of leaves initiated (number of leaves visible on the apex) was counted. Leaves were visible when their areas were approx. 0·001 mm2.
Individual leaf expansion, calculations of rate and duration of leaf expansion
Areas of leaves were measured at intervals of 1–3 d from initiation to the end of expansion for all rosette leaves produced during an experiment. From leaf initiation to leaf emergence, this was done by dissecting the apex of five plants in a drop of water under the microscope, the area of the excised leaf 6 was measured with image analysis software (Bioscan-Optimas V 4·10, Edmonds, WA, USA). After leaf emergence, leaf area of five plants per treatment was measured non-destructively with the aforementioned image analysis software on digital photographs until the end of leaf expansion.
A sigmoidal curve was fitted to the curve relating leaf area (y) to time (X):
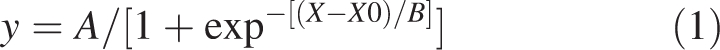 |
1 |
where B is the slope parameter and X0 is the curve inflection point parameter. Final leaf area was calculated as the upper asymptote (A, the plateau) of the sigmoidal curve (eqn 1). Leaf expansion was considered to begin at the time at which the leaf was initiated and to end when it reached 95 % of its final area as calculated from the sigmoidal curve (eqn 1). The maximum absolute expansion rate (LERmax) was calculated as the point of inflection of the fitted sigmoidal curve by the equation (adapted from Torres and Frutos, 1989):
| 2 |
Absolute leaf expansion rate at time j (LERj) was calculated from initiation to the end of expansion as the local slope of the relationship between leaf area and time. It was calculated by calculating the slope over three data points (j + and −2 or 3 d) which was plotted against the mean time over the period for which it was calculated.
Leaf relative expansion rate at time j (RERj) was calculated from initiation to the end of expansion as the local slope (at time j) of the relationship between the logarithm of leaf area (A) and time (see Granier and Tardieu, 1998a):
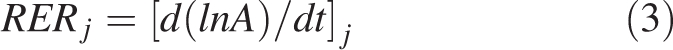 |
3 |
Mean initial relative leaf expansion rate (RERi) was calculated as the mean slope of the relationship between leaf area and time on a logarithmic scale during the period that this relationship is near-linear (see Granier et al., 2002).
Epidermal cell area and number
When the five leaves followed by digital pictures were fully expanded, a transparent negative film of the adaxial epidermis was obtained after evaporation of a varnish spread on the upper surface of the leaf. Films were placed under a microscope (Leica, Leitz DM RB, Wetzlar) coupled to a colour video camera (CCD IRIS/RGB, Sony, Japan) and the cell areas were measured using the aforementioned image analysis software. The area of a total of 100 cells per leaf was measured, corresponding to four patches of 25 neighbouring epidermal cells. One patch was located at the base of the leaf, one at the tip of the leaf, and two in the middle of the leaf on either side of the main vein. Epidermal cell number per leaf was calculated by dividing the final leaf area by mean epidermal cell area.
Statistical analysis of data
Differences in initial relative leaf expansion rate among the treatments were evaluated by the likehood ratio statistical test. All other statistical analyses were done using the computer package SPSS 11·0 for Windows (SPSS Inc., Chicago, USA) with a significance level of P = 0·05. Final leaf area, maximal absolute leaf expansion rate, the duration of leaf expansion and epidermal cell area were compared using a one-way ANOVA and epidermal cell area was analysed using a Mann–Whitney U-test.
RESULTS
Day length affects leaf number without affecting the rate of leaf initiation
Approximately ten leaves were formed on the rosette when A. thaliana Col-0 plants were grown under the long-day treatment of 16 h (Fig. 1A). The final rosette leaf number was increased by a short-day treatment of 12 h and reached around 16 leaves per rosette (Fig. 1A). In Ler, nine leaves were formed on the rosette when plants were grown under a 20-h day length and the final rosette leaf number reached around 21 leaves per rosette under the 10-h day-length treatment (Fig. 1B). In both ecotypes, this increase in leaf number in response to the decrease in day length was not related to the rate at which leaves were initiated on the apex, but was due to an increase in the duration of the phase with leaf production (Fig. 1A, B).
Fig. 1.
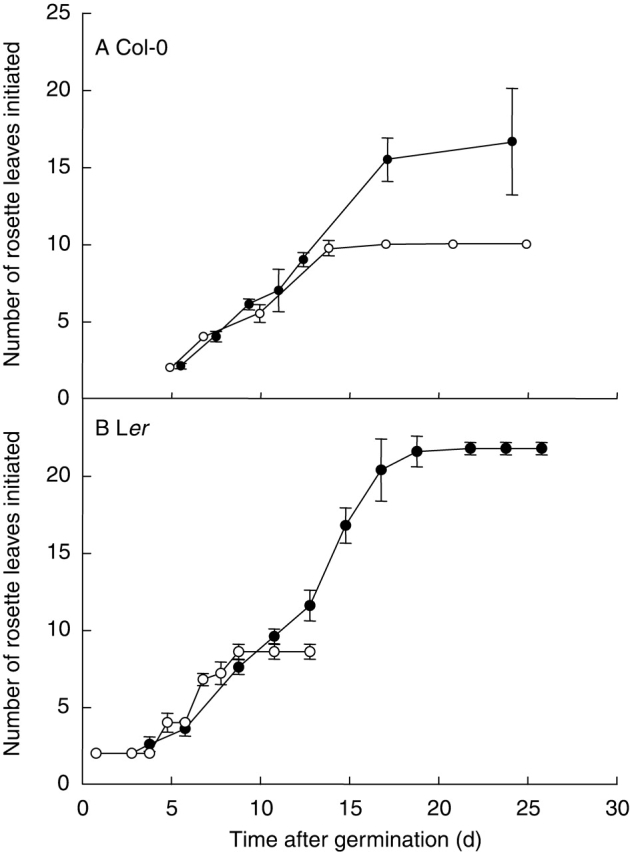
Number of initiated leaves over time in (A) Col-0 rosettes grown under a 12-h (closed symbols) and 16-h day length (open symbols) in expts 2 and 3, respectively, and in (B) Ler rosettes grown under a 10-h (closed symbols) and a 20-h day length (open symbols) in expt 4. Means shown with s.d. error bars (n = 5).
Day length only slightly affects final individual leaf area
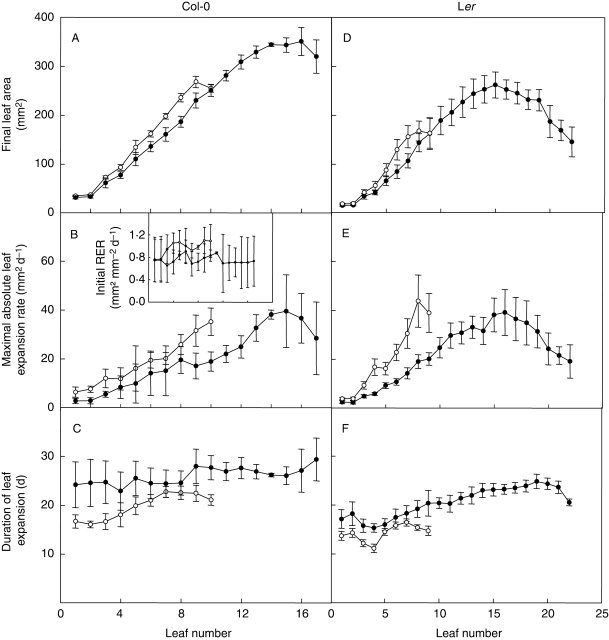
The profiles of final leaf area variation of plants grown under different day lengths show that leaf area varied depending on leaf position in the rosette both in Col-0 (Fig. 2A) and Ler (Fig. 2D and Supplementary Information). For all plants, independently of the day-length treatment, the first two leaves were of similar area and then most of the subsequently initiated rosette leaves increased in size until a maximum was reached. For plants grown in long days (16 h for Col-0 or 16 and 20 h for Ler), the leaf with maximal area was generally one of the last two leaves formed on the rosette (leaf 9 or 10). For plants grown in short days, leaf area increased with increasing leaf number for the first two-thirds of the leaves produced; for Col-0 at 12 h and Ler at 12 or 10 h this corresponded to leaves 16, 13 and 15, respectively, and the final few leaves were reduced in size. A short-day treatment had a tendency to cause a decrease in the final leaf areas of leaves emerging after leaf 2 both in Col-0 and Ler. This decrease was significant for leaves 5 and 7 when Col-0 plants were grown under a 12-h day length compared with those grown under 16 h (Fig. 2A), and significant for leaves 4–7 when Ler plants were grown under a 10-h day length compared with those grown under 20 h (Fig. 2D, and Fig. 3A for leaf 6).
Fig. 2.
The profiles of (A) final leaf area, (B) maximum absolute leaf expansion rate and (C) the duration of leaf expansion of each leaf of Col-0 plants grown under a 16-h (open symbols) or 12-h day length (closed symbols) during expts 2 and 3. Similar data for Ler plants grown in expt 4 (20 h, open symbols; 10 h, closed symbols) are presented in (D), (E) and (F), respectively. Insert: initial relative leaf expansion rate of each leaf of Col-0 plants grown in expts 2 and 3, symbols as (A), (B) and (C). Means with 95 % confidence intervals are given (n = 5 for expts 2 and 3; n = 10 for expt 4).
Fig. 3.
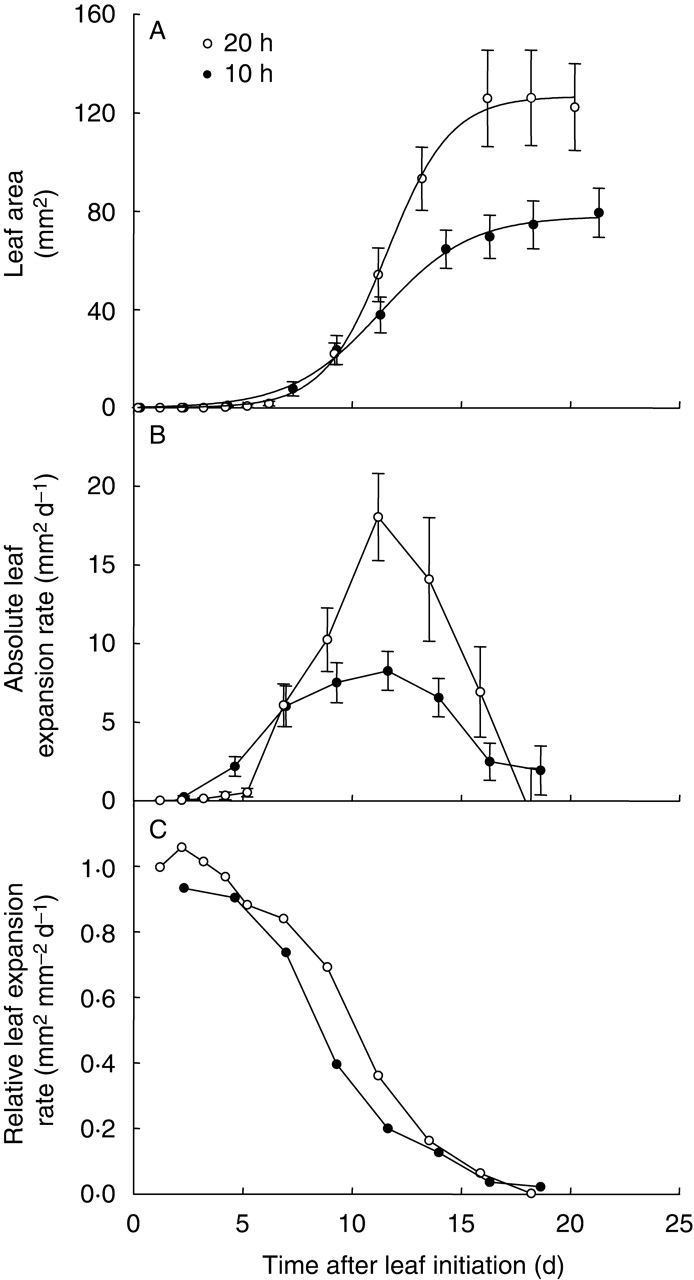
Changes with time of (A) leaf 6 area, (B) absolute leaf 6 expansion rate and (C) relative leaf 6 expansion rate of Ler plants grown in expt 4 under the day lengths of 20 h (open symbols) or 10 h (closed symbols). Means with 95 % confidence intervals are given, n = 5.
Day length affects the dynamics of individual leaf expansion
A detailed analysis of the dynamics of the expansion of the 6th leaf of Ler plants grown under 10- and 20-h day lengths is presented in Fig. 3. Leaf area expansion forms a sigmoid-shaped curve when plotted on a linear scale (Fig. 3A). This suggests that leaf expansion is slow during the early stage of leaf development and that it then accelerates with time until finally slowing down towards the end of leaf expansion. As a consequence, plotting absolute leaf expansion rate (the area formed per unit of time) against time produces a bell-shaped curve (Fig. 3B), the maximum of which can be quantified (maximal absolute leaf expansion rate; see Materials and methods). Maximal absolute leaf 6 expansion rate was significantly reduced when plants were grown under the short-day treatment (F1, 18 = 42·7, P < 0·0005; Fig. 3B). In contrast, when leaf expansion was expressed on a relative basis, relative leaf expansion rate (the area formed per unit of area and per time, mm2 mm−2 d−1) was high at the beginning of leaf expansion and then declined continuously until the end of expansion (Fig. 3C). The initial maximal relative leaf expansion rate was quantified (see Materials and methods) and it was significantly reduced when plants were grown under the short-day treatment (Fig. 3C).
Maximal absolute leaf expansion rate was also calculated for each leaf formed on the rosette of Ler plants grown under a 10-, 12-, 16- and 20-h day length and Col-0 plants grown under a 16- and a 12-h day length. The differences in maximal absolute leaf expansion rate among leaves under a given environmental treatment followed a pattern similar to that of final leaf area (Fig. 2A, B, D, E, and Supplementary Information). The short-day treatment of 12 h decreased the maximal absolute leaf expansion rate slightly in leaves 3–9 of Col-0 when compared with the long-day treatment of 16 h but this effect was not significant. In Ler the differences in maximal absolute leaf expansion rate between plants grown under a 16- or a 12-h day length were consistent but also not significant (Supplementary Information). However, in this ecotype, a shorter day-length treatment of 10 h decreased significantly the maximal absolute leaf expansion rate of all leaves after leaf 3 when compared with the long-day treatment of 20 h (Fig. 2E).
Relative leaf 6 expansion rate was slightly decreased by the short-day treatment during most of leaf 6 development. The rapid expansion of young leaves can be quantified by estimating the slope of the change in leaf area on the natural log scale against time when this relationship is near-linear (initial relative expansion rate). Initial relative leaf expansion rate was calculated for each leaf formed on Col-0 plants grown under a 12- and a 16-h day length (Fig. 2B insert). The short-day treatment (12 h in comparison with 16 h) reduced the initial relative leaf expansion rate in all leaves. The reduction was significant for all leaves except the first 2 and the 6th leaf (P < 0·05, likelihood ratio test).
The end of leaf expansion was slightly delayed by the short-day treatment, thus the leaves expanded for slightly longer. However, in Fig. 3 this effect on the duration of leaf expansion was not significant (F1, 18 = 3·0, P = 0·099; Fig. 3A and B).
The duration of leaf expansion was also calculated for each leaf formed on the rosette of Ler plants grown under a 10-, 12-, 16- and 20-h day length and Col-0 plants grown under a 16- and a 12-h day length. Short-day treatments caused an increase in the duration of leaf expansion for all leaves of the rosette both in Col-0 and Ler (16 h/12 h in Col-0; Fig. 2C; 20 h/10 h in Ler, Fig. 2F). These differences in the duration of leaf expansion were significant for leaves 1–3, 5, 9, 10 when comparing Col-0 plants grown under 16- and 12-h day lengths (Fig. 2C), and for leaves 1–4, 8 and 9 when comparing Ler plants grown under 20- and 10-h day lengths (Fig. 2F). Responses were conserved but they were less obvious when Ler plants were grown at 12- and 16-h day lengths (Supplementary Information).
Day length affects cellular processes underlying individual leaf expansion
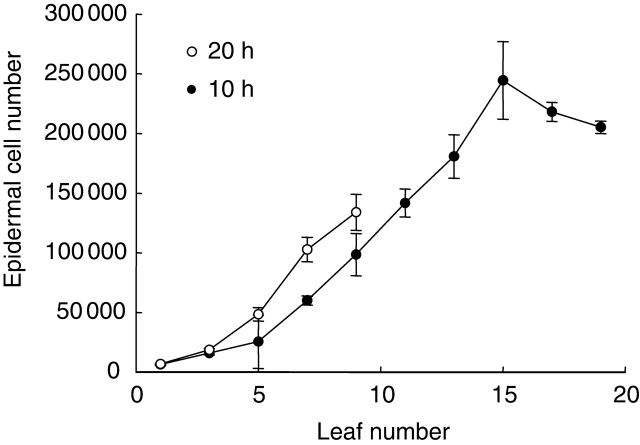
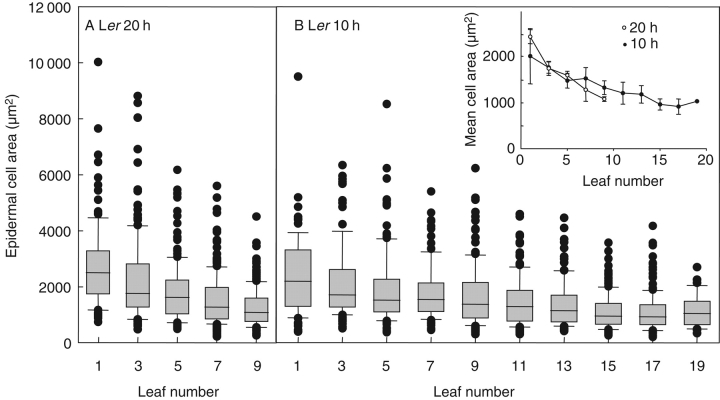
Final epidermal cell area and final epidermal cell number were estimated for each odd-numbered leaf formed on Ler rosettes grown under a 10- and 20-h day length. Epidermal cell number increased with increasing leaf number, except for the final leaves of the short day-grown plants (Fig. 4). The differences in epidermal cell number among leaves under a given environmental treatment followed a similar pattern to that of final leaf area (Figs 2D and 4). In contrast, median epidermal cell area decreased with increasing leaf number to a minimum mean cell area of 1000 µm2, which was reached by leaf 9 of plants grown under a 20-h day length and by leaf 15 of those grown under a 10-h day length (Fig. 5). The whole distribution of epidermal cell area was shifted towards lower values with increasing leaf number as shown by the box plot distributions of cell area in these leaves (Fig. 5). A short-day treatment decreased epidermal cell number when similar leaf numbers were compared after leaf 5 (Fig. 4). Epidermal cell area was not affected by the short-day treatment in leaves 1, 3 and 5 but was slightly increased for leaf 7 and significantly increased for leaf 9 (P = 0·0006; Fig. 5, inset).
Fig. 4.
Number of epidermal cells in leaves of Ler plants grown under day lengths of 20 h (open symbols) and 10 h (closed symbols) in expt 4. Means shown with s.d. error bars, n = 3.
Fig. 5.
Distribution of epidermal cell area in alternate leaves of Ler plants grown under day lengths of (A) 20 h and (B) 10 h in expt 4. Upper and lower dimensions of the boxes represent the third and first quartiles, the medians are indicated by bars inside the boxes and the circles represent outlying values below the 10th and above the 90th centile (n = 300, i.e. 100 cells of three leaves). Inset: the mean of the median values of epidermal cell area for three individual leaves was calculated and is shown with s.d. error bars for alternate leaves of Ler plants grown under day lengths of 20 h and 10 h.
Accelerating and delaying floral development affects cellular processes in the same fashion as long-day and short-day treatments, respectively
At the cellular level, long days had a tendency to cause a decrease in epidermal cell area and an increase in epidermal cell number of leaves at a given position on the rosette, as shown for leaf 9 of Ler plants grown at three different day lengths (10, 14 and 20 h; Fig. 6). When a Mann–Whitney U-test was done to compare the data statistically, the decrease in cell area was significant only when the 10-h and 20-h day-length treatments were compared (not for 10 h/4 h and 14 h/20 h).
Fig. 6.
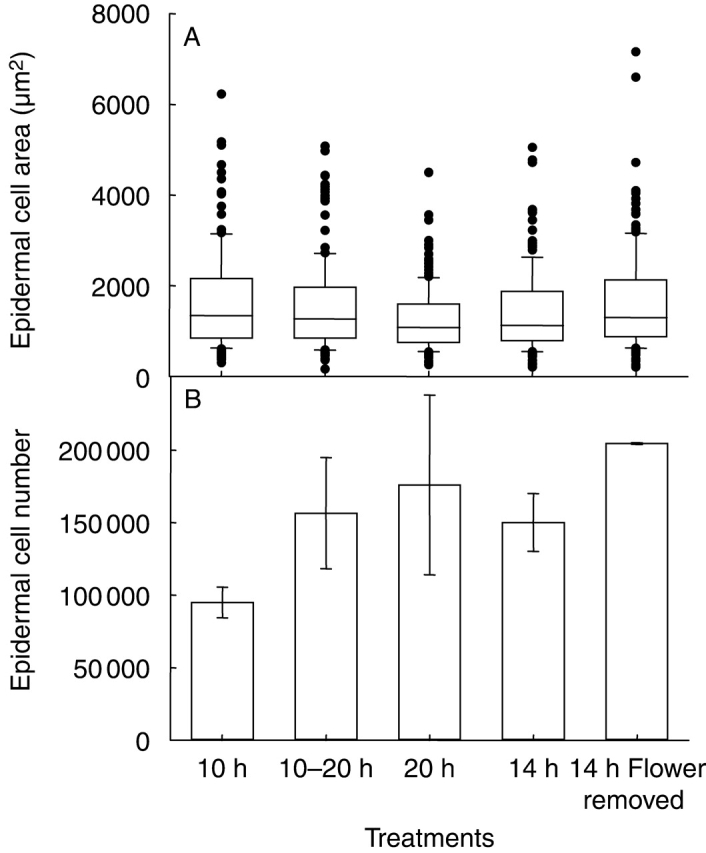
Epidermal cell area of leaf 9 of Ler plants grown under various day lengths, with or without the flower bud excised, during expts 4 and 5. Box plots (A) represent the distribution of the data around the median as in Fig. 5 and histogram (B) shows mean epidermal cell number with s.d. error bars, n = 3.
To test whether this result was specific to day-length perception or to an indirect effect of day length via an acceleration of flowering, two further treatments were imposed to accelerate or delay flower formation. During expt 4, half of the plants grown with a 10-h day length were transferred to a 20-h day length at the initiation of the 10th leaf (stage corresponding to the emergence of the 6th leaf). This treatment caused an acceleration of the onset of flowering as it reduced the duration of the phase during which leaves are initiated and consequently the final leaf number per plant (Table 2). Epidermal cell area was decreased in leaf 9 of plants subjected to this treatment when compared with plants grown during the whole experiment at a 10-h day length even if this decrease was not significant (Fig. 6A). This decrease in epidermal cell area occurred with an increase in epidermal cell number (Fig. 6A, B).
Table 2.
Effects of day length and the transfer of plants from a short to a long day on rosette development
| Treatments | No. of rosette leaves | Duration of leaf initiation (d) | Total rosette leaf area (mm2) |
|---|---|---|---|
| 20 h | 8·4 (± 0·5) | 6·8 | 815 (± 165) |
| Transfer 10 h→20 h | 12·8 (± 0·5) | 10·9 | 1388 (± 160) |
| 10 h | 21·8 (± 1·2) | 16·7 | 2822 (± 447) |
The number of rosette leaves at flowering, the duration of the phase with initiation of leaves and the final total rosette leaf area are given for each treatment. The 95 % interval of confidence is given in parenthesis.
During expt 5, Ler plants were continuously grown at a 14-h day length but in some plants, flower buds were excised at bolting to delay flower formation. Epidermal cell area was significantly increased in leaf 9 of plants subjected to this treatment when compared with those grown without flower bud excision (P = 0·032; Fig. 6A). Epidermal cell number was also increased (Fig. 6B).
Short-day effects on the cellular development in leaves could at least partly be mimicked by delaying flowering.
DISCUSSION
The day length responses could not be attributed to differences in other micro-meteorological conditions
The plant culture and micro-meteorological facilities used in this work allowed the precise imposition and characterization of different environmental conditions imposed. For the two most extreme treatments of 10 and 20 h, the total incident radiation received by the plants varied by only 0·05 mol m2 d−1 and the mean leaf temperature (measured every 10 min during the whole experiment) varied by only 0·06 °C. The changes in instantaneous light intensity were modified by neutral shading so that factors associated with changes in light quality did not impact upon the results. Reductions in light intensity are most often accompanied by a decrease in VPD and plant transpiration. Depending upon the interaction with other environmental conditions this could favour leaf expansion rate as demonstrated by the negative relationship obtained between leaf expansion rate and VPD in maize (Ben Haj Salah and Tardieu, 1996). Thus, here, VPD was maintained at low values in all of the treatments to accurately test an effect of day length. Similarly, soil water content was adjusted daily on a pot-to-pot basis in expts 1, 4 and 5 to avoid the establishment of different soil humidities among the treatments.
The maintenance of equal total daily incident PPFD received by the plants grown under different day lengths required a drastic reduction in the instantaneous PPFD. Such a reduction in PPFD could be perceived by the plants being examined as a ‘shade’ rather than an altered day-length treatment. However, the present results demonstrate that the responses observed were specific to the day-length treatments themselves and not due to shade responses. In fact, leaf expansion rate was increased and the duration of leaf expansion was decreased by long-day treatments (which in this study was accompanied by a decrease in instantaneous incident light). It is known that these growth variables are decreased and increased, respectively, in response to shading treatments (Chenu et al., 2005; Cookson and Granier, 2006). Similarly, at the cellular level, cell number was increased by a long-day treatment whereas it is decreased by shading, and cell area was decreased by a long-day treatment whereas it is increased by shading (Cookson and Granier, 2006). In summary, for both the dynamics and cellular bases of leaf expansion, the responses to an increase in day length reported were exactly the opposite of what has been reported in response to shade.
Day length does not affect the rate of leaf initiation but affects the dynamics of individual leaf expansion
There is controversy concerning the effect of day length on the rate of leaf initiation or appearance with very different results reported for different species studied under different environmental set ups (Pararajasingham and Hunt, 1995, and the references therein). The considerable genetic variation observed in the response of leaf appearance rate to day length among different wheat cultivars further complicates this issue (Pararajasingham and Hunt, 1995). In Ler, a day length of 10 h caused an increase in rosette leaf number by a factor of 2·3 in comparison with plants grown under a 20-h day length. This alteration occurred without affecting the rate of leaf initiation. Many studies of grasses grown under similarly well-controlled environmental conditions have reported analogous results (Cooper, 1951; Ryle, 1966; Østgård and Eagles, 1971; Hay and Pedersen, 1986).
In A. thaliana, a short-day treatment induced an increase in leaf number which occurred with a slight decrease in individual leaf area. When comparing the effects of day-length treatments of 16 and 12 h on plant development, leaf size differences were observed in Col-0, whereas in Ler the final leaf area of individual leaves was not affected. In Ler, leaf size differences were only observed when extremes of day length were analysed (here 20 h and 10 h). This result is similar to reports of the effects of day length on leaf expansion in wheat cultivars grown in growth chambers in which cultivars showed different degrees of final leaf size response (Pararajasingham and Hunt, 1995). Previous work has reported a direct influence of day length on individual leaf size (Arney, 1956; Newton, 1963). However, the dynamic analysis of leaf expansion of plants grown under different day-length regimes performed here reveals that the slight differences in final leafarea hide significant differences in the dynamics of leaf expansion (short-day treatments decrease the absolute leaf expansion rate and increase the duration of leaf expansion).
The antagonist effects of day length on the rate and duration of leaf expansion reported here have been described in response to other environmental conditions such as temperature (Granier and Tardieu, 1998b; Granier et al., 2002), light intensity (Chenu et al., 2005; Cookson and Granier, 2006) and moderate soil water-deficit treatments (Aguirrezabal et al., 2006). This phenomenon appears to be a general feature of how A. thaliana adapts to stress environmental conditions. How such an antagonistic behaviour could function remains nevertheless difficult to elucidate. Presumably an increase in the duration of leaf expansion when expansion rates are reduced is a mechanism of adaptation to increase leaf area under stress conditions. However, it does not seem to be a well-conserved property of leaf developmental plasticity between different plant species or genotypes as it was not observed, for example, in sunflower leaves grown either at different light intensities or soil water deficit treatments (Granier and Tardieu, 1999a, b). In addition, absolute leaf expansion rate could be reduced in some leaf growth mutants without alteration in duration of leaf expansion and vice versa (Cookson et al., 2005).
Although these antagonistic responses are evident when comparing different environmental treatments, if one compares all leaves of the rosette, under a given environmental treatment, they show remarkably similar initial relative leaf expansion rates and durations of expansion. This could suggest that there is a common plant-wide control of these developmental variables under unchanging environmental conditions.
The cellular basis of the day length-induced differences in leaf development is, at least partly, related to floral development
As shown for leaf growth responses to many other environmental conditions, cell number and leaf area were similarly affected by day length. In addition, epidermal cell number and final leaf area had similar profiles when all individual leaves of the rosette were considered successively. This work adds further support to the idea that both cell division and organ expansion are tightly related. In contrast, epidermal cell area was increased by a decrease in day length when cell number decreased. Cell area also decreased with increasing leaf number, whereas cell number increased. These antagonistic responses of cell number and cell size were also observed in A. thaliana plants subjected to shading and moderate soil water-deficit treatments (Aguirrezabal et al., 2006; Cookson and Granier, 2006).
The decline in cell area with increasing leaf position reported here has also been reported in other species, such as Ipomoea (Ashby, 1948) and sunflower (Granier and Tardieu, 1998a). In A. thaliana, a minimum epidermal cell area of approx. 1000 µm2 was measured in the last rosette leaf formed by the plant, independent of the number of leaves formed. The epidermal cell area results from plants grown under conditions that alter the timing of flowering, were in agreement with the ideas of Ashby (1948), who suggested that cell area depends on whole-plant control mechanisms related to flowering. Inducing flowering and delaying flowering mirrored the effects of short and long days, respectively, at the cellular level, suggesting that at least part of the day-length response of individual leaf development is mediated via whole-plant control mechanisms associated with floral development. It is now well-established that the day-length signal is perceived in the leaf, and that this signal is transduced to the shoot apex, where floral initiation occurs (Huang et al., 2005). The present results suggest that another signal from the shoot apex is involved in cell area regulation of growing leaves.
CONCLUSIONS
This is the first report of the effect of day length on dicotyledonous leaf expansion at the dynamic and cellular levels. Day length affects the dynamics of leaf development even when visible morphological changes are absent. The maximum absolute leaf expansion rate and cell number are decreased by short days, whereas the duration of leaf expansion and cell area are increased. Part of the increase in cell size caused by short-day treatments results from indirect effects of day length and is controlled by whole-plant mechanisms associated with the acceleration of flowering. The interaction between flowering transition and vegetative development shown here highlights the difficulty of morphological phenotyping of A. thaliana genotypes because many accessions, mutants or transgenic lines have different dates of flowering, and this could have an impact on leaf development in those lines.
SUPPLEMENTARY INFORMATION
A supplementary figure showing changes with time of leaf area, absolute leaf expansion rate and relative leaf expansion rate of Ler plants grown in expt 1 is available online at http://aob.oxfordjournals.org/.
ACKNOWLEDGEMENTS
This work was funded by the European Community Human Potential Program (HPRN-CT-2002-00267) as part of the DAGOLIGN (Development and growth of leaves: identification of genetic networks) Research Training Network. We thank A. Christophe and D. Combes for determining the light quality.
LITERATURE CITED
- Aguirrezabal L, Bouchier-Combaud S, Radziejwoski A, Dauzat M, Cookson SJ, Granier C. Plasticity to soil water deficit in Arabidopsis thaliana: dissection of leaf development into underlying growth dynamic and cellular variables reveals invisible phenotypes. Plant, Cell and Environment. 2006;29:2216–2227. doi: 10.1111/j.1365-3040.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Arney SE. Studies of growth and development in the genus Fragaria. VI. The effect of photoperiod and temperature on leaf size. Journal of Experimental Botany. 1956;7:65–79. [Google Scholar]
- Ashby E. Studies in the morphogenesis of leaves. 2. The area, cell size and cell number of leaves of Ipomoea in relation to their position on the shoot. New Phytologist. 1948;47:177–195. [Google Scholar]
- Ben Haj Salah H, Tardieu F. Quantitative analysis of the combined effects of temperature, evaporative demand and light on leaf elongation rate in well-watered field and laboratory-grown maize plants. Journal of Experimental Botany. 1996;47:1689–1698. [Google Scholar]
- Bulter TJ, Evers GW, Hussey MA, Ringer LJ. Rate of leaf appearance in crimson clover. Crop Science. 2002;42:237–241. doi: 10.2135/cropsci2002.2370. [DOI] [PubMed] [Google Scholar]
- Carré IA. Day-length perception and the day-lengthic regulation of flowering in Arabidopsis. Journal of Biological Rhythms. 2001;16:415–423. doi: 10.1177/074873001129002006. [DOI] [PubMed] [Google Scholar]
- Chenu K, Franck N, Dauzat J, Barczi JF, Rey H, Lecoeur J. Integrated responses of rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Functional Plant Biology. 2005;32:1123–1134. doi: 10.1071/FP05091. [DOI] [PubMed] [Google Scholar]
- Cookson SJ, Granier C. A dynamic analysis of the shade-induced plasticity in Arabidopsis thaliana rosette leaf development reveals new components of the shade-adaptative response. Annals of Botany. 2006;97:443–452. doi: 10.1093/aob/mcj047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson SJ, Van Lijsebettens M, Granier C. Correlation between leaf growth variables suggest intrinsic and early controls of leaf size in Arabidopsis thaliana. Plant, Cell and Environment. 2005;28:1355–1366. [Google Scholar]
- Cooper JP. Studies on growth and development in Lolium. II. Pattern of bud development of the shoot apex and its ecological significance. Journal of Ecology. 1951;39:228–270. [Google Scholar]
- Corbesier L, Coupland G. Photoperiodic flowering of Arabidopsis: integrating genetic and physiological approaches to characterization of the floral stimulus. Plant, Cell and Environment. 2005;28:54–66. [Google Scholar]
- Granier C, Tardieu F. Spatial and temporal analyses of expansion and cell cyle in sunflower leaves: a common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiology. 1998a;116:991–1001. doi: 10.1104/pp.116.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Is thermal time adequate for expressing the effects of temperature on sunflower leaf development? Plant, Cell and Environment. 1998b;21:695–703. [Google Scholar]
- Granier C, Tardieu F. Leaf expansion and cell division are affected by reducing absorbed light before but not after the decline in cell division rate in sunflower leaves. Plant, Cell and Environment. 1999a;22:1365–1376. [Google Scholar]
- Granier C, Tardieu F. Water deficit and spatial pattern of leaf development: variability in responses can be simulated using a simple model of leaf development. Plant Physiology. 1999b;119:609–620. doi: 10.1104/pp.119.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Massonnet C, Turc O, Muller B, Chenu K, Tardieu F. Individual leaf development in Arabidopsis thaliana: a stable thermal-time-based programme. Annals of Botany. 2002;89:595–604. doi: 10.1093/aob/mcf085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RKM. The influence of day-length on the dry matter accumulation of grasses and cereals. New Phytologist. 1990;116:233–254. [Google Scholar]
- Hay RKM, Pedersen K. Influence of long day-length on the growth of timothy (Phleum pratense L.) varieties from different latitudes in northern Europe. Grass Foliage Science. 1986;41:311–317. [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Californian Agricultural Experimental Station Circular. 1950;347:1–32. [Google Scholar]
- Huang T, Bohlenius H, Eriksson S, Parcy F, Nilsson O. The mRNA of the Arabidopsis gene FT moves from leaf to shoot apex and induces flowering. Science. 2005;309:1694–1696. doi: 10.1126/science.1117768. [DOI] [PubMed] [Google Scholar]
- Lempe J, Balasubramanian S, Sureshkumar S, Singh A, Schmid M, Weigel D. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 2005;1:e6. doi: 10.1371/journal.pgen.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P. Studies on the expansion of the leaf surface. II. The influence of light intensity and daylength. Journal of Experimental Botany. 1963;14:458–482. [Google Scholar]
- Onouchi H, Coupland G. The regulation of flowering time in response to daylength. Journal of Plant Research. 1998;111:271–275. [Google Scholar]
- Østgård O, Eagles CF. Variation in growth and development in natural populations of Dactylus glomerata from Norway and Portugal. II. Leaf development and tillering. Journal of Applied Ecology. 1971;8:383–391. [Google Scholar]
- Pararajasingham S, Hunt LA. Effects of day-length on leaf appearance rate and leaf dimensions in winter and spring wheats. Canadian Journal of Plant Science. 1995;76:43–50. [Google Scholar]
- Ryle GJA. Effects of day-lengths in growth cabinets on the growth of leaves and tillers in three perennial grasses. Annals of Applied Biology. 1966;57:269–279. [Google Scholar]
- Slafer GA, Connor DJ, Halloran GM. Rate of leaf appearance and final number of leaves in wheat: effects of duration and rate of change of day-length. Annals of Botany. 1994;74:427–436. [Google Scholar]
- Torres M, Frutos G. Analysis of germination curves of aged fennel seeds by mathematical models. Environmental and Experimental Botany. 1989;29:409–415. [Google Scholar]
- Wang Y, Henriksson E, Soderman E, Henriksson KN, Sundberg E, Engstrom P. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Developmental Biology. 2003;264:228–239. doi: 10.1016/j.ydbio.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Wilson GL. Studies on the expansion of the leaf surface. V-Cell division and expansion in a developing leaf as influenced by light and upper leaves. Journal of Experimental Botany. 1966;17:440–451. [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]