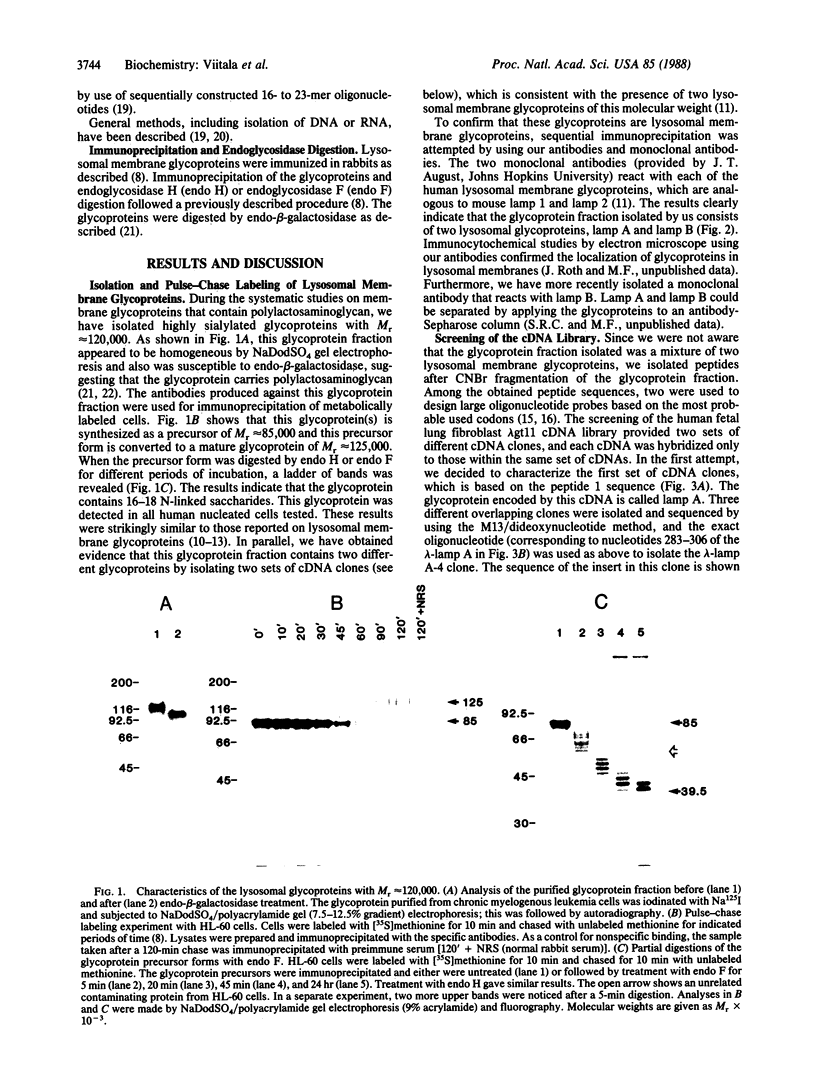
Abstract
Although several lysosomal membrane glycoproteins have been characterized by using specific antibodies, none of the studies so far elucidated the amino acid sequence of a lysosomal membrane glycoprotein. Here we describe cDNA clones encoding for one of the lysosome-associated membrane proteins with apparent Mr approximately equal to 120,000, lamp A. The amino acid sequence based on the fully coded cDNA shows that as many as 18 potential N-glycosylation sites can be found in the total of 385 amino acid residues. The results obtained by endoglycosidase F digestion support the conclusion that this glycoprotein contains 18 N-glycans. These N-glycosylation sites are clustered in two domains; one contains 10 and the other contains 8 N-glycosylation sites. These domains are separated by a (proline-serine)-rich region that has a distinct homology to the IgA hinge structure. The first N-glycosylated domain is elongated to a potential leader peptide toward the NH2-terminal end. The second N-glycosylated domain, on the other hand, is connected to a putative transmembrane portion consisting of hydrophobic amino acids. This segment, in turn, is elongated to a short cytoplasmic segment composed of 11 amino acid residues at the COOH-terminal end.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Brown W. J., Goodhouse J., Farquhar M. G. Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986 Oct;103(4):1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms N. M., Lobel P., Breitmeyer J., Chirgwin J. M., Kornfeld S. 46 kd mannose 6-phosphate receptor: cloning, expression, and homology to the 215 kd mannose 6-phosphate receptor. Cell. 1987 Jul 17;50(2):181–192. doi: 10.1016/0092-8674(87)90214-5. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780(2):119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J Biol Chem. 1984 Apr 25;259(8):4782–4791. [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. A., Sacchettini J. C., Welgus H. G. A collagenolytic serine protease with trypsin-like specificity from the fiddler crab Uca pugilator. Biochemistry. 1983 Jan 18;22(2):354–358. doi: 10.1021/bi00271a019. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel P., Dahms N. M., Breitmeyer J., Chirgwin J. M., Kornfeld S. Cloning of the bovine 215-kDa cation-independent mannose 6-phosphate receptor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2233–2237. doi: 10.1073/pnas.84.8.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Nagel G., Schmidt B., Stein M., Lorkowski G., Krentler C., Cully J., Meyer H. E., Grzeschik K. H., Mersmann G. Cloning of a cDNA encoding the human cation-dependent mannose 6-phosphate-specific receptor. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5575–5579. doi: 10.1073/pnas.84.16.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Bhat B. M., Wold W. S., Peterson P. A. A short sequence in the COOH-terminus makes an adenovirus membrane glycoprotein a resident of the endoplasmic reticulum. Cell. 1987 Jul 17;50(2):311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Isolation and characterization of human glycophorin A cDNA clones by a synthetic oligonucleotide approach: nucleotide sequence and mRNA structure. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1665–1669. doi: 10.1073/pnas.83.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P. D., Fukuda M. Molecular cloning of a human glycophorin B cDNA: nucleotide sequence and genomic relationship to glycophorin A. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6735–6739. doi: 10.1073/pnas.84.19.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzukida Y., Wang C. C., Putnam F. W. Structure of the A2m(1) allotype of human IgA--a recombinant molecule. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1104–1108. doi: 10.1073/pnas.76.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]