Abstract
Background and Aims
The relationship between ethylene production and both seed dormancy and germination was investigated using red rice (weedy rice) as a model species.
Methods
Both fully dormant and after-ripened (non-dormant) naked caryopses were incubated with or without inhibitors of ethylene synthesis [aminoethoxyvinylglycine (AVG)] and perception [silver thiosulfate (STS)], or in the presence of the natural ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). The kinetics of ethylene emissions were measured with a sensitive laser–photoacoustic system.
Key Results
Dormant red rice caryopses did not produce ethylene. In non-dormant caryopses, ethylene evolution never preceded the first visible stage of germination (pericarp splitting), and ethylene inhibitors completely blocked ethylene production, but not pericarp splitting. Accordingly, endogenous ACC appeared to be lacking before pericarp splitting. However, early seedling growth (radicle or coleoptile attaining the length of 1 mm) followed ethylene evolution and was delayed by the inhibitors. Wounding the dormant caryopses induced them to germinate and produce ethylene, but their germination was slow and pericarp splitting could be speeded up by ethylene.
Conclusions
The findings suggest that, in red rice, endogenous ethylene stimulates the growth of the nascent seedling, but does not affect seed dormancy or germination inception. Correspondingly, this phytohormone does not play a role in the dormancy breakage induced by wounding, but accelerates germination after such breakage has occurred.
Key words: Seed dormancy, red rice, Oryza sativa f. spontanea, ethylene inhibitors, wounding
INTRODUCTION
Red rice is a serious weed problem for rice production in many world areas, since its dispersal units remain dormant and may accumulate in the paddy soil to high population densities. Red rice is closely related to cultivated rice (Ferrero, 2003) and, apart from a few exceptions (Vaughan et al., 2001), is considered a weedy form of the crop species Oryza sativa (Gealy et al., 2002). In the literature, it has been referred to as f. spontanea (Chen et al., 2004), var. sylvatica (Vidotto et al., 2001) or without any sub-specific designation with respect to the cultivated species (Cohn and Hughes, 1986; Footitt and Cohn, 1995).
Seed dormancy is a state of developmental arrest that is very important in both crop and weedy plants (Bewley and Black, 1994; Finch-Savage and Leubner-Metzger, 2006), and red rice has been proposed as a model plant to elucidate the mechanisms of grass dormancy (Cohn, 1996; Chao et al., 2005). Although the biochemical bases of dormancy are not well understood, it is generally thought that plant hormones play a role in the induction, maintenance and breaking of dormancy (Bewley and Black, 1994; Kucera et al., 2005). Ethylene, the simplest unsaturated hydrocarbon, is a gaseous phytohormone produced in trace amounts by almost all higher plants, and is involved in the control of growth and developmental processes that range from germination to senescence (Abeles et al., 1992). In a number of species, ethylene stimulates germination in non-dormant seeds, and it is also reported to break dormancy in seeds that exhibit embryo dormancy (Kępczyński and Kępczyńska, 1997; Matilla, 2000; Kucera et al., 2005). The requirement for endogenous ethylene production in seed germination is supported by the close relationship between the amount of ethylene released and the percentage of germination (Machabée and Saini, 1991; Petruzzelli et al., 1995; Calvo et al., 2004), as well as the germination rate (Gorecki et al., 1991). However, it is difficult to tell if embryonic ethylene production triggers germination or, alternatively, if increasing ethylene production is the result of germination (Abeles et al., 1992).
An involvement of ethylene in seed germination has been verified in some species by using inhibitors of ethylene synthesis or perception (Kępczyński and Karssen, 1985; Lalonde and Saini, 1992; Petruzzelli et al., 1995; Kępczyński et al., 2003), and seed mutants having major alterations in the hormonal response (Siriwitayawan et al., 2003; Matilla et al., 2005). For Chenopodium album, Machabée and Saini (1991) suggested that the passage from dormancy to germination may involve two steps: an ethylene-requiring transition to the non-dormant state, followed by germination that does not have an absolute requirement for ethylene. Matilla et al. (2005) hypothesized that the alteration in the mechanism of ethylene signalling and action is one of the factors causing heterogeneity in germination among seeds, an adaptive strategy that increases the success of plant perpetuation. Thus, Kępczyński and Kępczyńska (1997) concluded that ethylene has a key role in dormancy release and seed germination of many plant species. However, seeds of some plants do not respond to ethylene or the promotive effects are very slight (Lalonde and Saini, 1992; Matilla, 2000). Moreover, some authors hold that ethylene production is a consequence of germination, rather than a requirement for such a process; therefore, the role of this gas remains controversial (Matilla, 2000).
Severe wounding of the dormant red rice caryopsis breaks its dormancy, and cutting away the embryo has been used to test the viability of dormant caryopses (Cohn and Hughes, 1986). Similarly, it has long been known that seed dormancy of wild oat caryopses can be released by piercing the seed coat or excising the embryo (Atwood, 1914), and the rate of germination is inversely related to the distance of the wound from the embryo (Hsiao et al., 1983). On the other hand, the embryo axis is the most relevant site of ethylene production in seeds (Abeles et al., 1992; Petruzzelli et al., 2000), and ethylene is involved in mechanical stimulation, such as cutting (Abeles et al., 1992). Moreover, Sung et al. (1987) proposed that the stimulating factor could be a volatile substance, although they were not able to detect ethylene. These observations led Cranston et al. (1996) to verify that inhibitors of ethylene synthesis or perception delayed or almost completely inhibited germination of embryos excised from dormant caryopses of one line of wild oat. These latter authors proposed that excising the embryo from the dormant oat caryopsis caused wound-induced ethylene production that was responsible for dormancy breaking and the consequent germination of such embryos.
The aim of this work was to study the relationship between germination and the evolution of ethylene from dormant and non-dormant red rice caryopses in the presence of inhibitors of ethylene synthesis and perception, or of ethylene precursors, in both intact and wounded caryopses.
MATERIALS AND METHODS
Materials
Straw-hulled red rice was grown in a paddy plot at Vercelli, a rice-growing area of the Po Valley, North Italy, in 2001. The dispersal units (caryopses covered by the hulls) were harvested by hand shattering, dried for 1 d at 35 °C and then stored in screw top jars at –18 °C to preserve dormancy. To obtain non-dormant dispersal units, a portion of the seeds were after-ripened in closed containers at 30 °C for 16 weeks. Dormant and after-ripened dispersal units were manually dehulled before the start of incubation, and naked caryopses were used in all the experiments.
The following chemicals (obtained from Sigma-Aldrich, St Louis, MO, USA) were used for their well-known effects (Abeles et al., 1992; Kępczyński and Kępczyńska, 1997; Matilla, 2000): 2-chloroethylphosphonic acid (ethephon) as an ethylene-releasing agent; 1-aminocyclopropane-1-carboxylic acid (ACC) as the immediate precursor of ethylene biosynthesis; aminoethoxyvinylglycine (AVG), an inhibitor of ethylene synthesis that blocks the conversion of S-adenosylmethionine to ACC; and silver nitrate and sodium thiosulfate were used to prepare silver thiosulfate (STS), which provides Ag+, an inhibitor of ethylene perception. In experiments where STS was used, the reported concentrations refer to Ag+, the active component. AVG and ACC solutions were prepared immediately before the start of each experiment by dilution from 50 mm stocks (in water), which were stored at –18 °C. Thiosulfate-complexed Ag+ solution was obtained by diluting a 10 mm Ag+ stock freshly prepared by slowly dropping 20 mm silver nitrate into an equal volume of 80 mm sodium thiosulfate to produce a 1:4 molecular ratio (Reid et al., 1980). Ethephon solutions were prepared just before the start of each experiment.
Germination tests
Germination tests in the presence of AVG and STS were performed in plastic Petri dishes (90 mm diameter; Sterilin) containing two sheets of filter paper (90 mm diameter, Schleicher & Schuell MicroScience) and 5 mL of incubation medium (water or aqueous solution of chemicals). Tests in the presence of ethephon were performed in 100 mL flasks closed with a rubber reversible sealing cap and containing one circle of Whatman 3MM paper (40 mm diameter) and 2 mL of ethephon solutions (buffered with 30 mm Na-phosphate pH 7·0). Three concentrations of AVG, 10 µm, 100 µm and 1 mm, and Ag+ (as STS), 75 µm, 1 mm and 5 mm, and two concentrations of ethephon, 1 and 10 mm, were tested. In these and all the subsequent experiments, incubations were carried out in the dark, but manipulations, germination recording and incubator inspections were performed under room light. Germination was recorded after 1 week of incubation at 25 °C, when rootlets or coleoptiles grew ≥1 mm (growth stage S1; Counce et al., 2000). According to Counce et al. (2000), coleoptile or radicle emergence is the first growth stage of rice (S1), and, depending on both genotype and environmental factors, in some cases the rice coleoptile emerges from the seed first and in other cases the radicle emerges first. When either emerges alone, the seedling growth stage is S1. For every treatment, two replications of 20 caryopses were used for each of two independent experiments, and the means from all four flasks are reported in Table 1. At the end of each experiment, non-germinated caryopses were tested for viability: they were transferred to Petri dishes (90 mm diameter; Sterilin) containing two sheets of filter paper (90 mm diameter, Schleicher & Schuell MicroScience) and 4 mL of water, and each caryopsis was cut longitudinally on one side along three-quarters of the endosperm with a scalpel blade to induce germination, before incubation for one additional week. Final cumulative germination (seedling attaining growth stage S1) was used as a measure of viability. Viability of both dormant and non-dormant caryopses averaged 99 %. The highest, non-toxic, concentrations of the inhibitors were chosen for the subsequent experiments to maximize their effectiveness.
Table 1.
Germination (seedling growth stage S1) of dormant and non-dormant caryopses after 7 d of incubation in water (Control) or in the presence of AVG (inhibitor of ethylene synthesis), STS (inhibitor of ethylene perception) or ethephon (ethylene releaser)
| Treatment | Dormant | Non-dormant |
|---|---|---|
| Control | 0 ± 0 | 100 ± 0 |
| 10 µm AVG | 0 ± 0 | 100 ± 0 |
| 100 µm AVG | 0 ± 0 | 98 ± 2 |
| 1000 µm AVG | 0 ± 0 | 97 ± 3 |
| 75 µm STS | 0 ± 0 | 98 ± 2 |
| 1 mm STS | 0 ± 0 | 97 ± 3 |
| 5 mm STS | 0 ± 0 | 99 ± 1 |
| 1 mm ethephon | 1 ± 1 | 100 ± 0 |
| 10 mm ethephon | 1 ± 1 | 100 ± 0 |
Means ± s.e. are reported (n = 4).
Experimental set-up for measurement of ethylene emission
In the experiments to determine the emission of ethylene from imbibed caryopses, 50 dehulled caryopses for each treatment were germinated on two layers of filter paper (55 mm diameter, Schleicher & Schuell MicroScience) placed in a germination plate containing the incubation medium. The inverted top of a 50 mm Petri dish (Sterilin) was used as germination plate. Each germination dish was positioned in a 150 mL cylindrical glass cuvette filled to 5/6 with glass beads and water. The cuvette was sealed with a glass top provided with inlet and outlet gas connections, leaving a small head space above the germination plate to permit free gas diffusion inside the cuvette. In this way, water saturated the cuvette atmosphere and prevented drying of the germination plate. Each cuvette was randomly assigned to one treatment, including one blank with water and no caryopses in the germination plate. The cuvettes were kept at 25 °C in an incubator, and, to measure ethylene emission, were connected to a gas flow-through system combined with a sensitive laser-based ethylene detector (Bijnen et al., 1996). Through the gas flow system, a source of purified air was connected to a mass flow controller (Brooks Instruments type 5850 S), and to a distribution valve which opened the air flow to each single cuvette every 2·5–3 h. Then, for a 20–25 min period, a constant airflow of 1·5 L h–1 was maintained through the cuvette to flush the accumulated gas to the ethylene detector. To eliminate interfering gases, a number of filters were introduced into the gas flow-through system. Any trace of external ethylene (or other hydrocarbons) was removed before the entrance of air into the cuvettes by a platinum-based catalyser. A scrubber with KOH (moist pellets) reduced the CO2 concentration in the air flow, a tube with CaCl2 (granules) decreased moisture, and a cooling trap (–150 °C) removed ethanol; these were inserted into the gas flow system just before the ethylene detector. Readings from the blank cuvette confirmed a low background signal. A detailed description of the detection system has been given elsewhere (Harren and Laarhoven, 2004). Briefly, the detector consisted of a line-tuneable CO2-laser emitting infrared radiation at specific wavelengths absorbed by ethylene, and a photoacoustic cell. The intermittent laser beam heats ethylene to generate pressure waves (i.e. sound) that are detected with a sensitive miniature microphone. The amplitude of the acoustic waves is directly proportional to the concentration of ethylene in the photoacoustic cell. The laser-based ethylene detector was calibrated with a certified mixture of 1 ppm ethylene in air (Air Liquide, Eindhoven, The Netherlands).
In all the experiments with normal or low emission levels, ethylene production of the rice caryopses was calculated by integrating the peaks of ethylene (released after the 2·5–3 h accumulation period), multiplying these values by the flow rate and then normalizing them to the emission of a single caryopsis in 1 h. The very high amounts of ethylene evolved in the presence of ACC were directly measured as real-time emissions after the initial, out-of-range, peak.
Incubation treatments with measurement of ethylene emission
Immediately before the start of each experiment, the caryopses were quickly rinsed with distilled water, blotted and transferred to the germination plate containing 2 mL of incubation solution. The control medium was sterile water, and the incubation treatments included: 1 mm AVG, 5 mm Ag+ (as STS) and 1 mm ACC. High concentrations of the chemicals were used to maximize effects on ethylene production, i.e. to work, presumably, in excess conditions. Preliminary tests indicated, and further experiments confirmed, that these concentrations were not toxic to the seedlings.
During incubation, two stages of germination were recorded: (1) pericarp splitting, the first visible sign of germination (Footitt and Cohn, 1995); and (2) first growth stage (S1, Counce et al., 2000), recorded when rootlets or coleoptiles were ≥1 mm (minimal visible seedling growth). Although the latter is the conventional stage at which germination is recorded, it is also the sign that, from a physiological point of view, this process has concluded (Bewley and Black, 1994; Kucera et al., 2005). Indeed, germination culminates in the elongation of the embryo axis that breaks through the covering layers (Finch-Savage and Leubner-Metzger, 2006). Germination in the strict sense would then terminate at pericarp splitting, close to the transition boundary between phases II and III of water uptake (Kucera et al., 2005). However, some minimal seedling growth makes completion of germination easier to see at growth stage S1, which therefore signals the onset of seedling growth as well as the conventional end of germination.
A few dormant caryopses that eventually started to germinate (<0·5 %) were recorded, but were promptly removed from their dishes. At the end of each experiment, radicle and coleoptile lengths were measured, and non-germinated caryopses were tested for viability: they were transferred to a Petri dish containing 2 mL of water and cut as indicated above, before incubation for one additional week. Final cumulative germination (seedling attaining growth stage S1) was used as a measure of viability. Viability of both dormant and non-dormant caryopses averaged 99 %.
To verify the effect of wounding itself on ethylene emission, either dry (un-imbibed) or pre-imbibed caryopses were cut and incubated in water, alone or in the presence of chemicals. In experiments where caryopses (either dormant or non-dormant) were cut before imbibition (dry), a transverse cut was performed (Cohn and Hughes, 1986), embryoless halves were removed and half caryopses with embryo were positioned with their cut surface down on the paper to allow direct access of solutions. When dormant caryopses were cut after some days of pre-imbibition, a longitudinal cut was performed as for the viability test. The effect of pre-imbibition was tested on non-dormant seed after cutting the dry caryopses and then incubating them, either in water or in the presence of inhibitors, for 5 d at 5 °C before testing germination.
To confirm that the action of inhibitors was really linked to ethylene, pre-imbibed dormant caryopses were wounded as above in the presence of AVG + STS and with or without ACC to verify reversion of the effects of the inhibitors on germination (Veen, 1985; Abeles et al., 1992).
Cumulative germination (growth stage S1) 1 week after wounding was 99 %, but for dormant caryopses cut when dry this was, on average, 76 %. All the treatments were replicated once in each of 2–4 independent experiments.
RESULTS
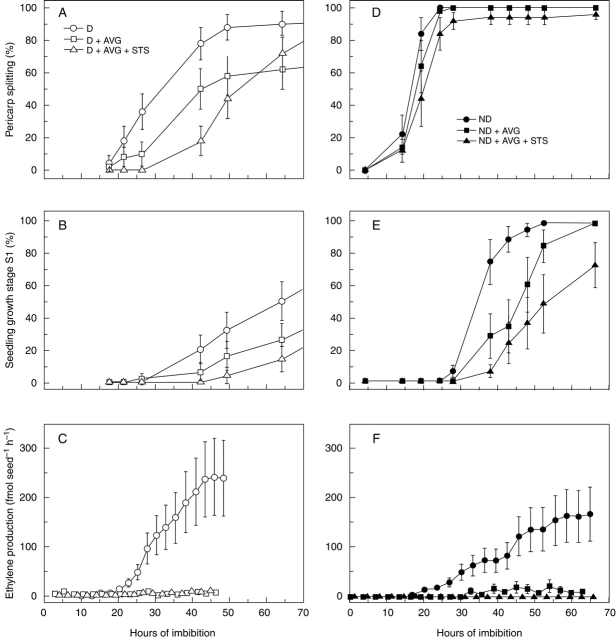
Germination tests showed no effect of AVG, STS or ethephon on the germinative capability of non-dormant red rice caryopses, or on the dormancy status of dormant caryopses (Table 1). In addition, flushing pre-imbibed (18 h) dormant caryopses for 2 h with 1 µL L–1 ethylene in air did not promote germination (data not shown). Similarly, during germination experiments with ethylene, dormant red rice caryopses did not germinate, whilst non-dormant caryopses promptly germinated at 25 °C, with most pericarp splitting occurring between 20 and 30 h, independently of the presence of ethylene inhibitors (Fig. 1A). Minimal seedling growth (stage S1) was accomplished between 35 and 55 h in water and with significant delay when AVG and, particularly, AVG + STS were present (Fig. 1B). No ethylene was produced by dormant caryopses or by non-dormant caryopses germinating in the presence of the ethylene biosynthesis inhibitor AVG (Fig. 1C). The detection limit of the laser-based photoacoustic system for ethylene was about 0·5 nL L–1. No toxic effects of the inhibitors were observed since non-dormant caryopses attained complete germination (growth stage S1) and dormant caryopses were fully viable after the treatments. Pericarp splitting started at 23 h, reached 50 % in 30 h and was completed in 45 h. Ethylene evolution commenced a few hours after pericarp splitting but prior to the onset of seedling growth (stage S1). Therefore, the adoption of pericarp splitting as an early sign of germination was useful to show that the start of ethylene production did not anticipate germination (as would appear by considering visible seedling growth at stage S1 as a first marker of germination) but was concomitant, or slightly subsequent, to it. Measurements of radicle and coleoptile lengths in germinating non-dormant caryopses at the end of the experiment (Table 2) indicated that: (a) inhibition of endogenous ethylene production strongly decreased radicle and coleoptile lengths; (b) STS showed an additive effect to AVG, notwithstanding that no further ethylene emission was detected during incubation with AVG alone; and (c) radicle growth appeared to be more sensitive to inhibition of endogenous ethylene than coleoptile growth. As a consequence of the last point, seedling growth stage S1 was attained by: mostly radicle growth in the absence of inhibitors, either radicle or coleoptile growth in the presence of AVG alone, and mostly coleoptile growth in the presence of AVG + STS.
Fig. 1.
Germination and ethylene production of dormant (D) and non-dormant (ND) dehulled red rice caryopses imbibed in water or in the presence of ethylene inhibitors. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. Standard errors between independent experiments are shown (n = 4).
Table 2.
Growth of radicle and coleoptile of non-dormant (ND) dehulled red rice caryopses after 5 d of incubation (i.e. at the end of the experiment reported in Fig. 1) either in water or in the presence of AVG or AVG + STS
| Treatment | Radicle (mm) | Coleoptile (mm) |
|---|---|---|
| ND | 24 ± 6 | 9 ± 4 |
| ND + AVG | 4 ± 2 | 5 ± 2 |
| ND + AVG + STS | <1 | 2 ± 1 |
Means ± s.e. are reported (n = 4).
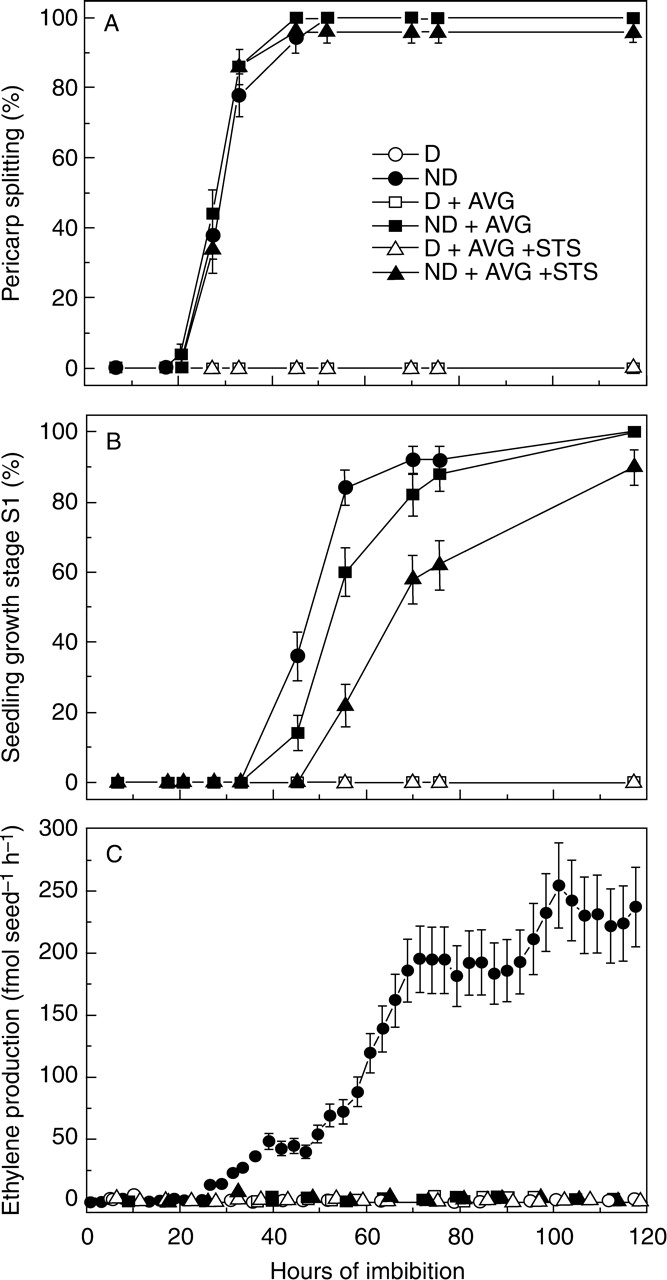
When caryopses were incubated in the presence of the ethylene precursor ACC, again non-dormant caryopses germinated and produced ethylene while dormant caryopses did not (Fig. 2). The sequence of events was confirmed to be: start of pericarp splitting, start of ethylene emission and then minimal growth. No effect of ACC on the timing of pericarp splitting was observed (Fig. 2A), whilst some delay of growth stage S1 was observed in the presence of ACC (Fig. 2B), possibly due to an inhibitory effect of the exceedingly high amounts of ethylene. The magnitude of ethylene production was greatly increased with respect to non-dormant caryopses germinating in water: after 40 h of incubation in the presence of ACC it was approx. 50 times higher than in the water control (Fig. 2C; Satler and Kende, 1985). Differences in ethylene evolution by non-dormant caryopses incubated with or without ACC are evidenced (Fig. 2C, inset).
Fig. 2.
Germination and ethylene production of dormant (D) and non-dormant (ND) dehulled red rice caryopses imbibed in water or in the presence of the ethylene precursor ACC. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. In the inset, data are replotted with a more detailed scale of emission (y-axis) to evidence ethylene production from non-dormant caryopses incubated in water. Standard errors between independent experiments are shown (n = 3).
When dry caryopses were cut before imbibition in water, alone or in the presence of AVG or AVG + STS, germination (pericarp splitting) occurred after 20 and 40 h for non-dormant and dormant caryopses, respectively (Fig. 3A). AVG showed no effect, but in the additional presence of STS a slight stimulatory effect seemed to occur after 65 h (Fig. 3A); however, it was not significant. Correspondingly, minimal seedling growth (stage S1) followed at 30 and 70 h for dormant and non-dormant caryopses, respectively (Fig. 3B). However, at this stage, some effect of the inhibitors became apparent, particularly for non-dormant caryopses which showed a significant delay of 10–20 h. The slow germination (both pericarp splitting and stage S1) of dormant caryopses contrasted with the fast germination of non-dormant ones (which was also completed more quickly than for intact caryopses), indicating that the former was not due to a general, deleterious, effect of cutting, but rather to residual effects of the initial dormancy. Ethylene was produced only in the absence of inhibitors, and in quite a lower amount by dormant than by non-dormant caryopses (Fig. 3C). In any case, the inhibitors did not prevent either pericarp splitting or the onset of seedling growth.
Fig. 3.
Germination and ethylene production of dormant (D) and non-dormant (ND) dehulled red rice caryopses that were cut transversally before imbibition of the embryonated half-seed in water or in the presence of ethylene inhibitors. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. Standard errors between independent experiments are shown (n = 2).
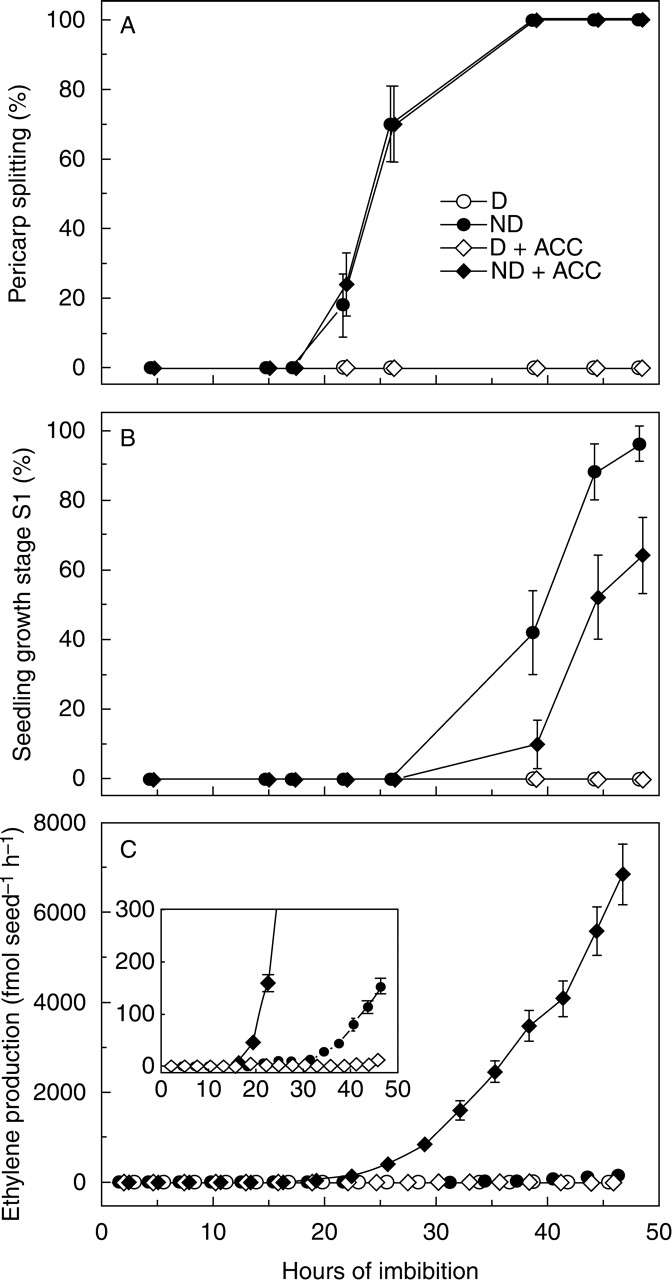
The effect of ACC was also tested on dormant caryopses that were induced to germinate by cutting them before imbibition (dry) or after they were pre-imbibed for 3–4 d (Fig. 4). Cutting pre-imbibed caryopses was more effective in stimulating germination than cutting the dry caryopses: in the former case, pericarp splitting and growth stage S1 started 20 and 30 h after the cut, respectively, whereas in the latter case there was a 20 h initial delay which increased with time of incubation (Fig. 4A, B). In addition, cutting the dry caryopses produced variable, and sometimes reduced (<70 %), final germination percentages (not shown). Longitudinal, incomplete, cutting along the caryopsis side was adopted just because of its greater efficacy; however, as it was hardly applicable to dry caryopses, it was used for pre-imbibed caryopses only. Caryopses wounded after pre-imbibition also showed a higher ethylene production compared with those cut when dry (Fig. 4C). Ethylene production started at about 20 and 55 h for the two cutting treatments, i.e. concomitantly with or well after pericarp splitting, respectively. ACC greatly increased ethylene emission and speeded up both pericarp splitting and the onset of seedling growth (stage S1). Also in this case, quicker germination (both stages) and ethylene production occurred when dormant caryopses were cut after pre-imbibition. In fact, in the presence of exogenous ACC, ethylene production started almost immediately, in 2–5 h after cutting the pre-imbibed caryopses and in 7–10 h after cutting the dry caryopses. In both cases it occurred before pericarp splitting.
Fig. 4.
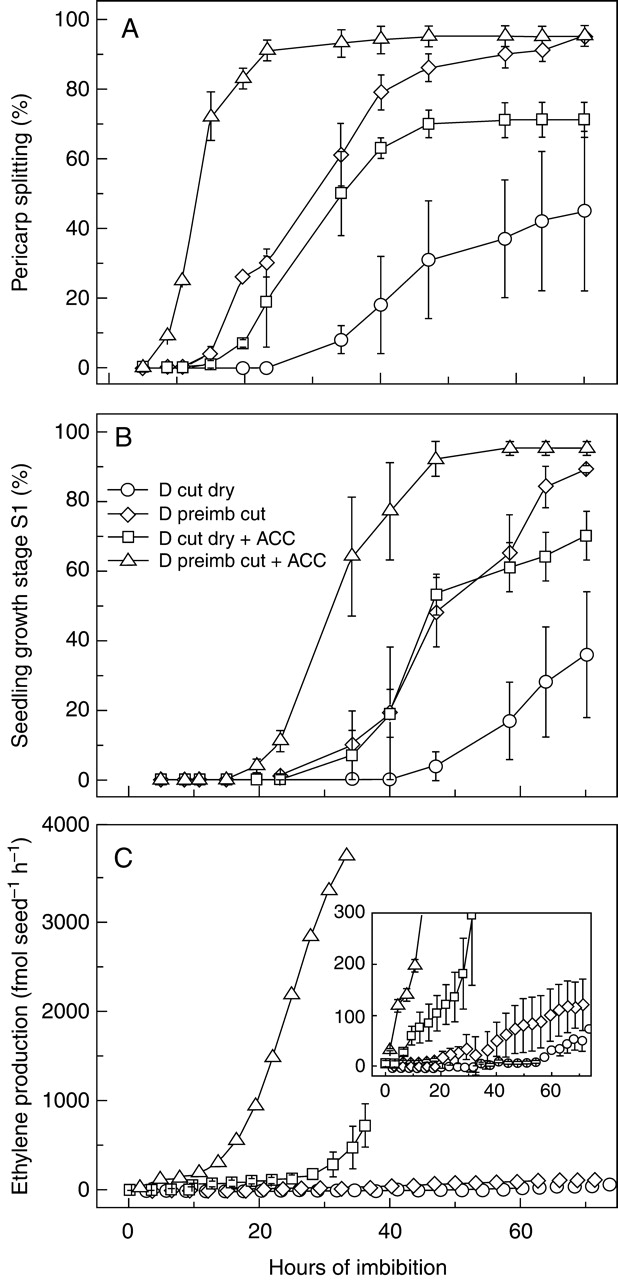
Germination and ethylene production of wounded dormant (D) dehulled red rice caryopses imbibed in water or in the presence of the ethylene precursor ACC. Dry caryopses were cut transversally before imbibition of the half-seed with embryo (time 0); pre-imbibed caryopses were wounded by longitudinal cutting (at time 0), after 3–4 d of pre-incubation. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. Standard errors between independent experiments are shown (n = 2).
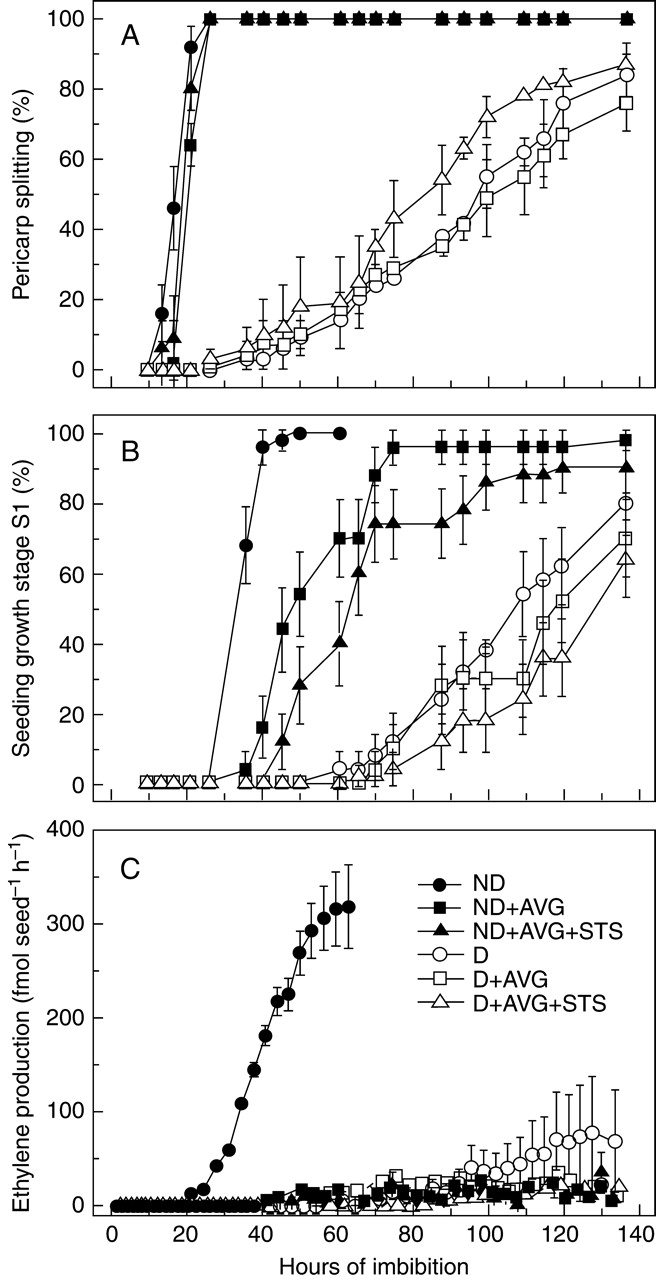
When dormant caryopses were pre-imbibed for 4 d and then wounded, pericarp splitting and the onset of seedling growth (stage S1) occurred in water with the same timing as observed in the previous experiment, but AVG and, particularly, AVG + STS inhibited both pericarp splitting and onset of seedling growth (Fig. 5A, B). Once more, ethylene production (Fig. 5C) only occurred in caryopses incubated without inhibitors (at a level similar to that of non-dormant caryopses: compare with Fig. 1C), and the emission started at the same time as pericarp splitting. As a control, non-dormant caryopses were pre-imbibed in the solutions for 5 d (at 5 °C) after cutting, to maintain the embryos in contact with the inhibitors before germination (Fig. 5D–F). After transfer to a temperature that allowed germination (25 °C), fast and simultaneous germination (pericarp splitting) occurred independently of the inhibitors (Fig. 5D), indicating that delayed pericarp splitting was a response to inhibitors that was restricted to initially dormant caryopses. In addition, the slow germination (at both stages) of cut caryopses was also confirmed to be specific to dormant caryopses rather than a general effect of cutting. Seedling growth (stage S1, Fig. 5E) was also rapid in non-dormant caryopses but it was slightly delayed by the inhibitors. Ethylene production (Fig. 5F) showed a timing consistent with that of other experiments.
Fig. 5.
Germination and ethylene production of wounded dormant (D) and non-dormant (ND) dehulled red rice caryopses imbibed in water or in the presence of ethylene inhibitors. Dormant caryopses were wounded by longitudinal cutting (at time 0), after 4 d of pre-imbibition (at 25 °C); non-dormant caryopses were cut transversally before imbibition of the half-seed with embryo for 5 d at 5 °C, then (at time 0) they were moved to 25 °C. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. Standard errors between independent experiments are reported (n = 3).
Dormant caryopses were pre-imbibed with or without ethylene inhibitors and wounded as above, but the additional presence of ACC was able to revert the slow germination (both stages, Fig. 6A, B), as well as the suppression of ethylene production (Fig. 6C), caused by the inhibitors. Actually, pericarp splitting was faster than in the untreated control, as already observed in Fig. 4. Reversibility of the action of the inhibitors on germination demonstrated that no toxic effects of the chemicals occurred at the concentrations used. Levels of ethylene emission were lower than in previous experiments with pre-imbibed caryopses, possibly due to the shorter pre-imbibition time.
Fig. 6.
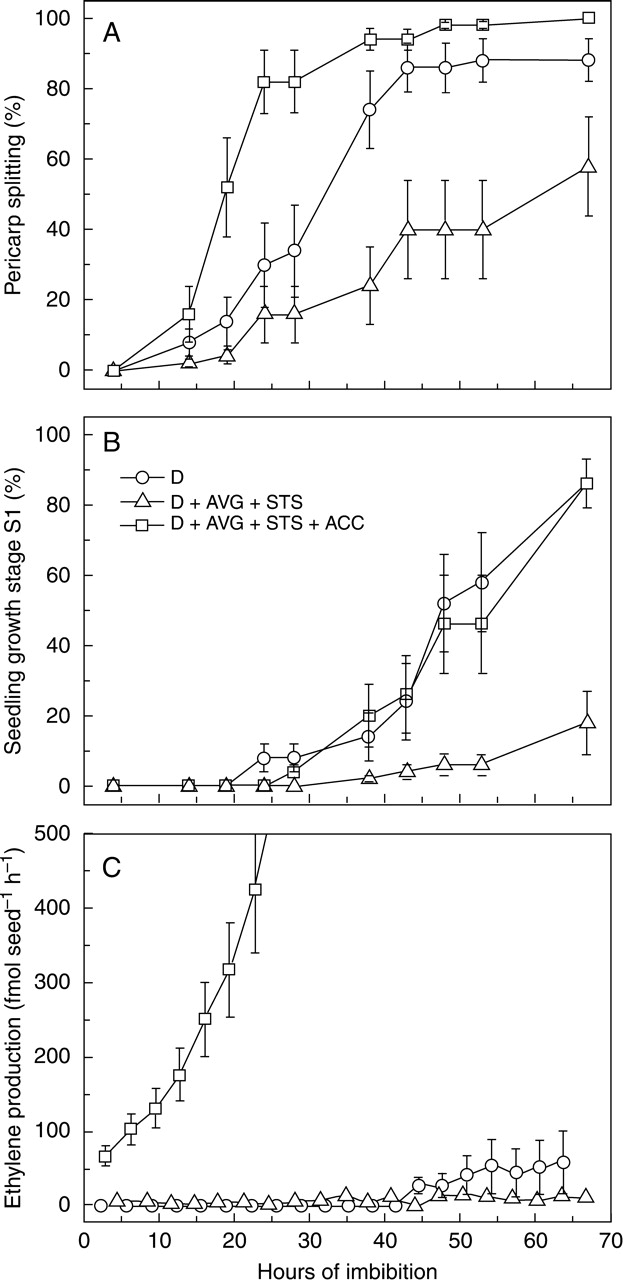
Germination and ethylene production of wounded dormant (D) dehulled red rice caryopses imbibed in water, or in the presence of ethylene inhibitors with or without the ethylene precursor ACC. All the caryopses were wounded by longitudinal cutting (at time 0), after 2 d of pre-imbibition. (A) Pericarp splitting (first visible stage of germination), (B) seedling growth stage S1, (C) rate of ethylene emission. Standard errors between independent experiments are reported (n = 2).
DISCUSSION
Preliminary germination tests (Table 1) showed no effect of AVG, STS and ethephon in dormant red rice caryopses. None of these agents prevented germination of non-dormant caryopses. This contrasts with barley where silver ions, unlike AVG, inhibited both germination and shoot growth (Locke et al., 2000). However, penetration of the inhibitors into the intact caryopsis prior to pericarp splitting is still an unsettled issue; hence, ethylene production was monitored in the subsequent experiments to confirm AVG effectiveness. STS was added to block the effects of any residual ethylene.
Dormant red rice caryopses did not emit ethylene, consistent with observations in wild oats (Adkins and Ross, 1981). In non-dormant caryopses, ethylene production started after the onset of pericarp splitting and increased as germination progressed (Fig. 1). A surge in ethylene production concomitant with, or shortly preceding, radicle emergence has been observed in a number of species (Machabée and Saini, 1991; Lalonde and Saini, 1992; Petruzzelli et al., 1994, 1995; Siriwitayawan et al., 2003; Puga-Hermida et al., 2006). However, ethylene inhibits subsequent stem elongation in many plants (Abeles et al., 1992), and peaks at the completion of germination in eudicots (Kucera et al., 2005). Instead, ethylene promotes seedling shoot growth in rice (Table 2; Satler and Kende, 1985; Abeles et al., 1992) and other cereals (Petruzzelli et al., 1994; Locke et al., 2000), and, in these species, its evolution increases during seedling development (Satler and Kende, 1985; Lalonde and Saini, 1992; Petruzzelli et al., 1994; Locke et al., 2000). Ethylene usually inhibits root elongation, so that stimulation of radicle growth after germination (Table 2) seems to be specific to rice (Abeles et al., 1992).
The role of ethylene in the removal of dormancy and initiation of germination is debated (Matilla, 2000). Indeed, the very close temporal association between ethylene evolution and germination suggests a physiological link, but makes it difficult to establish the cause–effect sequence without the use of inhibitors of ethylene synthesis and perception (Lalonde and Saini, 1992; Petruzzelli et al., 1995). Nevertheless, such a sequence can be analysed if the earliest signs of both the events are compared. The availability of a highly sensitive, laser-based, ethylene detection system to develop the precise kinetics of ethylene evolution (Petruzzelli et al., 1994), and the use of pericarp splitting as the first visible sign of germination (Footitt and Cohn, 1995), allowed determination of the fact that, in intact caryopses, the start of ethylene emission just precedes the onset of seedling growth (rice developmental stage S1; Counce et al., 2000) but not early germination (pericarp splitting; Cohn and Hughes, 1986). This supports the view that ethylene production is more related to post-germinative growth than to germination in the strict sense. Accordingly, suppression of ethylene synthesis by AVG did not prevent pericarp splitting although the presence of AVG and, particularly AVG + STS, delayed the onset of seedling growth (stage S1, Fig. 1). Further delay of growth stage S1 in the presence of AVG + STS over AVG alone was probably due to the block of any action of residual ethylene production below the detection threshold (approx. 5 fmol seed–1 h–1 in the accumulation experiments). In fact, the capability of ACC fully to reverse the slow germination induced by AVG + STS at both germination stages (Fig. 6) confirms that effects other than ethylene inhibition were not involved. Thus, the results indicate that ethylene is not required for dormancy maintenance or release, nor is it necessary to start germination. Corresponding results were reported by Adkins and Ross (1981) and Cranston et al. (1996) for intact oat caryopses, Kępczyński and Karssen (1985) for Amaranthus caudatus, Machabée and Saini (1991) for Chenopodium album, and Petruzzelli et al. (1995) for pea seeds. In barley, ethylene was found not to be an absolute requirement for germination, although it has a significant promotive action (Locke et al., 2000). Lalonde and Saini (1992) concluded that there is no general requirement for endogenous ethylene for dormancy breakage, and, in many species, germination does not strictly depend on the ethylene actually produced by the seed itself. On the other hand, ethylene inhibition strongly restricted radicle and coleoptile growth (Table 2), so that attainment of the length of 1 mm by radicle, or coleoptile, was always negatively affected. Analogously, ethylene accelerates radicle emergence in Arabidopsis and turnip (Siriwitayawan et al., 2003; Puga-Hermida et al., 2006). Ethylene production by germinating red rice caryopses is associated with the later, rather than the early, phase of germination, when the phythormone stimulates the speed of growth in the nascent seedling.
Non-dormant caryopses incubated in the presence of the ethylene precursor ACC (Fig. 2) showed an extremely high emission of ethylene after 16 h of imbibition, but did not anticipate pericarp splitting or seedling growth (stage S1). This result confirms that, in contrast to pea (Gorecki et al., 1991; Petruzzelli et al., 2000), the availability of endogenous ACC in the germinating rice seed limits ethylene production (Fig. 2; Satler and Kende, 1985). In particular, failure of non-dormant caryopses, incubated with the inhibitors, to produce ethylene (Fig. 1C) suggests that no endogenous ACC was initially available for ethylene production in ungerminated red rice seeds. Correspondingly, when dormant caryopses were cut before imbibition, they produced ethylene prior to pericarp splitting only if exogenous ACC was supplied (Fig. 4). In contrast, the delay of pericarp splitting caused by the inhibitors after cutting pre-imbibed dormant caryopses (Fig. 5A) suggests that, in these caryopses, an early production of ethylene (Fig. 5C) was speeding up pericarp splitting (as discussed in the next paragraphs) and some endogenous ACC was already present before this stage. Considering pericarp splitting as the first visible sign of germination, ACC synthesis appears to be a post-germinative event, at least in intact caryopses.
Germination (pericarp splitting) of dormant caryopses, whose dormancy had been broken by severe wounding (Figs 3 and 5), also occurred if ethylene production had been suppressed by the presence of AVG and STS, demonstrating that the phytohormone is not required in the transition from dormant to non-dormant state induced by wounding. However, in contrast to the fast germinating non-dormant caryopses (Fig. 1), dormant caryopses that were wounded, particularly if cut when dry, germinated slowly (Figs 3 and 5), suggesting that wounding can be effective in promoting the germinative capability of dormant caryopses, but leaves some residual effect of the initial dormancy. Slow germination of wounded caryopses can be speeded up by ethylene even at the stage of pericarp splitting, as demonstrated by the addition of exogenous ACC (Figs 4A and 6A). Furthermore, wounding dormant caryopses after pre-imbibition (Fig. 5) stimulated pericarp splitting more than when they were cut when dry (Fig. 3), but also made their early germination sensitive to AVG and STS (compare Figs 3A and 5A). This indicated that, in these pre-imbibed caryopses, ethylene was indeed speeding up pericarp splitting. Thus, at least in wounded caryopses, ethylene can speed up pericarp splitting if it is provided before this stage (as exogenous ACC or, endogenously, because of wounding after imbibition).
Acceleration of delayed pericarp splitting by endogenous ethylene in dormant caryopses that are wounded after pre-imbibition affects only the germinative kinetics and is an additional consequence of wounding, distinct from the primary effect of breaking dormancy and promoting germinative capability. In fact, wound-induced promotion of germinative capability occurs even if any detectable ethylene production is suppressed by the inhibitors and is, therefore, independent of ethylene. That wounding stimulates pericarp splitting, as well as stage S1, through both an ethylene-dependent and an ethylene-independent effect, is also made clear in Fig. 4 where the ability of ACC to speed up germination in caryopses that were wounded, either dry or pre-imbibed, demonstrates an ethylene-dependent stimulation. Conversely, the inability of dry cutting to promote germination, in the presence of excess ACC, to the same level as obtained by cutting pre-imbibed caryopses (Fig. 4), indicates an ethylene-independent action. An ethylene-independent effect is therefore apparent between the two cutting treatments (dry or after pre-imbibition), as well as between intact and wounded caryopses. Reduced dormancy-breaking effectiveness in caryopses cut when dry could be due either to the position of the wound (Hsiao et al., 1983) or to an attenuation of the wound signal before the caryopses are hydrated enough to be responsive. In either case, ethylene accelerates pericarp splitting in pre-imbibed dormant caryopses that are forced to germinate by cutting, but is not responsible for triggering their germination.
The present results suggest that: (a) in intact caryopses, ethylene is produced after pericarp splitting and accelerates the onset of seedling growth (stage S1); (b) wounding stimulates the germinative capability of dormant caryopses independently of ethylene, but (c) does not make them germinate as fast as the truly non-dormant seed; (d) once triggered, such slow germination can be speeded up by ethylene at the stage of pericarp splitting; and, indeed, (e) the faster pericarp splitting that occurs when dormant caryopses are wounded after pre-imbibition, rather than when dry, is due to a quick production of ethylene. Thus, once the seed starts to germinate because of wounding, the quicker ethylene is produced the faster the germination of initially dormant caryopses will be (Fig. 6). In this sense, the strong germination delay observed by Cranston et al. (1996) when they incubated excised embryos of a dormant wild oat line with ethylene inhibitors could be due to a requirement for ethylene to speed up the very slow growth of the radicle rather than to break dormancy and trigger germination. In fact, the attainment of a length of the radicle greater than the caryopsis diameter was used in those experiments as a criterion for germination. Indeed, minimal visible growth of the radicle (or, of the coleoptile in the case of rice) is the conventional stage at which germination is recorded, but, actually, it signals that narrow-sense germination is over (Bewley and Black, 1994; Kucera et al., 2005). Therefore, attribution of precise metabolic events, such as transition from dormancy to germination, to the conventionally recorded stage may bring about some ambiguity in the interpretation of germination experiments.
In conclusion, the results indicate that ethylene production in intact caryopses of red rice is activated during germination and is involved neither in dormancy maintenance nor in the breaking of dormancy by means of wounding.
ACKNOWLEDGEMENTS
We thank Dr Fulvia Rizza and Professor Alcide Bertani for providing useful hints to the discussion, and Steven Footitt as well as an anonymous referee for thoroughly reviewing the manuscript. This work was supported by the European Union (EU) Access to Research Infrastructure Action of the Improving Human Potential Programme.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME Jr. Ethylene in plant biology. 2nd edn. San Diego: Academic Press; 1992. [Google Scholar]
- Adkins SW, Ross JD. Studies in wild oat seed dormancy. 1. The role of ethylene in dormancy breakage and germination of wild oat seeds (Avena fatua L.) Plant Physiology. 1981;67:358–362. doi: 10.1104/pp.67.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood WM. A physiological study of the germination of. Avena fatua. Botanical Gazette. 1914;57:386–414. [Google Scholar]
- Bewley J, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. [Google Scholar]
- Bijnen FGC, Reuss J, Harren FJM. Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Review of Scientific Instruments. 1996;67:2914–2923. [Google Scholar]
- Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D. Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. Journal of Plant Growth Regulation. 2004;23:44–53. [Google Scholar]
- Chao WS, Horvath DP, Anderson JV, Foley ME. Potential model weeds to study genomics, ecology, and physiology in the 21st century. Weed Science. 2005;53:929–937. [Google Scholar]
- Chen LJ, Lee DS, Song ZP, Suh HS, Lu BR. Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Annals of Botany. 2004;93:67–73. doi: 10.1093/aob/mch006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MA. Chemical mechanisms of breaking seed dormancy. Seed Science Research. 1996;6:95–99. [Google Scholar]
- Cohn MA, Hughes JA. Seed dormancy in red rice. V. Response to azide, hydroxylamine, and cyanide. Plant Physiology. 1986;80:531–533. doi: 10.1104/pp.80.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counce PA, Keisling TC, Mitchell AJ. A uniform, objective, and adaptive system for expressing rice development. Crop Science. 2000;40:436–443. [Google Scholar]
- Cranston HJ, Kern AJ, Gerhardt SA, Dyer WE. Wound-induced ethylene and germination of embryos excised from dormant Avena fatua L. caryopses. International Journal of Plant Sciences. 1996;157:153–158. [Google Scholar]
- Ferrero A. Weedy rice, biological features and control. In: Labrada R, editor. Weed management for developing countries. Rome: Food and Agriculture Organization of the United Nations; 2003. pp. 89–107. FAO Plant Production and Protection Paper 120–Addendum 1. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Footitt S, Cohn MA. Seed dormancy in red rice (Oryza sativa). IX. Embryo fructose-2,6-bisphosphate during dormancy breaking and subsequent germination. Plant Physiology. 1995;107:1365–1370. doi: 10.1104/pp.107.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealy DR, Tai TH, Sneller CH. Identification of red rice, rice, and hybrid populations using microsatellite markers. Weed Science. 2002;50:333–339. [Google Scholar]
- Gorecki RJ, Ashino H, Satoh S, Esashi Y. Ethylene production in pea and cocklebur seeds of differing vigour. Journal of Experimental Botany. 1991;42:407–414. [Google Scholar]
- Harren FJM, Laarhoven LJJ. Photoacoustic Spectroscopy. In: Brown TG, Creath K, Kogelnik H, Kriss MA, Schmit J, Weber MJ, editors. The optics encyclopedia. Weinheim: Wiley-VCH; 2004. pp. 3021–3056. [Google Scholar]
- Hsiao AI, Mc Intyre GI, Hanes JA. Seed dormancy in Avena fatua. I. Induction of germination by mechanical injury. Botanical Gazette. 1983;144:217–222. [Google Scholar]
- Kępczyński J, Karssen CM. Requirement for the action of endogenous ethylene during germination of non-dormant seeds of. Amaranthus caudatus. Physiologia Plantarum. 1985;63:49–52. [Google Scholar]
- Kępczyński J, Kępczyńska E. Ethylene in seed dormancy and germination. Physiologia Plantarum. 1997;101:720–726. [Google Scholar]
- Kępczyński J, Kępczyńska E, Bihun M. The involvement of ethylene in the release of primary dormancy in Amaranthus retroflexus seeds. Plant Growth Regulation. 2003;39:57–62. [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Lalonde S, Saini HS. Comparative requirement for endogenous ethylene during seed germination. Annals of Botany. 1992;69:423–428. [Google Scholar]
- Locke JM, Bryce JH, Morris PC. Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.) Journal of Experimental Botany. 51:1843–1849. doi: 10.1093/jexbot/51.352.1843. [DOI] [PubMed] [Google Scholar]
- Machabée S, Saini HS. Differences in the requirement for endogenous ethylene during germination of dormant and non-dormant seeds of Chenopodium album L. Journal of Plant Physiology. 1991;138:97–101. [Google Scholar]
- Matilla AJ. Ethylene in seed formation and germination. Seed Science Research. 2000;10:111–126. [Google Scholar]
- Matilla A, Gallardo M, Puga-Hermida MI. Structural, physiological and molecular aspects of heterogeneity in seeds: a review. Seed Science Research. 2005;15:63–76. [Google Scholar]
- Petruzzelli L, Harren F, Reuss J. Patterns of C2H4 production during germination and seedling growth of pea and wheat as indicated by a laser-driven photoacoustic system. Environmental & Experimental Botany. 1994;34:55–61. [Google Scholar]
- Petruzzelli L, Harren F, Perrone C, Reuss J. On the role of ethylene in seed germination and early root growth of. Pisum sativum. Journal of Plant Physiology. 1995;145:83–86. [Google Scholar]
- Petruzzelli L, Coraggio I, Leubner-Metzger G. Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta. 2000;211:144–149. doi: 10.1007/s004250000274. [DOI] [PubMed] [Google Scholar]
- Puga-Hermida MI, Gallardo M, Rodriguez-Gacio MC, Matilla AJ. Polyamine contents, ethylene synthesis, and BrACO2 expression during turnip germination. Biologia Plantarum. 2006;50:574–580. [Google Scholar]
- Reid MS, Paul JL, Farhoomand MB, Kofranek AM, Staby GL. Pulse treatments with the silver thiosulfate complex extend the vase life of cut carnations. Journal of the American Society for Horticultural Science. 1980;105:25–27. [Google Scholar]
- Satler SO, Kende H. Ethylene and the growth of rice seedlings. Plant Physiology. 1985;79:194–198. doi: 10.1104/pp.79.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwitayawan G, Geneve RL, Downie AB. Seed germination of ethylene perception mutants of tomato and. Arabidopsis. Seed Science Research. 2003;13:303–314. [Google Scholar]
- Sung SS, Leather GR, Hale MG. Induction of germination in dormant barnyardgrass (Echinochloa crus-galli) seeds by wounding. Weed Science. 1987;35:753–757. [Google Scholar]
- Vaughan LK, Ottis BV, Prazak-Havey AM, Bormans CA, Sneller C, Chandler JM, Park WD. Is all red rice found in commercial rice really Oryza sativa. Weed Science. 2001;49:468–476. [Google Scholar]
- Veen H. Antagonistic effect of silver thiosulphate or 2,5-norbornadiene on 1-aminocyclopropane-1-carboxylic acid-stimulated growth of pistils in carnation buds. Physiologia Plantarum. 1985;65:2–8. [Google Scholar]
- Vidotto F, Ferrero A, Ducco C. A mathematical model to predict the population dynamics of Oryza sativa var. sylvatica. Weed Research. 2001;41:407–420. [Google Scholar]