Abstract
Background and Aims
Differences in the mating systems and the mechanisms of reproductive isolation between Chamaecrista desvauxii var. graminea and C. desvauxii var. latistipula were examined in the Chapada Diamantina, Brazil. These taxa occur sympatrically, and their populations demonstrate marked morphological differences. The objective of the present work was to determine if reproductive isolation mechanisms exist between these two populations of C. desvauxii, and to determine the influence of these putative mechanisms on their genetic differentiation.
Methods
Field observations were made of floral biology, phenology and floral visitation, and experiments on intra- and interpopulation pollination and germination rates of the resultant seeds were performed. A genetic examination of the populations was undertaken using four allozyme loci.
Key Results
The varieties examined demonstrated overlapping of flowering periods during the months of June to September. The main pollinator for both varieties was the bee Bombus brevivillus. Both varieties are self-compatible, and a large number of fruits are formed in cross-pollinations with high seed germination rates. Inter-taxa pollinations result in high levels of fruit production, but no seeds are formed. Two of the four loci examined were diagnostic for the varieties, and exclusive high-frequency alleles were encountered at the other loci, leading to a high genetic distance between the two populations (0·495).
Conclusions
Pre-zygotic barriers were not found between the two varieties, and these remain isolated due to post-zygotic events. The two varieties demonstrate marked differences in their morphology, floral biology, phenology and genetic make-up, all of which indicate that they should be treated as two distinct species. A complete revision involving the other varieties of the C. desvauxii complex will be necessary in order to define these two taxa formally.
Key words: Allozyme, campo rupestre, Chamaecrista desvauxii, Chapada Diamantina, floral biology, Leguminosae, mating systems, pollination, post-zygotic barriers, reproductive isolation, sympatric species
INTRODUCTION
Cross-breeding, resulting in genetic exchange and the formation of hybrids, is possible between sympatric species. Potential cross-breeding can be reduced, however, by pre-zygotic or post-zygotic mechanisms acting as reproductive barriers between taxa (Dobzhansky, 1937; Grant, 1981; Stace, 1989). The evolution of these isolation mechanisms has been widely discussed by evolutionary biologists (e.g. Schluter, 2001; Turelli et al., 2001; Servedio and Noor, 2003) who have sought to understand the processes leading to speciation. However, most of these studies have been carried out with animals (e.g. Dobzhansky, 1937; Coyne et al., 2002; Mendelson, 2003). It is widely agreed that an increase in reproductive isolation is generally associated with an increase in morphological and ecological differentiation (Moyle et al., 2004). According to Orr (2001), phenotypic evolution might often give rise to reproductive isolation, whereas reproductive isolation might often preserve phenotypic differences when taxa come into contact with each other. Although classical studies with plants have demonstrated a general association between reproductive isolation and the differentiation of some quantitative genetic features, the direct association between genetic distance and reproductive isolation has been examined in only a few species (e.g. Moyle et al., 2004).
The genus Chamaecrista (Leguminosae–Caesalpinioideae) comprises six sections with approx. 265 species, and is represented in the Americas by approx. 239 species (Irwin and Barneby, 1982). Irwin (1964) recognized 16 species in the section Xerocalyx (then in the genus Cassia) which were distinguished by their morphological, anatomical, biochemical and chromosomal characteristics. Since then, the section Xerocalyx has been subjected to a profound taxonomic reorganization, with only three species with 22 varieties, 17 of them in C. desvauxii, having been recognized (Irwin and Barneby, 1982), and more recently rearranged in ten species and 27 varieties by Fernandes and Nunes (2005).
This taxonomic instability is principally the result of disagreement over how to treat the complex patterns of morphological variation encountered within this group — either opting to recognize large polytypic species, or placing stricter limits on the species breadth. This problem has been largely approached on the basis of the analysis of herbarium specimens, a technique that does not permit the formulation of hypotheses concerning the biology and reproductive limits between the morphs observed in nature.
Chamaecrista desvauxii (Collad.) Killip is a Neotropical species with a distribution range from Mexico to northern Argentina. The varieties C. desvauxii var. graminea H. S. Irwin and Barneby, and C. desvauxii var. latistipula (Benth.) G. P. Lewis occur sympatrically in campo rupestre formations in the municipality of Mucugê, in the Chapada Diamantina mountain range, Bahia state, Brazil. Chamaecrista desvauxii var. graminea and C. desvauxii var. latistipula are distinguished from each other principally by their vegetative characteristics, having small stipules and lanceolate leaflets or large stipules and oblong leaflets, respectively (Fig. 1). The flowers of these two varieties show differences in size and colour pattern, although the latter characteristic is not preserved in herbarium material. In addition to morphological differences, there are also ecological differences between them that raise the hypothesis of whether these two varieties can hybridize, or whether they are in fact reproductively isolated and without genetic exchange, representing two distinct species.
Fig. 1.

(A, C, E) Chamaecrista desvauxii var. graminea: (A) habit; (C) flowers; (E) leaf. (B, D, F) Chamaecrista desvauxii var. latistipula: (B) habit; (D) flower; (F) leaf. Scale bars: A = 83 mm; B = 115 mm; C, E = 5 mm; D = 3 mm; F = 4 mm.
The objective of the present study was to ascertain the existence of reproductive isolation mechanisms between sympatric populations of two varieties of C. desvauxii (var. graminea and var. latistipula), and to examine the influence of these putative isolation mechanisms on genetic differentiation between the two populations through studies of reproductive biology and allozyme comparisons.
MATERIALS AND METHODS
Study site
The study was undertaken in the Mucugê Municipal Park, in the municipality of Mucugê, Bahia state, Brazil, in an area of approx. 140 000 m2. The park is located in the Chapada Diamantina mountain range, between the coordinates 12°59′02″ to 13°00′18″S and 41°20′41″ to 41°20′33″W, at altitudes near 1000 m above sea level. The predominant vegetation in the area is campo rupestre, which is composed principally of herbaceous and sub-shrub plants growing on sandy soils and herbaceous and shrub vegetation growing on rock outcrops (Giulietti and Pirani, 1988). The climate is mesothermic, type Cwb in the classification system of Köppen (1948), with an average annual temperature of 19·8 °C. Annual average rainfall varies between 830 and 1192 mm. Voucher specimens of the plants examined were stored in the herbarium of the Universidade Estadual de Feira de Santana (HUEFS): C. B. N. Costa 109 (C. desvauxii var. latistipula) and C. B. N. Costa 112 (C. desvauxii var. graminea).
Floral biology
Flowering phenology was followed on a monthly basis from July 2003 to December 2005, noting the presence or absence of flowers on marked individuals of C. desvauxii var. graminea (n = 100; randomly chosen) and C. desvauxii var. latistipula (n = 24). This difference in the sampling sizes is due to the rarity of individuals of C. desvauxii var. latistipula in the study area (all individuals were marked). Each taxon was considered as one population. Floral morphology was observed, the number of flowers per inflorescence, the duration of the flowers and their colour, as well as the time of anthesis. The stigmatic receptivity of approximately 20 flowers from each taxon was also examined in the field during different hours of the day using the hydrogen peroxide technique (Dafni, 1992). Pollen availability was confirmed by visual inspection of the anthers and by using a small tuning fork to liberate the pollen grains. Pollen viability was examined in five anthers of different flowers for each species using the nitroblue tetrazolium enzymatic test to measure dehydrogenase activity (Dafni, 1992). The presence of ultraviolet-absorbing pigments was investigated by exposing flowers to ammonium hydroxide vapours. Pollen morphology was examined using the acetolase process according to Erdtman (1960). The diameter of 25 randomly chosen pollen grains from each taxon was measured under a light microscope, calculating their average value and standard error. Pollen grains were further examined to determine the number of openings and their surface ornamentation.
Floral visitors were initially observed between 0530 h and 1800 h, although this period was later adjusted to 0530 h to 1300 h for C. desvauxii var. latistipula to cover only the time when the flowers were open. A total of 41 h of direct observations were realized with C. desvauxii var. graminea and 32 h with C. desvauxii var. latistipula, noting the types of visitors, their behaviour, their contact with the stigma, the frequency of visitation and the type of floral resource collected. Specimens of visiting species were collected, identified by specialists, and subsequently stored in the entomology collection of the Zoology Museum at the Universidade Estadual de Feira de Santana.
Self- and cross-compatibility experiments
The trichomes at the apex of the style form a stigmatic chamber in the genus Chamaecrista, making experimental pollination somewhat difficult. As such, a number of different techniques were tried in order to identify the best method of manual pollination: (a) simple transfer of pollen to the apex of the style; (b) pollination after removing the trichomes (in order to gain access to the stigma chamber); (c) pollination after vibrating the style with a tuning fork (in order to test for any possible physical effect of the vibrations on the fertilization process); and (d) pollination using a needle after manipulating the trichomes with a stiff-bristled paint brush (simulating the mechanical contact of the hairs of insect pollinators with the stigmatic trichomes). Ten flowers were used in each test, using pollen obtained by means of a turning fork to simulate the vibrations produced by bees. Technique (d) was found to be most effective, and was used in all subsequent pollination experiments.
Experiments of manual self-pollination, manual bi-directional intra- and inter-taxon cross-pollination, spontaneous self-pollination, as well as open pollination were performed for comparison with natural fruit formation. Intra-taxon cross-pollination was performed using pollen collected from plants that were separated by >100 m. Experimental flowers were covered while in the bud stage, subjected to the appropriate experimental pollination regime, and then covered again. An attempt was made to carry out a similar number of experimental pollinations among the individuals, and to cross as high a number of pairs of individuals as possible. However, this aim was not strictly achieved and the number of flowers used per treatment per individual was not uniform because of differences in the availability of flowers in different individuals, pollinations being carried out somewhat haphazardly. Even so, the pollinated flowers were considered independent events, in spite of the possibility of pseudo-replication, and it is felt that such an assumption does not qualitatively change the results.
Two to four pollinated flowers were collected 6, 10, 24 and 48 h after pollination and fixed in a 50 % solution of FAA in order to observe the growth of pollen tubes and/or the penetration of the ovules. Pistils were bleached with 5 % NaClO for 15–30 min, stained with 0·25 % aniline blue and observed under an epifluorescence microscope (modified from Martin, 1959).
Seeds from all experimentally produced fruits were weighed and tested for germination. Dormancy was broken by mechanical scarification of the seeds, which were then disinfected with 2·5 % NaClO for 5 min and washed with distilled water (modified from Gomes et al., 2001). Fifteen seeds for each experiment were placed in germination beds lined with filter paper moistened with distilled water and kept in a germination chamber under constant light at 27 ºC. Germination was examined daily for 30 d in order to measure the Germination Speed Index (GSI) of Maguire (1962) and the percentage of germination. Analysis of variance (ANOVA) was performed and the Tukey test employed to compare sample averages utilizing the BIOSTAT 3·0 software program (Ayres et al., 2003).
Allozyme variation
Allozyme variation was examined in order to verify the putative occurrence of exclusive alleles and/or exclusive loci for the varieties of C. desvauxii. Small sections of leaf tissue were crushed in 0·5 mL of grinding buffer (Lambert et al., 2006). Buffer systems #1 and #4 from Lambert et al. (2006) were used for electrodes and gels, and standard horizontal electrophoresis was performed according to these same authors. Three enzymatic systems were used: system 1 for malate dehydrogenase (Mdh; EC 1·1·1·37); and system 4 for acid phosphatase (Acp; EC 3·1·3·2) and phosphoglucose isomerase (Pgi; EC. 5·3·1·9). Staining procedures were similar to, but slightly modified from, Brune et al. (1998; Acp), Corrias et al. (1991; Pgi) and Soltis et al. (1983; Mdh). The exact recipes can be obtained from the authors on request. Enzymatic systems demonstrating more than one locus were numbered in ascending order, starting from the locus with the lowest mobility. Alleles were numbered according to their mobility relative to a standard allele, with the highest mobility present in all gels and designated as 100. The allelic frequencies were determined by manually counting the banding patterns of the homozygotes and heterozygotes stained in the gels. Genetic variability for every population was estimated by the following parameters: proportion of polymorphic loci (P; 0·95 criterion), mean number of alleles per locus (A), and observed (Ho) and expected (He) mean heterozygosity per locus. Partitioning of genetic diversity between the populations was estimated by FST (Wright, 1978), and the Nei's (1978; unbiased estimate) genetic distance between the two taxa was calculated. Data analysis was performed using the BIOSYS 1·0 software package (Swofford and Selander, 1989).
RESULTS
Flower morphology and phenology
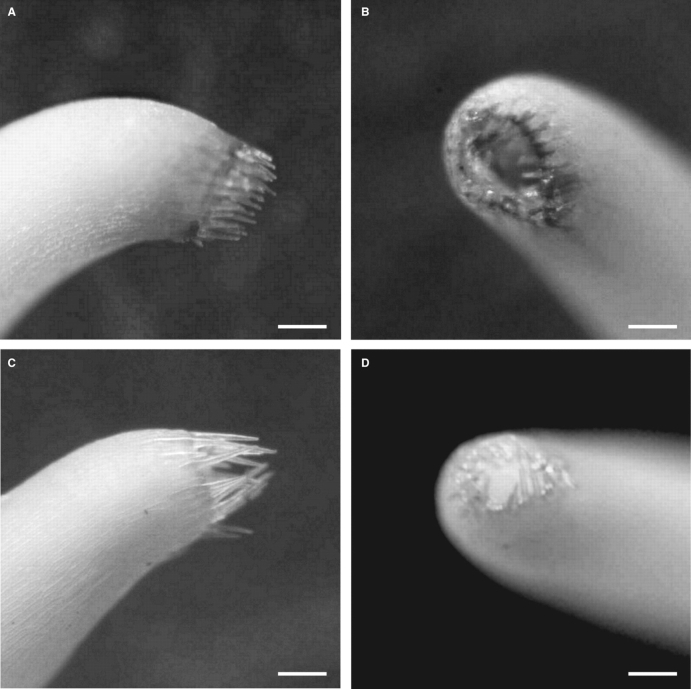
The inflorescence of C. desvauxii var. graminea has 1–2 yellow flowers, while that of C. desvauxii var. latistipula is unifloral, with yellow flower axillaries along the branch, and up to three flowers per branch (Fig. 1). The flowers of both taxa offer only pollen as a floral resource, the anthers opening by means of apical slits. The flowers do not have a perceptible odour and demonstrate enantiostyly, with two morphs being found on the same individual. The stigma is surrounded by a ring of trichomes, forming a stigmatic chamber at the apex of the style (Fig. 2).
Fig. 2.
Side (A, C) and frontal (B, D) view of the stigma of Chamaecrista desvauxii var. graminea (A, B) and C. desvauxii var. latistipula (C, D). Scale bars = 0·3 mm.
The flowers of C. desvauxii var. graminea are approx. 25 mm in diameter, with the petals being approx. 10 × 16 mm. The median petal is larger (22 × 13 mm) and is tinted red at the base. The anthers are yellow, three of them being approx. 8 mm long and seven being 4–5 mm long. All anthers have their openings facing towards the median petal. The pistil is green with a hairy ovary that contains 11–13 ovules.
The flowers of C. desvauxii var. latistipula are approx. 20 mm in diameter, with petals entirely yellow and approx. 8 × 12 mm. The median petal is larger and curved, however, being approx. 14 × 12 mm. The anthers are of uniform size (approx. 4–5 mm long), yellow-vinaceous, and directed towards the median petal. The pistil is also green; the ovary is densely hairy and it contains 12–13 ovules.
The flowers of C. desvauxii var. graminea last only a single day. Flowers begin to open at approx. 0330 h and are fully open by 0600 h when the stigma is receptive and pollen is available. Floral visits do not begin before 0530 h. Pollen availability is delayed on cloudy days, and the anther clefts remain closed. Although the anthers are of different sizes, there appears to be no significant difference between them in terms of pollen viability (larger anthers, 84·5 ± 3·1 %; smaller anthers, 85·2 ± 2·7 %; ANOVA: F = 0·1373 P > 0·05, d.f. = 1). Flowers close at approx. 1800 h, the stigma remaining receptive until that time. Anthesis in C. desvauxii var. latistipula occurs at about 0530 h and the flowers remain open until approx. 1200 of the same day. The stigma is receptive and pollen is available from the moment of anthesis until floral closing. The pollen of this taxon has a high viability (94·2 ± 2·34 %).
Pollen grains are essentially identical among these two varieties, both being tricolporate monads, with smooth external ornamentation. There is a slight variation in the average size of the pollen grains between the two taxa, but a small degree of overlap occurs. The pollen grains of C. desvauxii graminea are 47·5 ± 2·7 µm in polar diameter and 34·8 ± 2·69 µm in equatorial diameter, while the grains of C. desvauxii latistipula are 44·4 ± 3·0 µm and 29·4 ± 3·17 µm, respectively.
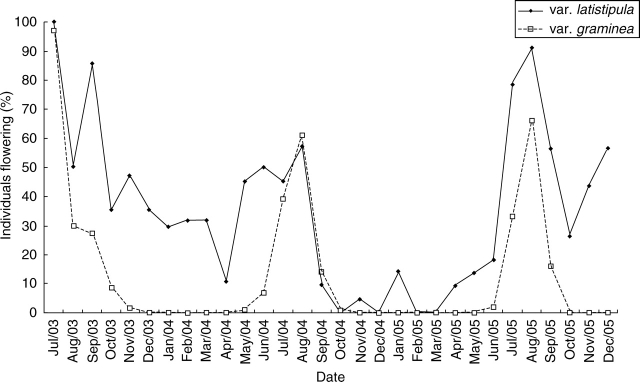
Chamaecrista desvauxii var. graminea demonstrates annual flowering, with a flowering peak in the dry season (June to September) (Fig. 3). Chamaecrista desvauxii var. latistipula flowers almost all year round, although with a peak of flowering during the months of July to September.
Fig. 3.
Flowering phenology of Chamaecrista desvauxii var. graminea and C. desvauxii var. latistipula in Mucugê, Bahia state, north-eastern Brazil.
Floral visitors
The principal floral visitor of both taxa examined was Bombus brevivillus Franklin, 1913 (Apidae) in light of its high frequency of visitation and its pollen-collecting behaviour (Table 1; Fig. 4A, B). In collecting the pollen, the bee lands in the centre of the flower, secures the anthers at their base and then initiates the process of buzz pollination. These bees were observed moving around within the flower in both plant species and vibrating from many different positions. These movements resulted in the pollen being deposited on all parts of their bodies, including the dorsal portion of their thorax, as the differentiated median petal directed the pollen flow. As a result of the bee's movements within the flower, different parts of its body come in contact with the stigma, resulting in pollination. Part of the pollen load deposited on the dorsal region of the thorax by the median petal is collected by these bees during the process of cleaning their bodies with their front legs.
Table 1.
Flower visitors and frequency of visits in Chamaecrista desvauxii var. graminea and Chamaecrista desvauxii var. latistipula in Mucugê, Bahia state, north-eastern Brazil
| Number of visits | ||
|---|---|---|
| Flower visitors | C. desvauxii var. graminea | C. desvauxii var. latistipula |
| Bombus brevivillus | 138 (79·8 %) | 124 (79·0 %) |
| Centris fuscata | 5 (2·9 %) | 5 (3·2 %) |
| Euglossa sp. | 7 (4·0 %) | 3 (1·9 %) |
| Pseudaugochlora sp1 | 1 (0·6%) | – |
| Pseudaugochlora sp2 | 9 (5·2 %) | 12 (7·6 %) |
| Trigona spinipes | 6 (3·5 %) | 4 (2·5 %) |
| Thygater sp. | 7 (4·0 %) | 9 (5·7 %) |
Fig. 4.

Bees visiting flowers of Chamaecrista desvauxii. (A, B) Bombus brevivillus collecting pollen in C. desvauxii var. graminea. (C–F) Chamaecrista desvauxii var. latistipula: (C) Halictidae on anther without contacting the stigma (arrow); (D) B. brevivillus collecting pollen; (E) B. brevivillus upside-down during a turning movement in the flower; (F) Thygater collecting pollen. Scale bars: A, B = 5·5 mm, C–F = 4·0 mm.
Other species of bees were also considered effective pollinators, such as Centris fuscata Lepeletier, 1841 and Euglossa sp. (Table 1), although these species visited the flowers more rapidly and left the flowers without demonstrating any variation in their behaviour. Contact with the stigmatic chamber was made by the lateral and dorsal portions of the bodies of these bees. Species of bees of the family Halictidae were also recorded visiting flowers of both taxa, although these smaller bees were not effective pollinators as they buzzed only one anther at a time, and then passed to another anther without entering into contact with the stigma (Fig. 4C).
Self- and cross-compatibility experiments
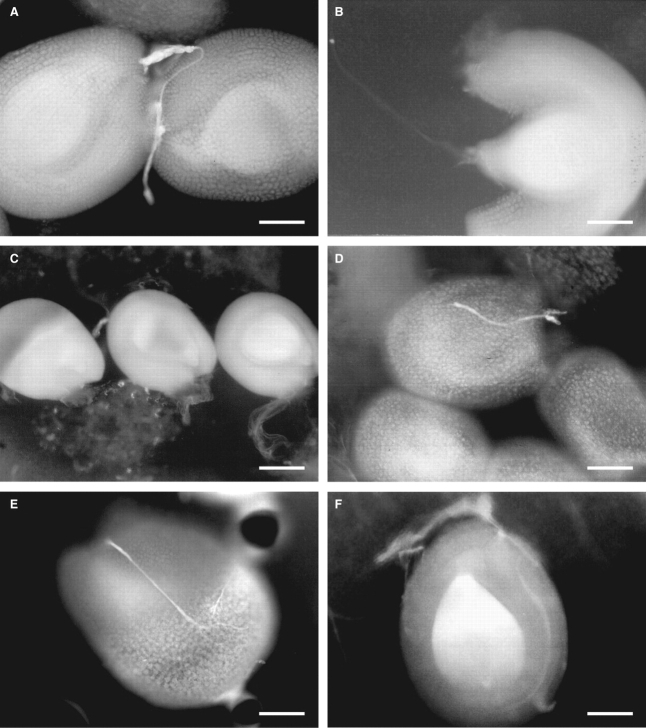
High fruit set was observed in both plant taxa following experimental self-pollination. The percentage values of fruiting were similar to those observed in intra-taxon cross-pollinations, indicating self-compatibility in these plants (Table 2). No significant difference among individuals occurred in any treatment of both taxa. No fruit formation by spontaneous self-pollination was observed in either taxon. A small difference in the rate of growth of pollen tubes was observed in C. desvauxii var. latistipula, with tubes reaching the ovules at the base of the ovary 6 h after cross-pollination, while in cases of self-pollination the pollen tubes reached only the more distal ovules within the same time frame (Fig. 5). With C. desvauxii var. graminea, penetration of the pollen tubes to the ovules required 10 h, but no differences in velocity were observed between cross- and self-pollination experiments. No difference in pollen tube morphology or speed of growth was observed between self- and cross-pollinations in either taxon.
Table 2.
Fruiting individuals, fruit and seed set, and seed germination in experimental pollinations in Chamaecrista desvauxii var. graminea and C. desvauxii var. latistipula
| Treatment | % of individuals fruiting (individuals fruiting/individuals used) | % fruit set (fruit/fower) | Seeds/fruit mean (min.–max.) | % germination | IVG |
|---|---|---|---|---|---|
| C. desvauxii graminea | |||||
| Open | 86·7 (13/15) | 44·4 (20/45) | 1·5 (0–10) | 71 | – |
| Spontaneous | 0 (0/5) | 0 (0/26) | – | – | – |
| Self-pollination | 100 (8/8) | 67 (21/31) | 0·8 (0–6) | 92·3 | 6·03 |
| Cross-pollination | 100 (11/11) | 69 (25/36) | 2·27 (0–7) | 82 | 6·15 |
| ×C. d. latistipula | 100 (12/12) | 83·3 (35/42) | 0 | – | – |
| C. desvauxii latistipula | |||||
| Open | 100 (10/10) | 63·3 (19/30) | 6·4 (0–13) | 100 | – |
| Spontaneous | 0 (0/10) | 0 (0/27) | – | – | |
| Self-pollination | 100 (7/7) | 59 (19/32) | 6 (0–11) | 92 | 21·0 |
| Cross-pollination | 100 (9/9) | 70 (21/30) | 7·6 (0–13) | 100 | 22·8 |
| ×C. d. graminea | 100 (8/8) | 52·4 (22/42) | 0 | – | – |
IVG = index of velocity of seed germination.
Fig. 5.
Pollen tubes penetrating ovules in Chamaecrista desvauxii. (A–D) Intra-taxon pollinations: (A, B) 6 h after cross- (A) and self-pollination (B) in C. desvauxii var. latistipula; (C, D) 10 h after cross- (C) and self-pollination (D) in C. desvauxii var. graminea. (E, F) Inter-taxa crossings between C. desvauxii var. graminea and C. desvauxii var. latistipula: (E) C. desvauxii var. latistipula as pollen receptor, 48 h; (F) C. desvauxii var. graminea as pollen receptor, 24 h. Scale bars: A, B, D–F = 0·15 mm; C = 0·2 mm.
Inter-taxa crosses resulted in a high level of fruit formation, superior even to those observed in intra-taxon cross-pollinations in C. desvauxii var. graminea (Table 2). The penetration of the pollen tubes to the ovules occurred 24 h after pollination in both taxa (Fig. 5). However, no viable seeds were formed by inter-taxa crosses, and only apparently unfertilized ovules and small aborted seeds without embryos were observed. No difference in pollen tube morphology or speed of growth was observed between intra- and inter-taxon cross-pollinations in either taxon.
The number of seeds formed per fruit varied according to the experimental crosses (Table 2), with the largest number of seeds resulting from cross pollination in C. desvauxii var. graminea (F = 5·5194, d.f. = 1, P < 0·05). There was no significant difference in the number of seeds resulting from either self-pollination or cross-pollination in C. desvauxii var. latistipula. Aborted seeds were found at the base, in the centre and in the apex of the fruits, indicating that there was no correlation between abortion and ovule position. No significant differences in seed weight were observed in the self-pollination and cross-pollination experiments, with an average of 5·4 (s.e. = 0·31) mg for seeds resulting from self-pollinations and 4·9 (s.e. = 0·32) mg for seeds resulting from cross-pollination in C. desvauxii var. latistipula, and 3·0 (s.e. = 0·3) mg and 3·4 (s.e. = 0·3) mg per seed in C. desvauxii var. graminea, respectively. Differences in seed weight between taxa were significant, however (F = 13·182, d.f. = 3, P < 0·01). All seeds demonstrated high levels of germination, and the germination velocity indices were similar for all treatments within the same taxon, but demonstrated differences between the taxa (Table 2).
Allozyme eletrophoresis
Four polymorphic loci were identified in the three systems tested, with each locus demonstrating two alleles (Table 3). Fixed exclusive alleles were observed for Pgi and Acp in each of the two plant varieties examined. Mdh-1 was monomorphic within the population of C. desvauxii var. latistipula, while nine of ten individuals of C. desvauxii var. graminea were heterozygotic. Chamaecrista desvauxii var. latistipula demonstrated 15 of 16 individuals as heterozygotic for Mdh-2, and a second allele was exclusive to this variety. The average heterozygostity observed in C. desvauxii var. latistipula was 0·143, and that in C. desvauxii var. graminea was 0·129. Both of these values were higher than that expected in a Hardy–Weinberg equilibrium. The genetic distance between the populations was high (0·495), and a high FST value was recorded for these species (0·720), indicating significant genetic structuring between the populations.
Table 3.
Allele frequencies in four allozymic loci in one population of Chamaecrista desvauxii var. latistipula and one population of C. desvauxii var. graminea occurring at the municipality of Mucugê, Chapada Diamantina, north-eastern Brazil
| Locus/allele | C. desvauxii var. latistipula | C. desvauxii var. graminea |
|---|---|---|
| Pgi | ||
| 62 | – | 1·000 |
| 100 | 1·000 | – |
| n | 17 | 14 |
| Acp | ||
| 80 | – | 1·000 |
| 100 | 1·000 | – |
| n | 16 | 14 |
| Mdh-1 | ||
| 76 | – | 0·550 |
| 100 | 1·000 | 0·450 |
| n | 6 | 10 |
| Mdh-1 | ||
| 91 | 0·500 | – |
| 100 | 0·500 | 1·000 |
| n | 16 | 14 |
DISCUSSION
Reproductive biology
The presence of a differentiated median petal, the formation of a stigmatic chamber and anthers with small cleft openings are common characteristics of the genus Chamaecrista (Gottsberger and Silberbauer-Gottsberger, 1988; Owens and Lewis, 1989). The anthers are functionally poricidal, which restricts pollination to those groups of bees that collect pollen by buzzing the anthers (Buchmann, 1983).
Ricochet pollination (Westerkamp, 2004) was observed by Gottsberger and Silberbauer-Gottsberger (1988) in C. hispidula. In this process, the median petal directs pollen to the dorsal portion of the thorax of the bee, a location that favours contact with the stigma and hence pollination. Dependence on this process is minimized by the bees' behaviour in the flower varieties studied here, because the movements of these insects ensure that numerous parts of their bodies come into contact with the stigma.
Flowering strategies differ between the two taxa examined, as C. desvauxii var. graminea demonstrates annual flowering of medium duration, while C. desvauxii var. latistipula demonstrates continuous flowering, with the production of flowers almost all year round (see Newstron, 1994). In spite of these different flowering strategies, however, there is a significant overlapping of flowering periods between the two taxa that could conceivably permit genetic exchange.
Chamaecrista desvauxii var. graminea and C. desvauxii var. latistipula are self-compatible, a characteristic observed in other species of this genus (Gottsberger and Silberbauer-Gottsberger, 1988; Fenster, 1995; Liu and Koptur, 2003). Self-compatibility seems to be common in Chamaecrista, while in other groups of Caesapinioideae gametophytic self-incompatibility (Bawa, 1974) and late-acting self-incompatibility (Lewis and Gibbs, 1999; Carvalho and Oliveira, 2003) are more common. The differences observed in pollen tube growth velocity in C. desvauxii var. latistipula would tend to favour cross-pollination, especially in view of the high germination rate of seeds produced by self-pollination. However, this mechanism does not seem to function in C. desvauxii var. graminea.
In spite of the apparent success of the pollen tubes in penetrating the ovules, both varieties show a high level of aborted seeds in both self-pollination and cross-pollination experiments. In the majority of plants, only a fraction of the ovules in any fruit actually become seeds (Lee, 1988) and, although self-pollination frequently results in fewer mature seeds per fruit than does cross-pollination, it can rarely be determined with any certainty if this reduction is due to a lack of fertilization or to post-zygotic abortion. Post-zygotic mechanisms, such as the presence of lethal recessive alleles, could also result in the diminished production of viable seeds as a result of high levels of spontaneous abortions in cases of self-pollination or of pollination between closely related individuals. This latter hypothesis is a plausible explanation for the results observed with C. desvauxii var. graminea.
Isolation mechanisms
Pre-zygotic gametophytic isolation mechanisms are commonly found in angiosperms (Stace, 1989), including the Leguminosae (Teixeira and Ranga, 2004); however, they were not found in the two taxa examined in the present work. These varieties demonstrated superposition of flowering periods, sharing of pollinators, similarities between floral events such as stigma receptivity and pollen release, similar floral morphology, and penetration of the ovules in interspecific crosses. The similarity in the morphology and growth speed of pollen tubes in intra- and inter-taxon crossings strengthens the evidences for the absence of pre-zygotic barriers. As such, seed incompatibility with death of the embryo or the endosperm is most probably the principal post-zygotic isolation mechanism in the two varieties studied. Similar mechanisms were seen in species of Lens (Leguminosae) (Ladizinsky, 1997).
The presence of exclusive alleles in each of the varieties, principally those fixed at the Pgi and Acph loci, is a strong indicator of the absence of gene flow between the two taxa, and this is supported by the results of the reproductive experiments (interspecific incompatibility). The genetic distance observed between the two varieties is consistent with that observed in other plant species (Thorpe, 1982; Crawford, 1989; van der Bank et al., 2001). Additionally, the observed genetic structuring is very high, reinforcing the idea of genetic isolation between the two taxa and characterizing them as two distinct species.
Taxonomic implications
Cassia latistipula was described by Bentham (1870), accepted by Irwin (1964) and later synonymized in Chamaecrista desvauxii (Collad.) Killip var. glauca (Hassler) H. S. Irwin and Barneby (Irwin and Barneby, 1982). A new combination was proposed by Lewis (1987), restoring the epithet latistipula for the variety. As such, the taxon came to be considered as C. desvauxii var. latistipula. Chamaecrista desvauxii var. graminea was described by Irwin and Barneby (1982).
Based on morphological, anatomical and chromosomal data, as well as chromatographic studies, Irwin (1964) raised Xerocalyx to the level of section, having been previously considered a sub-section of the section Chamaecrista of the genus Cassia. At a later date, Irwin and Barneby (1982) decided that the section Xerocalyx would be better viewed as a macro-species in which complex evolutionary processes were still occurring, but which were not advanced sufficiently to delimit true species. This vision resulted in a profound taxonomic shuffling, with the condensation of 16 species into only three species and 22 varieties. Fernandes and Nunes (2004) considered the delimitations proposed by Irwin and Barneby (1982) as excessively limited in their use of morphological characteristics, resulting in an elevated level of subjectivity in the taxonomic treatment of that section. However, these same authors proposed, in a relatively arbitrary manner, the reordering of the section Xerocalyx based exclusively on morphological characteristics, recognizing ten species and 27 varieties (Fernandes and Nunes, 2004). In this classification system, C. desvauxii var. latistipula was recognized as a species, while C. desvauxii var. graminea was considered a variety of C. linearis.
The varieties ‘latistipula’ and ‘graminea’ of C. desvauxii demonstrate marked differences in habit and leaf morphology. They are also reproductively isolated and show unique ecological characteristics in terms of their floral longevity, flowering period and microhabitat, which all reinforce the hypothesis of reproductive isolation. As such, based on all of the ecological, geographical, morphological, reproductive and genetic data available for C. desvauxii var. graminea and C. desvauxii var. latistipula, it is reasonable to affirm that they represent distinct species. This conclusion partially supports the taxonomic proposal of Fernandes and Nunes (2004), who considered some of the taxa that Irwin and Barneby (1982) classified as varieties as being true species. On the other hand, Fernandes and Nunes (2004) maintained many distinct morphs as varieties of the species they recognized. As such, the results of the present work indicate the necessity for a much wider examination of the section Xerocalyx, using ecological and genetic data and focusing on the reproductive isolation among the taxa, in order to establish a more stable and natural taxonomic ordering for this group.
ACKNOWLEDGEMENTS
We thank Jorge A. S. Costa for help on field work and improvements to the manuscript, the staff of the Parque Municipal de Mucugê for logistic support, and Favízia F. Oliveira for identification of the bees. This work was supported by a grant from Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB). C.B.N.C. received a schoolarship from FAPESB. E.L.B. and L.P.Q. are supported by a grant (PQ2) from CNPq.
LITERATURE CITED
- Ayres M, Ayres JM, Ayres DL, Santos AS. BioEstat 3·0. Belém: Sociedade Civil Mamirauá; 2003. [Google Scholar]
- van der Bank H, van der Bank M, van Wyk B. A review of the use of allozyme electrophoresis in plant systematics. Biochemical Systematics and Ecology. 2001;29:469–483. doi: 10.1016/s0305-1978(00)00086-7. [DOI] [PubMed] [Google Scholar]
- Bawa KS. Breeding systems of tree species of a lowland community. Evolution. 1974;28:85–92. doi: 10.1111/j.1558-5646.1974.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Bentham G. von Martius CEP, editor. Leguminosae II et III. Swartzieae, Caesalpinieae, Mimoseae — tribus XVII. Cynometreae. Flora Brasiliensis. 1870;15:239–248. [Google Scholar]
- Brune W, Alfenas AC, Junghans TG. Identificações específicas de enzimas em géis. In: Alfenas AC, editor. Eletroforese de isoenzimas e proteínas afins: fundamentos e aplicações em plantas e microorganismos. Viçosa: Universidade Federal de Viçosa; 1998. pp. 201–328. [Google Scholar]
- Buchmann SL. Buzz pollination in angiosperms. In: Jones LE, Little RJ, editors. Handbook of experimental pollination biology. New York: Scientific and Academic Editions; 1983. pp. 73–113. [Google Scholar]
- Carvalho DA, Oliveira PE. Biologia reprodutiva e polinização de Senna sylvestris (Vell.) H. S. Irwin and Barneby (Leguminosae, Caesalpinioideae) Revista Brasileira de Botânica. 2003;26:319–328. [Google Scholar]
- Corrias B, Rossi W, Arduino P, Cianchi R, Bullini L. Orchis longicornu Poiret in Sardinia: genetic, morphological and chorological data. Webbia. 1991;45:71–101. [Google Scholar]
- Coyne JA, Kim SY, Chang AS, Lachaise D, Elwyn S. Sexual isolation between two sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution. 2002;56:2424–2434. doi: 10.1111/j.0014-3820.2002.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Crawford DJ. Enzyme eletrophoresis and plant systematics. In: Soltis ED, Soltis PS, editors. Isozymes in plant biology. Portland, OR: Dioscorides Press; 1989. pp. 146–164. [Google Scholar]
- Dafni A. Pollination ecology — a practical approach. New York: Oxford University Press; 1992. [Google Scholar]
- Dobzhansky T. Genetics and the origin of species. New York: Columbia University Press; 1937. [Google Scholar]
- Erdtman G. The acetolysis method. A revised description. Svensk Botanisk Tidskrift. 1960;54:561–564. [Google Scholar]
- Fenster CB. Mirror image and their effect on outcrossing rate in Chamaecrista fasciculata (Leguminosae) American Journal of Botany. 1995;82:46–50. [Google Scholar]
- Fernandes A, Nunes EP. Registros botânicos. Fortaleza: Edições Livro Técnico; 2005. [Google Scholar]
- Giulietti AM, Pirani JR. Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Heyer WR, Vanzolini PE, editors. Proceedings of a workshop on neotropical distribution patterns. Rio de Janeiro: Academia Brasileira de Ciências; 1988. pp. 39–69. [Google Scholar]
- Gomes V, Madeira JA, Fernandes GW, Lemos Filho JP. Seed dormancy and germination of sympatric species of Chamaecrista (Leguminosae) in a rupestrian field. International Journal of Ecology and Environment Sciences. 2001;27:191–197. [Google Scholar]
- Gottsberger G, Silberbauer-Gottsberger I. Evolution of flower structures and pollination in neotropical Cassiinae (Caesalpiniaceae) species. Phyton. 1988;28:293–320. [Google Scholar]
- Grant V. Plant speciation. New York: Columbia University Press; 1981. [Google Scholar]
- Irwin HS. Monographic studies in Cassia (Leguminosae-Caesalpinioideae). I section Xerocalyx. Memoirs of the New York Botanical Garden. 1964;12:1–114. [Google Scholar]
- Irwin HS, Barneby RC. The American Cassinae. A synoptical revision of Leguminosae tribe Cassieae subtribe Cassinae in the New World. Memoirs of the New York Botanical Garden. 1982;35:636–895. [Google Scholar]
- Koppen W. Climatologia con un estudio de los climas de la tierra (transl. by Peres PRH) Mexico City: Fondo de Cultura Económica; 1948. [Google Scholar]
- Ladizinsky G. A new species of Lens from south-east Turkey. Botanical Journal of the Linnean Society. 1997;123:257–260. [Google Scholar]
- Lambert SM, Borba EL, Machado MC, Andrade SCS. Allozyme diversity and morphometrics of Melocactus paucispinus (Cactaceae) and evidence for hybridization with M. concinnus in the Chapada Diamantina, North-eastern Brazil. Annals of Botany. 2006;97:389–403. doi: 10.1093/aob/mcj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD. Patterns of fruit and seed production. In: Doust JL, Doust L., editors. Plant reproductive ecology, patterns and strategies. Oxford: Oxford University Press; 1988. pp. 179–202. [Google Scholar]
- Lewis GP. Legumes of Bahia. Royal Botanic Gardens: Kew; 1987. [Google Scholar]
- Lewis GP, Gibbs PE. Reproductive biology of Caesalpinia calycina and C. pluviosa (Leguminosae) of the caatinga of north-eastern Brazil. Plant Systematics and Evolution. 1999;217:43–53. [Google Scholar]
- Liu H, Koptur S. Breeding system and pollination of a narrowly endemic herb of the Lower Florida Keys: impacts of the urban–wildland interface. American Journal of Botany. 2003;90:1180–1187. doi: 10.3732/ajb.90.8.1180. [DOI] [PubMed] [Google Scholar]
- Maguire JO. Speed of germination: aid in selection and evaluation for seedling emergence and vigor. Crop Science. 1962;2:176–177. [Google Scholar]
- Martin FW. Staining and observing pollen tubes in the style by means of florescence. Stain Technology. 1959;34:125–128. doi: 10.3109/10520295909114663. [DOI] [PubMed] [Google Scholar]
- Mendelson TC. Sexual isolation evolves faster than hybrid inviability in a diverse and sexually dimorphic genus of fish (Percidae: Etheostoma) Evolution. 2003;57:317–327. doi: 10.1111/j.0014-3820.2003.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Moyle CL, Olson MS, Tiffin P. Patterns of reproductive isolation in three angiosperm genera. Evolution. 2004;58:1195–1208. doi: 10.1111/j.0014-3820.2004.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Newstrom LE, Frankie GW, Baker HG. A new classification for plant phenology based on flowering plants in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica. 1994;26:141–159. [Google Scholar]
- Orr HA. The genetics of species differences. Trends in Ecology and Evolution. 2001;16:343–350. doi: 10.1016/s0169-5347(01)02167-x. [DOI] [PubMed] [Google Scholar]
- Owens SJ, Lewis GP. Taxonomic and functional implications of stigma morphology in species of Cassia, Chamaecrista and Senna (Leguminosae: Caesalpinioideae) Plant Systematics and Evolution. 1989;163:93–105. [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends in Ecology and Evolution. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annual Review of Ecology, Evolution and Systematics. 2003;34:339–364. [Google Scholar]
- Soltis DE, Haufler CH, Darrow DC, Gastony GJ. Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedule. American Fern Journal. 1983;73:9–27. [Google Scholar]
- Stace CA. Plant taxonomy and biosystematics. London: Edward Arnold; 1989. [Google Scholar]
- Swofford DL, Selander RB. BIOSYS-1: computer program for the analysis of allelic variation in population genetics and biochemical systematics. Champaign: Illinois Natural History Survey; 1989. [Google Scholar]
- Teixeira SP, Ranga NT. Biosystematics of the genus Dahlstedtia Malme (Leguminosae, Papilionoideae, Millettieae) Revista Brasileira de Botânica. 2004;27:37–45. [Google Scholar]
- Thorpe JP. The molecular clock hypothesis: biochemical evolution, genetic differentiation and systematics. Annual Review of Ecology and Systematics. 1982;13:139–168. [Google Scholar]
- Turelli M, Barton NH, Coyne JA. Theory and speciation. Trends in Ecology and Evolution. 2001;16:330–343. doi: 10.1016/s0169-5347(01)02177-2. [DOI] [PubMed] [Google Scholar]
- Westerkamp C. Ricochet pollination in Cassias – and how bees explain enantiostyly. In: Magalhães FB, Pereira JO, editors. Solitary bees: conservation, rearing and management for pollination. Fortaleza: Universidade Federal do Ceará; 2004. pp. 225–230. [Google Scholar]
- Wright S. Evolution and the genetics of populations – variability within and among natural populations. Vol. 4. Chicago: University of Chicago Press; 1978. [Google Scholar]