Abstract
Background and Aims
The architecture of a plant depends on the nature and relative arrangement of each of its parts; it is, at any given time, the expression of an equilibrium between endogenous growth processes and exogenous constraints exerted by the environment. The aim of architectural analysis is, by means of observation and sometimes experimentation, to identify and understand these endogenous processes and to separate them from the plasticity of their expression resulting from external influences.
Scope
Using the identification of several morphological criteria and considering the plant as a whole, from germination to death, architectural analysis is essentially a detailed, multilevel, comprehensive and dynamic approach to plant development. Despite their recent origin, architectural concepts and analysis methods provide a powerful tool for studying plant form and ontogeny. Completed by precise morphological observations and appropriated quantitative methods of analysis, recent researches in this field have greatly increased our understanding of plant structure and development and have led to the establishment of a real conceptual and methodological framework for plant form and structure analysis and representation. This paper is a summarized update of current knowledge on plant architecture and morphology; its implication and possible role in various aspects of modern plant biology is also discussed.
Key words: Plant morphology, plant architecture, level of organization, growth, branching, differentiation, morphogenetic gradients, physiological age, meristem, annual shoot, phenotypic plasticity, ontogeny, phase change
INTRODUCTION
Progress in our understanding of plants has increased dramatically in recent decades and research in this domain has given rise to analytical, methodological and theoretical innovations on various aspects of plant science (Sattler and Rutishauser, 1997; Hedden et al., 2002; Turnbull, 2005).
Plant form, development and evolution have been analysed under the functional view of biomechanics (Niklas, 1992, 2005; Rowe and Speck, 2005) and pollination ecology or population biology (White, 1979; Harper, 1985; Diggle, 2003; Friedman and Harder, 2004, 2005). The importance of phenotypic plasticity, phenology and crown architecture in evolutionary plant ecology or in the understanding of community and stand structure, functioning and production has been stressed by several authors (Givnish, 1984; King, 1998; Diggle, 1999, 2002; Huber et al., 1999; Alpert and Simms, 2002; Novoplansky, 2002; Wright et al., 2002; Oborny, 2004; Sachs, 2004; Damascos et al., 2005; de Kroon et al., 2005; Pearcy et al., 2005; Wolfe and Mazer, 2005).
Since its introduction and definition by von Goethe (1790), plant morphology has had a successful history and it is commonly accepted that plants are modular organisms that develop by the repetition of elementary botanical entities whose morphological, dimensional, functional and anatomical features change during ontogeny and according to several processes variously called heteroblasty, phase change, life stages, maturation, ageing, age states or morphogenetic progression (Goebel, 1900; Troll and Rauh, 1950; Robbins, 1957; Wareing, 1959, 1961; Nozeran et al., 1971, 1982; Nozeran, 1978, 1984; Gatsuk et al., 1980; Greenwood, 1987, 1995; Poethig, 1990; Jones, 1999, 2001; Claßen-Bockoff, 2001; Kaplan, 2001). As plant morphology deals with plant form and/or structure and with their temporal and/or topological changes during ontogeny and even phylogeny, it is therefore relevant to practically all the disciplines of modern plant biology cited above (Sattler, 1978; Roloff, 1988; Sattler and Rutishauser, 1997; Gleißner, 1998; Claßen-Bockhoff, 2000, 2005; Kaplan 2001; Scotland et al., 2003; Wiens, 2004; Mueller, 2006).
The study of plant architecture emerged as a new scientific discipline some 30 years ago, and derived, in several ways, from earlier works on plant morphology (Hallé and Oldeman, 1970; Oldeman, 1974; Hallé et al., 1978). An original feature of architectural studies is that they were initiated in tropical regions and were, at first, concerned with the analysis of the aerial vegetative structure of tropical trees (Hallé and Oldeman, 1970). Since their definition, architectural concepts have provided powerful tools for studying plant form or even tropical forest structure and the understanding of its dynamics (Oldeman, 1974, 1983, 1990; Hallé et al., 1978; Vester, 1997). Investigations based on these concepts quickly spread to temperate species (Edelin, 1981; Caraglio and Edelin, 1990; Gleißner, 1998; Nicolini, 1998; Grosfeld et al., 1999; Millet et al., 1999; Sabatier and Barthélémy, 1999; Claßen-Bockhoff, 2000; Stecconi et al., 2000), herbs (Jeannoda-Robinson, 1977; Blanc, 1978; de Castro e Santos, 1981; Gay, 1993; Cremers and Edelin, 1995; Rua and Gróttola, 1997; Perreta et al., 2000), lianas (Cremers, 1973; Coudurier, 1992; Caballé, 1998) and root systems (Atger and Edelin, 1994a, b; Jourdan and Rey,1997a, b). The architecture of a plant depends on the nature and on the relative arrangement of each of its parts; it is, at any given time, the expression of an equilibrium between endogenous growth processes and exogenous constraints exerted by the environment. The aim of architectural analysis is to identify these endogenous processes and to separate them from the plasticity of their expression resulting from external influences by means of observation and sometimes experimentation. Considering the plant as a whole, from germination to death, architectural analysis is essentially a global, multilevel and dynamic approach to plant development. For each species, at each stage of development and in each environmental condition, careful qualitative and quantitative morphological or even anatomical observations are made on varying numbers of individuals, depending on the complexity of the architecture. Small plants can be analysed, observed and manipulated directly but this is hardly possible when plants reach several metres high. For the highest and biggest trees, some qualitative observations can be carried out from ground level (Barthélémy et al., 1989, 1991; Millet et al.,1998b; Nicolini, 1998) but the use and practice of destructive methods are most generally necessary for more precise analysis and data collection (Heuret et al., 2000, 2002; Passo et al., 2002). Results of field observations are summarized in a series of diagrams that symbolize successive growth stages or developmental steps, whereas quantitative analyses are grouped and made according to this qualitative knowledge. The validity of these diagrams and analyses is then checked by comparing them with reality and they must apply to the architecture of any individual of the same species encountered in the field for the study to be considered as complete.
Applicable to any kind of plant, architectural analysis has proved to be one of the most efficient means currently available for the study of the organization of complex arborescent plants. Architectural concepts appear to be of particular interest for the understanding of crown construction in trees. Completed by precise morphological observations and innovative computational aspects, recent research in this field has greatly increased our understanding of plant structure and development and has led to the establishment of a real conceptual and methodological framework for plant form analysis and understanding (Barthélémy et al.,1997a; Bouchon et al., 1997; Caraglio and Barthélémy, 1997; de Reffye and Houllier, 1997; Godin and Caraglio, 1998; de Reffye et al., 1998; Guédon et al., 2001, 2003; Hu and Jaeger, 2003; Yan et al., 2004).
The present paper describes major concepts and notions that are currently used in plant architecture and morphology description. It aims to provide a summarized update of our knowledge in this field. The possible applications, implications and roles of plant architecture in modern and current plant research are discussed.
MORPHOLOGICAL BASES AND CRITERIA FOR PLANT ARCHITECTURE DESCRIPTION AND ANALYSIS
Plant morphology, in its historical and broader sense and as a synthetic discipline (see, for example, Sattler, 1978; Claßen-Bockoff, 2001; Kaplan, 2001; Mueller, 2006), may be considered as one of the main ‘inspiring soul’ of plant architecture studies. In this section, however, we will only illustrate the main morphological traits that are commonly used in plant architectural analysis (for a more comprehensive and general illustration and survey of other morphological traits we refer the reader to Troll, 1937 or Bell, 1991). These traits are well documented in previous synthetic works (Hallé and Oldeman, 1970; Hallé et al., 1978) and they may be grouped according to four major categories: (1) growth process, (2) branching process, (3) the morphological differentiation of axes and (4) the position (lateral vs. terminal) of reproductive structures. Although they correspond to basic morphological concepts, their associated terminology proved to be sometimes confused and led to incorrect interpetations. They were thus recently discussed and sometimes redefined (Caraglio and Barthélémy, 1997, and see below).
Growth process
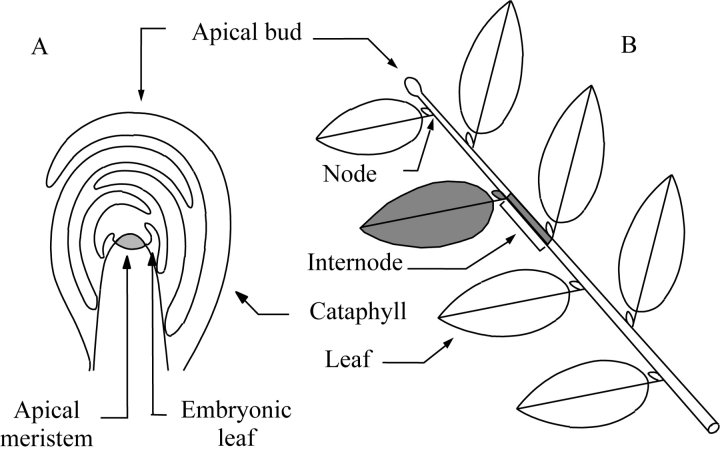
The primary growth of a plant is the result of several processes that can be grouped into two distinct, but co-ordinated morphogenetic events: organogenesis and extension (Champagnat et al., 1986). The inception of new organs (organogenesis) results from the functioning of undifferentiated cells constituting the apical meristem (Fig. 1A). Located at the tip of a stem, this meristem forms, when in an active phase, small cell masses with different potentialities (Lyndon, 1988; Nougarède, 2001); these masses develop into embryonic leaves, then leaves on elongated stems. The insertion zone of a leaf on the stem is referred to as a node and the stem portion which separates two successive nodes is called the internode. According to species and phyllotaxis, one, two or more leaves may be inserted at one node and one to several, named supernumerary, buds (see Fig. 8) may develop at the axil of each individual leaf. A stem can thus be considered as a succession of internodes and the entity formed by a node, associated with its leaf (or leaves) and axillary bud(s) plus the subtending internode, represents the basic structural unit of the plant body commonly called the metamer or phytomer (White, 1979; Barlow, 1989). During growth, the superposition and repetition of this elementary entity builds up the leafy axis (Fig. 1B).
Fig. 1.
Shoot apex (A) and stem (B) organization. Each leafy axis (B) ends in an apical meristem frequently protected in an apical bud (A). Each stem comprises a succession of metamers, i.e. the set composed by (1) one internode, (2) the node (i.e. insertion point of the leaves on a stem) located at its tip and (3) the corresponding one or several leaves and associated lateral buds (in grey on A; White, 1979; Caraglio and Barthélémy, 1997).
Plant growth may be considered in several ways according to the kind of organ and/or level of organization concerned (growth of a leaf, a stem, a fruit, the whole plant, etc.). As we are dealing here with the topological edification of the stem and macroscopic aspects of plant growth, we will focus mainly on the extension process of the leafy axis or shoot and will neither detail organogenesis processes nor consider secondary (sensu Fahn, 1967) or radial growth.
Determinate vs. indeterminate growth (Fig. 2)
Fig. 2.
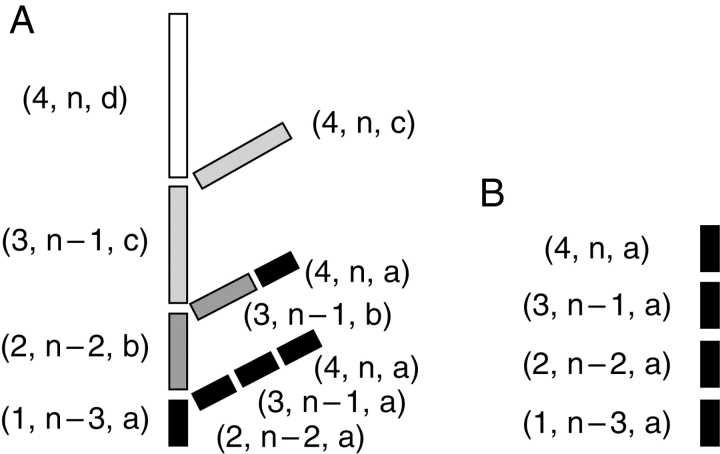
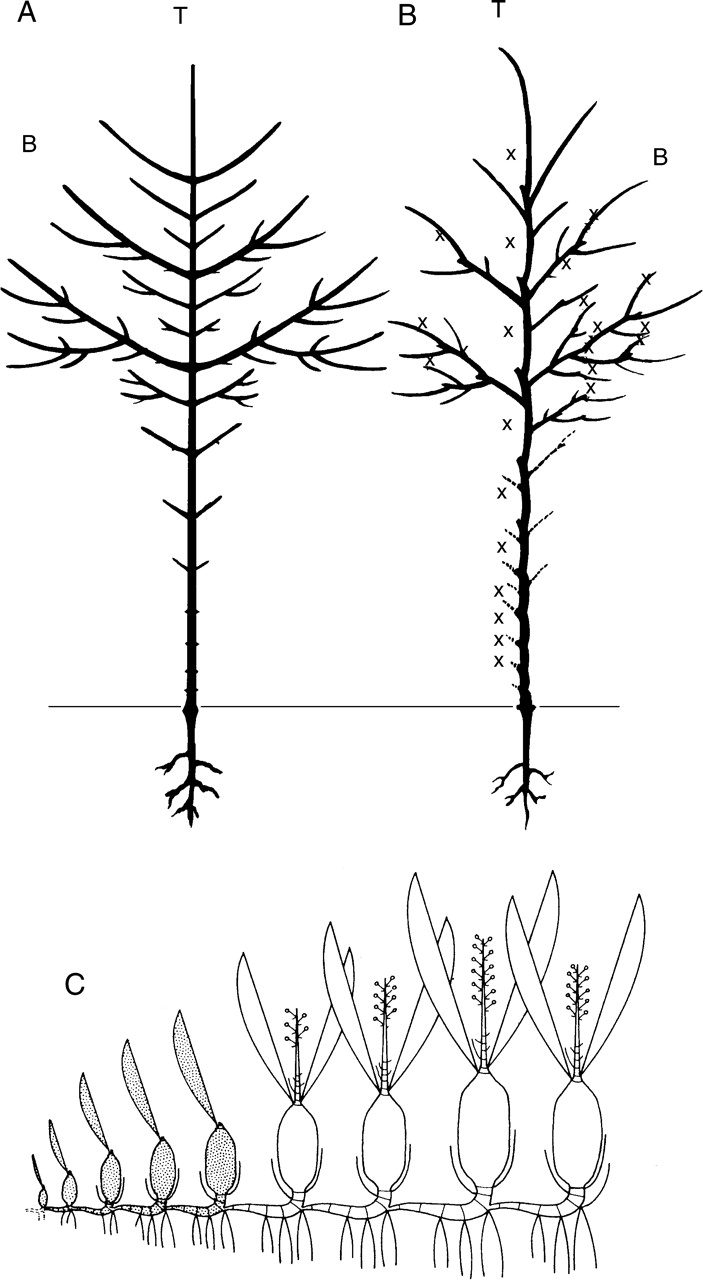
Determinate growth corresponds to an irreversible transformation of the apical meristem, which can be due to (A) apical flowering as in Nerium oleander, (B) parenchymatization (arrow) of the apical meristem as in Alstonia sp. or (C) apical death or abscission (‘X’) as in Castanea sativa. Indeterminate growth corresponds to permanent apical meristem functioning, as illustrated by the main stem of Picea excelsa (D).
In many plant species, the apex may abscise or abort after some period of functioning (Garrison and Wetmore, 1961; Millington, 1963; Puntieri et al., 1998, 1999) or it may transform into a specialized structure (flower, inflorescence, spine, tendril, parenchymatous cells, etc.) lacking further extension capacity. In these cases, the axis is considered to have a determinate growth (Fig. 2A–C; Hallé et al., 1978; ‘definite extension’, Bell, 1991). By contrast, indeterminate growth (Hallé et al., 1978) or indefinite extension (Bell, 1991) refers to an axis on which apical meristem indefinitely maintains its growth potential (Fig. 2D). As the indefinite functioning of an apex is always limited at least by the limited life span of the plant it belongs to, this ultimate term is somewhat ‘theoretical’ and a misuse of the language (Guinochet, 1965). Nonetheless, this notion is useful and justified by the necessity to describe and name this phenomenon.
Rhythmic vs. continuous growth
Hallé et al. (1978) distinguished shoots which have no marked endogenous cessation of extension (continuous growth) from shoots which have marked endogenous periodicity and cessation of extension (rhythmic growth; Hallé and Martin, 1968). Although organogenesis may be considered or known, these two growth patterns are generally more concerned with extension.
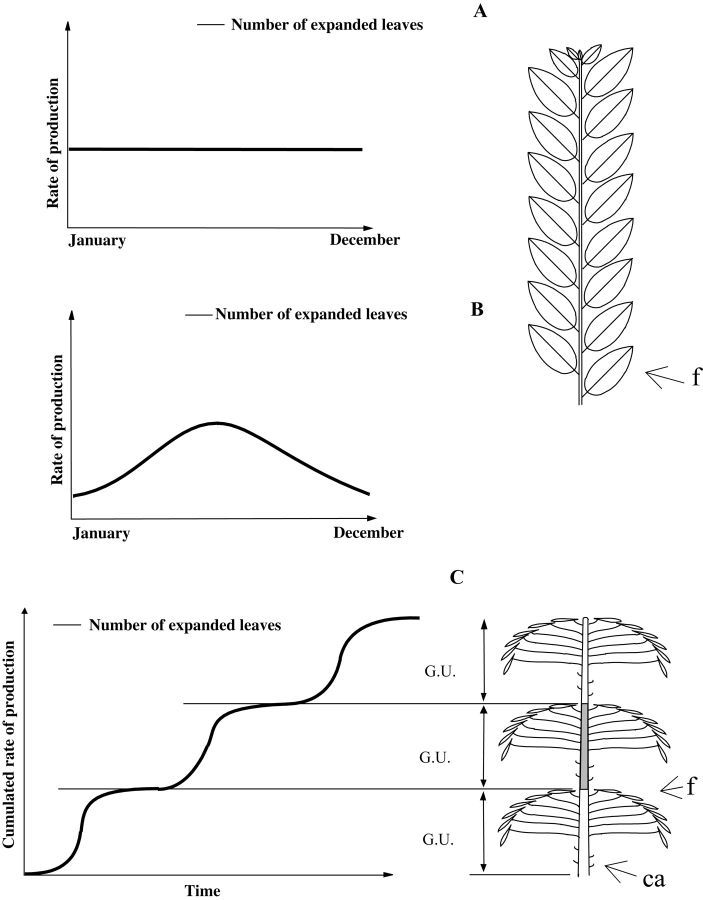
Continuous extension (Fig. 3A, B) is quite a rare phenomenon in the field and has generally been observed and described mostly in uniform equatorial climates or environments (mainly palm or mangrove trees; Venkatanarayana, 1957; Rees, 1964; Gill and Tomlinson, 1971) or for some herbs native to temperate (Bell, 1991) or tropical (Blanc, 2002) regions. In all these cases, plants exhibit a more or less constant production of leaves and/or shoots throughout the year. For the mangrove trees Rhizophora mangle (Gill and Tomlinson, 1971), growing in the subtropical climate of Florida, it was shown that the extension of each axis may have a fluctuating rate according to the seasonal climatic conditions, without ever being interrupted completely (Fig. 3B). In some cases, the endogenous nature of continuous extension may be ‘masked’ by fluctuating environmental conditions in the field and can be revealed experimentally by growing plants in favourable and constant conditions, as was demonstrated for several temperate Cupressaceae from the Southern Hemisphere (Grosfeld and Barthélémy, 2004). It has been shown that plants with continuous extension may also present a continuous production of new embryonic leaves, i.e. organogenesis (Gill and Tomlinson, 1971). As far as it has been documented, when extension is continuous the resulting stem is generally quite homogeneous and successive metamers and their constitutive elements are more or less of the same type and size along the elongated axis (Fig. 3A, B, right; Tomlinson and Gill, 1973; Hallé et al., 1978).
Fig. 3.
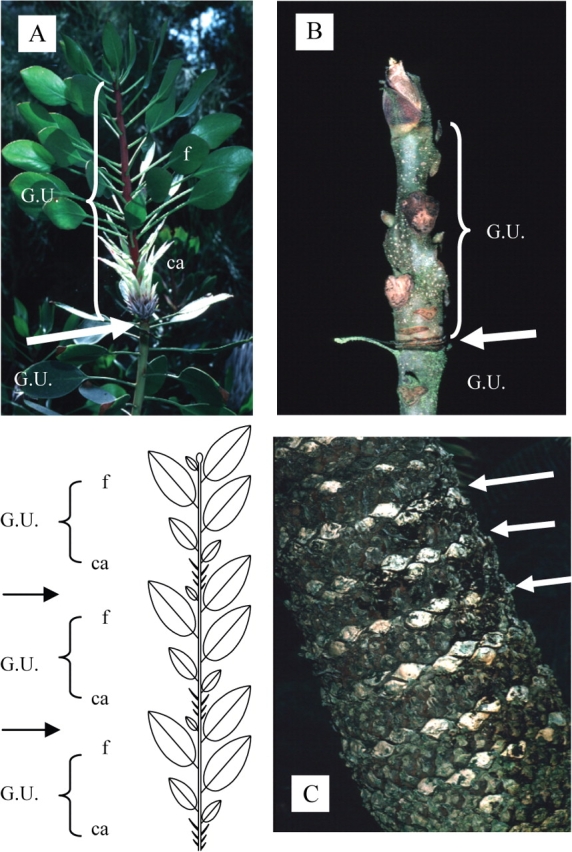
Leaf extension rate in continuous (A and B) vs. rhythmic (C) growth and structure of the resulting stems (modified from Hallé et al., 1978). (A) Constant leaf extension in a theoretical case (i.e. several palm trees) and (B) fluctuations in the leaf extension rate of Rhizophora mangle correlated with climatic fluctuations. (C) Rhythmic cumulative rate of leaf extension in Hevea brasiliensis. G.U., growth unit; f, leaf; ca, cataphyll.
Rhythmic extension of leafy axes is a far more frequent growth pattern in plants, regardless of their geographical origin, and is expressed by an alternation of periods of rest in meristem activity and periods of active shoot extension or ‘growth flushes’ (Fig. 3C). This rhythmic growth has been thoroughly studied by numerous authors (Alvim, 1964; Kozlowski, 1971; Hallé et al., 1978; Puntieri et al., 1998; Sabatier and Barthélémy, 1999).Hallé and Martin (1968), in their detailed study of the tropical tree Hevea brasiliensis, defined the ‘growth unit’ as the portion of an axis which develops during an uninterrupted period of extension. A growth unit is generally easy to identify on the elongated stem as the limit between two growth units is usually marked by a zone of short internodes and/or cataphylls (i.e. scale leaves) corresponding to the protective organs of the bud from which it derives (Fig. 4). Even if very common in rhythmically growing axes, this alternation of cataphylls and leaves (termed articulate growth by Tomlinson and Gill, 1973; Fig. 3C right, Fig. 4) may not be obvious in some species, and other morphological or macro-anatomical markers may sometimes be used in addition, for the a posteriori identification of a rhythmically elongating axis (Fig. 5).
Fig. 4.
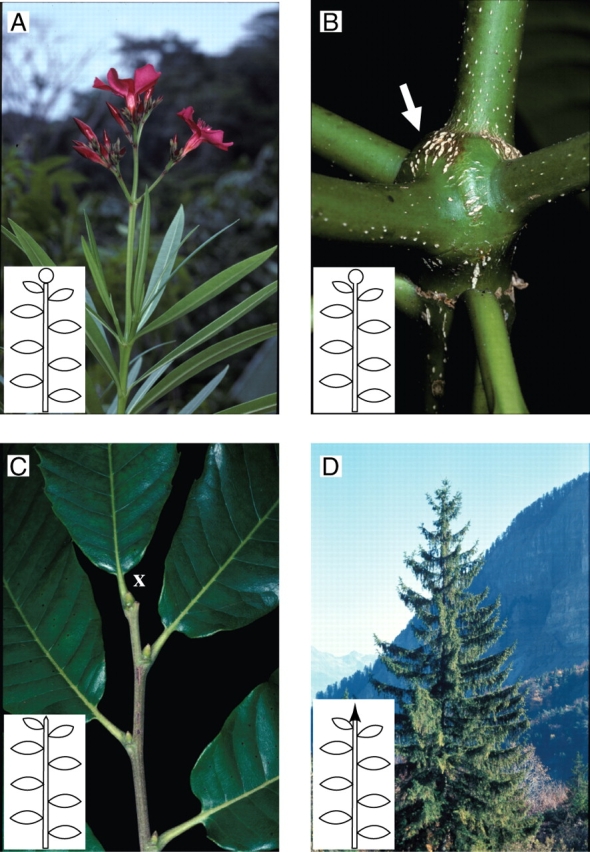
Morphological markers of rhythmic extension. Growth cessation phases (arrows) and delimitation of successive growth units (G.U.) as revealed a posteriori by an alternation of cataphylls (ca) and photosynthetic leaves (f) in Protea cynaroïdes (A) or their scars (Carya laciniosa, B, or Cycas pectinata, C).
Fig. 5.
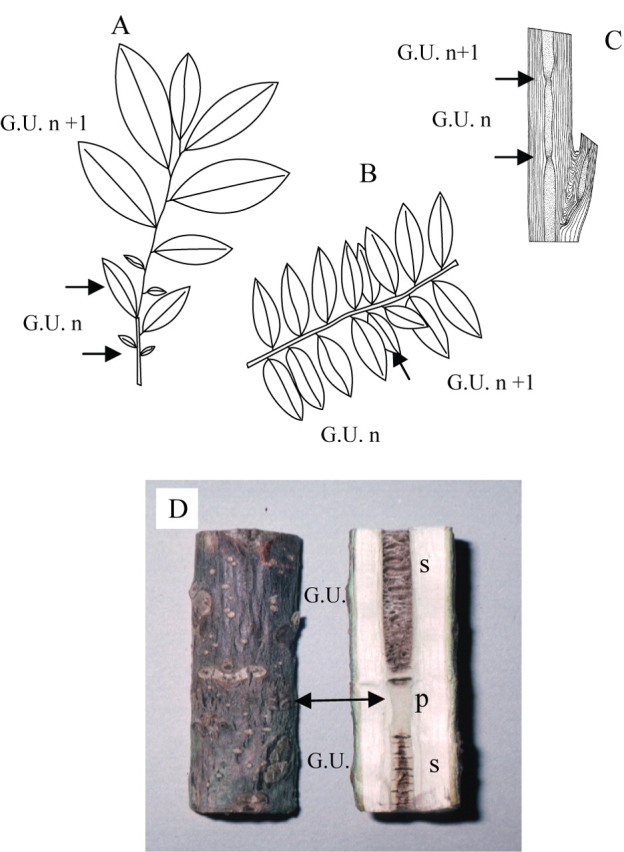
Successive growth units may be delimited (arrows) only by more or less marked changes in leaf size (Virola michelii, A; Virola surinamensis, B; drawings from Edelin, 1993). In some cases the limit (arrow) between two growth units is indicated by a decrease in the pith diameter (Carapa procera, C; drawings from Edelin, 1993) and/or even by pith structure as in Juglans sp. (D). G.U., growth unit; ‘n’, ‘n + 1’, successive theoretical years of growth; p, plain pith; s, septate pith.
As with continuous growth, the identification of the endogenous nature of rhythmic growth, for plants naturally growing in seasonal climates, requires experimental verification in controlled, constant and favourable environmental conditions (Greathouse et al., 1971; Lavarenne, 1971; Payan, 1982; Parisot, 1985). Regardless, the rhythmic (or continuous) pattern of a shoot or axis growth must be checked by periodic measurement of its length. In practice, most often only the extension component of growth is known, such that some authors have proposed the use of the term ‘unit of extension’ (Gill and Tomlinson, 1971; Hallé et al., 1978) rather than growth unit, even though the two terms are synonymous. When organogenesis is known, it has been shown that the rhythmic extension of the stem may be combined either with a continuous (Bond, 1942) or more frequently, a rhythmic (Hallé and Martin, 1968; Gill, 1971; Puntieri et al., 2002a; Sabatier et al., 2003b) organogenesis pattern. In the latter case, it has been proposed that the ‘unit of morphogenesis’ is the axis portion corresponding to an uninterrupted phase of organogenesis (Hallé and Martin, 1968). Despite its relevance for the understanding of growth patterns in plants, the nature of the unit of morphogenesis is unknown in most cases partly owing to the long and tedious experimental work needed for its identification.
Preformation and neoformation
In the case of rhythmic growth, all the metamers and organs of the future elongated shoot may be present at an embryonic stage in a bud before the elongation of the shoot deriving from it; in this case the shoot is referred to as ‘preformed’ and its constitutive organs as ‘preformed organs’ or ‘preformation’ (Hallé et al., 1978; ‘early leaves’, Critchfield, 1960; ‘fixed growth’, Lanner, 1976). The duration of preformed organs at an embryonic stage in a bud may vary from several days or weeks (Hallé and Martin, 1968; Sabatier et al., 1995) to several years (Aydelotte and Diggle, 1997; Diggle, 1997; Meloche and Diggle, 2001). In other cases, more organs than those included at an embryonic stage in the bud are elongated: these supplementary, non-preformed elements are referred to as ‘neoformed organs’ (i.e. ‘neoformation’ sensu Hallé et al., 1978, or ‘late leaves’, Critchfield, 1960; ‘free growth’, Jablanczy, 1971; Lanner, 1976). As a consequence stems or shoots may comprise only preformed metamers (Critchfield, 1960; Rivals, 1965, 1966; Gill, 1971; Roloff, 1985; Sabatier et al., 1995; Nicolini, 1998; Puntieri et al., 2000, 2002a, b; Souza et al., 2000) or, more rarely, may be entirely neoformed (Borchert, 1969; El-Morsy, 1991). In many cases, a preformed part can be followed by a neoformed part and thus give rise to a mixed shoot (Critchfield, 1960; Hallé and Martin, 1968; Kozlowski, 1971; Nitta and Ohsawa, 1998; Puntieri et al., 2000, 2002b; Souza et al., 2000; Seleznyova et al., 2002; Costes et al., 2003; Gordon et al., 2006). As discussed in recent studies, the amount of preformation or the relative extent of preformation and neoformation in shoots may vary both between and within species according to external or internal parameters. For a number of tree species, preformation seems to be more relevant than neoformation in the mean number of organs developed by a shoot at a specific position within a tree's architecture (Remphrey and Davidson, 1994; Puntieri et al., 2000, 2002b; Souza et al., 2000). Neoformation responses within a specific position of a tree would be involved in the plastic response of trees to factors acting locally at the time of shoot extension (Remphrey and Powell, 1984; Gordon et al., 2006; Guédon et al., 2006).
Annual shoot (Fig. 6)
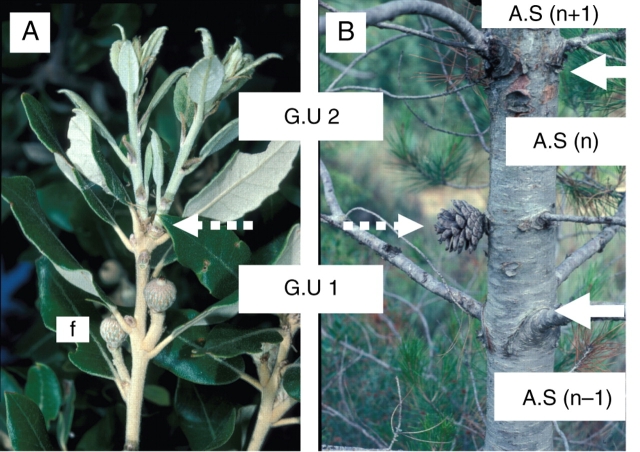
Fig. 6.
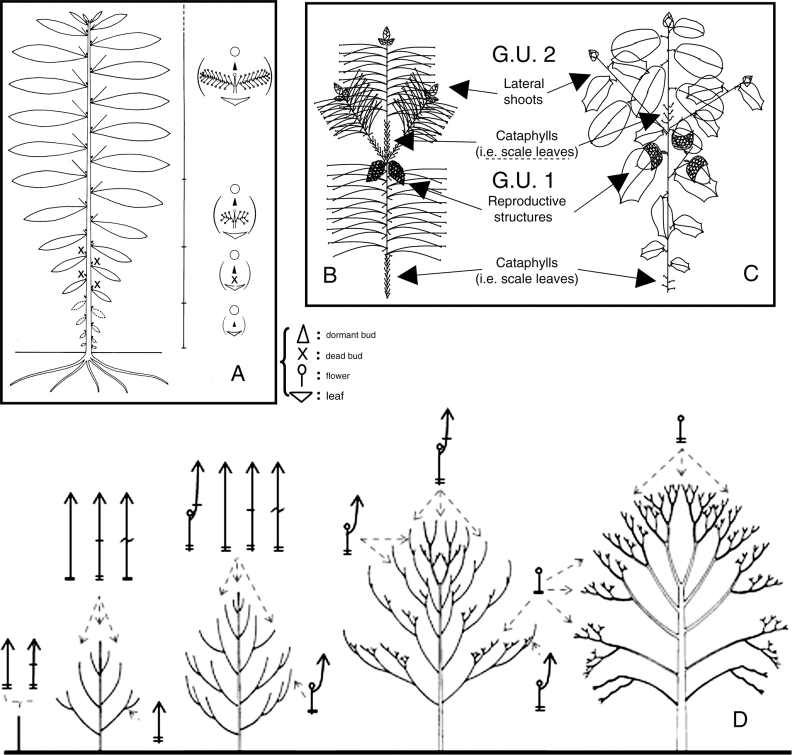
Stem extension may occur more than once during a same calendar year. The set of growth units produced in one year is then called an annual shoot (A.S.). In Quercus ilex (A) or Pinus halepensis (B) bicyclic shoots, the first growth unit (G.U.1) may produce reproductive organs whereas the second (G.U.2) is vegetative. On old stems, the presence of female cones or fruits on the first growth unit and the major development of branches borne on the second growth unit of such bicyclic annual shoots distinguishes these first and second growth units, respectively, as the a posteriori delimitation of successive annual shoots (B). ‘n-1’, ‘n’, ‘n + 1’, successive theoretical years of growth; solid white arrow, limit of an annual shoot; dashed white arrow, limit of a growth unit.
In rubber trees growing in equatorial conditions (Hallé and Martin, 1968), an axis with indeterminate growth forms a new growth unit about every 45 d and these successive units are morphologically identical (cf. Fig. 3C) so that the growth unit level itself is pertinent to describe the infrastructure of an axis and its morphological heterogeneity. In other tropical species, however, the timing of rhythmic extension during a whole calendar year is more complex. In Ryania speciosa var. subuliflora (Comte, 1993), for instance, the annual growth pattern corresponds to the extension of two growth units in a relatively short time, followed by a long resting phase, which again is followed by the rapid emission of a succession of two growth units. In some temperate species, shoot extension may occur in one, two or more successive events in a same growing season giving rise to an ‘annual shoot’ made up of one or a succession of several growth units or growth cycles (respectively monocyclism vs. polycyclism, i.e. Bugnon and Bugnon, 1951 or Lanner 1976, and see Caraglio and Barthélémy, 1997, for a critical and historical revision of these terms) occurring in a vegetative cycle (period between two marked seasons or two winters, Lanner, 1976). When several successive growth units are formed in the same annual vegetative cycle these growth units most often present distinctive features (Fig. 6); spring shoots and summer or additional shoots are frequently distinguished (Späth, 1912; Kozlowski, 1971; Cannell et al., 1976). In this situation, successive growth units produced in a same year are thus not identical and it is useful and pertinent to distinguish an annual-shoot level of organization.
According to the number of constitutive growth units of an annual shoot and according to the indeterminate vs. determinate nature and to the relative extent of preformation and neoformation in each of the constitutive growth units, several combinations may exist for different species or even for a single species, depending on external and/or internal conditions (Lanner, 1971, 1976; de Reffye et al., 1991; Costes, 1993; Fontaine et al., 1999; Heuret et al., 2000, 2003, 2006; Puntieri et al., 2000; Isik et al., 2001).
Branching process
Although the architecture of some vascular plants consists only of a single vegetative axis during their whole life span, most display a more complex architecture consisting of several axes, one derived from another by a repetitive process known as branching.
Terminal vs. lateral branching
An apical meristem (McManus and Veit, 2002) or an initial apical cell (Gifford, 1983) can directly split and give rise to two or more new sibling axes, which results in dichotomy or polytomy, respectively (Emberger, 1960). Frequently encountered in ferns and mosses (Emberger, 1960), this terminal branching (Gatin, 1924) is rarely expressed in angiosperms (see for monocotyledons: Schoute, 1909; Tomlinson, 1971; Fisher, 1976; Tomlinson and Posluszny, 1977; and for dicotyledons: Nolan, 1969; Boke, 1976; Iwamoto et al., 2005). It is important to mention that this phenomenon is, as far as we know, not encountered within gymnosperms.
In other cases, the branching process relies on the delimitation of a zone of embryonic cells just aside the initiated leaf, i.e. the axillary meristem. This lateral branching process is the most common one among angiosperms and gymnosperms. The resulting lateral branch is characterized by the presence of one or two (in respectively monocotyledons or dicotyledons) prophylls: the first foliar organs of the lateral axis (see Fig. 7A, B for their particular location).
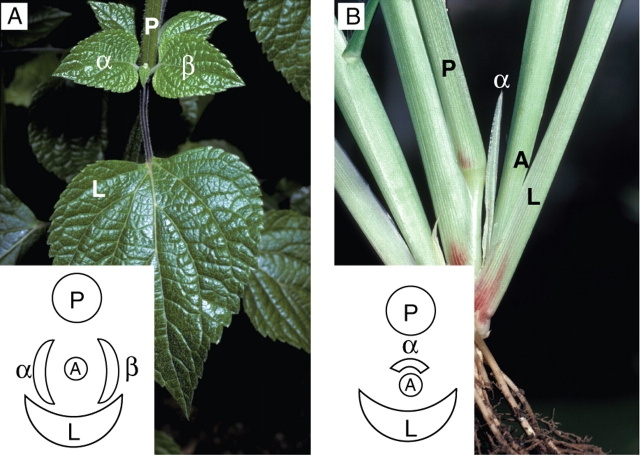
Fig. 7.
The leaf, or leaves, of the first (proximal) node of a lateral shoot (A) are referred to as prophylls. In dicotyledons prophyll α and prophyll β are mainly in opposite and lateral position with respect to the plan formed by the axillary leaf (L) and the parent axis (P) (Salvia guaranitica, A). In monocotyledons, the first leaf (prophyll α) is often bicarinate and shows a particular arrangement (unidentified Poaceae, B): it is located in adaxial position between the lateral shoot (A) and its parent axis (P).
In some plants, there are, at a single leaf axil, more than one axillary meristem, which are then called supernumerary or accessory buds (Sandt, 1925; Troll, 1937; Espagnac and Neuville, 1969; Altman and Goren, 1978; Bell, 1991; Fig. 8A, B). In this case, each individual lateral meristem may be identified by the position of its prophylls, which also allows the distinction between this situation and the development of condensed and more complex lateral branching systems (i.e. complexes of secondary buds, Hallé et al., 1978), where second-order lateral buds may be present in the axils of main lateral bud's prophylls (Fig. 8C).
Fig. 8.
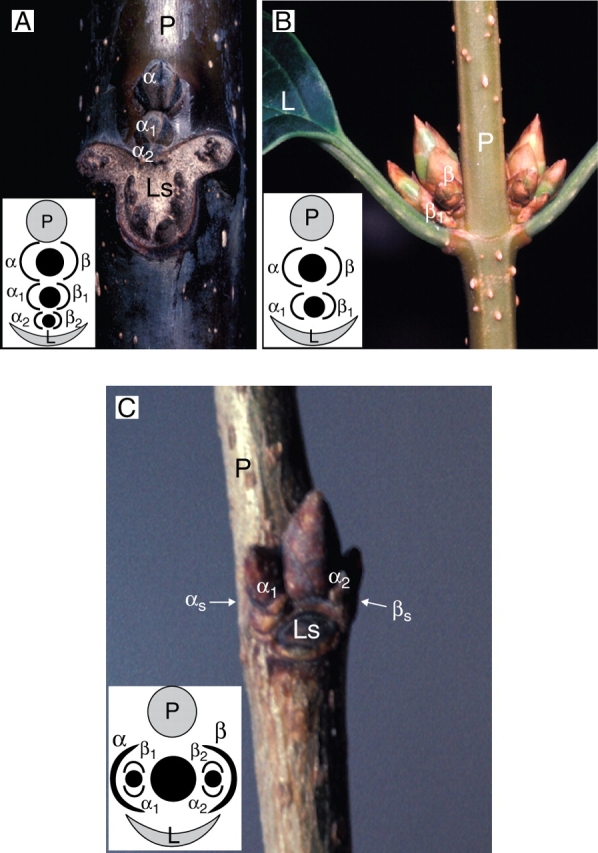
Vertical succession of supernumerary (or accessory) buds in Juglans regia (A) and in Forsythia vulgaris (B). The arrangement of the prophylls (diagrams) distinguishes supernumerary buds from reduced branching systems as illustrated in Zelkova serrata (C). L, axillary leaf; Ls, leaf scar; P, parent axis; prophylls α and β or their scars, αs and βs, after abscission.
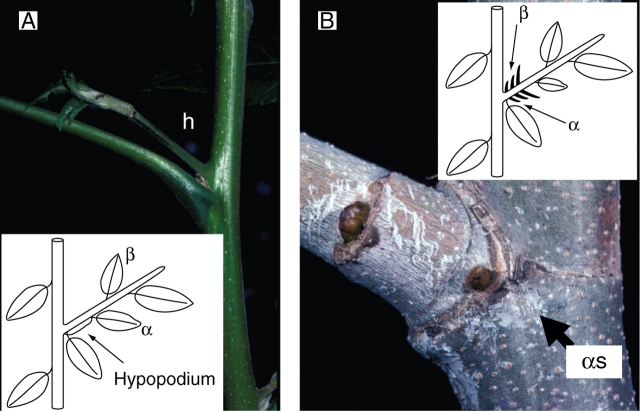
Immediate vs. delayed branching (Fig. 9)
Fig. 9.
In the case of immediate branching (Juglans regia, A), the first internode is generally long and termed the hypopodium (h). Delayed branching refers to a system where lateral branching follows a resting phase of the lateral meristem during which it is frequently included in a bud. When elongated, such delayed branching lateral shoots frequently show a short first internode and proximal scale leaves or bud scale scars when abscissed (Platanus sp., B). x, apical mortality; α, prophyll alpha; αs, scar of abscissed prophyll alpha.
Once initiated, an axillary meristem may remain dormant or can develop into a lateral axis. A lateral axis may elongate immediately after lateral meristem initiation or after a phase during which the lateral meristem remains inactive and very often protected in a lateral bud. These branching patterns are referred to, respectively, as immediate (i.e. ‘sylleptic’ sensu Hallé et al., 1978; Müller-Doblies and Weberling, 1984; Wu and Hinckley, 2001) or delayed (‘proleptic’ sensu Hallé et al., 1978; ‘prolepsis’, Bell, 1991) branching. The terms immediate vs delayed branching should be preferred to the more traditional ‘sylleptic’ vs. ‘proleptic’ branching (sensu Hallé et al., 1978) because of etymological and historical reasons, which have rendered the latter two terms ambiguous (see Caraglio and Barthélémy, 1997, for a critical review).
Morphologically, these two branching patterns may generally be identified a posteriori by the observation of the base of the lateral axis. Because of the immediate extension of lateral organs, branches with immediate development generally lack proximal cataphylls and present a relatively long most proximal internode termed a hypopodium (Fig. 9A; Tomlinson and Gill, 1973). Irrespective of the delay length, delayed branches present very short internodes and one or several cataphylls in their proximal portion, close to the point of insertion (Fig. 9B; Hallé et al., 1978). Thorough observation of the proximal part of lateral axes and a periodic observation and measurement of lateral meristem or bud size normally leaves no doubt as to the immediate or delayed nature of branching. In some cases, however, because lateral resting buds may be ‘hidden’, during the resting phase, in the axils of persistent basal foliar organs of the terminal bud (Guédès, 1975, 1980) or because lateral axes in an embryonic stage may already exist inside a resting bud (Champagnat, 1965; Roloff, 1985), the interpretation may be complicated or may lead to a misuse of the terminology (Caraglio and Barthélémy, 1997). Finally, in delayed branching it has been shown that the duration of the delay may be of several weeks to a year (Sabatier et al., 1995, 1998, 2003b; Puntieri et al., 1998; Heuret et al., 2003) and even several years (Fink, 1983; Nicolini et al., 2001).
Monopodial vs. sympodial branching
Depending on the indeterminate or determinate growth pattern of an axis, its branching pattern may respectively be monopodial (Emberger, 1960; or ‘monopodic’, Sachs, 1874), or sympodial (Emberger, 1960; or ‘sympodic’, Sachs, 1874). In the latter case, one, two or more branches may develop after the death, abscission, abortion or transformation of the apex, and the resulting sympodial branching pattern be qualified respectively as mono-, di- or polychasial. In plant architecture, the concept of module (‘article’ in French) was defined for the first time by Prévost (1967) in her study of tropical Apocynaceae and then applied to ‘a leafy axis in which the entire sequence of differentiation is carried out from the initiation of the meristem that builds up the axis to the sexual differentiation of its apex’ (Hallé, 1986). Because of the various causes of determinate growth (see above), this definition is, however, restrictive and the term ‘module’ increasingly refers to the portion of an axis edified by a single terminal meristem, which corresponds also to a ‘sympodial unit’ as used by Bell (1991).
Involving one or more lateral axes, a sympodial branching system is very often three dimensional. In some cases, however, a sympode may imply a linear succession of ‘modules’ or ‘sympodial units’ forming a so-called ‘pseudomonopodium’ as termed by German mophologists (Troll, 1937; Rauh, 1939). The resulting rectilinear structure mimics an axis edified by a single meristem with indeterminate growth and, if reproduced on all axes, may give rise to a totally sympodial branching system that resembles a monopodial one (Caraglio and Edelin, 1990; Bell, 1991). As a consequence, a rectilinear stem may thus be composed of a succession of metamers or growth units or annual shoots all produced by a single meristem or by a linear succession of sympodial units or modules. In a broader sense and following Room et al. (1994), the term ‘leafy axis’ or ‘axis’ will identify not only a structure edified by a single meristem as initially considered by Hallé et al. (1978) but also a rectilinear stem, whatever its intrinsic mode of construction.
Branched system and branching order (Fig. 10)
Fig. 10.
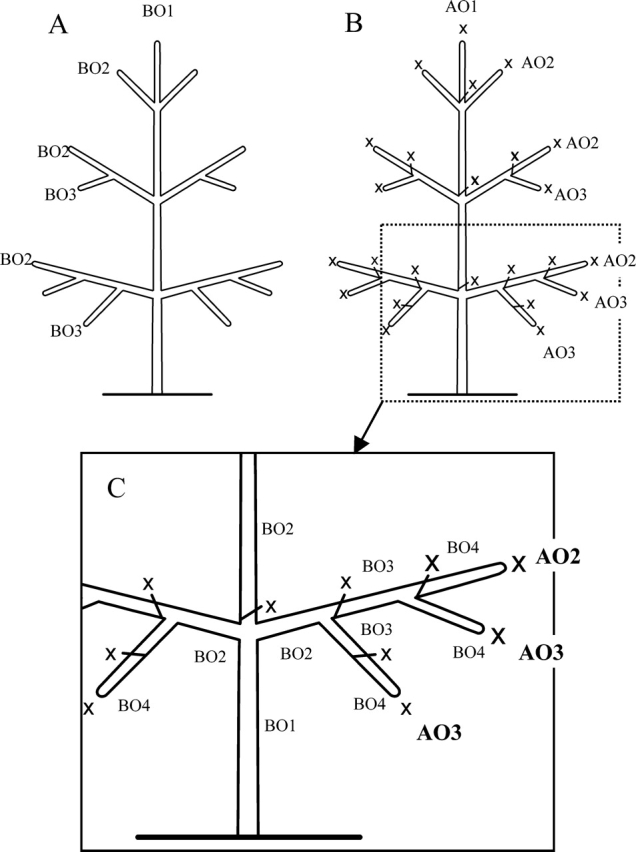
As a result of branching, sibling axes succeed topologically from a parent axis. This spatial succession is referred to as ‘branching order’ (BO). The first axis (branching order one, BO1) bears a lateral one (branching order 2, BO2) and so on as illustrated diagrammatically for a monopodial branching system (A). In a sympodial branching system, the branching order may increase rapidly (C). When successive sympodial units (each resulting from the functioning of a single meristem) are more or less in a rectilinear disposition (B), it can be considered that the general spatial direction of such a succession constitutes an ‘apparent branching order’ (AO) as in a monopodial system (pseudomonopodium sensu Troll, 1937). x, apical mortality; AOx, ‘apparent branching order’ number x.
The description of a branched system implies the use of a precise topological terminology. In plant architecture analysis it is usual to use ordinal numbers and to consider the main stem arising from seed as the order 1 axis (Fig. 10A; Hallé et al., 1978; Barthélémy et al., 1989, 1991) whereas the axes it gives rise to are referred to order 2 axes and so on. In a sympodial system, a rigorous use of this terminology will lead to the reference of the successive sympodial units as axis orders 1, 2, 3, etc. In order to be coherent with our broad axis definition (see above), each rectilinear succession of modules, even though not strictly edified by a single meristem, will be considered as an axis and will represent an ‘apparent branching order’ (Fig. 10A, C).
Rhythmic vs. continuous or diffuse branching
Defined and used for the first time for plant architecture description, these terms contribute to the characterization and definition of architectural models (Hallé and Oldeman, 1970; Hallé et al., 1978) and take into account the topological distribution of sibling axes on a parent axis. Depending on whether all the axillary meristems of a stem develop into lateral axes, or whether lateral axes are grouped as distinct tiers with an obvious regular alternation of a succession of unbranched and branched nodes on the parent stem, branching is respectively referred to as continuous or rhythmic. In some cases, neither all nodes of a parent axis are associated with a lateral axis nor is there an obvious regular distribution of branches in tiers, and the branching pattern is then called ‘diffuse’. As revealed in Cupressaceae by qualitative observations (Courtot and Baillaud, 1961; Baillaud, 1999) and, in recent years by sophisticated mathematical methods (Guédon et al., 2001; Grosfeld, 2002; Heuret et al., 2002), a diffuse branching pattern may not mean an unorganized distribution of sibling shoots on a parent shoot but may indicate a predictable, precise and subtle branching organization.
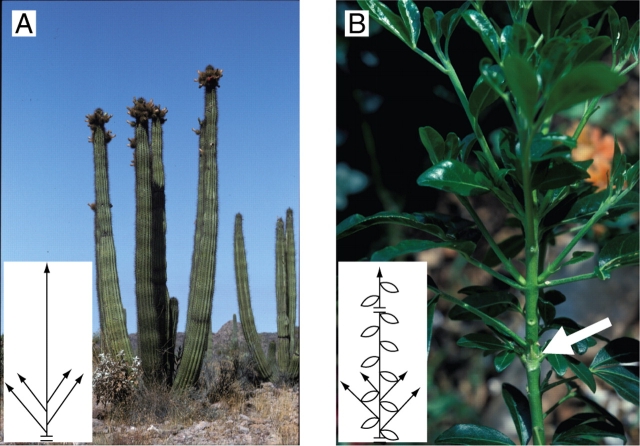
Acrotonic vs. mesotonic or basitonic branching (Fig. 11)
Fig. 11.
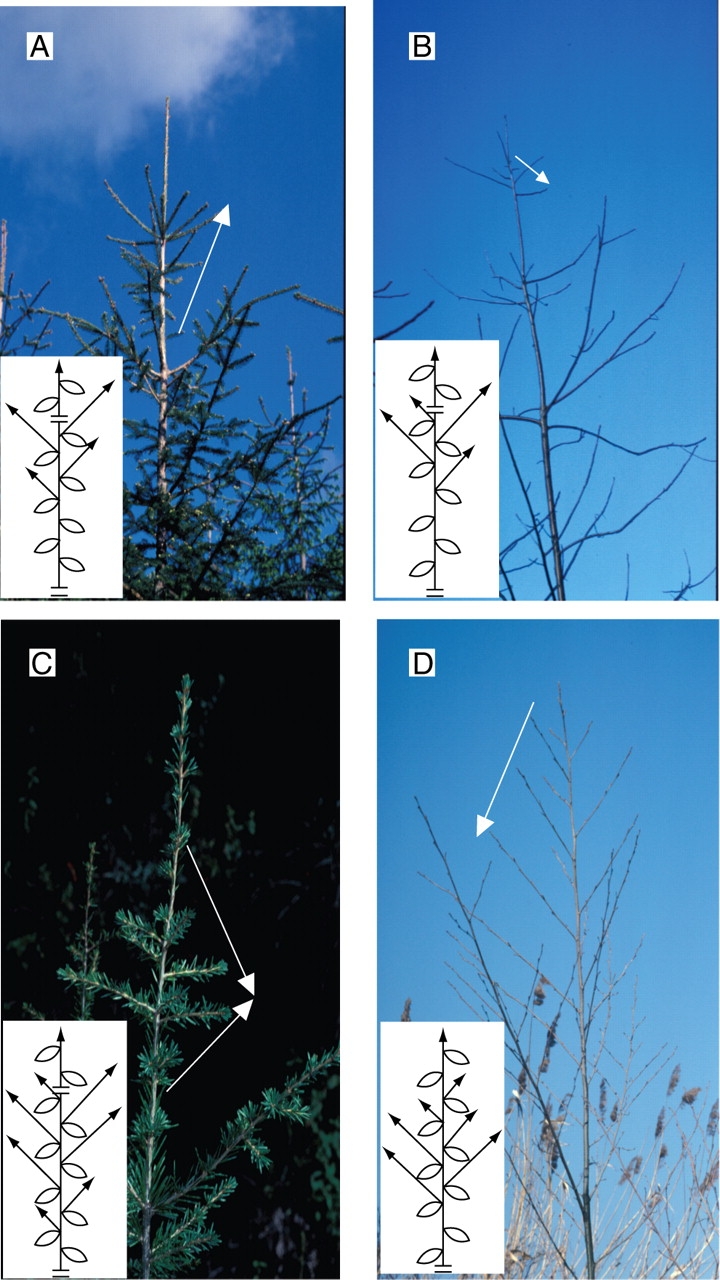
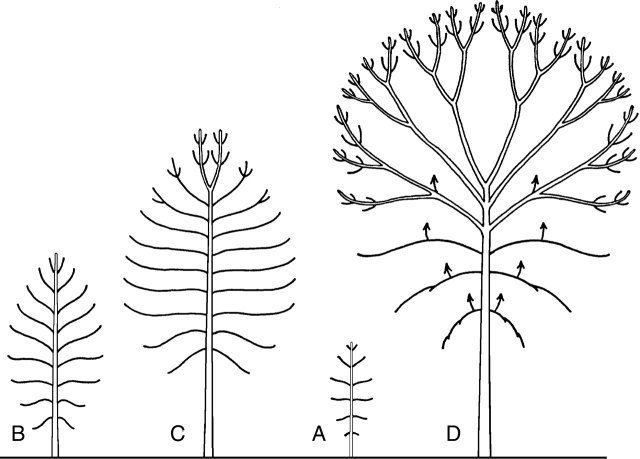
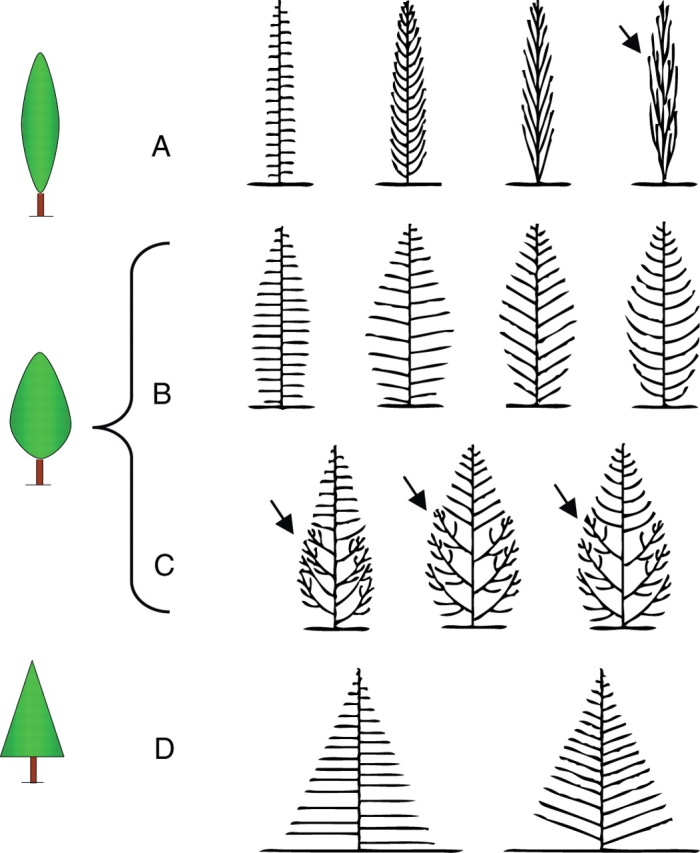
Privileged repartition of sibling shoots on a vertical parent shoot or axis. Acrotony is the preferred development of lateral axes in the distal part of a parent axis or shoot (A and B). The topological lateral arrangement of branches along the parent axis may be associated with an increasing (Abies sp., A) or decreasing (Juglans nigra, B) gradient in length and/or vigour of the branches. Mesotony refers to a privileged development of branches in the median part of a shoot or axis. The topological lateral arrangement of branches along the parent axis may be associated with a distal to proximal increasing and then decreasing (Cedrus atlantica, C) or a decreasing (Alnus glutinosa, D) gradient in length and/or vigour of the branches. White arrows indicate the increasing gradient in length of branches. On the diagrams, the break represents the limit of an annual shoot.
In order to describe the positional preferential development of lateral axes on a vertical parent axis or shoot, Troll (1937) and Rauh (1939) distinguished three modalities that were grouped under the German expression ‘longitudinale Symmetrie’. Acrotony (Fig. 11A, B) is the prevalent development of lateral axes in the distal part of a parent axis or shoot, and depending on whether branching is monopodial or sympodial, Rauh (1939) and Champagnat (1947) termed it, respectively, ‘primary’ or ‘secondary’ acrotony. In the initial definition of acrotony, the parent axis was always longer than the sibling ones, but Bell (1991) used the term ‘acrotonic branching’ for the distal position of the largest lateral branch, independent of its size relative to parent stem. Basitony (Fig. 12) was at first (Troll, 1937) considered as the preferential development of lateral axes in the basal part of a vertical stem. Bell (1991) used the term ‘basitonic branching’ when the proximal branches grow larger than the distal ones. Finally, mesotony (Fig. 11C, D) is used for a privileged development of branches in the median part of a shoot or axis.
Fig. 12.
Privileged repartition of sibling shoots on a vertical parent shoot or axis. Basitony is the privileged development of lateral axes in the basal part of a vertical stem or shoot. This may involve the whole plant level as for the shrubby plant Stenocereus thurberi (A) or the growth unit level only (Choysia ternatea, B). White arrow, limit of a growth unit. On the diagrams, the break is the limit of a growth unit.
Clearly included in the definition (Bell, 1991) or implicitly shown in the illustrations (Troll, 1937; Rauh, 1939), the topological lateral arrangement of branches along the parent axis is often associated with an increasing or decreasing gradient in length and/or vigour of the branches. As discussed by Caraglio and Barthélémy (1997), these two points must nevertheless be considered separately as the diversity of their expression in plants shows that there is neither automatic nor direct correlation between the privileged position and the relative vigour of lateral branches (compare Fig. 11A with Fig. 11B, or Fig. 11C with Fig. 11D). As all kinds of combinations may be found in the plant kingdom, we would prefer the terms acrotony, basitony and mesotony to be used only in reference to the privileged localization of branches on a parent shoot (respectively distal part, proximal part and median position) without reference to their relative vigour or length, which can be given in precise terms in addition.
Acrotony or basitony are frequently considered as two fundamental phenomena underlying, respectively, the ‘arborescent’ or ‘bushy’ growth habit (Troll, 1937; Rauh, 1939; Champagnat, 1947; Barnola and Crabbé, 1991). Nevertheless these authors refer mainly to the acrotonic branching of growth units or annual shoots in the arborescent case whereas they consider the proximal branching at the base of the whole individual when considering bushy plants. As discussed by Caraglio and Barthélémy (1997) either acrotonic or basitonic branching may characterize the growth units or shoots of either tree or bush, whereas basal sprouts may also be an adaptative strategy of some trees (Fig. 12). Therefore, these terms should be used only at the growth unit annual shoot or axis levels or, at least, the plant level of organization under consideration must be specified when using these terms.
Hypotony, epitony and amphitony (Fig. 13)
Fig. 13.
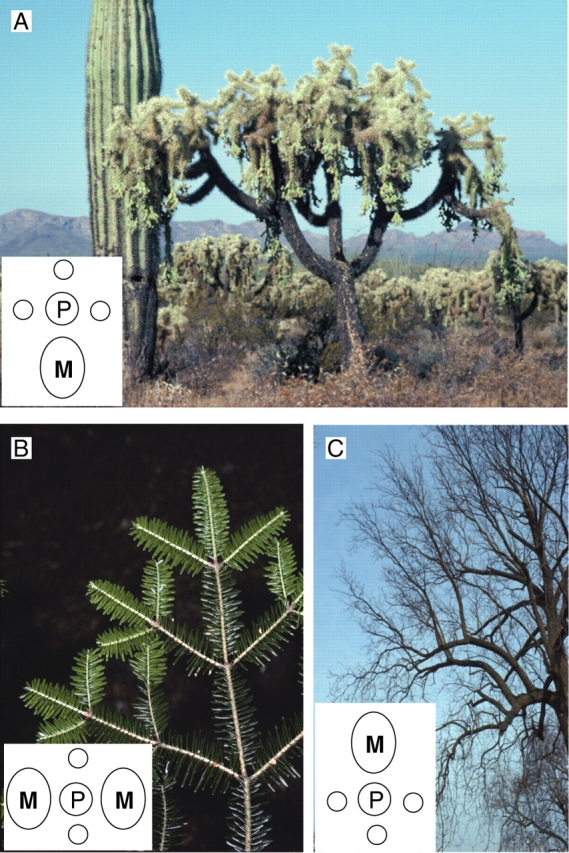
Privileged repartition of sibling shoots on a slanted or horizontal parent shoot or axis. Hypotony refers to the privileged development of branches on a basal position on a parent axis (Opuntia fulgida, A). Mesotony refers to the privileged development of branches on a lateral position on a parent axis (branches of Abies sp. from above, B). Epitony refers to the privileged development of branches on upper positions on a parent axis (Juglans nigra, C). P, parent axis; M, privileged lateral branch.
In order to describe the privileged arrangement of lateral axes on a horizontal, curved or slanted parent axis, Troll (1937) defined three modalities of so-called ‘laterale Symmetrie’.
According to this terminology the privileged development of branches on the upper, lateral or basal position of a parent axis is referred to, respectively, as epitony, amphitony or hypotony. Epitony is a very common process in many fruit trees (Costes et al., 2006) and in plants belonging to such architectural models as those of Champagnat, Troll or Mangenot (Hallé et al., 1978); it is also often associated with the survival of old branches in the canopy of old trees (Fig. 13C). Hypotony (Fig. 13A) is frequently marked by a privileged development of lateral axes in the curvature zone of a slanted branch whereas amphitony (Fig. 13B) is frequent on rectilinear horizontal or slightly slanted branches. Hypotony and amphitony may be combined in slanted and curved branches and their incidence in the expansion of lateral branch complexes is of the utmost importance in the crown architecture of many woody plants. Finally, it is noticeable that amphitony is a frequent feature in rectilinear branches whereas epitony and hypotony are characterized by the predominant development of lateral axes on the convex side of the curved, downwardly or upwardly orientated branches (Caraglio and Barthélémy, 1997; and compare Fig. 13A, B and C). As highly influenced by axis orientation, these phenomena are frequently combined with the previously described topological arrangement along axes (acrotony, basitony, mesotony) and these combinations have considerable relevance to bud fate according to their topological position and space orientation within a plant crown.
Morphological differentiation of axes
The general orientation of a leafy axis and the spatial disposition of its leaves are of major importance in the growth strategy of a plant. Within a single plant, some of these axes are essential in plant skeleton edification; some are aerials whereas others may grow underground; some are involved in space exploration whereas others are more related to reproductive dissemination activities or in environment exploitation via photosynthesis. This axis polymorphism is frequent in plants and represents a true morphological differentiation related to meristem expression and activity (Hallé and Oldeman, 1970).
Orthotropy, plagiotropy and mixed axes (Fig. 14)
Fig. 14.
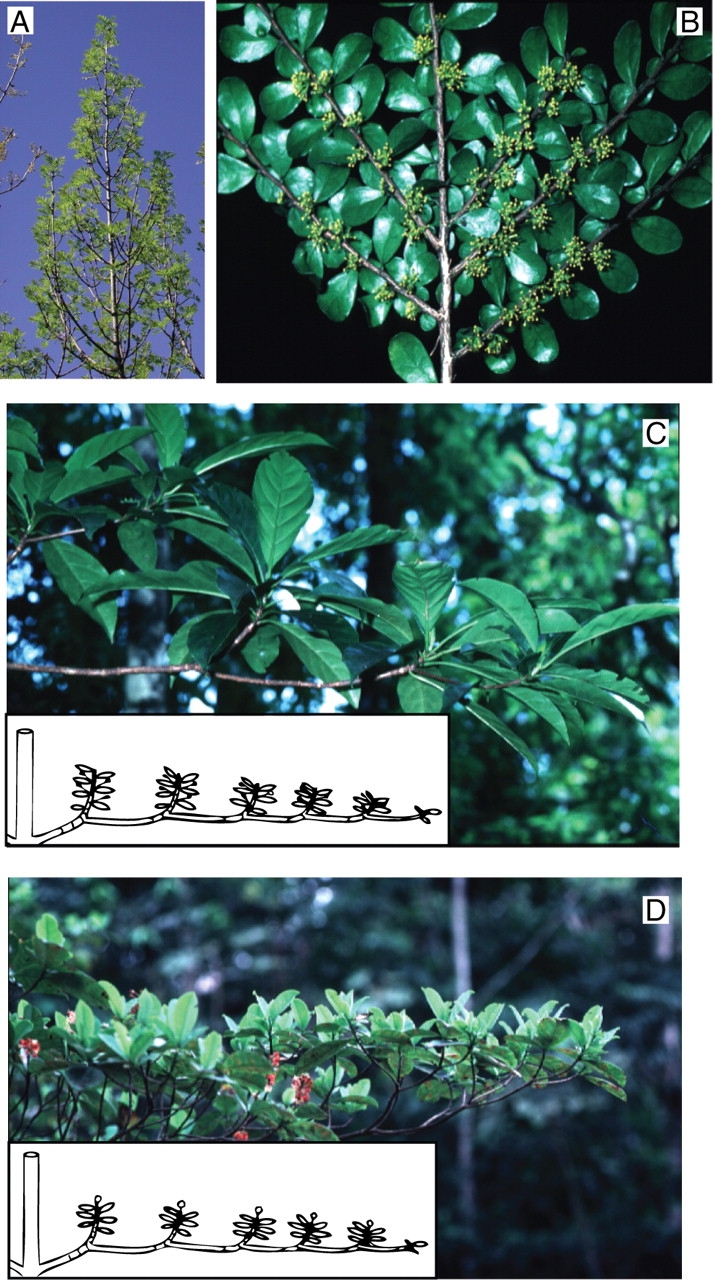
Orthotropic axes are generally erect to vertical with a radial symmetry, bear large leaves and long lateral axes (Fraxinus oxyphylla, A). By contrast, horizontal axes tend to exhibit a bilateral symmetry frequently associated with a high reproductive and photosynthetic strategy: they represent plagiotropic axes (Azara microphylla, B). Particular kinds of plagiotropic axes correspond to an immediate hypotonic sympodial branching system of successive indeterminate (plagiotropy by apposition: unidentified Sapotaceae, C) or determinate (plagiotropy by substitution: Byrsonima densa, D) sympodial units.
On most plants and more evidently in trees, two major types of axes may be distinguished according to their erect or horizontal general orientation. Orthotropy (Fig. 14A; Frank, 1868; Koriba, 1958) refers to axes whose general orientation is vertical and whose symmetry is radial, with leaves in a spiral, opposite or verticillate disposition, and associated lateral branches arranged in all spatial directions. Plagiotropic axes (Fig. 14B; Frank, 1868; Koriba, 1958) have a general horizontal to slanted orientation and a bilateral symmetry owing to leaves (distichous phyllotaxis) and branches being generally arranged in one plane.
Therefore, both kinds of axes are defined not just by their orientation but rather by a set or ‘syndrome’ of features (Edelin, 1984). In addition, some axes may have intermediate features and/or secondary orientation of axes may sometimes occur thus complicating their exact characterization. In many plants, a single meristem may give rise to an axis with mixed properties. Sometimes an axis may have an orthotropic proximal portion and a plagiotropic distal end; the superposition of such ‘mixed axes’ is a distinctive feature of the trunk edification in some plants (Mangenot's architectural model for instance; Hallé and Oldeman, 1970). Other mixed axes may present the reverse configuration, i.e. a proximal plagiotropic portion followed by a distal orthotropic end. In such cases, the modules are formed by successive hypotonic branching, giving rise to horizontal branched systems named plagiotropy by apposition (Fig. 14C; Hallé et al., 1978; apposing growth of Koriba, 1958; apposition growth of Roux, 1968) or plagiotropy by substitution (Fig. 14D; Hallé et al., 1978; substituting growth of Koriba, 1958) depending on whether modules are of, respectively, indeterminate or determinate growth.
For a given species, all axes may be of the same kind or several axis types may coexist on the same individual. Different axis types may even coexist at the same foliar axil. On the main stem of Coffea trees for instance, the more distal axillary meristem develops in an immediate plagiotropic branch whereas a more proximal reserve (supernumerary) bud (Varossieau, 1940; Moens, 1963) in the same leaf axil may develop as a delayed orthotropic axis. The coexistence of buds with different fates on the same node leads to many questions regarding the physiological control and genetic determinism of plagiotropy (Tomlinson, 1986, 1987) and more generally branching differentiation.
Short vs. long axes (Fig. 15)
Fig. 15.
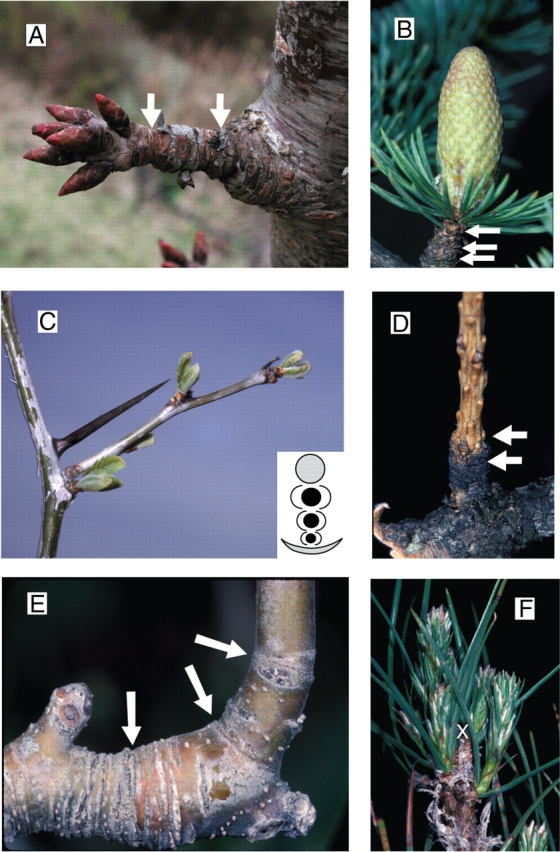
Short shoots are characterized by short internodes and successive growth units (Prunus avium, A). They are frequently associated with lateral (A) or terminal (Cedrus atlantica, B) reproductive organs. Short shoot type may be linked to position in the leaf axil in the case of supernumerary buds (Gleditsia triacanthos, C). In some conditions a short shoot can dedifferentiate into a long shoot (Larix decidua, D; Malus domestica, E). Even the very specialized brachyblast of Pinus species (P. nigra, F) may transform into a long shoot after stem traumatism (white cross). White arrows indicate the transition between two successive growth units.
In the vegetative aerial part of most woody plants, orthotropy is generally associated with plant skeleton construction and the colonization or exploration of the vertical space, whereas plagiotropy is generally more concerned with exploration and exploitation of the horizontal space and reproductive functions (photosynthesis, flowering).
In many cases, axis differentiation is also related to axis size and very often long and short axes, respectively, specialized in environmental exploration and exploitation (Fig. 15A and B) may be identified in a plant species (Champagnat, 1965; Rivals, 1965, 1966; Kozlowski, 1971; Zimmerman and Brown, 1971). Here again the differentiation of axes and bud fate may be highly specialized and very different structures (i.e. flowers, inflorescences, spines, long axis, etc.) may be found in a single leaf axil and in a precise position, as is the case in several species (see Fig. 15C). In all these cases, however, differentiation of an axis may not be an irreversible process, and according to modifications of internal or external conditions or after architectural traumatism, reversion of axis differentiation is very often possible (see Fig. 15D–F), indicating that shoot differentiation and bud fate are controlled by a whole plant network of correlations (Champagnat, 1961; Nozeran et al., 1984; Greenwood, 1987, 1995) and environmental conditions.
Position (terminal vs. lateral) of sexuality and reproductive organs
As architectural studies have historically focused mainly on the vegetative structure of the plant body, reproductive structures are considered as a whole and according to the impact they have on plant growth and branching. Because of their incidence on growth and branching, the lateral or terminal position of reproductive structures will be considered, i.e. whether they result from the transformation of a lateral or terminal meristem, respectively. Readers seeking more precise terminology and descriptions of the structure of reproductive organs or inflorescences in angiosperms should refer to more general (Bell, 1991) or dedicated synthetic works dealing with inflorescence typology or shoot typology according to the arrangement of reproductive or floral elements (Parkin, 1914; Pilger, 1921; Troll, 1957; Briggs and Johnson, 1979; Müller-Doblies and Weberling, 1984; Weberling, 1989; Claßen-Bockhoff, 2000, 2005).
THE CONCEPT OF ARCHITECTURAL MODEL
For a tree species the growth pattern which determines the successive architectural phases is called its architectural model, or shorter, its model (Hallé and Oldeman, 1970). The architectural model is an inherent growth strategy that defines both the manner in which the plant elaborates its form and the resulting architecture. It expresses the nature and the sequence of activity of the endogenous morphogenetic processes of the organism, and corresponds to the fundamental growth programme on which the entire architecture is established. The identification of the architectural model of any given plant is based on the observation of the features belonging to the four major groups of simple morphological features presented above: (1) the growth pattern, i.e. determinate vs. indeterminate growth and rhythmic vs. continuous growth; (2) the branching pattern, i.e. terminal vs. lateral branching vs. no branching, monopodial vs. sympodial branching, rhythmic vs. continuous vs. diffuse branching, immediate vs. delayed branching; (3) the morphological differentiation of axes, i.e. orthotropic vs. plagiotropic vs. axes with mixed morphological and/or geometrical features (with plagiotropic and orthotropic portions); and (4) lateral vs. terminal flowering.
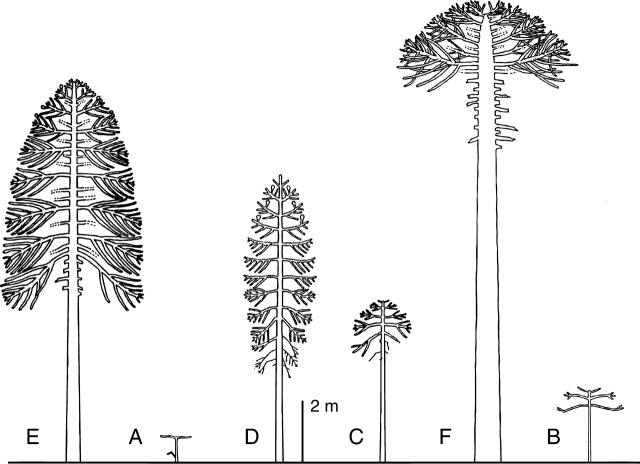
Each architectural model is defined by a particular combination of these simple morphological features and named after a well-known botanist (Fig. 16). Although the number of these combinations is theoretically very high, there are apparently only 23 architectural models found in nature. Each of these models applies equally to arborescent or herbaceous plants, from tropical or temperate regions, and which can belong to closely related or distant taxa.
Fig. 16.
Some architectural models. Corner's model (A) concerns unbranched plants with lateral inflorescences. Leeuwenberg's model (B) consists of a sympodial succession of equivalent sympodial units, each of which is orthotropic and determinate in its growth. Rauh's model (C) is represented by numerous woody plants where growth and branching are rhythmic, all axes are monopodial and sexuality is lateral. Illustrations after Hallé and Oldeman (1970), Hallé et al. (1978) and Barthélémy (1991).
The reader will find detailed information on each architectural model in Hallé and Oldeman (1970) and Hallé et al. (1978).
Growth patterns defined by the architectural models are genetically determined. Only under extreme ecological conditions is their expression affected by the environment (Hallé, 1978; Barthélémy, 1986; Barthélémy et al., 1995, 1997b, 1999; Grosfeld et al., 1999; Grosfeld, 2002; Stecconi, 2006). Different models can be represented by plants belonging to closely related species (Edelin, 1977; Temple, 1977; Edelin and Hallé, 1985; Vester, 1999; Hallé, 2004). Architectural analysis also shows that some plants frequently exhibit morphological features that are apparently related to two or three models (Hallé and Ng, 1981; Edelin, 1977, 1984; Veillon, 1978, 1980; Grosfeld et al., 1999). These intermediate forms indicate that there is no real disjunction between the models. On the contrary, it must be considered that all architectures are theoretically possible and that there could be a gradual transition from one to another. Among this ‘architectural continuum’ (Edelin, 1977, 1981; Hallé et al., 1978), the models themselves represent the forms that are the most stable and the most frequent, i.e. the most probable biologically. As an architectural model is defined by few and simple morphological features, it gives only an idea of the elementary developmental pattern of a species. Nonetheless, this level of representation of the plant could be well adapted to describe the evolutionary pattern or phylogenetic or taxonomic distribution of plant architecture (Johnson, 2003; Hallé, 2004) and may help to unravel the ecological importance of these patterns (Foresta, 1983; Vester, 1997, 1999).
THE ARCHITECTURAL UNIT
The architectural model represents the basic growth strategy of a plant, and this concept has allowed the definition of a typology of main plant growth strategies. Nevertheless, the characters used in its identification are far too general to describe the complete and precise architecture of a plant. For any given plant, the specific expression of its model has been called its ‘architectural unit’ (Barthélémy et al., 1989, 1991; originally called ‘diagramme architectural’ in French: Edelin, 1977).
The architecture of a plant can be seen as a hierarchical branched system in which the axes can be grouped into categories according to their morphological, anatomical or functional distinctive features (Fig. 17).
Fig. 17.
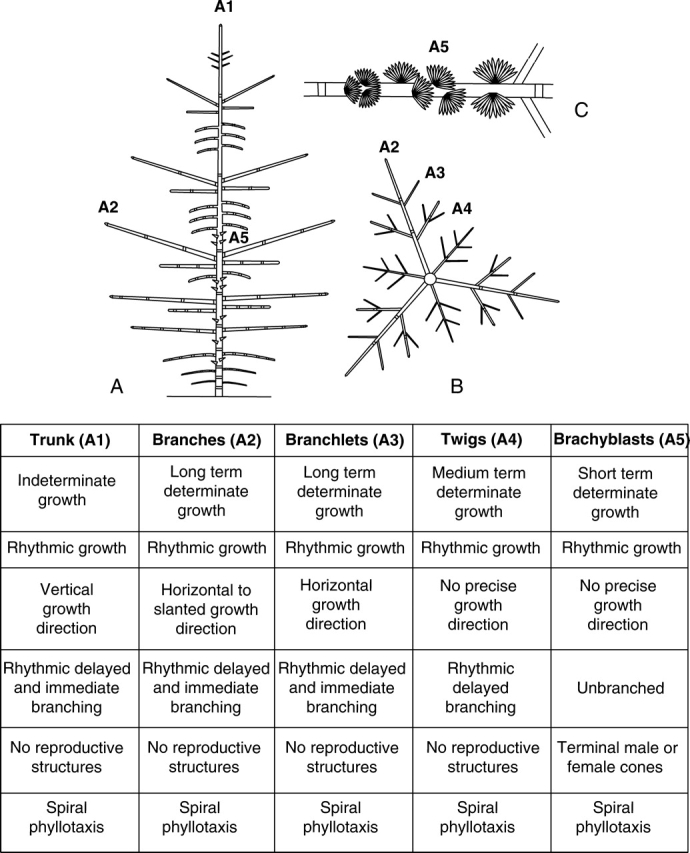
The architectural unit of Cedrus atlantica (Pinaceae) is composed of five axis categories (A1 to A5). (A) Diagrammatic representation of the tree (view in elevation) representing the relative position of main axis categories; (B) diagrammatic representation of a tier of branches (view from above); (C): diagram of a twig annual shoot bearing several short shoots. The break symbol indicates the limit between two successive annual shoots (from Sabatier and Barthélémy, 1999). The table summarizes the morphological features of all axis categories.
The structure and function of each category of axis may be described by a non-limitative series of features. For each of them, the observation of all the architectural characteristics previously described is necessary, but the observations have to be as exhaustive as possible and may concern any elementary level of organization (i.e. metamer, growth unit, annual shoot, module) and any kind of morphological feature (e.g. precise growth direction, phyllotaxis, pre- or neoformation, immediate or delayed branching, form and size of foliar organs, presence, absence and position of sexuality).
The results may be summed up in a table and diagrams that describe and define the specific elementary architecture of each plant, i.e. its architectural unit. Within the context of a general organization, the differences between architectural units are thus represented by the number of categories of axes, their functional and morphological features, and their relative positions.
For each species it has been shown that the number of categories of axes is finite (Edelin, 1977; Caraglio and Edelin, 1990; Caraglio, 1997; Grosfeld et al., 1999; Sabatier, 1999; Grosfeld, 2002; Stecconi, 2006) and generally small (no more than five or six in some Cupressaceae according to the above cited studies). This indicates that the architecture of a fully established branched system, whatever its complexity, can be summarized in terms of a very simple sequence of axes which represents its fundamental organization. In this sequence, leading from axis 1 to the ultimate axis category, following the specific branching pattern, each branch is the expression of a particular state of meristematic activity and the branch series as a whole can be considered to be tracking the overall activity. In this sense, the architectural unit represents the fundamental architectural and functional elementary unit of any given species.
For structurally complex woody plants in particular, strong axis specialization is frequent: for example, some axes have a more structural and/or exploration function (like the main axis and major branches), and others (short shoots, twigs, etc.) are more concerned with photosynthesis and reproduction. Depending on the species, the differentiation of axes may be strong or not. In some species, axes of different categories have similar features (e.g. ability to flower on all axis categories) whereas some other species have more specialized categories of axes, each presenting very distinctive and exclusive features. Similarly, the relative arrangement of the categories of axes may depend on the degree of differentiation among them, which, in turn, may be related to particular morphological processes such as acrotony. Thus, the categories of axes may or may not be superposed to the notion of branching order (Fig. 18).
Fig. 18.
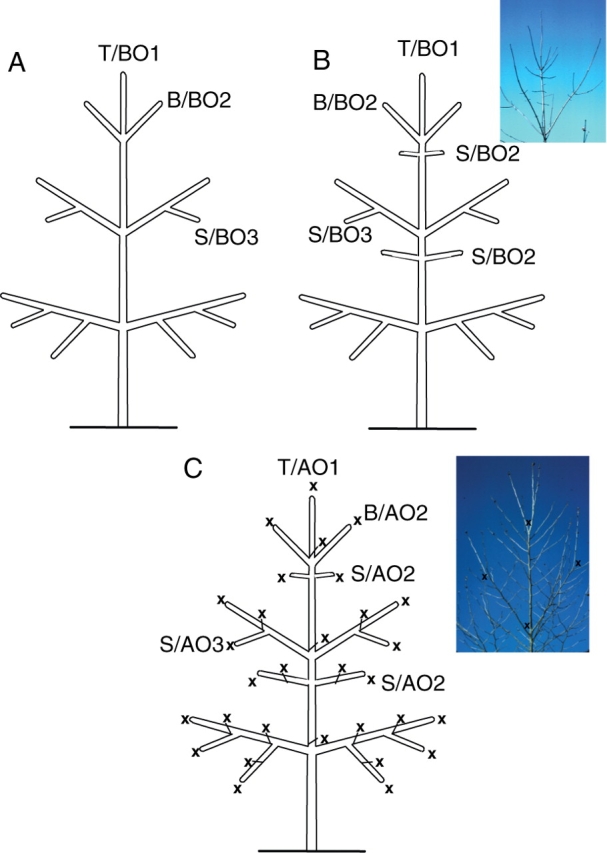
Category of axis vs. branching order. The relative arrangement of categories of axes (T, trunk; B, branch; S, short shoot) may (A, i.e. Araucaria araucana) or may not (B and C) be superposed to the notion of branching order, in either monopodial (B, i.e. Acer sp.) or sympodial (C, i.e. Platanus sp.; Caraglio and Edelin, 1990) branching pattern. x, apical mortality; BOn, branching order n; AOn, apparent branching order n.
According to a species' developmental pattern, the expression of the architectural unit may be different. Whereas the relative arrangement of categories of axes is generally clear in most woody plants and especially in trees, the total expression of the architectural unit in some herbs or sympodial plants may include the whole succession of modules (Fig. 19).
Fig. 19.
Each category of axis (trunk, T; branch, B) results from the succession of shoots in a monopodial system (A, Acer sp., redrawn from Troll, 1937) or the succession of modules in sympodial trees (B, Ulmus sp., redrawn from Troll, 1937; dotted lines represent self shed branches; x, apical mortality) or herbs (C, Encyclia vespa, from Barthélémy, 1988; dotted sympodial units represent those that are naturally shed for the developmental stage diagrammatically represented).
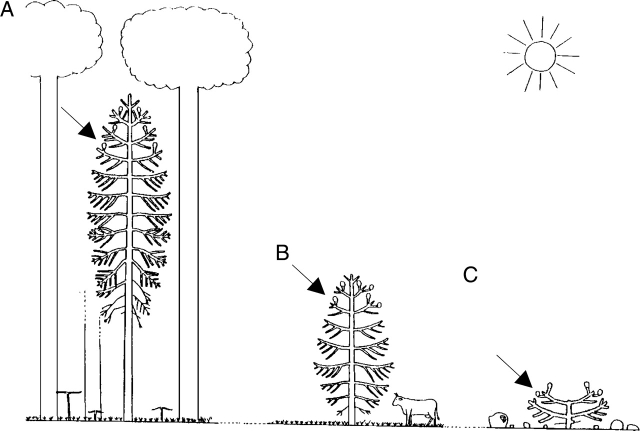
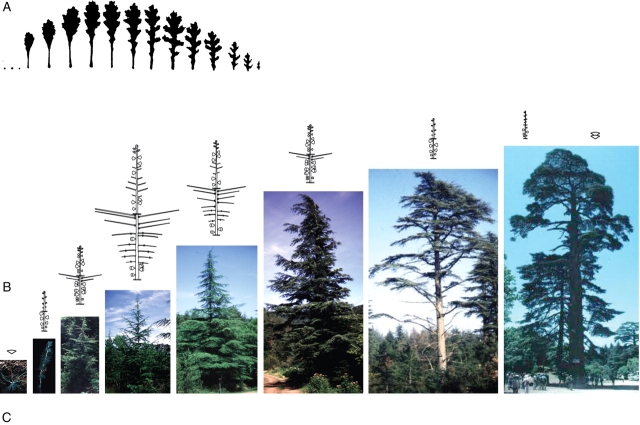
In a particular species the architectural unit is a very stable and endogenous feature, as shown for several species in which different environmental conditions affect the external form of individuals but change only quantitatively the expression of the species' endogenous morphogenetic sequence (Fig. 20; Grosfeld et al., 1999).
Fig. 20.
Variation in crown physiognomy and architecture in relation to environmental conditions at the time of architectural unit expression in Araucaria araucana. (A) In forest stands, the mature tree expressing its architectural unit has a 15–20-m-high trunk which bears at its top a large conical crown composed of up to 20 tiers of living branches. (B) In full sun and with favourable soil and precipitation conditions, the tree has, at first cone production, a typical pyramidal crown. The trunk is 6–8 m high and most branches are alive. (C) In full sun and poor soil and precipitation conditions, the first production of cones may occur in a tree no more than 4 m high. The crown has a typical ‘umbrella form’ and most of the branches are alive (Grosfeld et al., 1999). Black arrows indicate terminal female cones.
Finally, for species such as Cupressus sempervirens, for which several ‘forms’ are traditionally well known by foresters and horticulturists (i.e. fastigiated, horizontal and intermediate), it was also shown that there were only minor qualitative differences between the elementary architecture or architectural sequence of these three forms. In this case, the observed phenotypic variability could be explained by the different expressions of several basic morphological and geometrical features such as (1) the relative main stem/branch length, (2) the straightness or gradual straightening-up status of the branches, (3) the insertion angle, (4) homogeneity or heterogeneity of branch types within a single tree and (5) the occurrence, early manifestation and importance of the reiteration process (Fig. 21; Barthélémy et al.,1999).
Fig. 21.
Architectural types of Cupressus sempervirens observed in ‘fastigiated’ (A), ‘intermediate’ (B and C) and ‘horizontal’ (D) crown shape groups (from Barthélémy et al.,1999). Distinctive features of these architectural types are: the length of branches (compare A left and D), their straightness (A and B left) or straightening up (A and B right), their initial insertion angle (differences between D left and D right), homogeneity (A, B and D) or heterogeneity (C) of branch types within a single individual, the occurrence and importance of the reiteration process (A right and C). Black arrows indicate reiterated complexes.
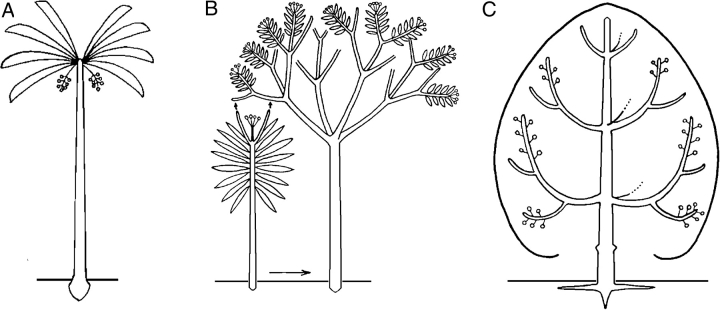
THE CONCEPT OF REITERATION
Although some plants conform to their architectural unit during their whole life span (Fig. 22), most plants repeat their architectural unit during their development, late in ontogeny, or under particular conditions. Oldeman, (1974) named this process ‘reiteration’ and defined it as a morphogenetic process through which the organism duplicates its own elementary architecture, i.e. its architectural unit. The result of this process is called a ‘reiterated complex’ (Hallé et al., 1978; Barthélémy et al., 1989, 1991) or a ‘reiterate’ (Millet et al.,1998a). Reiteration encompasses several aspects (sprouts, root-suckers, etc.) that have been known incidentally by botanists for a long time. The fundamental interest of this concept resides on it regrouping all these phenomena into a coherent whole, to bring out a common morphogenetic event. Oldeman, (1974) and others (Hallé et al., 1978; Edelin, 1984; Nicolini, 1997; Vester, 1997) hypothesized about the factors triggering this process, and distinguished several types of reiteration.
Fig. 22.
Architectural sequence of development in Araucaria araucana (from Grosfeld, 2002). In this temperate South American species, the plant expresses step by step (A–D) its architectural unit composed of three axis categories (D). The following stages of development (E and F) are only marked by quantitative modifications, and the tree remains conform to its architectural unit, without any reiteration, up to the end of its life.
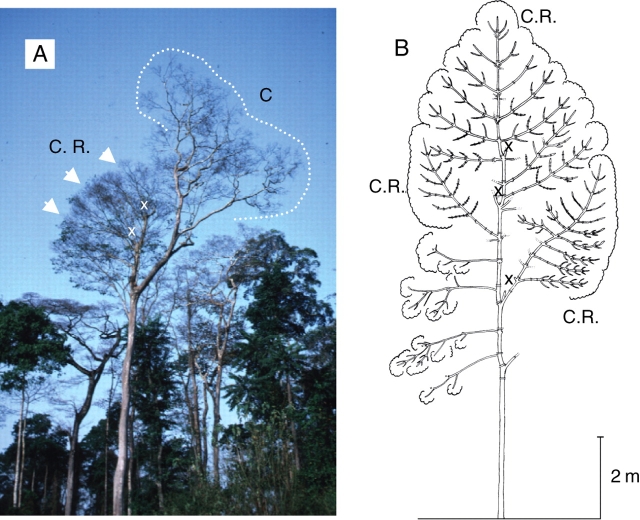
As first stated by Oldeman, (1974) the reiteration process may involve the expression of the total architectural unit from axis 1 to the most differentiated axis category (‘complete’ or ‘total’ reiteration), or the expression of part of the developmental sequence duplicating only part of the species' architectural unit (‘partial reiteration’): see Fig. 23A.
Fig. 23.
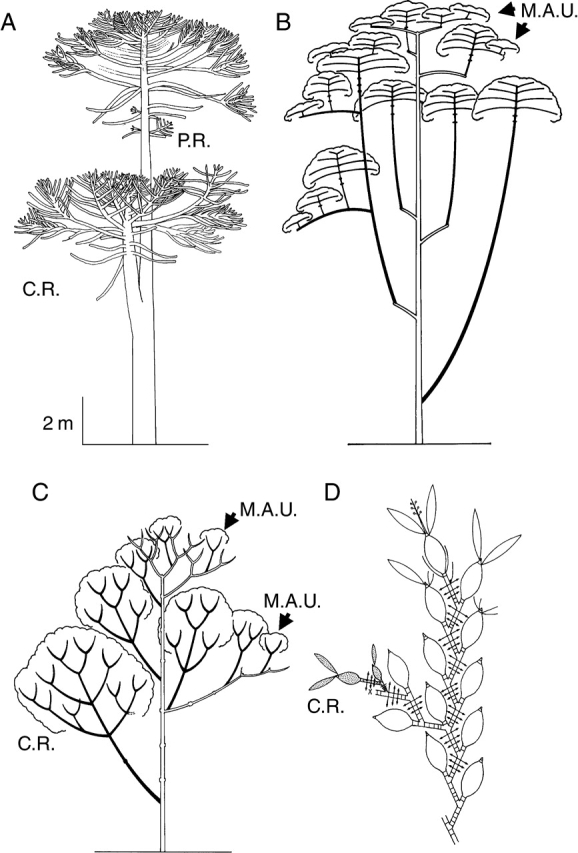
Opportunistic reiteration and structure of the reiterated complexes. (A) Opportunistic, ‘partial’ (P.R.) and ‘complete’ (C.R.) reiteration in an individual of Araucaria araucana (Araucariaceae; from Grosfeld et al., 1999). Diagrammatic representation of reiterated complexes (in black) according to their location on the tree in (B) Symphonia globulifera (from Barthélémy, 1988) and in (C) Isertia coccinea (from Barthélémy, 1988). All complete reiterated complexes result from the development of a previously dormant bud (delayed reiteration). They all duplicate the original sequence of differentiation of the original individual but the duplication is smaller and more ‘pauperized’ according to their insertion from the base of the trunk to the ‘periphery’ of the crown. At the top of the tree and in the most peripheral part of the crown, pauperization of the duplication is the highest and reiterated complexes all have a reduced and minimal specific structure (in the case of Symphonia globulifera, a small trunk bearing only one flowering tier of plagiotropic branches, and in the case of Isertia coccinea, a succession of small sympodial units only branched below the terminal inflorescence) named ‘Minimal Architectural Unit’ (M.A.U.) by Barthélémy (1988). (D) Opportunistic, ‘complete’ (C.R.) reiteration (dotted units) in old parts of a traumatically cut (x) sympodial herb (Encyclia vespa, Orchidaceae, from Barthélémy, 1988). Double arrows, roots.
Reiterated complexes may originate from dormant meristems and reiteration in this case is called ‘proleptic’ or ‘delayed’. By contrast, reiteration may result from a shift in the functioning of the apical meristem of a growing shoot that will finally produce a ‘less differentiated structure’, i.e. a branch apex that after some time of functioning gives rise to a ‘supernumerary trunk’. In this case, the reiteration is described as ‘sylleptic’ (or better ‘immediate’) or ‘reiteration by dedifferentiation’ (Fig. 24). Either of these two types of reiterations may be qualified as total or partial.
Fig. 24.
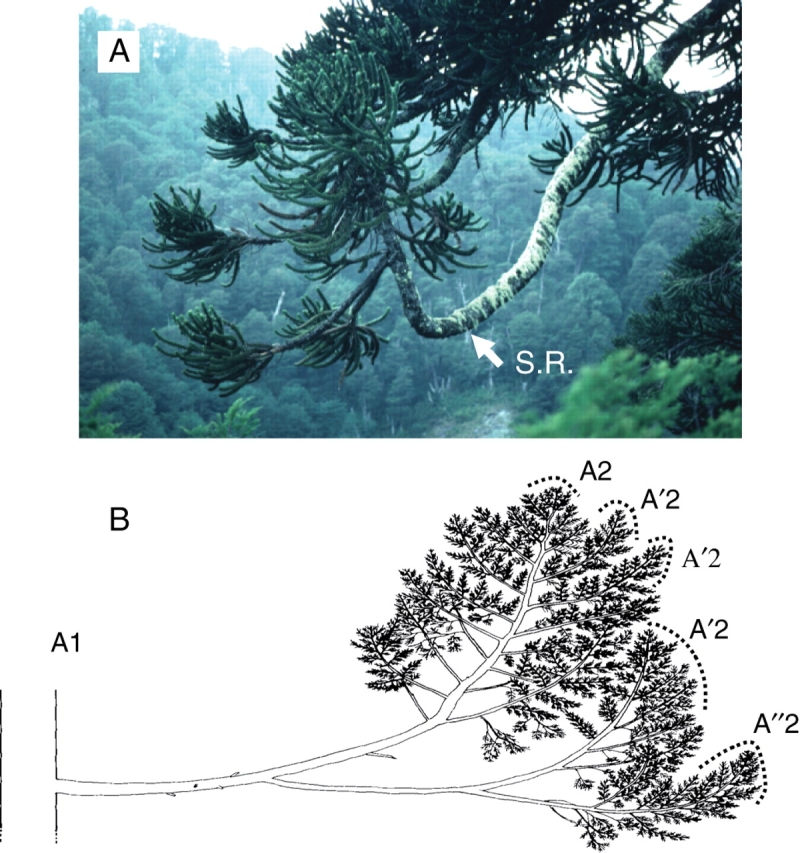
(A) Total immediate reiteration (S.R.) expressed on the distal part of a branch of Araucaria araucana. (B) Successive immediate and partial reiterated complexes on a branch of Austrocedrus chilensis (from Grosfeld, 2002). A1, main stem; A'2, first order of reiteration of A2 category of axis; A''2, second order of reiteration.
Reiteration at first was considered as an opportunistic (non-automatic) process (Oldeman, 1974; Hallé et al., 1978). ‘Opportunistic reiteration’ may today be considered as any kind of reiteration linked to the individual history of each tree and may have two main origins: (1) ‘adaptive reiteration’ is a response to an increase in resource levels whereas (2) ‘traumatic reiteration’ is a response of a plant after it has been damaged and lost a major part of its structure (Fig. 25). More recent investigations (de Castro e Santos, 1980; Edelin, 1984; Sanoja, 1992; Nicolini, 1997; Sabatier, 1999; Vester, 2001; Grosfeld, 2002; Stecconi, 2006), however, have demonstrated that, beside these cases of opportunistic reiteration, the same process of repetition may be involved in the inherent growth pattern of a species and occur automatically during plant development after a definite threshold of differentiation (Fig. 26). This latter case is a common feature of tree development and crown construction and is referred to as ‘automatic’ (Edelin, 1984) or ‘sequential’ reiteration (Nicolini, 1997).
Fig. 25.
(A) Damaged tree crown of an unidentified tropical tree comprising a part of the initial crown (C) and a set of reiterated complexes (C.R.) on the broken part (x). (B) Adaptive reiterated complexes (C.R.) can occur following local structural changes in the crown of an individual of Fraxinus excelsior (Barthélémy et al.,1997a) as caused by traumatism along the main stem (x); compare with Fig. 26.
Fig. 26.
Diagrammatic representation of the architectural sequence of development in Fraxinus excelsior (from Barthélémy et al.,1997a; highest category axes are not represented). The young plant expresses step by step (A and B) its architectural unit (in B) and then duplicates it automatically in the following stages of development (C and D) finally to give rise to a complex mature crown made of a succession of reiterated complexes (D).
As already noted, the development of a plant conforming to its model implies the notion of a differentiation sequence in the activity of the whole set of meristems of the plant (see also following sections). In the case of opportunistic reiteration (cf. Fig. 23) the occurrence of reiterated complexes seems to be a move backwards within the plant's developmental sequence, i.e. a real ‘dedifferentiation’. A supernumerary trunk (or branch), resulting from the transformation of a growing branch (Fig. 24, ‘immediate reiteration’), or from the development of a branch from a dormant meristem (Fig. 23, ‘delayed reiteration’), implies that the plant expresses again the juvenile growth pattern of the organism developed from seed. This is well illustrated in cases of regeneration in which, when a trunk is cut, sprouts resembling young trunks are formed from the stump, whereas reiterated complexes that develop after a branch has been damaged have an architecture similar to that of this branch. More generally speaking, it has been demonstrated that, in this case, the real degree of dedifferentiation of a reiterated complex depends on the location of the reiterated complex in the whole plant architecture and on the ontogenetic stage of the plant at the moment reiteration occurs (Barthélémy, 1988; Fig. 23B, C).
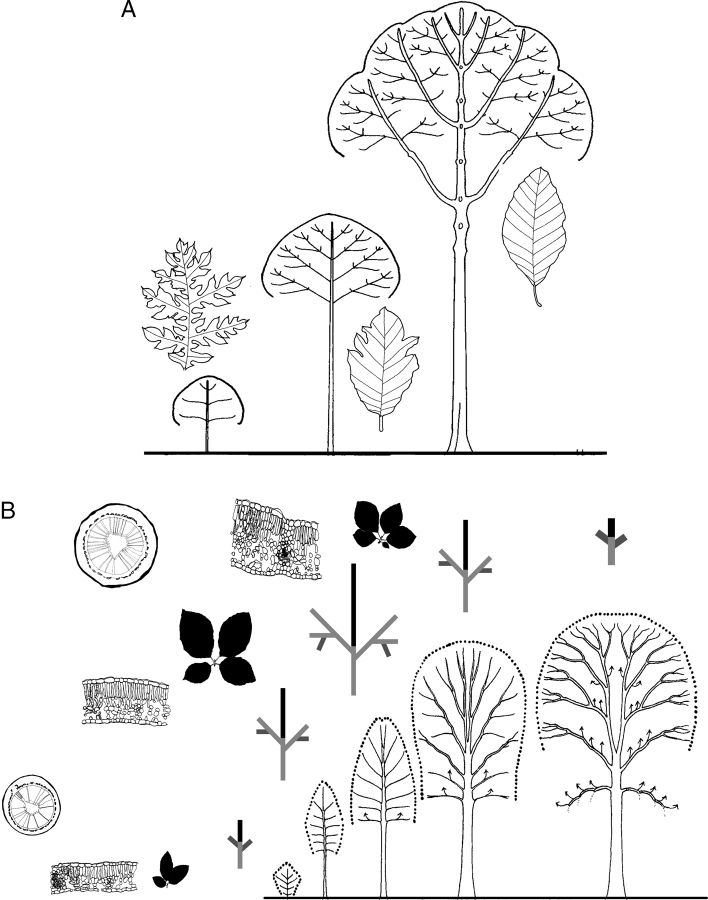
‘Automatic’ or ‘sequential’ reiteration has been intensively studied in the last two decades (Edelin, 1984; Caraglio and Edelin, 1990; Barthélémy et al., 1991; Durand, 1997; Nicolini, 1997; Vester, 1997; Sabatier, 1999; Grosfeld, 2002; Stecconi, 2006) and proved to be a very common and major morphogenetic process underlying crown construction in most forest trees. In these studies, it was suggested that sequential reiteration must not be interpreted as a move backwards within the developmental sequence of the original organism, but rather as part of this sequence as illustrated by the continuous and gradual trends in morphological and/or anatomical parameters observed during the whole sequence of development (Fig. 27).
Fig. 27.
Sequential (‘automatic’) reiteration and gradual trends in architectural, morphological and anatomical features according to stages of development. (A) Trends in leaf size and form for the main axis during the ontogeny of Artocarpus elasticus (from Edelin, 1984). (B) From left to right: trends in stem and leaf anatomy, in leaf and short shoot size and structure, in a main stem branching complexity (branching grade) and size (length of the latest G.U. in black and position of short shoots in dark grey on maximum expanded branching system in grey) and in architecture according to successive stages of development in Fagus sylvatica (after Nicolini, 1997; Nicolini and Chanson, 1999).
LEVELS OF ORGANIZATION, REPETITION PHENOMENA AND SEQUENCES OF DIFFERENTIATION
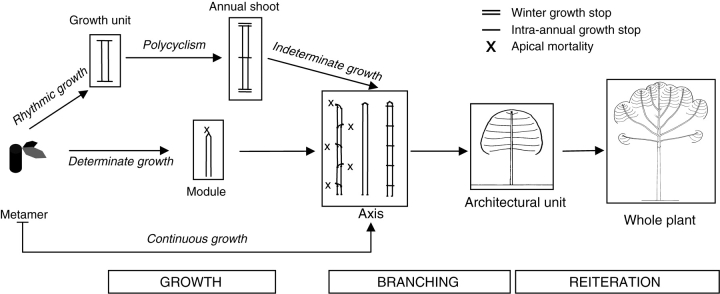
Since the pioneering work of von Goethe (1790), observations of many authors on numerous plant species (e.g. White, 1979; Barthélémy, 1986, 1991; Bell, 1991; Room et al., 1994; Caraglio and Barthélémy, 1997; Gleißner, 1998) have confirmed that plants are modular organisms developing by the repetition of elementary botanical entities or construction units. In seed plants, considering their huge species diversity, the number of these entities is actually relatively small. They correspond, in increasing order of complexity or integration, to: metamer, growth unit, sympodial unit, annual shoot, axis, architectural unit, and whole reiterated organism (Barthélémy, 1991; Barthélémy et al.,1997a; Caraglio and Barthélémy, 1997). From the most elementary to the most global and integrative, these units represent as many nested ‘levels of organization’. During ontogeny, they progressively derive from one another by three main and fundamental morphogenetic processes or ‘repetition phenomena’, namely growth, branching and reiteration, involving the repetition of respectively more complex and integrative elementary entities (Fig. 28).
Fig. 28.
Diagrammatic representation of main levels of organization (construction units) and repetition phenomena (in italics or terms in boxes) in seed plants (synthesis from Barthélémy, 1991; Barthélémy et al.,1997a; Caraglio and Barthélémy, 1997). x, apical mortality.
As stated earlier, the development of a plant may be seen as the expression of a precise and ordered sequence of morphogenetic events underlying a strong differentiation process. Whatever the botanical entity concerned, all the above cited studies and others (Goebel, 1900; Troll and Rauh, 1950; Poethig, 1990; Gleißner, 1998; Claßen-Bockhoff, 2000, 2001; Kaplan, 2001) show that its repetition, by one or another repetition phenomenon (i.e. growth, branching, reiteration), always induces either abrupt or progressive changes in its morphological features (Barthélémy et al.,1997a; Caraglio and Barthélémy, 1997). Whatever their qualitative or quantitative nature these variations always lead to a ‘differentiation’ of the different repeated botanical entities. For each level of organization, this differentiation is most often related to an ordered and precise series of transformations that represent a real ‘sequence of differentiation’, the morphological expression of which may be observable at any organizational level, from the most elementary, i.e. the metamer, to the most global, i.e. the whole reiterated individual (Barthélémy, 1988; Barthélémy et al.,1997a; Fig. 29, but see also Figs 23 and 27).
Fig. 29.
Differentiation processes and levels of organization in seed plants. (A) Coordinate and related trends in leaf and internode structure and in size and nature of foliar axil products along the unique axis of a herbaceous tropical plant native to French Guyana: Noisettia longifolia (after Barthélémy, 1988). (B and C) Differentiation at the level of the growth unit (G.U.) and annual bicyclic shoot in the Mediterranean trees Pinus halepensis (B) and Quercus ilex (C, after Caraglio and Barthélémy, 1997, see also Fig. 6). In both cases, differentiation at the level of each growth unit is marked by the nature of foliar organs (cataphylls vs. photosynthetic leaves) or axillary products (dormant bud vs. lateral shoot or vs. reproductive organs) for G.U.1. For both species, at the bicyclic annual shoot level, differentiation between the two successive G.U.s (i.e. G.U.1 vs. G.U.2) is revealed by the presence of reproductive structures and lateral shoots on G.U.1 only and by differences in leaf size and structure according to their bearing G.U., i.e. G.U.1 vs. G.U.2. (D) Differentiation at the comprehensive level of the whole ontogeny of a plant is illustrated in the case of common walnut (Juglans regia, after Sabatier, 1999) by the architectural trend from young to mature tree (left to right) and by the associated annual shoot structure trends (diagrammatically represented here at each stage for the main stem and some lateral branches). Break symbol (=), winter growth stop; – or ∼, intra-annual growth stop or decrease in growth speed; o, terminal female flower.
Owing to the widespread effect of differentiation at each level of organization, the specific and exact structure of a particular botanical entity in a given location within the architecture of a plant may be seen as the result of the concomitant influence of several ontogenetic and morphogenetic factors that affect all levels of organization of the organism, at each stage of its development and during its whole life span. Although environmental factors may ‘modulate’ these sequences of differentiation (Fig. 20), it was shown (Barthélémy et al., 1995, 1997a, b; Nicolini 1997; Sabatier, 1999; Claßen-Bockhoff, 2000; Heuret et al., 2000; Guérard et al., 2001) that they almost never (except probably in extreme conditions) modify the inherent morphogenetic and ontogenetic constructional rules of plant organization.
THE NOTION OF ‘MORPHOGENETIC GRADIENTS’
In the last two decades, coupled with precise morphological observations, architectural analyses of several plant species (Caraglio and Edelin, 1990; Barthélémy et al., 1992, 1995; Drénou, 1994; Nicolini and Caraglio, 1995; Caraglio and Barthélémy, 1997; Nicolini, 1997, 1998, 2000; Gleißner, 1998; Puntieri et al., 1998, 1999, 2000, 2001, 2002a, b; Genoyer et al., 1999; Nicolini and Chanson, 1999; Sabatier and Barthélémy, 1999, 2001a, b; Sabatier et al., 1999, 2003a, b; Claßen-Bockhoff, 2000; Heuret et al., 2000; Nicolini et al., 2000; Souza et al., 2000; Stecconi et al., 2000; Guérard et al., 2001; Passo et al., 2002) revealed that, under given environmental conditions, the structure and features of a particular elementary botanical entity (metamer, growth unit, annual shoot) are predictable and strongly dependent on both (1) its topological location in the comprehensive architecture of a plant and (2) the ontogenetic stage of the organism.
At the level of the whole plant, the ‘morphogenetic gradients’ notion was defined (Barthélémy et al.,1997a) in order to take into account the intrinsic organization rules of plant structure and was shown to be a powerful concept (Prusinkiewicz et al., 2001) to explain the observed structure and series of modifications of botanical entities during the ontogeny of any plant species (Fig. 30).
Fig. 30.
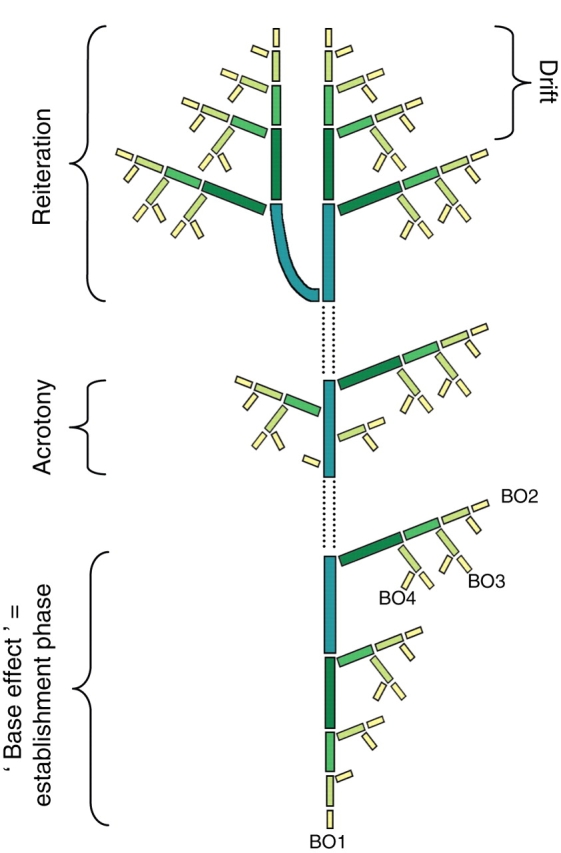
Morphogenetic gradients and physiological age of meristems (after Barthélémy et al.,1997a). Theoretical and diagrammatic representation of the distribution of elementary botanical entities with similar characteristics (i.e. presenting the same ‘physiological age’ and represented by the same size and colour rectangle on the diagram) according to some main morphogenetic gradients very commonly observed in seed plants: for the initial structure and reiterated complexes four ‘branching orders’ (BO1–BO4), BO1 representing the main axis; ‘base effect’ is a gradient linked to the ‘establishment growth phase’ of any plant grown from seed; ‘acrotony’, with increasing acropetal gradient of vigour of lateral axes, is a common feature of the annual shoots and growth units of most rhythmically growing trees; ‘drift’ is a general feature linked with axis ageing; sequential ‘reiteration’, in this case, represented by the automatic duplication of the sequence of development and associated gradients of the main axis by another axis.
These morphogenetic gradients reflect the various processes of differentiation related to morphogenetic repetition phenomena that can be identified in plant construction (see previous paragraph). Their observable number in a plant largely depends on its specific structural complexity and on the number of levels of organization it is able to express. It is at a minimum for a monocaulous continuously growing plant in which morphogenetic gradients will only concern the successive metamers (and their individual constitutive parts) whereas it will be at a maximum in a polycyclic, branched and reiterated, rhythmically and sympodially growing plant where these gradients will reflect, at several levels, the complex topological and geometrical structural nested organization.
Some of these gradients are very widespread in the plant kingdom and have a very comprehensive and general expression during the development as a whole as they are associated with either the first successive steps of the ontogeny or the ageing of axes or plants. These gradients were respectively called ‘base effect’, or ‘drift’ (Barthélémy et al.,1997a) and take into account the respective ontogenetic increase or decrease in several parameters along the main stem of any plant, or even along lateral axes of branched plants. They encompass several phenomena that have been known and described for a long time but that very often were considered or defined independently or separately according to the observer's interest (plant structural edification for botanists, main stem height and radial growth for foresters) and the phylogenetic status (seed plant or not, actual or fossil plant, monocotyledons or dicotyledons, etc.) or the particular growth pattern of the plant, or plant group (i.e. monopodial or sympodial, herbaceous annual or woody perennial plants) under study. According to the specific complexity and/or growth pattern, these gradients may be described using different parameters. Among these parameters are the size, nature, and/or internal and external structure of the successive metamers, growth units, annual shoots or modules in sympodial plants or even appendages and sibling lateral axes if any (Fig. 31), but also on the growth rate and the various portions of the sigmoidal growth curve of all plants and axes (Pressler, 1865; Pardé and Bouchon, 1988) or even on the ‘rhythms of primary thickening growth’ (Kaplan, 2001) described by various authors (‘Erstarkungswachstum’ of Troll and Rauh, 1950; ‘establishment growth’ of Tomlinson and Zimmerman, 1966; Tomlinson and Esler, 1973; ‘epidogenesis’ and ‘apoxogenesis’ of Eggert, 1961, 1962; Daviero et al., 1996; Soria and Meyer-Berthaud, 2004). These rhythms are often associated with the obconic shape of primary structures (Martinez and De La Sota, 2001; Hueber and Galtier, 2002; Isnard et al., 2005) during the first stages of shoot (or even plant) ontogeny and/or with the diameter of the shoot apex (Steeves and Sussex, 1989). Whatever their specific and particular expression and because of their very wide occurrence in any actual or fossil plant, these gradients or ‘rhythms of primary thickening growth’ may be considered as one of the few fundamental principles of plant shoot organization (Kaplan, 2001).
Fig. 31.
(A) Shape and size of successive leaves along a stem of a herbaceous Senecio sp. (redrawn from Baillaud and Courtot, 1955). (B) Organization of the two last successive annual shoots of the main stem of Cedrus atlantica during its life and (C) the corresponding global tree structure (from Sabatier and Barthélémy, 1995).
‘Base effect’ and ‘drift’ are commonly expressed at the axis level and thus affect the main axis of any plant and all the lateral axes of branched ones. According to these gradients, the exact structure of a lateral axis depends on its topological and ontogenetic position on the parent axis (Fig. 30). The effect of ‘branching order’ may be superimposed on these previous gradients at a whole plant level. In general terms, the higher the branching order of an axis, the higher its degree of differentiation. At a more local scale in the architecture of a branched plant or system and according to the expression of the specific branching pattern, gradients, linked to shoot structure and precise branching pattern (acrotony, basitony, mesotony) or linked to shoot spatial orientation and/or geometry (hypotony, epitony, amphitony), may be superimposed on previous ones, which explains the observed heterogeneity of axes or shoots in close topological positions.
Because of its duplicative and repetitive nature, the reiteration process reproduces the morphogenetic gradients of the non-reiterated parent plant (Fig. 30) and the structure of the reiterated complexes resembles that of the whole plant (Fig. 23B, C; Barthélémy, 1988).
As previously stated, all these morphogenetic gradients have a strong intrinsic basis and allow in a given environmental condition the prediction of the precise structure of a particular elementary entity according to its precise architectural position and to the developmental stage of the plant. Their expression, however, may be modulated by environmental or technical factors. Where constant but different conditions prevail during the whole ontogeny, this may lead to very different final structures as development in each condition may be related to a particular ‘reading’ of the intrinsic sequence of differentiation or in other words a particular ontogenic trajectory (Fig. 17). In fluctuating conditions, in case of a trauma or according to localized and temporally limited perturbations resulting from environmental or technical causes, knowledge of these gradients and the possible a posteriori identification of their local or partial alteration or modification and the induced local perturbation of the sequence of differentiation may also serve as a basis for a very precise diagnosis of a plant's particular history (Fig. 25B).
THE NOTION OF ‘PHYSIOLOGICAL AGE OF A MERISTEM’
The notion of morphogenetic gradients illustrates and takes into account the commonly observed situations where, for a given plant species or even individual, (1) at a given time and/or for a given stage of development, homologous botanical entities with very different features (or ‘states of differentiation’) coexist on the same individual (e.g. short vs. long shoots or reproductive vs. vegetative shoots; for instance see Figs 19, 21, 23 and 27) whereas, by contrast, (2) similar elementary botanical entities with the same morphological features (e.g. short floriferous shoots for instance; e.g. see Figs 15 and 29) may be encountered for very different plant ages or stages of development.
These observations complemented by others led to the definition of ‘physiological age of a meristem’ (Barthélémy et al.,1997a) that may generally be characterized and defined by a particular combination of several morphological, anatomical and/or functional attributes of a given botanical entity derived from this meristem (Fig. 32).
Fig. 32.
Diagrammatic representation of trends in the value of some morphological parameters according to annual shoot rank of successive annual shoots of branches borne on the same ontogenetical age parent shoots of the main stem of a set of 22-year-old dominant to co-dominant Pinus pinaster individuals grown on a same stand in the south-west of France (unpublished data; Coudurier et al., 1995; Heuret et al., 2006), where a is the mean annual shoot length (in percentage of maximum length), b is the percentage of polycyclic shoots, c is the percentage of branched shoots, and d is the percentage of shoots with male cones. For each shoot rank the particular combination of the value of the morphological parameters allows a strict characterization of the physiological age of meristem activity that produced it.
This physiological age of a meristem is thus determined, a posteriori, by the morphological analysis of the elementary botanical entity it produces, and may not be an intrinsic property of this meristem itself but the result of all morphogenetic interactions between plant parts that are related to differentiation processes and result in the expression of morphogenetic gradients.
As previously discussed, the physiological age of a meristem (or elementary botanical entity) depends on its precise location in the plant architecture and on the stage of development of the organism, and its expression may be modulated by environmental factors. The functioning of a meristem, or the elementary botanical entities it produces, can thus be characterized by three different ages (Barthélémy et al.,1997a; Fig. 33):
the calendar or chronological age corresponds to the period (i.e. year, month, week or day of formation for instance) in which the elementary botanical entity has been edified (metamer, growth unit or annual shoot according to specific and relevant growth pattern and plant complexity);
the ontogenetical age refers to the elapsed time after seed germination (the ontogenetic time unit considered may be a year, a day or a growth cycle according to the specific complexity and growth pattern of the species);
the physiological age of a meristem relates to the degree of differentiation of the structures it produced. It may be estimated a posteriori by a non-limitative series of qualitative and quantitative criteria. For example, the short axes of many trees are typical features of ‘physiologically aged’ structures: growth units are short, bear flowers and have a short lifetime. These highly differentiated axes may be considered as ‘physiologically old’ whatever their moment of appearance. By contrast, main axes consisting of vigorous growth units and/or annual shoots may be considered as ‘physiologically young’ products and generally appear only in the young tree.
Fig. 33.
Diagrammatic representation of a theoretical plant whose elementary botanical entities produced by the meristems (for instance annual shoots, represented by rectangles) may encompass four different physiological ages (a–d). The ‘plant’ grows from seed in four steps (possible ontogenetic ages 1–4), each step corresponding to one year of growth (n – 3 to n). It is hypothetically represented as growing in ‘good’ environmental conditions (where the main stem expresses the four possible physiological ages successively and in relation to successive four ontogenetic ages and where branches fully express morphogenetic gradients as shown in Fig. 30: case ‘A’), or in suppressed condition (as for the growth of Araucaria araucana in Fig. 20; case ‘B’). Each entity is characterized by a trinom – where the first element represents ontogenetic age (1–4), the second element represents calendar age (n – 3 to n) and the third element represents physiological age (a–d) – that allows us to understand the structure of each entity in the whole architecture of the plant (represented in A and B 4 years after germination).
The identification for a botanical entity of these three ages is fundamental in order to understand the comprehensive architecture of a plant or even its plasticity, i.e. the effects of the environment on its development and structure. It permits the precise characterization of all elementary levels of organization within the more integrative individual architecture and allows a precise multilevel description of plant architecture and organization.
CONCLUSIONS
Any plant may be recognized by its general form, but this physiognomy is always the result of a very precise structure that underlies the existence of a strong inherent organizational pattern in plant architecture construction.
Architectural studies that have been carried out for some 30 years have led to the definition of several concepts and notions that provide powerful tools for studying plant form and intrinsic morphogenetic and ontogenetic rules. Combined with architectural concepts, the recognition and definition of the notions of ‘levels of organization’, ‘repetition phenomena’, ‘sequence of differentiation’, ‘morphogenetic gradients’ and ‘physiological age of meristems’ provide a general and robust framework for the understanding and interpretation of plant morphogenesis. This framework has applications in agronomy, botany, forestry and plant or landscape management, owing to its contribution in the study of: the diagnosis of the physiological status of a plant (Barthélémy et al., 1992; Millet et al., 1998a, b, 1999; Génoyer et al., 1999; Nicolini et al., 2003), the effect of environmental factors and architectural plasticity (Grosfeld et al., 1999; Nicolini et al., 2000; Guérard et al., 2001; Stecconi, 2006), technical practices (Caraglio et al., 2000; Sabatier et al., 2000; Leroy and Caraglio, 2003), architectural and genetic variation, plant breeding and selection (Barthélémy et al.,1999; Corradini et al., 2002; Sabatier et al., 2003a) and landscape management (Auclair et al., 2001), among other issues.
Based on this botanical background and with the development of high-power computers, a concomitant and complementary computational and numerical approach to the modelling and computer visualization of plant architecture has gained importance in recent decades. Mathematical models of growth, branching and architecture have been developed on the basis of qualitative botanical knowledge, e.g. models and quantitative data on the demography, size and positions of plant components (Godin et al., 1997, 1999; Godin and Caraglio, 1998; Ferraro and Godin, 2000, 2003; Godin, 2000; Guédon et al., 2001; Costes and Guédon, 2002; Heuret et al., 2002, 2003; Costes et al., 2003; Durand et al., 2005; Ferraro et al., 2005; Godin et al., 2005). This new multidisciplinary approach offers new possibilities to understand or quantify the genetic basis or regulation of plant architecture (Barbier de Reuille et al., 2005, 2006; McSteen and Leyser, 2005; Mündermann et al., 2005; Wang and Li, 2005) and will probably renew various aspects of developmental biology studies (Turnbull, 2005).
Results of these mathematical models are used to simulate plant development and architecture (de Reffye and Houllier, 1997). In these simulations much emphasis has been given to the changes in the physiological age of plant components and in the three-dimensional structure during plant development (Bouchon et al., 1997; Yan et al., 2003; Zhao et al., 2003). This methodology may be used for any kind of plant (Barczi et al., 1997) and has also been successfully used to simulate root systems (Jourdan and Rey,1997a, b, c; Danjon et al.,1999a, b, 2005; Dupuy et al., 2005a, b, c, 2006). It can also be used to evaluate developmental changes such as growth responses to controlled environments, aerial biomass, volume prospected in soil by roots, radiant energy transfer, retrodiffusion of the canopy for calibrating remote-sensing techniques, and the mechanical status of a tree (de Reffye and Houllier, 1997; Castel et al., 2002; Hu and Jaeger, 2003; Soler et al., 2003; Picard et al., 2004; Chenu et al., 2005; Sellier et al., 2006). Finally, the models may be linked to eco-physiological models, leading to structural functional models (Godin and Sinoquet, 2005) or process-based architectural models, i.e. morphogenetic models of tree architecture directly driven by eco-physiological processes, with possible applications in environmental and genetic control of morphogenesis in crops and phenotypic plasticity modelling and simulation (de Reffye et al., 1997; Yan et al., 2004; Dingkuhn et al., 2005; Guo et al., 2006).
Because of its economic importance for agronomic production, plant reproduction or vegetative propagation, acquisition of reproductive capacities during ontogeny has been extensively studied (Goebel, 1900; Passecker, 1944, 1958; Chouard, 1950; Rémy, 1951; Stokes and Verkerk, 1951; Doorenbos, 1954; Robbins, 1957, 1961; Wareing, 1959, 1961; Zeevaart, 1962; Stoutmeyer, 1964; Visser, 1964; Doorenbos, 1965; Picard, 1965; Visser and De Vries, 1970; Zimmerman, 1972; Borchert, 1976; Schwabé, 1976; Greenwood, 1995). With regard to this potential, four main phases of plant development have been proposed: the embryonic phase, in which the shoot and root meristem are established; the juvenile phase, in which there is no sexual reproduction; the adult vegetative phase, in which reproductive capacity is established; and finally the adult reproductive phase (Greenwood, 1995; Poethig, 2003). The transition between these phases is currently referred to as phase change (Jones, 1999). In addition to reproductive function, ontogenetic changes in several other parameters and morphological, anatomical and/or functional features has long been described in plants (Koch, 1873; Carrière, 1880; Hochtetter, 1880; Beissner, 1888; Cockayne, 1912; Frost, 1938; Webber and Batchelor, 1948; Ashby, 1949; Doorenbos, 1954; Schaffalitzky de Muckadell, 1954, 1959; Brink, 1962; Trippi, 1963; Wardle, 1963; Greenwood and Atkinson, 1977; Poethig, 1990, 2003; Jones, 1999; Kaplan, 2001) and these changes may be simultaneous with the appearance of reproductive capacity during development. At the whole individual level, plant development may thus be viewed as a succession of phases, each characterized by a set of several features or parameters. Under given conditions, the duration of these phases may be more or less fixed for a species (Wareing, 1959; Zimmerman, 1972; Hackett, 1985) and it is thus possible to identify a mean specific age in which a plant will be able or unable to express a particular feature. According to plant species, this ‘chronological’ (Ritterbusch, 1990) or ‘calendar’ (Gatsuk et al., 1980) age may be expressed in days or years after germination and it is known that it may be strongly modified by environmental factors (Doorenbos, 1954; Zimmerman, 1972; Gatsuk et al., 1980). At a given moment, a plant may thus be characterized not only by its ‘chronological’ or ‘calendar’ age but also by a set of biological criteria that indicates its ‘stage’ of development variously referred to as ‘biological age’ (Levin, 1966; Roloff, 1989; Gleißner, 1998), ‘physiological age’ (Robbins, 1957; Schaffalitzky de Muckadell, 1959; Grubb, 1977), ‘ontogenic age’ (Passecker, 1977) or ‘age state’ (Uranov, 1975; Gatsuk et al., 1980). The ‘physiological age’ of the individual may merge with the metamer level and may be defined or even quantified by the features or parameters of the successive constitutive metamers or leaves in small, herbaceous monocaulous continuously growing plants, as variously demonstrated (Ashby, 1949; Baillaud and Courtot, 1955; Poethig, 2003; Fig. 31A), because the whole plant structure corresponds to an elementary botanical entity. In more complex plants with several nested levels of organization we have demonstrated that metamer level is not sufficient to take into account the complete heterogeneity of plant parts either at a given stage or during the whole ontogeny. In the latter cases, the idea of a ‘physiological age’ better corresponds to the growth unit and/or sympodial unit and/or annual shoot levels (Fig. 31B). The general description of plant parts heterogeneity thus implies the use of this notion at the meristem level and the pertinent elementary levels it produces in order to take into account plant architecture diversity. Coupled with the description of ‘morphogenetic gradients’, the notion of physiological age of a meristem (as applied to the pertinent level of organization) may allow a generic description of the multiple and diverse expressions of ‘ontogenetic contingency’ (Diggle, 1994, 1997) observed in the plant kingdom (Kaplan, 2001). Furthermore, as these notions allow a multilevel description of the influence of the environment on several features and their expression along ontogeny and according to architectural position, they may provide a powerful tool to separate ontogenetic variations from environmental plasticity or to separate traits or ontogenetic trajectories plasticity (Wu and Stettler, 1998; Huber et al., 1999; Wu and Hinckley, 2001; Alpert and Simms, 2002; Diggle, 2002; Novoplansky, 2002; Wright et al., 2002; Sachs, 2004; de Kroon et al., 2005; Wolfe and Mazer, 2005).
Despite the frequently stressed importance of considering plant morphology and/or architecture to understand their phylogeny, very few studies actually describe the link between phylogeny and architecture (Sanoja, 1992; Johnson, 2003; Hallé, 2004) and here also we probably require more data and a multilevel consideration of plant structure.
Finally, the dynamic, multilevel and comprehensive approach of plant form and ontogeny have proved to be fruitful but the number of questions raised shows that some of the answers will also, we hope, come from a multidisciplinary approach and by the combination of different approaches to the same plant object.
ACKNOWLEDGEMENTS
We thank Brigitte Meyer Berthaud, Daniel Auclair, Patrick Heuret, Javier Grosfeld, Yann Guédon and Javier Puntieri for stimulating discussions and for critical and valuable comments on the manuscript. We are also very grateful to Sylvie Sabatier for providing some of the cedar photographs for Fig. 31.
LITERATURE CITED
- Alpert P, Simms EL. The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evolutionary Ecology. 2002;16:285–297. [Google Scholar]
- Altman A, Goren R. Development of Citrus bud explants in culture. Journal of the American Society for Horticultural Science. 1978;103:120–123. [Google Scholar]
- Alvim P de T. Tree growth periodicity in tropical climates. In: Zimmermann MH, editor. Formation of wood in forest trees. New York: Academic Press; 1964. pp. 479–495. [Google Scholar]
- Ashby E. De la forme des feuilles et de leur rapport avec l'âge physiologique des plantes. Endeavour. 1949;T 8(29):18–25. [Google Scholar]
- Atger C, Edelin C. Premières données sur l'architecture comparée des systèmes racinaires et caulinaires. Canadian Journal of Botany. 1994a;72:963–975. [Google Scholar]
- Atger C, Edelin C. Stratégies d'occupation du milieu souterrain par les systèmes racinaires des arbres. Revue d'Ecologie (Terre Vie) 1994b;49:343–356. [Google Scholar]
- Auclair D, Barczi J-F, Borne F, Etienne M. Assessing the visual impact of agroforestry management with landscape design software. Landscape Research. 2001;26:397–406. [Google Scholar]
- Aydelotte AR, Diggle PK. Analysis of developmental preformation in the alpine herb Caltha leptosepala (Ranunculaceae) American Journal of Botany. 1997;84:1646–1657. [PubMed] [Google Scholar]
- Baillaud L. Structures répétitives spatiales et spatio-temporelles des plantes. Phytomorphology. 1999;49:377–404. [Google Scholar]
- Baillaud L, Courtot Y. Temps et rythmes chez les végétaux. Annales Françaises de Chronométrie. 1955;9:87–102. [Google Scholar]
- Barbier de Reuille P, Bohn-Courseau I, Godin C, Traas J. A protocol to analyse cellular dynamics during plant development. Plant Journal. 2005;44:1045–1053. doi: 10.1111/j.1365-313X.2005.02576.x. [DOI] [PubMed] [Google Scholar]
- Barbier de Reuille P, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J. Computer simulations reveal novel properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proceedings of the National Academy of Science. 2006;103:1627–1632. doi: 10.1073/pnas.0510130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczi JF, de Reffye P, Caraglio Y. Essai sur l'identification et la mise en oeuvre des paramètres nécessaires à la simulation d'une architecture végétale: le logiciel AMAPsim. In: Bouchon J, de Reffye P, Barthélémy D, editors. Modélisation et Simulation de l'Architecture Des Végétaux. Paris: INRA Editions; 1997. pp. 205–254. Science Update. [Google Scholar]
- Barlow PW. Meristems, metamers and modules and the development of shoot and root systems. Botanical Journal of the Linnean Society. 1989;100:255–279. [Google Scholar]
- Barnola P, Crabbé J. La basitonie chez les végétaux ligneux. Déterminisme et variabilité d'expression. Comptes rendus du 2ème Colloque International sur l'Arbre, Montpellier, 10–15 Septembre 1990, Naturalia Monspeliensia, No. Hors Série. 1991:381–396. [Google Scholar]
- Barthélémy D. Harper JL, Rosen BR, White J, editors. Establishment of modular growth in a tropical tree: Isertia coccinea Vahl. (Rubiaceae) The growth and form of modular organisms. Philosophical Transactions of the Royal Society of London, sér. 1986;313:89–94. [Google Scholar]
- Barthélémy D. Architecture et sexualité chez quelques plantes tropicales: le concept de floraison automatique. University Montpellier 2; 1988. PhD thesis. [Google Scholar]
- Barthélémy D. Levels of organization and repetition phenomena in seeds plants. Acta Biotheoretica. 1991;39:309–323. [Google Scholar]
- Barthélémy D, Edelin C, Hallé F. Architectural concepts for tropical trees. In: Holm-Nielsen LB, Balslev H, editors. Tropical forests: botanical dynamics, speciation and diversity. London: Academic Press; 1989. pp. 89–100. [Google Scholar]
- Barthélémy D, Edelin C, Hallé F. Canopy architecture. In: Raghavendra AS, editor. Physiology of trees. Chichester: John Wiley and Sons; 1991. pp. 1–20. [Google Scholar]
- Barthélémy D, Caraglio Y, Drénou C, Figureau C. Architecture et sénescence des arbres. Forêt Entreprise. 1992;83:15–35. [Google Scholar]
- Barthélémy D, Sabatier S, Pascal O. Le développement architectural du Noyer commun Juglans regia L. (Juglandaceae) Forêt Entreprise. 1995;103:61–68. [Google Scholar]
- Barthélémy D, Caraglio Y, Costes E. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon J, de Reffye P, Barthélémy D, editors. Modélisation et simulation de l'architecture des végétaux. Paris: Editions INRA; 1997a. pp. 89–136. Sciences Update. [Google Scholar]
- Barthélémy D, Sabatier S, Pascal O. Le développement architectural du Noyer noir Juglans nigra L. (Juglandaceae) Forêt Entreprise. 1997b;115:40–47. [Google Scholar]
- Barthélémy D, Grosfeld J, Bouroulet-Hallard F, Ducatillion C. Biology, growth and development. In: Teissier du Cros E, editor. Cypress: a practical handbook. Florence: Studio Leonardo; 1999. pp. 26–33. [Google Scholar]
- Beissner L. Über Jugendformen von Pflanzen. speciell Coniferen. Berichte der Deutschen Botanischen Gesellschaft. 1888;6:83–86. [Google Scholar]
- Bell A. Plant form an illustrated guide to flowering plant morphology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Blanc P. Aspects de la ramification chez des aracées tropicales. University Paris VI; 1978. PhD thesis. [Google Scholar]
- Blanc P. Etre plante à l'ombre des forêts tropicales. Paris: editions Nathan; 2002. [Google Scholar]
- Boke N. Dichotomous branching in Mammillaria (Cactaceae) American Journal of Botany. 1976;63:1380–1384. [Google Scholar]
- Bond TET. Studies in the vegetative growth and anatomy of the Tea plant (Camellia thea Link.) with special reference to the phloem. I. The flush shoot. Annals of Botany. 1942;9:183–216. [Google Scholar]
- Borchert R. Unusual shoot growth pattern in a tropical tree, Oreopanax (Araliaceae) American Journal of Botany. 1969;56:1033–1041. [Google Scholar]
- Borchert R. The concept of juvenility in woody plants. Acta Horticulturae. 1976;56:21–33. [Google Scholar]
- Bouchon J, de Reffye P, Barthélémy D. Modélisation et simulation de l'architecture des végétaux. Inra Editeur; 1997. Sciences Update. [Google Scholar]
- Briggs B, Johnson L. Evolution in the Myrtaceae – evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales. 1979;192:157–272. [Google Scholar]
- Brink RA. Phase change in higher plants and somatic cell heredity. Quarterly Review of Biology. 1962;37:1–22. [Google Scholar]
- Bugnon P, Bugnon F. Feuilles juvéniles et pousses multinodales chez le Pin maritime. Bulletin de la Société d'Histoire Naturelle de Toulouse. 1951;86:18–23. [Google Scholar]
- Caballé G. Le port autoportant des lianes tropicales: une synthèse des stratégies de croissance. Canadian Journal of Botany. 1998;76:1703–1716. [Google Scholar]
- Cannell MGR, Thompson S, Lines R. An analysis of inherent differences in shoot growth within some north temperate conifers. In: Cannell MGR, Last FT, editors. Tree physiology and yield improvement. New York: Academic Press; 1976. pp. 223–243. [Google Scholar]
- Caraglio Y. Le développement architectural. In: Le Merisier B, Boulet-Gercourt , editors. Les guides du sylviculteur. Paris: IDF; 1997. pp. 19–23. [Google Scholar]
- Caraglio Y, Barthélémy D. Revue critique des termes relatifs à la croissance et à la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye Ph, Barthélémy D, editors. Modélisation et simulation de l'Architecture des végétaux. Editions Inra; 1997. pp. 11–88. Sciences Update. [Google Scholar]
- Caraglio Y, Edelin C. Architecture et dynamique de la croissance du platane, Platanus hybrida Brot. (Platanaceae) {syn. Platanus acerifolia (Aiton) Willd.} Bulletin de la Société Botanique de France, Lettres Botaniques. 1990;137:279–291. [Google Scholar]
- Caraglio Y, Becquey J, Gallois F, Vidal C. Réaction de jeunes merisiers à deux modalités de taille. Forêt Entreprise. 2000;132:25–29. [Google Scholar]
- Carrière EA. Revue du genre. Paris: Retinospora; 1880. [Google Scholar]
- Castel T, Guerra F, Caraglio Y, Houllier F. Retrieval biomass of a large Venezuelan pine plantation using JERS-1 SAR data. Analysis of forest structure impact on radar signature. Remote Sensing of Environment. 2002;79:30–41. [Google Scholar]
- de Castro e Santos A. Essai de classification des arbres tropicaux selon leur capacité de réitération. Biotropica. 1980;12:187–194. [Google Scholar]
- de Castro e Santos A. L'appareil végétatif des monocotylédones. Un essai de synthèse. University Montpellier 2; 1981. PhD thesis. [Google Scholar]
- Champagnat P. Les principes généraux de la ramification des végétaux ligneux. Revue Horticole. 1947;2143:335–341. [Google Scholar]
- Champagnat P. Dominance apicale. Tropisme. Epinastie. In: Rühland W, editor. Encyclopedia of plant physiology. Vol. 14. Berlin: Springer Verlag; 1961. pp. 872–908. [Google Scholar]
- Champagnat P. Physiologie de la croissance et de l'inhibition des bourgeons: dominance apicale et phénomènes analogues. In: Rühland W, editor. Encyclopedia of plant physiology. 1. Vol. 15. Berlin: Springer-Verlag; 1965. pp. 1106–1171. [Google Scholar]
- Champagnat P, Barnola P, Lavarenne S. Quelques modalités de la croissance rythmique endogène des tiges chez les végétaux ligneux. Comptes rendus du Colloque International sur l'Arbre, Montpellier, 9–14 Septembre 1985, Naturalia Monspeliensia, No. Hors Série. 1986:279–302. [Google Scholar]
- Chenu K, Franck N, Dauzat J, Barczi JF, Rey H, Lecoeur J. Integrated response for rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Functional Plant Biology. 2005;32:1123–1134. doi: 10.1071/FP05091. [DOI] [PubMed] [Google Scholar]
- Chouard P. Pourquoi fleurissent les plantes. Conférence faite au Palais de la Découverte; 29 October 1949; University of Paris; 1950. [Google Scholar]
- Claßen-Bockhoff R. Inflorescences in Bruniaceae. With general comments on inflorescences in woody plants. Opera Botanica Belgica. 2000;12:5–310. [Google Scholar]
- Claßen-Bockhoff R. Plant morphology: the historic concepts of Wilhelm Troll, Walter Zimmermann and Agnes Arber. Annals of Botany. 2001;88:1153–1172. [Google Scholar]
- Claßen-Bockhoff R. Aspekte, Typifikationsverfahren und Asussagen der Pflanzenmorphologie. In: Harlan V, editor. Wert und Grenzen des Typus in der Botanischen Morphologie. Galunder: Nümbrecht; 2005. pp. 31–52. [Google Scholar]
- Cockayne L. Observations concerning evolution, derived from ecological studies in New Zealand. Transactions and Proceedings of the New Zealand Institute. 1912;44:1–50. [Google Scholar]
- Comte L. Rythmes de croissance et structures spatiales périodiques d'arbres tropicaux. Exemple de cinq espèces de forêt équatoriale. University Montpellier 2; 1993. PhD thesis. [Google Scholar]
- Corradini P, Edelin C, Bruneau A, Bouchard A. Architectural and genotypic variation in the clonal shrub Taxus canadensis as determined from random amplified polymorphic DNA and amplified fragment length polymorphism. Canadian Journal of Botany. 2002;80:205–219. [Google Scholar]
- Costes E. Architecture aérienne de l'Abricotier en développement libre. Acta Botanica Gallica. 1993;140:249–261. [Google Scholar]
- Costes E, Guédon Y. Modelling branching patterns on one-year-old trunks of apple cultivars. Annals of Botany. 2002;89:513–523. doi: 10.1093/aob/mcf078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes E, Sinoquet H, Kelner JJ, Godin C. Exploring within tree architectural development of two apple tree cultivars over 6 years. Annals of Botany. 2003;91:91–104. doi: 10.1093/aob/mcg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes E, Lauri PE, Regnard JL. Analyzing fruit tree architecture: implications for tree management and fruit production. In: Janick J, editor. Horticultural reviews. Vol. 32. New York: John Wiley and Sons; 2006. pp. 1–61. [Google Scholar]
- Coudurier T. Sur la place des lianes dans la forêt guyanaise. Une approche qui utilise l'architecture végétale. University Montpellier 2; 1992. PhD thesis. [Google Scholar]
- Coudurier T, Barthélémy D, Chanson B, Courdier F, Loup C. Modélisation de l'architecture du Pin maritime Pinus pinaster Ait. (Pinaceae): premiers résultats. In: Bouchon J, editor. Architecture des Arbres Fruitiers et Forestiers, Montpellier (FRA), 23–25 Novembre 1993. INRA editions; 1995. pp. 305–321. Les Colloques no. 74. [Google Scholar]
- Courtot Y, Baillaud L. Sur la ramification d'un Cupressus. Annales des Sciences de l'Université de Besançon. Botanique. 1961;17:69–72. [Google Scholar]
- Cremers G. Architecture de quelques lianes d'Afrique tropicale. Candollea. 1973;28:249–280. [Google Scholar]
- Cremers G, Edelin C. Study on aerial architecture of some tropical plants with basitone branching – for a revision of the Tomlinson model. Canadian Journal of Botany. 1995;73:1490–1503. [Google Scholar]
- Critchfield WB. Leaf dimorphism in Populus trichocarpa. American Journal of Botany. 1960;47:699–711. [Google Scholar]
- Damascos MA, Prado CH, Ronquim CC. Bud composition, branching patterns and leaf phenology in cerrado woody species. Annals of Botany. 2005;96:1075–1084. doi: 10.1093/aob/mci258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjon F, Bert D, Godin C, Trichet P. Structural root architecture of 5-year-old Pinus pinaster measured by 3D digitising and analysed with AMAPmod. Plant and Soil. 1999a;217:49–63. [Google Scholar]
- Danjon F, Sinoquet Y, Godin C, Colin F, Drexhage M. Characterisation of structural tree root architecture using 3D digitising and AMAPmod software. Plant and Soil. 1999b;211:241–258. [Google Scholar]
- Danjon F, Fourcaud T, Bert D. Root architecture and windfirmness of mature Pinus pinaster Aït. New Phytologist. 2005;168:387–400. doi: 10.1111/j.1469-8137.2005.01497.x. [DOI] [PubMed] [Google Scholar]
- Daviero V, Meyer-Berthaud B, Lecoustre R. A morphometric approach to the architecture and ontogeny of the extant sphenopsid Equisetum telmateia Ehrh. International Journal of Plant Science. 1996;157:567–581. [Google Scholar]
- Diggle P. The expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenetic contingency. American Journal of Botany. 1994;81:1354–1365. [Google Scholar]
- Diggle PK. Extreme preformation in Alpine Polygonum viviparum: an architectural and developmental analysis. American Journal of Botany. 1997;84:154–169. [PubMed] [Google Scholar]
- Diggle PK. Heteroblasty and the evolution of flowering phenologies. International Journal of Plant Sciences. 1999;160:S123–S134. doi: 10.1086/314217. [DOI] [PubMed] [Google Scholar]
- Diggle PK. A developmental morphologist's perspective on plasticity. Evolutionary Ecology. 2002;16:267–283. [Google Scholar]
- Diggle PK. Architectural effects on floral form and function: a review. In: Stuessy T, Hörandl E, Mayer V, editors. Deep morphology: toward a renaissance of morphology in plant systematics. Königstein: Koeltz; 2003. pp. 000–000. [Google Scholar]
- Dingkuhn M, Luquet D, Quilot B, de Reffye Ph. Environmental and genetic control of morphogenesis in crops: towards models simulating phenotypic plasticity. Australian Journal of Agricultural Research. 2005;56:1–14. [Google Scholar]
- Doorenbos J. Rejuvenation of Hedera helix in grafts combinations. Proceedings Koninklijke Nederlandse Akademie van Wetenschappen. Sér C. 1954;57:99–102. [Google Scholar]
- Doorenbos J. Juvenile and adult phases in woody plants. In: Rühland W, editor. Handbuch der Pflanzenphysiologie. 1. Vol. 15. Berlin: Springer-Verlag; 1965. pp. 1222–1235. [Google Scholar]
- Drénou C. Approche architecturale de la sénescence des arbres. Le cas de quelques angiospermes tempérées et tropicales. PhD thesis: University Montpellier 2; 1994. [Google Scholar]
- Dupuy L, Fourcaud T, Stokes A. A numerical investigation into factors affecting the anchorage of roots in tension. European Journal of Soil Science. 2005a;56:319–327. [Google Scholar]
- Dupuy L, Fourcaud T, Stokes A. A numerical investigation into the influence of soil type and root architecture on tree anchorage. Plant and Soil. 2005b;278:119–134. [Google Scholar]
- Dupuy L, Fourcaud T, Stokes A, Danjon F. A density based approach for the modelling of root architecture: application to Maritime pine (Pinus pinaster Ait) root systems. Journal of Theoretical Biology. 2005c;236:323–334. doi: 10.1016/j.jtbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Durand M. Pondy Papers in Ecology. Pondichery: IFP; 1997. Architecture and growth strategy of two evergreen species of the Western Ghats (South Inda) Knema attenuata (Myristicaceae) and Vateria indica (Dipterocarpaceae) [Google Scholar]
- Durand JB, Guédon Y, Caraglio Y, Costes E. Analysis of the plant architecture via tree-structured statistical models: the hidden Markov tree models. New Phytologist. 2005;166:813–825. doi: 10.1111/j.1469-8137.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- Edelin C. Images de l'architecture des conifères. University Montpellier 2; 1977. PhD thesis. [Google Scholar]
- Edelin C. Quelques aspects de l'architecture végétative des Conifères. Bulletin de la Société Botanique de France, Lettres Botaniques. 1981;128:177–188. [Google Scholar]
- Edelin C. L'architecture monopodiale: l'exemple de quelques arbres d'Asie Tropicale. University Montpellier 2; 1984. Thesis Doct. Etat. [Google Scholar]
- Edelin C. Aspects morphologiques de la croissance rythmique chez les arbres tropicaux. Compte Rendu du Séminaire du groupe d'Etude de l'Arbre: le rythme de croissance, base de l'organisation temporelle de l'arbre. 1993:13–23. Angers, 25–26 Mars 1993. [Google Scholar]
- Edelin C, Hallé F. Architecture et évolution chez deux genres d'arbres tropicaux: Diospyros et Cordia. Actes du 110e Congrès National des Sociétés Savantes. Montpellier Sciences. 1985;II:255–265. [Google Scholar]
- Eggert DA. The ontogeny of carboniferous arborescent Lycopsida. Paleontographica (Part B) 1961;108:43–92. [Google Scholar]
- Eggert DA. The ontogeny of Carboniferous arborescent Sphenopsida. Palaeontographica, (Part B) 1962;110:99–127. [Google Scholar]
- El-Morsy AA. Croissance rythmique et micropropagation in vitro chez le bigaradier (Citrus aurantium) et le mandarinier (Citrus deliciosa) Besançon: University Franche-Comté; 1991. PhD thesis. [Google Scholar]
- Emberger L. Les Végétaux Vasculaires. Tome II du Traité de Botanique Systématique, Chadefaud M. & Emberger L. Paris: Masson; 1960. [Google Scholar]
- Espagnac H, Neuville P. Feuilles et aisselles doubles chez Olea europaea L. Bulletin de la Société Botanique de France. 1969;116:57–70. [Google Scholar]
- Fahn A. Plant anatomy. Oxford: Pergamon Press; 1967. [Google Scholar]
- Ferraro P, Godin C. A distance measure between plant architectures. Annals of Forest Science. 2000;57:445–461. [Google Scholar]
- Ferraro P, Godin C. An edit distance between quotiented trees. Algorithmica. 2003;36:1–39. [Google Scholar]
- Ferraro P, Godin C, Prusinkiewicz P. Toward a quantification of self-similarity in plants. Fractals. 2005;13:91–109. [Google Scholar]
- Fink S. The occurrence of adventitious and preventitious buds within the bark of some temperate and tropical trees. American Journal of Botany. 1983;70:532–542. [Google Scholar]
- Fisher JB. Development of dichotomous branching and axillary buds in Strelitzia (Monocotyledonae) Canadian Journal of Botany. 1976;54:578–592. [Google Scholar]
- Fontaine F, Chaar H, Colin F, Clément Ch, Burrus M, Druelle J-L. Preformation and neoformation of growth units on 3-year-old seedlings of Quercus petraea. Canadian Journal of Botany. 1999;77:1623–1631. [Google Scholar]
- de Foresta H. Le spectre architectural: application à l'étude des relations entre architecture des arbres et écologie forestière. Bulletin du Muséum National d'Histoire Naturelle. 1983;5:295–302. [Google Scholar]
- Franck AB. Über die Einwirkung der Gravitation auf das Wachsthum einiger Pflanzentheile. Botanik Zeitung. 1868;26:873–882. [Google Scholar]
- Friedman J, Harder LD. Inflorescence architecture and wind pollination in six grass species. Functional Ecology. 2004;18:851–860. [Google Scholar]
- Friedman J, Harder LD. Functional associations of floret and inflorescence traits among grass species. American Journal of Botany. 2005;92:1862–1870. doi: 10.3732/ajb.92.11.1862. [DOI] [PubMed] [Google Scholar]
- Frost HB. Nucellar embryony and juvenile characters in clonal varieties of Citrus. Journal of Heredity. 1938;29:423–432. [Google Scholar]
- Garrison R, Wetmore RH. Studies in shoot-tip abortion: Syringa vulgaris. American Journal of Botany. 1961;48:789–795. [Google Scholar]
- Gatin C-L. Dictionnaire de fotanique. Paris: Lechevalier; 1924. [Google Scholar]
- Gatsuk LE, Smirnova OV, Vorontzova LI, Zaugolnova LB, Zhukova LA. Age states of plants of various growth forms: a review. Journal of Ecology. 1980;68:675–696. [Google Scholar]
- Gay H. The architecture of a dimorphic clonal fern, Lomagramma guianensis (Aublet) Ching (Dryopteridaceae) Botanical Journal of the Linnean Society. 1993;111:343–358. [Google Scholar]
- Genoyer P, Atger C, Edelin C, Caraglio Y. Some architectural markers of plane tree development (Platanus x acerifolia (Aiton) Willd.): contribution to the establishment of an ontogenic based diagnosis. Acta Horticulturae. 1999;496:209–220. [Google Scholar]
- Gifford EM. Concept of apical cells in Bryophytes and Pteridophytes. Annual Review of Plant Physiology. 1983;34:419–440. [Google Scholar]
- Gill AM. The formation, growth and fate of buds of Fraxinus americana L. in Central Massachusetts. Harvard Forest Paper. 1971;20:1–16. [Google Scholar]
- Gill AM, Tomlinson PB. Studies on the growth of Red Mangrove (Rhizophora mangle L.). 3. Phenology of the shoot. Biotropica. 1971;3:109–124. [Google Scholar]
- Givnish TJ. Leaf and canopy adaptations in tropical trees. In: Medina E, Mooney HA, Vazquez-Yanes C, editors. Physiological ecology of plants of the wet tropics. The Hague: Dr W. Junk; 1984. pp. 51–84. [Google Scholar]
- Gleißner P. Das Verzweigungsmuster ausgewählter Laubbaumarten und seine Veränderung durch nicht-pathogene Schädigungen. Palmarum Hortus Francofurtensis. 1998;6:3–132. [Google Scholar]
- Godin C. Representing and encoding plant architecture: a review. Annals of Forest Science. 2000;57:413–438. [Google Scholar]
- Godin C, Caraglio Y. A multiscale model of plant topological structures. Journal of Theoretical Biology. 1998;191:1–46. doi: 10.1006/jtbi.1997.0561. [DOI] [PubMed] [Google Scholar]
- Godin C, Sinoquet H. Functional–structural plant modelling. New Phytologist. 2005;166:705–708. doi: 10.1111/j.1469-8137.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- Godin C, Costes E, Caraglio Y. Exploring plant topology structure with the AMAPmod software: an outline. Silva Fennica. 1997;31:355–366. [Google Scholar]
- Godin C, Costes E, Sinoquet H. A method for describing plant architecture which integrates topology and geometry. Annals of Botany. 1999;84:343–357. [Google Scholar]
- Godin C, Costes E, Sinoquet H. Plant architecture modelling – virtual plants, dynamic and complex systems. In: Turnbull C, editor. Plant architecture and its manipulation. Annual plant reviews. Vol. 17. Oxford: Blackwell Publishing; 2005. pp. 238–287. [Google Scholar]
- Goebel K. Organography of plants. Part I. General organography (translated by IB Balfour) Oxford: The Clarendon Press; 1900. [Google Scholar]
- von Goethe JW. Lamétamorphose des plantes. Traduction de Bideau H. 1975. Paris: Editions Triades; 1790. [Google Scholar]
- Greathouse DC, Laetsch WM, Phinney BO. The shoot growth rhythm of a tropical tree. Theobroma cacao. American Journal of Botany. 1971;58:281–299. [Google Scholar]
- Greenwood MS. Rejuvenation of forest trees. Plant Growth Regulation. 1987;6:1–12. [Google Scholar]
- Greenwood MS. Juvenility and maturation in conifers: current concepts. Tree Physiology. 1995;15:433–438. doi: 10.1093/treephys/15.7-8.433. [DOI] [PubMed] [Google Scholar]
- Greenwood RM, Atkinson IAE. Evolution of divaricating plants in New Zealand, in relation to moa browsing. Proceedings of the New Zealand Ecological Society. 1977;24:21–33. [Google Scholar]
- Grosfeld J. Análisis de la variabilidad morfológica y arquitectural de Austrocedrus chilensis (D. Don) Florin y Boutleje, Fitzroya cupressoides (Mol.) Johnson, Pilgerodendron uviferum (D. Don) y Cupressus sempervirens. L (Cupressaceae) Bariloche: University Nacional del Comahue; 2002. PhD thesis. [Google Scholar]
- Grosfeld J, Barthélémy D. Primary growth and morphological markers of intra-annual growth limits in Cupressaceae from Patagonia. Botanical Journal of the Linnean Society. 2004;146:285–293. [Google Scholar]
- Grosfeld J, Barthélémy D, Brion C. Architectural variations of Araucaria araucana (Molina) K. Koch (Araucariaceae) in its natural habitat. In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. Kew: Royal Botanic Gardens; 1999. pp. 109–122. [Google Scholar]
- Grubb PJ. Maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Review. 1977;52:107–145. [Google Scholar]
- Guédès M. Modalités du développement sympodial chez quelques végétaux ligneux tempérés. Comptes Rendus de l'Académie des Sciences, Paris. 1975;281:527–530. [Google Scholar]
- Guédès M. Endorhythmic development in Choisya ternata Kunth (Rutaceae), with a further elucidation of lammas shoots, as well as sylleptic and proleptic shoots. Botanical Journal of the Linnean Society. 1980;7:109–155. [Google Scholar]
- Guédon Y, Barthélémy D, Caraglio Y, Costes E. Pattern analysis in branching and axillary flowering sequences. Journal of Theoretical Biology. 2001;212:481–520. doi: 10.1006/jtbi.2001.2392. [DOI] [PubMed] [Google Scholar]
- Guédon Y, Heuret P, Costes E. Comparison methods for branching and axillary flowering sequences. Journal of Theoretical Biology. 2003;225:301–325. doi: 10.1016/s0022-5193(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Guédon Y, Puntieri J, Sabatier S, Barthélémy D. Relative extents of preformation and neoformation in tree shoots: analysis by a deconvolution method. Annals of Botany. 2006;98:835–844. doi: 10.1093/aob/mcl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérard N, Barthélémy D, Cabanettes A, Courdier F, Trichet P, Willm J. Influence de la compétition herbacée sur la croissance et le développement de jeunes Chênes rouges d'Amérique (Quercus rubra L., Fagaceae) en plantation. Annals of Forest Sciences. 2001;58:395–410. [Google Scholar]
- Guinochet M. Notions fondamentales de botanique générale. Paris: Masson; 1965. [Google Scholar]
- Guo Y, Ma Y, Zhan Z, Li B, Dingkuhn M, Luquet D, de Reffye P. Parameter optimization and field validation of the functional–structural model GREENLAB for maize. Annals of Botany. 2006;97:217–230. doi: 10.1093/aob/mcj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TWP. Juvenility, maturation, and rejuvenation in woody plants. Horticultural Reviews. 1985;7:109–155. [Google Scholar]
- Hallé F. Architectural variation at specific level of tropical trees. In: Tomlinson PB, Zimmermann MH, editors. Tropical trees as living systems. Cambridge: Cambridge University Press; 1978. pp. 209–221. [Google Scholar]
- Hallé F. Harper JL, Rosen BR, White J, editors. Modular growth in seed plants. The growth and form of modular organisms. Philosophical Transactions of the Royal Society of London, Series B. 1986;313:77–87. [Google Scholar]
- Hallé F. Eloge de la plante. Pour une nouvelle biologie. Paris: Editions du Seuil; 2004. [Google Scholar]
- Hallé F, Martin R. Etude de la croissance rythmique chez Hevea brasiliensis Müll. Arg (Euphorbiaceae – Crotonoïdées) Adansonia, Série 2. 1968;8:475–503. [Google Scholar]
- Hallé F, Ng FSP. Crown construction in mature Dipterocarp trees. Malaysian Forester. 1981;44:222–223. [Google Scholar]
- Hallé F, Oldeman RAA. Essai sur l'architecture et la dynamique de croissance des arbres tropicaux. Paris: Masson; 1970. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests. Berlin: Springer-Verlag; 1978. [Google Scholar]
- Harper JL. Population biology and evolution of clonal organisms. New Haven, CT: Yale University Press; 1985. Modules, branches, and the capture of resources; pp. 1–33. [Google Scholar]
- Hedden P, Phillips AL, Rojas MC, Carrera E, Tudzynski B. Gibberellin biosynthesis in plants and fungi: a case for convergent evolution? Journal of Plant Growth Regulators. 2002;20:319–331. doi: 10.1007/s003440010037. [DOI] [PubMed] [Google Scholar]
- Heuret P, Barthélémy D, Nicolini E, Atger C. Analyse des composantes de la croissance en hauteur et de la formation du tronc chez le chêne sessile (Quercus petraea (Matt.) Liebl., Fagaceae) en sylviculture dynamique. Canadian Journal of Botany. 2000;78:361–373. [Google Scholar]
- Heuret P, Barthélémy D, Guédon Y, Coulmier X, Tangre J. Synchronism in growth, branching and flowering processes on the individual and stand level in the South American tropical tree Cecropia obtusa Trécul (Cecropiaceae) American Journal of Botany. 2002;89:1180–1187. doi: 10.3732/ajb.89.7.1180. [DOI] [PubMed] [Google Scholar]
- Heuret P, Guédon Y, Guérard N, Barthélémy D. Analysing branching pattern in plantations of young red oak trees (Quercus rubra L., Fagaceae) Annals of Botany. 2003;91:479–492. doi: 10.1093/aob/mcg046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuret P, Meredieu C, Coudurier T, Courdier F, Barthélémy D. Ontogenetic trends in the morphological features of main stem annual shoots of Pinus pinaster Ait. (Pinaceae) American Journal of Botany. 2006;93:1577–1587. doi: 10.3732/ajb.93.11.1577. [DOI] [PubMed] [Google Scholar]
- Hochtetter W. Die sogenannten Retinospora-Arten der Gärten. Regels Gartenflora. 1880;29:362. [Google Scholar]
- Hu B-G, Jaeger M. Plant growth modelling and applications (PMA03); Proceedings of the 2003 International Symposium on Plant Growth Modeling, Simulation, Visualization and Their Applications; 13–16 October 2003; Beijing, Chine. Beijing: Tsinghua University Press, Springer; 2003. [Google Scholar]
- Huber H, Lukács S, Watson MA. Spatial structure of stoloniferous herbs: an interplay between architectural blue-print, ontogeny and phenotypic plasticity. Plant Ecology. 1999;141:107–115. [Google Scholar]
- Hueber FM, Galtier J. Symplocopteris wyattii n. gen. et n. sp. A zygopterid fern with a false trunk from the Tournaisian (Lower Carboniferous) of Queensland, Australia. Review of Palaeobotany and Palynology. 2002;119:241–273. [Google Scholar]
- Isik F, Isik K, Yildrim T, Li B. Annual shoot growth components related to growth of Pinus brutia. Tree Physiology. 2001;22:51–58. doi: 10.1093/treephys/22.1.51. [DOI] [PubMed] [Google Scholar]
- Isnard S, Speck T, Rowe N. Biomechanics and development of the climbing habit in two species of the South American palm genus Desmoncus (Arecaceae) American Journal of Botany. 2005;92:1444–1456. doi: 10.3732/ajb.92.9.1444. [DOI] [PubMed] [Google Scholar]
- Iwamoto A, Matsumura Y, Ohba H, Murata J, Imaichi R. Development and structure of trichotomous branching in Edgeworthia chrysantha (Thymeleaceae) American Journal of Botany. 2005;92:1350–1358. doi: 10.3732/ajb.92.8.1350. [DOI] [PubMed] [Google Scholar]
- Jablanczy A. Changes due to age in apical development in spruce and fir. Canadian Forest Services, Bi-Monthly Results Notes. 1971;27:10. [Google Scholar]
- Jeannoda-Robinson V. Contribution à l'étude de l'architecture des herbes. University Montpellier 2; 1977. PhD thesis. [Google Scholar]
- Johnson DM. Phylogenetic significance of spiral and distichous architecture in the Annonaceae. Systematic Botany. 2003;28:503–511. [Google Scholar]
- Jones CS. An essay on juvenility, phase change and heteroblasty in seed plants. International Journal of Plant Science. 1999;160:S105–S111. doi: 10.1086/314215. [DOI] [PubMed] [Google Scholar]
- Jones CS. The functional correlates of heteroblastic variation in leaves: changes in form and ecophysiology with whole plant ontogeny. Boletin de la Sociedad Argentina de Botanica. 2001;36:171–184. [Google Scholar]
- Jourdan C, Rey H. Architecture and development of the oil-palm (Elaeis guineensis Jacq.) root system. Plant and Soil. 1997a;189:33–48. [Google Scholar]
- Jourdan C, Rey H. Modelling and simulation of the architecture and development of the oil-palm (Elaeis guineensis Jacq.) root system. I. The model. Plant and Soil. 1997b;190:217–233. [Google Scholar]
- Jourdan C, Rey H. Modelling and simulation of the architecture and development of the oil-palm (Elaeis guineensis Jacq.) root system. II. Estimation of root parameters using the RACINES postprocessor. Plant and Soil. 1997c;190:235–246. [Google Scholar]
- Kaplan DR. The science of plant morphology: definition, history and role in modern biology. American Journal of Botany. 2001;88:1711–1741. [PubMed] [Google Scholar]
- Koch K. Dendrologie. 1873 Erlangen. [Google Scholar]
- Koriba K. On the periodicity of tree growth in the tropics, with reference to the mode of branching, the leaf-fall, and the formation of the resting bud. Garden's Bulletin, Singapore. 1958;17:11–81. [Google Scholar]
- King DA. Influence of leaf size on tree architecture: first branch height and crown dimensions in tropical rain forest trees. Trees. 1998;12:438–445. [Google Scholar]
- Kozlowski TT. Growth and development in trees, vol. 1: seed germination, ontogeny, and shoot growth, vol. 2: cambial growth, root growth, and reproductive growth. New York: Academic Press; 1971. [Google Scholar]
- de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytologist. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Lanner RM. Patterns of shoot development in Pinus and their relation to growth potential. In: Cannell MGR, Last FT, editors. Tree physiology and yield improvement. New York: Academic Press; 1976. pp. 223–243. [Google Scholar]
- Lavarenne S, Champagnat P, Barnola P. Croissance rythmique de quelques végétaux ligneux de régions tempérées cultivés en chambres climatisées à température élevée et constante et sous diverses photopériodes. Bulletin de la Société Française de Physiologie Végétale. 1971;118:131–162. [Google Scholar]
- Leroy C, Caraglio Y. Effects of tube shelters on the growth of young Turkish pines (Pinus brutia Ten., Pinaceae) Annals of Forest Science. 2003;60:539–547. [Google Scholar]
- Levin GG. Age changes in plants. Botanicheskij Zhurnal S.S.S.R. 1966;51:1774–1795. [Google Scholar]
- Lyndon RF. The shoot apical meristem. Its growth and development. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Martinez OG, De La Sota ER. La obconidad caulinar de Megalastrum pulverulentum (Pteridophyta-Dryopteridaceae) Boletin de la Sociedad Argentina de Botanica. 2001;36:105–109. [Google Scholar]
- McManus MT, Veit BE. Meristematic tissues in plant growth and development. Sheffield: Academic Press; 2002. Sheffield Biological series. [Google Scholar]
- McSteen P, Leyser O. Shoot branching. Annual Review of Plant Biology. 2005;56:353–374. doi: 10.1146/annurev.arplant.56.032604.144122. [DOI] [PubMed] [Google Scholar]
- Meloche CG, Diggle PK. Preformation, architectural complexity, and developmental flexibility in Acomastylis rossii (Rosaceae) American Journal of Botany. 2001;88:980–991. [PubMed] [Google Scholar]
- Millet J, Bouchard A, Edelin C. Plagiotropic architectural development and successional status of four tree species of the temperate forest. Canadian Journal of Botany. 1998a;76:2100–2118. [Google Scholar]
- Millet J, Bouchard A, Edelin C. Plant succession and tree architecture an attempt at reconciling two scales of analysis of vegetation dynamics. Acta Biotheoretica. 1998b;46:1–22. [Google Scholar]
- Millet P, Bouchard A, Edelin C. Architecture and successional status of trees in a temperate deciduous forest. Ecoscience. 1999;6:187–203. [Google Scholar]
- Millington WF. Shoot tip abortion in Ulmus americana. American Journal of Botany. 1963;50:371–378. [Google Scholar]
- Moens P. Les bourgeons végétatifs et génératifs de Coffea canephora Pierre. La Cellule. 1963;63:165–244. [Google Scholar]
- Mueller RJ. Ask the plant: investigating and teaching plant structure. Botanical Journal of the Linnean Society. 2006;150:73–78. [Google Scholar]
- Müller-Doblies D, Weberling F. Über Prolepsis und verwandte Begriffe. Beitrage zur Biologie der Pflanzen. 1984;59:121–144. [Google Scholar]
- Mündermann L, Erasmus Y, Lane B, Coen E, Prusinkiewicz P. Quantitative modeling of Arabidopsis development. Plant Physiology. 2005;139:960–968. doi: 10.1104/pp.105.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini E. Approche morphologique du développement du hêtre (Fagus sylvatica L.) University Montpellier 2; 1997. PhD thesis. [Google Scholar]
- Nicolini E. Architecture et gradients morphogénétiques chez de jeunes hêtres (Fagus sylvatica L. Fagaceae) en milieu forestier. Canadian Journal of Botany. 1998;76:1232–1244. [Google Scholar]
- Nicolini E. Nouvelles observations sur la morphologie des unités de croissance du hêtre (Fagus sylvatica L.). Symétrie des pousses, reflets de la vigueur des arbres. Canadian Journal of Botany. 2000;78:77–87. [Google Scholar]
- Nicolini E, Caraglio Y. L'influence de divers caractères architecturaux sur l'apparition de la fourche chez le Fagus sylvatica, en fonction de l'absence ou de la présence d'un couvert. Canadian Journal of Botany. 1995;72:1723–1734. [Google Scholar]
- Nicolini E, Chanson B. La pousse courte feuillée, un indicateur du degré de différenciation chez le Hêtre (Fagus sylvatica L.) Canadian Journal of Botany. 1999;77:1539–1550. [Google Scholar]
- Nicolini E, Barthélémy D, Heuret P. Influence de l'intensité du couvert forestier sur le développement de jeunes chênes sessiles Quercus petraea (Matt.) Liebl. Canadian Journal of Botany. 2000;78:1531–1544. [Google Scholar]
- Nicolini E, Chanson B, Bonne F. Stem growth and epicormic branch formation in understorey Beech trees (Fagus sylvatica L.) Annals of Botany. 2001;87:737–750. [Google Scholar]
- Nicolini E, Caraglio Y, Pélissier R, Leroy C, Roggy JC. Epicormic branches: a growth indicator for the tropical forest tree, Dicorynia guianensis Amshoff (Caesalpiniaceae) Annals of Botany. 2003;92:97–105. doi: 10.1093/aob/mcg119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. Plant biomechanics. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Niklas KJ. Modelling below- and above-ground biomass for non-woody and woody plants. Annals of Botany. 2005;95:315–321. doi: 10.1093/aob/mci028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta I, Ohsawa M. Bud structure and shoot architecture of canopy and understorey evergreen broad-leaved trees at their northern limit in East Asia. Annals of Botany. 1998;81:115–129. [Google Scholar]
- Nolan JR. Bifurcation of the stem apex in Asclepias syriaca. American Journal of Botany. 1969;56:603–609. [Google Scholar]
- Nougarède A. Le méristème caulinaire des Angiospermes: nouveaux outils, nouvelles interpretations. Acta Botanica Gallica. 2001;148:3–77. [Google Scholar]
- Novoplansky A. Developmental plasticity in plants: implications of non-cognitive behaviour. Evolutionary Ecology. 2002;16:177–188. [Google Scholar]
- Nozeran R. Réflexions sur les enchaînements de fonctionnements au cours du cycle des végétaux supérieurs. Bulletin de la Société Botanique de France. 1978;125:263–280. [Google Scholar]
- Nozeran R. Integration of organismal development. In: Barlow PW, Carr DJ, editors. Positional control in plant development. Cambridge: Cambridge University Press; 1984. pp. 375–401. [Google Scholar]
- Nozeran R, Bancilhon L, Neuville P. Intervention of internal correlations in morphogenesis of higher plants. Advances in Morphogenesis. 1971;9:1–66. doi: 10.1016/b978-0-12-028609-6.50005-4. [DOI] [PubMed] [Google Scholar]
- Nozeran R, Ducreux G, Rossignol-Bancilhon L. Réflexions sur les problèmes de rajeunissements chez les végétaux. Bulletin de la Société Botanique de France, Lettres Botanique. 1982;129:107–130. [Google Scholar]
- Nozeran R, Rossignol-Bancilhon L, Mangenot G. Les recherches sur le genre Phyllanthus (Euphorbiaceae): acquis et perspectives. Botanica Helvetica. 1984;94:199–233. [Google Scholar]
- Oborny B. External and internal control in plant development. Complexity. 2004;9:22–28. [Google Scholar]
- Oldeman RAA. L'architecture de la forêt guyanaise. Paris: O.R.S.T.O.M; 1974. Mémoire no., 73. [Google Scholar]
- Oldeman RAA. Tropical rain forest, architecture, silvigenesis and diversity. In: Sutton SL, Whitmore TC, Chadwick AC, editors. Tropical rain forest: ecology and management. Oxford: Blackwell; 1983. pp. 139–150. [Google Scholar]
- Oldeman RAA. Forests: elements of silvology. Berlin: Springer-Verlag; 1990. [Google Scholar]
- Pardé J, Bouchon J. Dendrométrie, 2ième édition. Nancy: Ecole Nationale du Génie Rural, des Eaux et des Forêts. 1988 [Google Scholar]
- Parisot E. Etude de la croissance rythmique chez les jeunes Manguiers (Mangifera indica) University Clermont-Ferrand 2; 1985. PhD thesis. [Google Scholar]
- Parkin J. The evolution of the inflorescence. Linnean Society of Botany of London. 1914;42:511–563. [Google Scholar]
- Passecker F. Jugend und Altersformen bei den Obstgehölzen. Gartenbauwissenschaft. 1944;18:219–230. [Google Scholar]
- Passecker F. Zur Frage des ‘primären’ und ‘fertilen’ Stadiums bei Apfelsämlingen. Mitteilungen Klosterneuburg, Series B. 1958;8:116–117. [Google Scholar]
- Passecker F. Theorie der ontogenetischen Evolution und Alterung holziger Gewächse. Bodenkultur. 1977;28:277–294. [Google Scholar]
- Passo A, Puntieri J, Barthélémy D. Trunk and main-branch development in Nothofagus pumilio (Nothofagaceae): a retrospective analysis of tree growth based on the size and structure of its annual shoots. Canadian Journal of Botany. 2002;80:763–772. [Google Scholar]
- Payan E. Contribution à l'étude de la croissance rythmique chez de jeunes Chênes pédonculés (Quercus pedunculata Ehrh.) University Clermont-Ferrand 2; 1982. PhD thesis. [Google Scholar]
- Pearcy RW, Muraoka H, Valladares F. Crown architecture in sun and shade environments: assessing function and tradeoffs with a 3-D simulation model. New Phytologist. 2005;166:791–800. doi: 10.1111/j.1469-8137.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- Perreta MG, Tivano JC, Vegetti AC. Forma de crecimiento en Leptochloa chloridiformis (Poaceae) Darwiniana. 2000;38:219–226. [Google Scholar]
- Picard C. Contribution à la connaissance de la vernalisation, de ses particularités et de sa signification chez Oenothera biennis var. sulfura De Vries. Annales des Sciences Naturelles, Botanique. 1965;12:197–314. [Google Scholar]
- Picard G, Le Toan T, Quegan S, Caraglio Y, Castel T. Radiative transfer modeling of cross-polarized backscatter from a pine forest using the discrete ordinate and eigenvalue method. IEEE Transactions on Geoscience and Remote Sensing. 2004;42:1720–1730. [Google Scholar]
- Pilger R. Bemerkungen zur phylogenetischen Entwicklung der Blütenstände. Bericht der Freien Vereinigung für Pflanzen Geographyraphie und Systematische Botanik für das Jahr. 1921:69–77. [Google Scholar]
- Poethig RS. Phase change and the regulation of shoot morphogenesis in plants. Science. 1990;250:923–930. doi: 10.1126/science.250.4983.923. [DOI] [PubMed] [Google Scholar]
- Poethig RS. Phase change and the regulation of developmental timing in plants. Science. 2003;301:334–336. doi: 10.1126/science.1085328. [DOI] [PubMed] [Google Scholar]
- Pressler R. Das Gesetz der Stammbildung. Leipzig: Arnloldische Buchhandlung; 1865. [Google Scholar]
- Prévost MF. Architecture de quelques Apocynacées ligneuses. Bulletin de la Société Botanique de France. Lettres Botaniques. 1967;114:24–36. [Google Scholar]
- Prusinkiewicz P, Muendermann L, Karkowski R, Lane B. The use of positional information in the modelling of plants. Proceedings of SIGGRAPH. 2001:289–300. [Google Scholar]
- Puntieri J, Barthélémy D, Martinez P, Raffaele E, Brion C. Annual–shoot growth and branching patterns in Nothofagus dombeyi (Fagaceae) Canadian Journal of Botany. 1998;76:673–685. [Google Scholar]
- Puntieri J, Raffaele E, Martinez P, Barthélémy D, Brion C. Morphological and architectural features of young Nothofagus pumilio (Poepp. et Endl.) Krasser (Fagaceae) plants. Botanical Journal of the Linnean Society. 1999;130:395–410. [Google Scholar]
- Puntieri J, Souza MS, Barthélémy D, Brion C, Nuñez M, Mazzini C. Preformation, neoformation and shoot structure in Nothofagus dombeyi (Mirb.) Blume (Nothofagaceae) Canadian Journal of Botany. 2000;78:1044–1054. [Google Scholar]
- Puntieri J, Brion C, Barthélémy D, Souza MS. Variaciones en el tamaño y la composición de las yemas de Nothofagus pumilio y N. dombeyi (Fagaceae) Darwiniana. 2001;39:1–10. [Google Scholar]
- Puntieri J, Barthélémy D, Mazzini C, Brion C. Periods of organogenesis in shoots of Nothofagus dombeyi (Mirb.) Oersted (Nothofagaceae) Annals of Botany. 2002a;89:115–124. doi: 10.1093/aob/mcf012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntieri J, Stecconi M, Barthélémy D. Preformation and neoformation in shoots of Nothofagus antarctica (G. Forster) Oerst. (Nothofagaceae) shrubs from northern Patagonia. Annals of Botany. 2002b;89:665–673. doi: 10.1093/aob/mcf108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh W. Über die Gesetzmässigkeit der Verzweigung und deren Bedeutung für die Wuchsformen der Pflanzen. Mitteilungen Deutsche Dendrologische Gesellschaft. 1939;52:86–111. [Google Scholar]
- Rees AR. The apical organization and phyllotaxis of the Oil Palm. Annals of Botany (N.S.) 1964;28:209–269. [Google Scholar]
- de Reffye P, Houllier F. Modelling plant growth and architecture: some recent advances and applications to agronomy and forestry. Current Science. 1997;73:984–992. [Google Scholar]
- de Reffye P, Hu B-G. Relevant qualitative and quantitative choices for building an efficient dynamic plant growth model: GreenLab case. In: Hu B, Jaeger M, editors. Plant Growth Modelling and Applications (PMA03); Proceedings of the 2003 International Symposium on Plant Growth Modeling, Simulation, Visualization and Their Applications; 13–16 October 2003; Beijing, China. Beijing: Tsinghua University Press, Springer; 2003. pp. 87–107. [Google Scholar]
- de Reffye Ph, Dinouard P, Barthélémy D. Comptes Rendus du 2ème Colloque International Sur l'Arbre, Montpellier, 10–15 Septembre 1990, Naturalia Monspeliensia, No. Hors Série. 1991. Modélisation et simulation de l'architecture de l'Orme du Japon Zelkova serrata (Thunb.) Makino (Ulmaceae): la notion d'axe de référence; pp. 251–266. [Google Scholar]
- de Reffye P, Fourcaud T, Blaise F, Barthélémy D, Houllier F. A functional model of tree growth and tree architecture. Silva Fennica. 1997;31:297–311. [Google Scholar]
- de Reffye P, Houllier F, Blaise F. Modelling plant growth and architecture: some recent advances and applications to agronomy and forestry. In: Marcelis LFM, editor. 2nd International Symposium on Models for Plant Growth, Environmental Control and Farm Management in Protected Cultivation. Vol. 456. Wageningen: ISHS. Acta Horticulturae; 1998. pp. 105–116. [Google Scholar]
- Remphrey WR, Davidson CG. Shoot preformation in clones of Fraxinus pennsylvanica in relation to site and year of bud formation. Trees. 1994;8:126–131. [Google Scholar]
- Remphrey WR, Powell GR. Crown architecture of Larix laricina saplings: shoot preformation and neoformation and their relationships to shoot vigour. Canadian Journal of Botany. 1984;62:2181–2192. [Google Scholar]
- Remy P. L'étude de la période juvénile chez les arbres fruitiers. Revue Horticole. 1951;123:543–547. [Google Scholar]
- Ritterbusch A. The measure of biological age in plant modular systems. Acta Biotheoretica. 1990;38:113–124. [Google Scholar]
- Rivals P. Essai sur la croissance des arbres et sur leurs systèmes de floraison (Application aux espèces fruitières) Journal d'Agronomie Tropicale et de Botanique Appliquée. 1965;12:655–686. [Google Scholar]
- Rivals P. Essai sur la croissance des arbres et sur leurs systèmes de floraison (Application aux espèces fruitières) Journal d'Agronomie Tropicale et de Botanique Appliquée. 1966;13:91–122. [Google Scholar]
- Robbins WJ. Physiological aspects of aging in plants. American Journal of Botany. 1957;44:289–294. [Google Scholar]
- Robbins WJ. Juvenility and the induction of flowering. Recent Advances in Botany. 1961;2:1647–1652. [Google Scholar]
- Roloff A. Morphologie der Kronenentwicklung von Fagus sylvatica L. (Rotbuche) unter besonderer Berücksichtigung möglicherweise neuartiger Veränderungen. Gottingen: Georg-August University; 1985. PhD thesis. [Google Scholar]
- Roloff A. Morphologie der Kronenentwicklung von Fagus silvatica L. (Rotbuche) unter besonderer Berücksichtigung neuartiger Veränderungen. II. Strategie der Luftraumeroberung und Veränderung durch Umwelteinflüsse. Flora. 1988;180:297–338. [Google Scholar]
- Roloff A. Kronenentwicklung und Vitalitätsbeurteilung Ausgewählter Baumarten der Gemäigten Breiten. Frankfurt am Main: J.D. Sauerländer's Verlag; 1989. [Google Scholar]
- Room PM, Maillette L, Hanan JS. Module and metamer dynamics and virtual plants. Advances in Ecological Research. 1994;25:105–157. [Google Scholar]
- Roux J. Sur le comportement des axes aériens chez quelques plantes à rameaux végétatifs polymorphes. Le concept de rameau plagiotrope. Annales des Sciences Naturelles, Botanique. 1968;12:109–256. [Google Scholar]
- Rowe NP, Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytologist. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Rua GH, Gróttola MC. Growth form models within the genus Paspalum L. (Poaceae, Paniceae) Flora. 1997;192:65–80. [Google Scholar]
- Sabatier S. Variabilité morphologique et architecturale de deux espèces de Noyers: Juglans regia L., Juglans nigra L. et de deux Noyers hybrides interspécifiques. University Montpellier 2; 1999. PhD thesis. [Google Scholar]
- Sabatier S, Barthélémy D. Architecture du Cèdre de l'Atlas, Cedrus atlantica (Endl.) Manetti ex Carrière (Pinaceae) In: Bouchon J, editor. Architecture des Arbres Fruitiers et Forestiers, Montpellier (FRA), 23–25 Novembre 1993. Paris: INRA Editions; 1995. pp. 157–173. Les Colloques no. 74. [Google Scholar]
- Sabatier S, Barthélémy D. Growth dynamics and morphology of annual shoots according to their architectural position in young Cedrus atlantica (Endl.) Manetti ex Carrière (Pinaceae) Annals of Botany. 1999;84:387–392. [Google Scholar]
- Sabatier S, Barthélémy D. Bud content in relation to shoot morphology and position on vegetative shoots of Juglans regia L. (Juglandaceae) Annals of Botany. 2001a;87:117–123. [Google Scholar]
- Sabatier S, Barthélémy D. Annual shoot morphology and architecture in Persian Walnut, Juglans regia L. (Juglandaceae) Acta Horticulturae. 2001b;544:255–264. [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I, Germain E. Nature de la pousse annuelle chez le Noyer commun, Juglans regia L. var. Lara (Juglandaceae): préformation hivernale et printanière. In: Bouchon J, editor. Architecture des Arbres Fruitiers et Forestiers. Paris: INRA Editions; 1995. pp. 109–124. Les colloques no. 74. [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I, Germain E. Modalités d'allongement et morphologie des pousses annuelles chez le noyer commun, Juglans regia L. cv. ‘Lara’ (Juglandaceae) Canadian Journal of Botany. 1998;76:1253–1264. [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I, Germain E. Allongement et morphologie de pousses annuelles issues de greffe chez le Noyer commun, Juglans regia L. cv. Lara (Juglandaceae) Canadian Journal of Botany. 1999;77:1595–1603. [Google Scholar]
- Sabatier S, Barthélémy D, Becquey J, Perrier S. Taille et architecture chez de jeunes noyers hybrides. Forêt Entreprise. 2000;132:54–58. [Google Scholar]
- Sabatier S, Baradat P, Barthélémy D. Intra- and interspecific variations of polycyclism in young trees of Cedrus atlantica (Endl.) Manetti and Cedrus libani A. Rich (Pinaceae) Annals of Forest Science. 2003a;60:19–29. [Google Scholar]
- Sabatier S, Barthélémy D, Ducousso I. Periods of organogenesis in mono- and bicyclic annual shoots of Juglans regia L. (Juglandaceae) Annals of Botany. 2003b;9:1–8. doi: 10.1093/aob/mcg127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. Traité de Botanique, conforme à l'état présent de la science. Paris: Librairie F. SAVY; 1874. Traduit par Van Tieghem. [Google Scholar]
- Sachs T. Self–organization of tree form: a model for complex social systems. Journal of Theoretical Biology. 2004;230:197–202. doi: 10.1016/j.jtbi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sandt W. Zur Kenntnis der Beiknospen. Zugleich ein Beitrag zum Korrelationsproblem. Botanische Abhandlungen. 1925;7:1–160. [Google Scholar]
- Sanoja E. Essai d'application de l'architecture végétale à la systématique. L'exemple de la famille des Vochysiaceae. University Montpellier 2; 1992. PhD thesis. [Google Scholar]
- Sattler R. What is theoretical plant morphology? Acta Biotheoretica. 1978;27:5–20. [Google Scholar]
- Sattler R, Rutishauser R. The fundamental relevance of morphology and morphogenesis to plant research. Annals of Botany. 1997;80:571–582. [Google Scholar]
- Schaffalitzky de Muckadell M. Juvenile stages in woody plants. Physiologia Plantarum. 1954;7:782–796. [Google Scholar]
- Schaffalitzky de Muckadell M. Investigations on ageing of apical meristems in woody plants and its importance in sylviculture. Forstl Forsogsvags. 1959;25:310–455. [Google Scholar]
- Schoute JC. Uber die Verästelung bei monokotylen Bäumen. II. Die Verästelung von Hyphaene. Recents Travaux Botaniques Néerlandais. 1909;15:211–232. [Google Scholar]
- Schwabé WW. Applied aspects of juvenility and some theoretical considerations. Acta Horticulturae. 1976;56:45–56. [Google Scholar]
- Scotland RW, Olmstead RG, Bennett J. Phylogeny reconstruction: the role of morphology. Systematic Biology. 2003;52:539–548. doi: 10.1080/10635150390223613. [DOI] [PubMed] [Google Scholar]
- Sellier D, Fourcaud T, Lac P. A finite element model for investigating effects of aerial architecture on tree oscillations. Tree Physiology. 2006;26:799–806. doi: 10.1093/treephys/26.6.799. [DOI] [PubMed] [Google Scholar]
- Soler C, Sillion FX, Blaise F, de Reffye Ph. An efficient instantiation algorithm for simulating radiant energy transfer in plant models. ACM Transactions on Graphics. 2003;22:204–233. [Google Scholar]
- Soria A, Meyer-Berthaud B. Tree fern growth strategy in the Late Devonian cladoxylopsid species Pietzschia levis from the study of its stem and root system. American Journal of Botany. 2004;91:10–23. doi: 10.3732/ajb.91.1.10. [DOI] [PubMed] [Google Scholar]
- Souza MS, Puntieri J, Barthélémy D, Brion C. Bud leaf primordia content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Fagaceae) Annals of Botany. 2000;85:547–555. [Google Scholar]
- Späth HL. Der Johannistrieb. Berlin: 1912. Inaugural-Dissertation zur Erlangung der Doktorwürde, Friedrich-Wilhelms-Universität. [Google Scholar]
- Stecconi M. Variabilidad arquitectural de especies nativas de Nothofagus de la Patagonia (N. antarctica, N. pumilio, N. dombeyi) Argentina: Regional Universitario Bariloche – Universidad Nacional del Comahue; 2006. PhD thesis in Biology, Centro. [Google Scholar]
- Stecconi M, Puntieri J, Barthélémy D. Annual–shoot growth in Nothofagus antarctica (G. Forster) Oersted (Fagaceae) from northern Patagonia. Trees, Structure and Function. 2000;14:289–296. [Google Scholar]
- Steeves TA, Sussex IM. Patterns in plant development. 2nd edn. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Stokes P, Verkerk K. Flower formation in Brussels sprouts. Medelingen van de Landbouwhogeschoological te Wageningen. 1951;50:143–160. [Google Scholar]
- Stoutmeyer VT. Juvenility and flowering potential in woody plants. American Horticultural Magazine. 1964;43:161–167. [Google Scholar]
- Temple A. Ericaceae: polymorphisme architectural d'une famille des régions tempérées et tropicales d'altitude. Comptes Rendus de l'Académie des Sciences. Paris, Sér. D. 1977;284:163–166. [Google Scholar]
- Tomlinson PB. The shoot apex and its dichotomous branching in the Nypa palm. Annals of Botany. 1971;35:865–879. [Google Scholar]
- Tomlinson PB. Plagiotropy. Comptes Rendus du Colloque International sur l'Arbre, Montpellier, 9–14 Septembre 1985, Naturalia Monspeliensia, No. Hors Série. 1986:451–463. [Google Scholar]
- Tomlinson PB. Architecture of tropical plants. Annual Review of Ecology and Systematics. 1987;18:1–21. [Google Scholar]
- Tomlinson PB, Esler AE. Establishment growth in woody monocotyledons native to New Zealand. Principes. 1973;19(C):83–99. [Google Scholar]
- Tomlinson PB, Gill AM. Growth habit of tropical trees: some guiding principles. In: Meggers JB, Ayensu ES, Duckworth WD, editors. Tropical forest ecosystems in Africa and South America: a comparative review. Washington: Smithsonian Institute Press; 1973. pp. 129–143. [Google Scholar]
- Tomlinson PB, Posluszny U. Features of dichotomizing apices in Flagellaria. American Journal of Botany. 1977;64:1057–1065. [Google Scholar]
- Tomlinson PB, Zimmermann MH. Anatomy of the palm Rhapis excelsa III. Juvenile phase. Journal of the Arnold Arboretum. 1966;47:301–312. [Google Scholar]
- Trippi VS. Studies on ontogeny and senility in plants. III. Changes in proliferative capacity in vitro during ontogeny in Robinia pseudoacacia and Castanea vulgaris and in adult and juvenile clones of R. pseudoacacia. Phyton. 1963;20:153–159. [Google Scholar]
- Troll W. Vergleichende Morphologie der höheren Pflanzen. Berlin: Borntraeger; 1937. [Google Scholar]
- Troll W. Praktische Einführung in die Pflanzenmorphologie 2 Teil: die blühende Pflanze. Jena: Fischer Verlag; 1957. [Google Scholar]
- Troll W, Rauh W. Sitzungsberichte der Heidelberger Akademie der Wissenschaften. Abhandlung: 1950. Das Erstarkungswachstum krautiger Dikotylen, mit besonderer Berücksichtigung der primären Verdickungsvorgänge; pp. 1–86. Mathematisch- naturwissentschaftliche Klasse. Jahrgang 1950. 1. [Google Scholar]
- Turnbull C. Plant architecture and its manipulation. Annual plant reviews. Vol. 17. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- Uranov AA. Age spectrum of the phytocoenopopulation as a function of time and energetic wave processes. Biologicheskie Nauki. 1975;2:7–34. [Google Scholar]
- Van den Berg D, Lanner RM. Bud development in lodgepole pine. Forest Science. 1971;17:479–486. [Google Scholar]
- Varossieau WW. On the development of the stem and the formation of leaves in Coffea species. Annales du Jardin Botanique de Buitenzorg. 1940;50:115–198. [Google Scholar]
- Veillon JM. Architecture of the New Caledonian species of Araucaria. In: Tomlinson PB, Zimmermann MH, editors. Tropical trees as living systems. Cambridge: Cambridge University Press; 1978. pp. 233–245. [Google Scholar]
- Veillon JM. Architecture des espèces néo-calédoniennes du genre Araucaria. Candollea. 1980;35:609–640. [Google Scholar]
- Venkatanarayana G. On certain aspects of the development of the leaf of Cocos nucifera L. Phytomorphology. 1957;7:297–305. [Google Scholar]
- Vester H. The trees and the forest. The role of tree architecture in canopy development; a case study in secondary forests (Araracuara, Colombia). University Amsterdam; 1997. PhD thesis. [Google Scholar]
- Vester HFM. Tree Temperaments. In: Labrecque M, Director , editors. L'arbre 2000, the Tree. Canada: IQ Collectif, Institut de Recherche en Biologie Végétale; 2001. pp. 25–30. [Google Scholar]
- Vester HFM. Architectural diversification within the genus Vismia (Clusiaceae) in the Amazonian rain forest (Araracuara, Colombia) In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. Kew: Royal Botanical Gardens; 1999. pp. 147–158. [Google Scholar]
- Visser T. Juvenile phase and growth of apple and pear seedlings. Euphytica. 1964;13:119–129. [Google Scholar]
- Visser T, De Vries DP. Precocity and productivity of propagated apple and pear seedlings as dependent on the juvenile period. Euphytica. 1970;19:141–144. [Google Scholar]
- Wang Y, Li J. Genes controlling plant architecture. Current Opinion in Biotechnology. 2005;17:1–7. doi: 10.1016/j.copbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wardle P. Evolution and distribution of the New Zealand flora as affected by Quaternary climates. New Zealand Journal of Botany. 1963;1:3–17. [Google Scholar]
- Wareing PF. Problems of juvenility and flowering in trees. Journal of the Linnean Society of London, Botany. 1959;56:282–289. [Google Scholar]
- Wareing PF. Juvenility and induction of flowering. Recent Advances in Botany. 1961;2:1652–1654. [Google Scholar]
- Webber HJ, Batchelor LD. The citrus industry, Vol. I. History, botany and breeding. Berkeley: University of California Press; 1948. [Google Scholar]
- Weberling S. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- White J. The plant as a metapopulation. Annual Review of Ecology and Systematics. 1979;10:109–145. [Google Scholar]
- Wiens JJ. The role of morphological data in phylogeny reconstruction. Systematic Biology. 2004;53:653–661. doi: 10.1080/10635150490472959. [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Mazer SJ. Responses to environmental heterogeneity: fitness consequences of phenotypic stability vs. sensitivity in wild radish (Raphanus sativus: Brassicaceae) International Journal of Plant Sciences. 2005;166:631–640. [Google Scholar]
- Wright IJ, Westoby M, Reich PB. Convergence towards higher leaf mass per area in dry- and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology. 2002;90:534–543. [Google Scholar]
- Wu R, Hinckley TM. Phenotypic plasticity of sylleptic branching: genetic design of tree architecture. Critical Reviews in Plant Science. 2001;20:467–485. [Google Scholar]
- Wu R, Stettler RF. Quantitative genetics of growth and development in Populus. III. Phenotypic plasticity of crown structure and function. Heredity. 1998;81:299–310. [Google Scholar]
- Yan HP, de Reffye P, Pan CH, Hu BG. Fast construction of plant architectural models based on substructure decomposition. Journal of Computer Science and Technology. 2003;18:780–787. [Google Scholar]
- Yan H-P, Kang MZ, de Reffye P, Dingkuhn M. A dynamic, architectural plant model simulating resource-dependent growth. Annals of Botany. 2004;93:591–602. doi: 10.1093/aob/mch078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD. The juvenile phase in Bryophyllum daigremontianum. Planta. 1962;58:543–547. [Google Scholar]
- Zhao M-Z, de Reffye P, Barthélémy D, Hu B-G. Interactive simulation of plant architecture based on a dual-scale automaton model. In: Hu B, Jaeger M, editors. Plant growth modelling and applications (PMA03); Proceedings of the 2003 International Symposium on Plant Growth Modeling, Simulation, Visualization and Their Applications; 13–16 October 2003; Beijing, China. Beijing: Tsinghua University Press, Springer; 2003. pp. 144–153. [Google Scholar]
- Zimmerman RH. Juvenility and flowering in woody plants: a review. Hortscience. 1972;7:447–455. [Google Scholar]
- Zimmerman MH, Brown CL. Trees: structure and function. Berlin: Springer-Verlag; 1971. [Google Scholar]