Abstract
Background and Aims
Daytime CO2 efflux rates (FCO2) from tree stems are often reported to be lower than expected from the exponential relationship between temperature and respiration. Explanations of daytime depression in FCO2 have focused on the possible role of internal CO2 transport in the xylem. However, another possible cause that has been overlooked is the daily dynamics of the water status in the living stem tissues and its influence on stem growth rate and thus respiration. The objective of this study was to assess the daily dynamics of stem water status and growth rate and to determine the extent to which they may be linked to daily variations in stem FCO2.
Methods
FCO2 of young beech and oak stems were measured under controlled conditions. Relative stem turgor pressure (Ψp), obtained from simulations with the ‘RCGro’ model, was used as an indicator of the water status in the living stem tissues. Daily dynamics of stem growth were derived from Ψp: growth was assumed to occur when Ψp exceeded a relative threshold value.
Key Results
There was a strong correspondence between fluctuations in FCO2 and simulated Ψp. The non-growth conditions during daytime coincided with depressions in FCO2. Moreover, FCO2 responded to changes in Ψp in the absence of growth, indicating also that maintenance processes were influenced by the water status in the living stem tissues.
Conclusions
Daytime depressions in stem FCO2 correlate with the daily dynamics of turgor, as a measure of the water status in the living stem tissues: it is suggested that water status of tree stems is a potentially important determinant of stem FCO2, as it influences the rate of growth and maintenance processes in the living tissues of the stem.
Key words: Fagus sylvatica (beech), Quercus robur (oak), CO2 efflux, diameter variations, dynamic model, radial growth, plant–water relations, sap flow, stem respiration, stem turgor pressure
INTRODUCTION
Respiration rates of woody tissues are commonly measured by enclosing the tissue in a cuvette, and measuring the rate of CO2 efflux from the tissue with an infrared gas analyser (IRGA). It is assumed that all the CO2 respired by the woody tissue enclosed in the cuvette diffuses laterally from the stem interior into the cuvette. Several studies (e.g. Negisi, 1975, 1978, 1982; Lavigne, 1987; Kakubari, 1988) have shown that on warm sunny days, measured stem CO2 efflux rate (FCO2) was lower than that expected from the exponential relationship between respiration and temperature (Amthor, 1989):
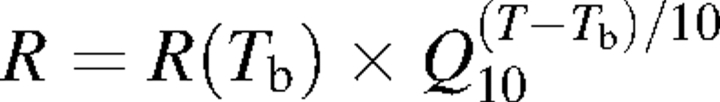 |
1 |
with R the respiration rate at temperature T, R(Tb) the respiration rate at a basal temperature Tb, and Q10 the relative increase in respiration rate with a 10 °C rise in temperature. This phenomenon is the so-called daytime depression in stem FCO2. Several authors have suggested that sap flow rate (FH2O) might have an influence on stem FCO2 (Negisi, 1979; Ryan, 1990; Sprugel, 1990; Hari et al., 1991; Martin et al., 1994; McGuire and Teskey, 2004; Bowman et al., 2005). This explanation is based on the high solubility of CO2, H2CO3 and HCO3– in water, so that a portion of the CO2 evolved by the respiring cells of woody tissues might dissolve in the sap and be transported vertically in the xylem along with the transpiration stream instead of moving laterally through the stem into the cuvette (Hari et al., 1991; Kaipiainen et al., 1998). Hence, measured values of stem CO2 efflux may be affected by the rate of CO2 diffusion in xylem sap rather than by respiratory processes per se.
However, transport of dissolved CO2 in the xylem is only one consequence of sap flow. According to the cohesion tension theory, water in the xylem of transpiring trees is under considerable tension as it is pulled from soil to leaves along a complex pathway of water-conducting elements, which together have a large hydraulic resistance (Irvine and Grace, 1997). When tension develops in the xylem, a water potential gradient develops between the phloem and xylem, which leads to a water flux from the phloem across the cambium towards the xylem (Garnier and Berger, 1986). Hence, water reserves of the living tissues external to the xylem are depleted during the daytime, resulting in stem diameter shrinkage. These living tissues respire to provide energy for growth and maintenance processes. When the water reserves in the living tissues external to the xylem are depleted, water deficits may occur, temporarily reducing rates of growth and maintenance processes and the respiratory processes that support them (Lavigne, 1987; Kakubari, 1988; Wang et al., 2003; Daudet et al., 2005). In particular, expansion growth may be reduced by water deficit: it is one of the most sensitive of all plant processes to drought stress (Hsiao, 1973). However, it is difficult to actually determine the occurrence of water deficit in the stem tissue. Wang et al. (2003) measured xylem water potential of Larix gmelini branches, which was greater (less negative) in the morning than afternoon, but this is not evidence that growth and/or maintenance processes in the living tissues are actually suppressed. It is not the xylem water potential, but the turgor pressure (Ψp) in the stem tissue that reflects the water status in the living cells of the stem (phloem, cambium and parenchyma; Bradford and Hsiao, 1982). Growth processes, such as cell formation, cell-wall expansion and deposition of new wall material are more dependent on Ψp and cell volume than on water potential (Boyer, 1968; Hsiao et al., 1976; Ray, 1987; Proseus and Boyer, 2006). The cell turgor is in direct proportion to the water potential only if the osmotic potential of the cell remains constant. However, turgor and cell volume can be maintained by osmotic adjustment (i.e. the active accumulation of solutes in the symplast), which may serve to sustain growth (Woodruff et al., 2004). Hence, it is the daily course of Ψp that can reveal when cell growth is likely to occur, and not the daily course of the water potential. A widely used model of cell expansion, developed by Lockhart (1965), relates relative cell expansion to cell Ψp, cell-wall extensibility (ϕ) and a threshold Ψp at which wall yielding occurs (Γ):
 |
2 |
Ψp must be above this threshold value for the cell to expand irreversibly. This idea has been incorporated into a mathematical model linking sap-flow dynamics in trees to daily fluctuations in stem diameter and radial growth (Steppe et al., 2006). This model, which requires only transpiration rate of the whole tree as input variable, simulates the change of Ψp relative to the maximum Ψp, occurring at zero FH2O.
In order to explain daytime depressions in FCO2, previous studies have examined the link between FH2O and stem FCO2 to test the hypothesis that sap flow directly determines internal CO2 transport in the xylem. However, sap flow also determines the course of Ψp in the living stem tissues, which, as discussed, is a very important driving variable for cell growth and, consequently, for the associated energy demand. We know of no studies that have attempted to evaluate the effects of Ψp and cell growth on the daily course of stem FCO2. Therefore, the objective of this study was to assess the daily dynamics of Ψp and growth, and to determine their association with daytime depressions in stem FCO2. The present work does not aim at refuting previous explanations for daytime depressions in stem FCO2, but introduces strong, if circumstantial evidence for the role of Ψp and the daily dynamics of cell growth as a cause of daytime depressions in stem FCO2.
MATERIALS AND METHODS
Plant material and growth chamber
Two different tree species, a ring-porous oak (Quercus robur L.) and a diffuse-porous beech (Fagus sylvatica L.), were studied in order to examine the possible link between daytime depressions of FCO2 and Ψp. A 3-year-old beech tree, previously grown outdoors, was planted at the beginning of February 2004 in a 50-L container, filled with potting mixture. The tree was 1·55 m high and the stem diameter at the soil surface was 16·4 mm. A 3-year-old oak tree was studied in 2005; it was 1·6 m high and had a stem diameter at the soil surface of 19·2 mm. The trees were placed in a growth chamber with dimensions 2 × 1·5 × 2 m (height × width × length) in order to control radiation and air temperature. Light was from densely packed fluorescent lamps (‘TL'D 80, Philips Lighting NV, Brussels, Belgium), producing a photon flux (400–700 nm) of photosynthetically active radiation (PAR) of approximately 470 µmol m−2 s−1 at the top of the trees (measured with a quantum sensor, type Li-190S, Li-COR, Lincoln, TE, USA). The trees were watered weekly and fertilized monthly with a NPK plus micronutrient mix (Substral, Sint-Niklaas, Belgium). Measurements were performed during the beginning of the growing season of 2004 for the beech tree and 2005 for the oak tree. During the measurement periods, the leaf area of the trees rapidly increased.
On one day during the experiment (day 154), from 1400 h until 1800 h, the leaves of the oak tree were enclosed in large transparent plastic bags in order to decrease the transpiration rate, while the light and temperature in the chamber were kept constant.
CO2 efflux measurements
Gas exchange was measured on a 13-cm long segment of each stem, approximately 0·7 m above the soil surface. The lower and upper diameter of the segments were 8·3 and 8·0 mm, respectively, for the beech, and 11·0 and 10·7 mm, respectively, for the oak stem. The segments were enclosed in opaque, air-tight cylindrical PVC cuvettes with a diameter of 6 cm, which prevented all light from reaching the tissue to avoid corticular photosynthesis. To minimize short-term fluctuations in CO2 concentration within the stem cuvettes, air from the growth chamber was pumped by a membrane pump (type N 86.KN 18, KNF Verder, Aartselaar, Belgium) at a flow rate of 1 L min−1 into a 50-L buffer tank before entering the cuvettes. Air leaving the cuvettes was first partially dried at 4 °C with a gas cooler (CG/G 73–4, Hartmann and Braun AG, Germany), before the CO2 concentration was measured with an infra-red gas analyzer (IRGA; Binos 100-4P, Fisher-Rosemount, Hasselroth, Germany), as the difference between the air leaving the stem cuvette and a reference cuvette, which did not contain a stem segment. The system was automatically zeroed every 450 s and 360 s for the beech and oak tree, respectively, by passing air from the reference cuvette through the reference and measuring cells of the IRGA. Since only living cells are producing CO2 and the majority of living cells in small trees are located close to the surface (phloem and cambium; Stockfors and Linder, 1998), CO2 efflux rates (FCO2) were expressed per unit of stem surface area.
Stem diameter measurement
Stem diameter (D) was measured using a linear variable displacement transducer (LVDT) and transducer bridge (respectively LBB, 375-PA-100 and 8C-35, Schaevitz, Hampton, VA, USA), placed 1 cm below the cuvette. The LVDT was supported by a stainless steel holder: tests with a 12-mm diameter aluminium rod showed that no temperature correction was required.
Sap flow measurements
Sap flow rates (FH2O) at the stem base and on a second-order branch (at the tree top), were measured with flow sensors based on the heat-balance principle (Models SGB16 and SGA5, Dynamax Inc., Houston, TX, USA), and installed according to the operation manual, as was calculation of FH2O (van Bavel and van Bavel, 1990). The sensors were thermally insulated with several layers of aluminium foil. Sheath conductance of the gauge was recalculated daily using minimum values in darkness between 0400 h and 0700 h. The value for thermal conductance of woody stems of 0·42 W m−1 °C−1 was taken from Steinberg et al. (1989).
Data acquisition
All signals from sensors and devices were logged (HP 34970A, Hewlett Packard, Palo Alto, CA, USA) at 10 s intervals, and monitored continuously (Hewlett-Packard programme VEE). All sensor signals were averaged over 450 s and 360 s periods for the beech and oak tree, respectively, and recorded by a computer.
Temperature correction for CO2 efflux rates
Air temperature (Ta) and stem temperature (Tst) were measured with copper–constantan thermocouples (Omega, Amstelveen, Netherlands). For Tst a 1-mm diameter hole, 7 mm deep was drilled in the stems into which the thermocouple was inserted. Although a constant temperature was set for the growth chamber, there were small variations, so the measured FCO2 was adjusted to Tst of 20 °C using the following equation (based on eqn 1):
 |
3 |
with FCO2(20) the calculated CO2 efflux rate at 20 °C (expressed in μmol m−2 s−1). Q10 was estimated by ordinary least squares, based on measurements of FCO2 on days where Ta was altered stepwise (four temperature steps of 1 h: 24 → 21 → 17 → 21 °C) during the dark period of day 121 for beech and during the dark period of day 148 for oak. Calculation of Q10 was based on FCO2 data for the dark period, since then no sap was flowing and it was assumed that the stem tissue was fully hydrated.
Simulation of stem turgor pressure
Non-destructive measurement of Ψp is difficult, so the mechanistic flow and storage model ‘RCGro’, developed by Steppe et al. (2006), was applied to simulate the change of stem Ψp relative to the maximum Ψp, at zero FH2O (Fig. 1). The model enables simulation of tree FH2O dynamics (water transport submodel), which are directly linked to variations in stem diameter (D) (stem diameter variation submodel), using the radial flow of water (see Fig. 1) between the xylem (considered as a continuous rigid cylinder) and the stem storage compartment (i.e. the living, extensible cells external to the xylem). This radial flow causes changes in the water content of the living tissues, and hence in Ψp. D varies due to reversible stem shrinkage/swelling and irreversible radial stem growth. If Ψp is smaller than the threshold Ψp at which wall yielding occurs (Γ), then variations in D only reflect reversible shrinkage/swelling. If Ψp is larger than Γ, then irreversible radial growth occurs, in addition to shrinkage/swelling (Fig. 1). For a detailed description of the model see Steppe et al. (2006). Whole-tree transpiration rate, which is the input variable for the model, is normally determined by multiplying measured branch FH2O (not stem FH2O since this lags behind transpiration) by the ratio of total leaf area/leaf area upstream of the sap flow sensor (Steppe et al., 2006). However, since the leaf area of both trees rapidly increased during the measurement period, it would have been necessary to measure total leaf area every day, which is a very laborious task. Therefore, FH2O measured at the top of each tree was increased each day by multiplying by a scaling factor, namely the ratio between the daily sum of stem FH2O and branch FH2O. This up-scaled branch FH2O was used as the input variable for the model. Stem FH2O and variations in D were selected as suitable variables for model calibration, using the simplex method (Nelder and Mead, 1965) by minimizing the sum of squared errors between the simulated values and the measured data of stem FH2O and variations in D (Steppe et al., 2006). The model was calibrated for beech and oak separately, and allowed us to simulate relative changes in stem Ψp throughout the day, as an indicator of the water status in the living tissues of the stem. Simulated relative Ψp was continuously compared with the relative threshold value Γ (i.e. expressed as a percentage of maximum Ψp occurring at zero FH2O) in order to estimate during which periods of the day irreversible radial stem growth occurred (see eqn 2).
Fig. 1.
Schematic of the model (algebraic and differential equations) linking the dynamics of tree sap flow and storage to changes in stem diameter and growth. The direct linkage referred to in the text is indicated by the arrow between the two submodels. Ψ = total water potential of storage compartment; Ψx = total water potential of xylem compartment; W = water content of storage compartment; C = capacitance of storage compartment; R = flow resistance between xylem and storage compartment; Rx = flow resistance in xylem compartment; ρw = density of water; A = surface area of the virtual membrane separating the stem storage compartment from the xylem compartment; L = hydraulic conductivity of the membrane; f = water flow between xylem and storage compartment; E = transpiration; F = water flow in a xylem compartment; V = volume of storage compartment; d = thickness of storage compartment; Di = inner diameter of stem segment; D = outer diameter of stem segment; l = length of stem segment; Ψp = turgor pressure potential; ε0 = proportionality constant; Γ = threshold Ψp at which wall-yielding occurs; ϕ = cell wall extensibility; a and b = allometric parameters; Ψπ = osmotic potential.
RESULTS
Daytime depression in CO2 efflux rate
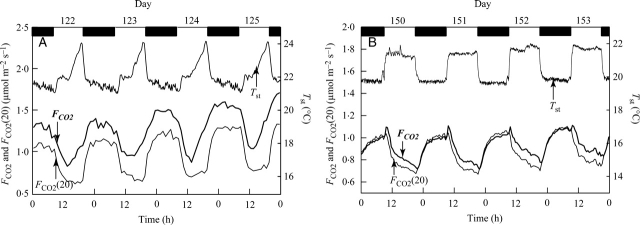
Despite the higher Tst during the day than night, the daytime FCO2 of both tree stems were lower compared with night values (Fig. 2). When FCO2 was adjusted for variations in Tst using eqn (3) [FCO2(20)], with estimated Q10 values of 3·2 (± 0·20 s.e.) and 2·0 (± 0·17) for beech and oak, respectively, the daytime depressions in FCO2 became even more pronounced (Fig. 2).
Fig. 2.
Daily patterns of measured temperature (Tst), CO2 efflux rate (FCO2) and CO2 efflux rate normalized to 20 °C [FCO2(20)] for stems of (A) beech (days 122–125), and (B) oak (days 150–153). Dark periods are indicated by black boxes. Values of FCO2(20) were obtained using eqn (3), with estimated Q10 values of 3·2 and 2·0 for beech and oak, respectively.
When the leaves of the oak tree were enclosed in plastic bags for 4 h on day 154, transpiration decreased substantially, although temperature and light conditions were not altered. An increment in stem FCO2(20) was observed (Fig. 3). Hence, factors other than temperature variations appear to exert a control over stem FCO2.
Fig. 3.
Time course of CO2 efflux rate from stems, normalized to 20 °C [FCO2(20)], obtained using eqn (3), (Q10 = 2·0), and sap flow rate at the stem base (FH2O) for the oak tree on day 154, when the leaves where enclosed in transparent plastic bags from 1400 h until 1800 h. Black boxes indicate the dark periods.
Model input and outputs
The RCGro model used the up-scaled branch FH2O as the only input variable. The values of the estimated model parameters (Table 1) were similar to those obtained by Steppe (2004), Steppe and Lemeur (2006) and Steppe et al. (2006) for young beech and oak trees. Figure 4 shows how well the output variables D and stem FH2O of the RCGro model fit the measured values for both trees. Coefficients of determination (r2) of the linear regression between measured and simulated data were very high for both trees.
Table 1.
Values of the estimated model parameters for beech and oak
| Parameter | Beech | Oak |
|---|---|---|
| Rx (MPa s mg−1) | 0·1478 | 0·1701 |
| C(stem) (mg MPa−1) | 638·6 | 1158·4 |
| C(crown) (mg MPa−1) | 350·3 | 5063·4 |
| ϕ (MPa−1 s−1) | 6·95 × 10−7 | 5·16 × 10–7 |
| Relative Γ (% of full turgor) | 92·86 | 95·66 |
Rx = flow resistance in xylem compartment; C(stem) = capacitance of stem storage compartment; C(crown) = capacitance of crown storage compartment; ϕ = cell wall extensibility; Γ = threshold Ψp at which wall yielding occurs.
Fig. 4.
Comparison between measured (thick lines) and simulated (thin lines) sap flow rates (FH2O) at the stem base for (A) beech and (B) for oak, and measured and simulated stem diameter (D) for (C) beech and (D) oak. Dark periods are indicated by black boxes. The coefficients of determination (r2) of the linear regression between measured and simulated values are given.
Stem turgor pressure versus CO2 efflux rate normalized at 20 °C
Changes in Ψp, simulated with the calibrated RCGro model, and FCO2(20) during the period day 122–125 for beech and day 150–154 for oak are shown in Fig. 5. At the onset of each light period, Ψp decreased sharply, due to the depletion of the water reserves in the stem tissues. This is also observed in the decrease of diameter (D; Fig, 4C, D). During the light periods, Ψp became lower than the critical wall-yielding threshold value needed for irreversible radial stem growth. At the beginning of each dark period, Ψp increased sharply due to the replenishment of the water reserves in the stem. The replenishment is also observed as an increase of D (Fig. 4C, D). During darkness, Ψp exceeded the wall-yielding threshold value. The diurnal variations of Ψp corresponded closely with the temperature-independent variations of FCO2. Upon enclosure of the oak foliage in plastic bags on day 154, Ψp in the stem increased but remained lower than the wall-yielding threshold value necessary for irreversible growth (Fig. 5B). Nevertheless, FCO2(20) increased. Closer inspection of the data for FCO2(20) and Ψp (Fig. 6A, B), showed that FCO2(20) lagged behind Ψp and hysteresis, following a counter-clockwise time course, is clearly visible when FCO2(20) is plotted as a function of Ψp (Fig. 6C, D).
Fig. 5.
Daily patterns of simulated stem turgor pressure (Ψp), expressed as a percentage of maximum Ψp occurring at zero FH2O, and calculated CO2 efflux rate normalized to 20 °C [FCO2(20)] during the period days 122–125 for beech (A), and 150–154 for oak (B). Ψp was simulated with the calibrated RCGro model. Values of FCO2(20) were calculated using eqn (3), with a Q10 of 3·2 and 2·0 for beech and oak, respectively. The horizontal dotted line represents the relative wall-yielding threshold value for Ψp above which radial stem growth occurs. Black boxes indicate dark periods.
Fig. 6.
Time-course of simulated stem turgor pressure (Ψp), expressed as a percentage of maximum Ψp occurring at zero FH2O, and CO2 efflux rate normalized to 20 °C [FCO2(20)] on day 122 for beech (A), and 153 for oak (B). Values of FCO2(20) were calculated using eqn (3), with a Q10 of 3·2 and 2·0 for beech and oak, respectively. Black boxes indicate dark periods. Hysteresis occurs between Ψp and FCO2(20) for beech (C) and oak (D). Measurement symbols are connected chronologically, with the arrows indicating hysteresis.
DISCUSSION
Clear daytime depressions were observed in stem FCO2 compared with the night despite a higher Tst, suggesting that factors other than temperature controlled FCO2 (Fig. 2). The experiments were conducted during spring, when the tree growth rate was high, and growth respiration is the dominant contributor to total stem respiration (e.g. Stockfors and Linder, 1998). Growth processes are very sensitive to drought stress (Hsiao, 1973) and so very probably depend on the dynamics of the water status in the living stem tissues. This study used a simulation model approach to estimate Ψp, which is a good indicator of the water status of the living tissues. Ψp fluctuated diurnally due to the dynamics (depletion and replenishment) of water reserves in the stem. As a result, relative radial stem growth rate fluctuated diurnally, with the highest growth rate occurring during the night when Ψp exceeded the wall-yielding threshold value (Γ) necessary for cell growth (Fig. 5). Simulated and measured stem diameter patterns both illustrate that irreversible radial stem growth occurred during the night: D increased continuously (Fig. 4C, 4D). Differences in plant growth rate during day and night have been studied by several authors. Boyer (1968) observed that enlargement of leaves of a well-watered sunflower plant (Helianthus annus) was five to six times higher at night than during the day. Schurr et al. (2000) found that leaf growth rates in Ricinus communis peaked during the late night and were minimal in the late afternoon. Halter et al. (1996) observed that root elongation rates in Eucalyptus nitens and E. pauciflora seedlings were 60 and 67 % higher, respectively, during the night than during the day. In our study, daytime Ψp was lower than the wall-yielding threshold value that must be exceeded for expansion growth (Fig. 5), indicating that the stem growth rate of both trees was zero during the daytime. Measurements of stem diameter confirm this: D was constant during the day (Fig. 4C, D). Hence, the higher FCO2(20) during darkness compared with the light can be explained at least partially by the higher energy demand to support growth in the stem tissue.
When transpiration of the oak tree was decreased by enclosure of the leaves (Figs 3 and 5B), Ψp increased but did not exceed the wall-yielding threshold value. Hence, cell growth was not expected to occur during this period. Nevertheless, FCO2(20) increased. This indicates that FCO2 increased with any improvement of the water status in the living stem tissues, even when stem expansion growth did not take place. A plausible explanation is that with any increase in Ψp, and hence an improved water status, the rate of maintenance metabolism (such as protein turnover, membrane repair, etc.) increases. In contrast with growth, maintenance metabolism always occurs, even under stress conditions. However, when conditions become more favourable (e.g. an enhanced water status in the living stem tissues), the rate of maintenance metabolism, and hence FCO2, will be enhanced. Unfortunately, we are unaware of any studies on the relationship between turgor and rates of maintenance processes. It is also important to note that cell wall expansion is not the only growth process in stems. Growth also includes processes such as cell wall deposition and assembly. Proseus and Boyer (2006) recently demonstrated that by decreasing Ψp, wall deposition and assembly in Chara corallina cells decreased. Hence, not only wall expansion is affected by changes in Ψp. However, it was not investigated whether a threshold Ψp exists for these processes to occur.
Changes in FCO2(20) were clearly related to simulated changes in Ψp. However, the response of FCO2(20) lagged behind changes in Ψp (Fig. 6C, D). A possible explanation is that when CO2 production by the living cells increases due to improved water status, its radial diffusion through the stem into the cuvette is delayed by the large resistance in the stem. A substantial restriction to radial gas movement in Pinus strobus branches was demonstrated by Eklund and Lavigne (1995).
As mentioned in the Introduction, an explanation for daytime depressions in FCO2 is that the sap flowing in the xylem of transpiring trees transports respired CO2 away from the stem. These studies therefore link FCO2 to FH2O. Our study links daytime depressions in FCO2 to changes in Ψp, and hence indirectly also to FH2O: changes in Ψp reflect changes in the water status in the living stem tissues, which result from changes in FH2O. Until now, the effect of FH2O on stem turgor, and hence on stem growth rate and respiration, has been ignored. With our dataset we could not distinguish between the effects of CO2 transport in the xylem and those of turgor dynamics in the living stem tissues. Thus our work does not refute previous explanations; rather it points out that in addition to the idea that respired CO2 is transported with the transpiration stream, the daily dynamics of Ψp and growth might equally well explain daytime depressions in stem FCO2. Further studies need to be conducted in which the effects on stem FCO2 of CO2 transport in the xylem and those of turgor and growth dynamics in the tissues external to the xylem are separated, and in which the relative importance of both processes on stem FCO2 are investigated. It is possible that processes in the external tissues have a larger impact on stem FCO2 than CO2 transport in the xylem. Some facts support this hypothesis. The cambium is quite impermeable to gases (Hook et al., 1972), which is demonstrated by the large difference in CO2 concentrations between xylem and bark tissues: xylem CO2 concentrations have been reported to be as high as 26 % (MacDougal and Working, 1933), while CO2 concentrations in bark tissues are reported to be around 0·06 to 0·17 % (Cernusak and Marshall, 2000; Wittmann et al., 2006). Furthermore, Maier and Clinton (2006) measured xylem CO2 concentrations and FCO2 in young Pinus taeda tree stems during spring and found that, after partial removal of the canopy, xylem CO2 concentrations increased but there was no apparent change in stem FCO2. They assumed that during spring the cambium and phloem meristems are likely to respire at a much higher rate than the xylem parenchyma and thus would be a major source of CO2 in the stem.
Another challenge will be to verify that respiration (i.e. not FCO2) of the tissues exterior to the xylem is actually slowed during the day. One possibility might be using the method of Pruyn et al. (2002), who examined tissue respiratory potential by sampling stem tissues, placing them in vials and measuring the difference in CO2 concentration after closing the vial and after an incubation period. However, an important problem with this method is that the samples lost water during the incubation period. Before their method can be used for our purpose, it needs to be improved so that water losses during sampling and incubation are completely prevented in order to maintain the Ψp of the living tissue at the same level.
CONCLUSIONS
This study demonstrates that the loss of turgor in the living stem tissues during daytime is quantitatively consistent with a slowing, or even cessation, of growth processes in these tissues. Since growth respiration is an important component in total stem respiration, daytime depressions in stem FCO2 (i.e. lower compared with what would be expected from the exponential temperature function) can at least partially be explained by the restricted growth during the daytime. However, since stem FCO2 also responded to changes in turgor when the turgor was still lower than the wall-yielding threshold value for growth, it was suggested that not only growth rate, but also the rate of maintenance metabolism fluctuates diurnally, due to the daily dynamics in the water status of the living stem tissues.
This work did not aim at distinguishing between the effects of turgor and the effects of transport of dissolved CO2 in the xylem. It rather sort to demonstrate that the daily dynamics of water status in the living stem tissues might explain daytime depressions in stem FCO2 just as well as the idea that the transpiration stream is transporting CO2 in the xylem. It will be a challenge in further research to separate the effects of both processes and to unravel to what extent both processes might influence stem FCO2.
ACKNOWLEDGEMENTS
The authors thank the Special Research Fund (B.O.F.) of Ghent University for the Ph.D. funding granted to the first author. We are also indebted to Philip Deman for his accurate and enthusiastic technical support.
LITERATURE CITED
- Amthor JS. Respiration and crop productivity. New York: Springer-Verlag; 1989. [Google Scholar]
- van Bavel MG, van Bavel CHM. Dynagage installation and operation manual. Houston, TX: Dynamax Inc; 1990. [Google Scholar]
- Bowman WP, Barbour MM, Turnbull MH, Tissue DT, Whitehead D, Griffin KL. Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytologist. 2005;167:815–828. doi: 10.1111/j.1469-8137.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Relationship of water potential to growth of leaves. Plant Physiology. 1968;43:1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ, Hsiao TC. Physiological responses to moderate water stress. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology. New series, Physiological plant ecology II: Water relations and carbon assimilation. Berlin: Springer-Verlag; 1982. pp. 263–324. [Google Scholar]
- Cernusak LA, Marshall JD. Photosynthetic refixation in branches of western white pine. Functional Ecology. 2000;14:300–311. [Google Scholar]
- Daudet FA, Ameglio T, Cochard H, Archilla O, Lacointe A. Experimental analysis of the role of water and carbon in tree stem diameter variations. Journal of Experimental Botany. 2005;56:135–144. doi: 10.1093/jxb/eri026. [DOI] [PubMed] [Google Scholar]
- Eklund L, Lavigne MB. Restricted lateral gas movement in Pinus strobus branches. Trees. 1995;10:83–85. [Google Scholar]
- Garnier E, Berger A. Effect of water stress on stem diameter changes of peach trees growing in the field. Journal of Applied Ecology. 1986;23:193–209. [Google Scholar]
- Halter R, Sands R, Sadanandannambiar EK, Ashton DH. Elongation of Eucalyptus roots during day and night. Tree Physiology. 1996;16:877–881. doi: 10.1093/treephys/16.11-12.877. [DOI] [PubMed] [Google Scholar]
- Hari P, Nygren P, Korpilathi E. Internal circulation of carbon within a tree. Canadian Journal of Forest Research. 1991;21:514–515. [Google Scholar]
- Hook DD, Brown CL, Wetmore RH. Aeration in trees. Botanical Gazette. 1972;133:443–454. [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annual Review of Plant Physiology. 1973;24:519–570. [Google Scholar]
- Hsiao TC, Acevedo E, Fereres E, Henderson DW. Stress metabolism: water stress, growth and osmotic adjustment. Philosophical Transactions of the Royal Society of London, Series B. 1976;273:479–500. [Google Scholar]
- Irvine J, Grace J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of the wood. Planta. 1997;202:455–461. [Google Scholar]
- Kaipiainen LK, Sofronova GI, Hari P, Yalynskaya EE. The role of xylem in CO2 exchange in Pinus sylvestris woody stems. Russian Journal of Plant Physiology. 1998;45:500–505. [Google Scholar]
- Kakubari Y. Diurnal and seasonal fluctuations in the bark respiration of standing Fagus sylvatica trees at Solling, West Germany. Journal of the Japanese Forestry Society. 1988;70:64–70. [Google Scholar]
- Lavigne MB. Differences in stem respiration responses to temperature between balsam fir trees in thinned and unthinned stands. Tree Physiology. 1987;3:225–233. doi: 10.1093/treephys/3.3.225. [DOI] [PubMed] [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. Journal of Theoretical Biology. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- MacDougal DT, Working EB. The pneumatic system of plants, especially trees. Washington, DC: Carnegie Insitute of Washington; 1933. [Google Scholar]
- Martin TA, Teskey RO, Dougherty PM. Movement of respiratory CO2 in stems of loblolly pine (Pinus taeda L.) seedlings. Tree Physiology. 1994;14:481–495. doi: 10.1093/treephys/14.5.481. [DOI] [PubMed] [Google Scholar]
- McGuire MA, Teskey RO. Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiology. 2004;24:571–578. doi: 10.1093/treephys/24.5.571. [DOI] [PubMed] [Google Scholar]
- Negisi K. Diurnal fluctuation of CO2 release from the stem bark of standing young Pinus densiflora trees. Journal of the Japanese Forestry Society. 1975;57:375–383. [Google Scholar]
- Negisi K. Daytime depression in bark respiration and radial shrinkage in stem of a standing young Pinus densiflora tree. Journal of the Japanese Forest Society. 1978;60:380–382. [Google Scholar]
- Negisi K. Bark respiration rate in stem segments detached from young Pinus densiflora trees in relation to velocity of artificial sap flow. Journal of the Japanese Forestry Society. 1979;61:88–93. [Google Scholar]
- Negisi K. Diurnal fluctuations of the stem bark respiration in relationship to the wood temperature in standing young Pinus densiflora, Chamaecyparis obtusa and Quercus myrsinaefolia trees. Journal of the Japanese Forestry Society. 1982;64:315–319. [Google Scholar]
- Nelder JA, Mead R. A simplex method for function minimization. Computer Journal. 1965;7:308–313. [Google Scholar]
- Proseus TE, Boyer JS. Periplasm turgor pressure controls wall deposition and assembly in growing Chara corallina cells. Annals of Botany. 2006;98:93–105. doi: 10.1093/aob/mcl098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyn ML, Gartner BL, Harmon ME. Respiratory potential in sapwood of old versus young ponderosa pine trees in the Pacific Northwest. Tree Physiology. 2000;22:105–116. doi: 10.1093/treephys/22.2-3.105. [DOI] [PubMed] [Google Scholar]
- Ray PM. Principles of plant cell expansion. In: Cosgrove DJ, Knievel DP, editors. Physiology of cell expansion during plant growth. Rockville, MD: American Society of Plant Physiologists; 1987. pp. 1–17. [Google Scholar]
- Ryan MG. Growth and maintenance respiration in stems of Pinus contorta and Picea engelmannii. Canadian Journal of Forest Research. 1990;20:48–57. [Google Scholar]
- Schurr U, Heckenberger U, Herdel K, Walter A, Feil RL. Leaf development in Ricinus communis during drought stress: dynamics of growth processes, of cellular structure and of sink–source transition. Journal of Experimental Botany. 2000;51:1515–1529. doi: 10.1093/jexbot/51.350.1515. [DOI] [PubMed] [Google Scholar]
- Sprugel DG. Components of woody-tissue respiration in young Abies amabilis trees. Trees. 1990;4:88–98. [Google Scholar]
- Steinberg S, van Bavel CHM, McFarland MJ. A gauge to measure mass flow rate of sap in stems and trunks of woody plants. Journal of the American Society for Horticultural Science. 1989;114:466–472. [Google Scholar]
- Steppe K. Diurnal dynamics of water flow through trees: design and validation of a mathematical flow and storage model. Belgium: PhD Thesis, Ghent University; 2004. [Google Scholar]
- Steppe K, Lemeur R. Effects of ring-porous and diffuse-porous stem wood anatomy on the hydraulic parameters used in a water flow and storage model. Tree Physiology. 2006;27:43–52. doi: 10.1093/treephys/27.1.43. [DOI] [PubMed] [Google Scholar]
- Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem PA. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiology. 2006;26:257–273. doi: 10.1093/treephys/26.3.257. [DOI] [PubMed] [Google Scholar]
- Stockfors J, Linder S. Effect of nitrogen on the seasonal course of growth and maintenance respiration in stems of Norway spruce trees. Tree Physiology. 1998;18:155–166. doi: 10.1093/treephys/18.3.155. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Yang FJ, Zu YG, Wang HM, Takagi K, Sasa K, Koike T. Stem respiration of a Larch (Larix gmelini) plantation in Northeast China. Acta Botanica Sinica. 2003;45:1387–1397. [Google Scholar]
- Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G. Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant, Cell and Environment. 2006;29:1149–1158. doi: 10.1111/j.1365-3040.2006.01495.x. [DOI] [PubMed] [Google Scholar]
- Woodruff DR, Bond BJ, Meinzer FC. Does turgor limit growth in tall trees? Plant, Cell and Environment. 2004;27:229–236. [Google Scholar]