Abstract
Background and Aims
Cassava (Manihot esculenta) has three adventitious root types: primary and secondary fibrous roots, and storage roots. Different adventitious root types can also regenerate from in vitro cultured segments. The aim of this study was to investigate aspects of in vitro production of storage roots.
Methods
Morphological and anatomical analyses were performed to identify and differentiate each root type. Twenty-nine clones were assayed to determine the effect of genotype on the capacity to form storage roots in vitro. The effects of cytokinins and auxins on the formation of storage roots in vitro were also examined.
Key Results
Primary roots formed in vitro and in vivo had similar tissue kinds; however, storage roots formed in vitro exhibited physiological specialization for storing starch. The only consistent diagnostic feature between secondary fibrous and storage roots was their functional differentiation. Anatomical analysis of the storage roots formed in vitro showed that radial expansion as a consequence of massive proliferation and enlargement of parenchymatous cells occurred in the middle cortex, but not from cambial activity as in roots formed in vivo. Cortical expansion could be related to dilatation growth favoured by hormone treatments. Starch deposition of storage roots formed in vitro was confined to cortical tissue and occurred earlier than in storage roots formed in vivo. Auxin and cytokinin supplementation were absolutely required for in vitro storage root regeneration; these roots were not able to develop secondary growth, but formed a tissue competent for starch storing. MS medium with 5 % sucrose plus 0·54 μm 1-naphthaleneacetic acid and 0·44 μm 6-benzylaminopurine was one of the most effective in stimulating the storage root formation. Genotypes differed significantly in their capacity to produce storage roots in vitro. Storage root formation was considerably affected by the segment's primary position and strongly influenced by hormone treatments.
Conclusions
The storage root formation system reported here is a first approach to develop a tuberization model, and additional efforts are required to improve it. Although it was not possible to achieve root secondary growth, after this work it will be feasible to advance in some aspects of in vitro cassava tuberization.
Key words: Auxins, cassava, cytokinins, in vitro root regeneration, Manihot esculenta, morphogenesis, storage organs, tuberization
INTRODUCTION
Root and tuber crops are important to the agroeconomy of several tropical countries. In addition to their role in contributing to household food needs, these crops are commonly marketed as fresh or processed products. Foremost among them, in terms of aggregate output and estimated value of production, are cassava (Manihot esculenta), potato (Solanum tuberosum), sweet potato (Ipomoea batatas) and yams (Dioscorea spp.).
Cassava has been traditionally considered a subsistence crop, but nowadays there is evidence from Africa, Asia and Latin America that documents its global emergence as a cash crop. The main value of root and tuber crops resides in the production of more edible energy per hectare per day than other crops and the capacity to generate high yields under conditions where other crops might fail (Scott et al., 2000).
The Manihot genus belongs to the Euphorbiaceae family and has about 100 species, among which cassava is the only one commercially cultivated (Alves, 2002). Cassava is a heliophile and perennial woody shrub, cultivated mainly in the tropics for its starchy tuberous roots. This crop presents easy propagation systems, high drought tolerance, satisfactory yields – even growing in low-fertility soils – low exigency for sophisticated cultural requirements, potential pest and disease resistance or tolerance, high root starch contents and good mechanization prospects (Cereda and Vilpoux, 2003). In addition, it represents the fourth greatest source of calories in the world, after rice (Oryza sativa), sugarcane (Saccharum officinarum) and maize (Zea mays) (Puonti Kaerlas, 1998).
The cassava root system is distinguished by different adventitious root types: fibrous roots (FRs) that absorb water and mineral salts or provide a support function, and storage roots (SRs), which accumulate starch as a reserve compound (Domínguez et al., 1982). The physiological process by which a stem section or a root undergoes morphological changes to become a special storage organ is termed tuberization (Melis and van Staden, 1984).
Potato is the most widely studied tuber crop, and most research on tuberization has been done on this species (Melis and van Staden, 1985; Struik et al., 1999). However, systematic information on the biology, physiology and biochemistry of the SR is scarce.
Numerous papers on cultivation and utilization of Manihot have been published, but they have little bearing on the in vivo tuberization process. The first complete study of the anatomy of cassava stem and root was conducted by Rateaver (1951), who also included experimental trials on organ regeneration. Indira and Kurian (1977) classified the roots into normal and tuber forming, based on an analysis of the anatomical changes undergone during cassava root tuberization. Mogilner et al. (1967a) reported the effect of photoperiod and segment primary position on yield capacity of the SRs. On the other hand, Mogilner et al. (1967b) assayed reciprocal grafts of hardy tapioca (Manihot flabellifolia) and cassava and their influence on SR formation and growth of aerial parts. Melis and van Staden (1985) studied the nature and distribution of cytokinins and abscisic acid in cassava tuberous roots. Carvalho et al. (1993) analysed, in cassava roots of two clones, the protein content and profile of the roots at different tuberization stages, and the activities of the enzymes related to starch biosynthesis in different fractions during amyloplast isolation. More recently it has been shown, using SDS–PAGE (Cabral and Carvalho, 2000) and two-dimensional gel electrophoresis (Cabral and Carvalho, 2001), that variation in the protein pattern complexity was correlated with root type. Batista de Souza et al. (2004) revealed five genes with a higher expression level in SRs than in FRs.
However, at present, only two studies have mentioned sporadic in vitro SR formation. One of them reported a protocol to regenerate a virus-free plant, in which occasional SR differentiation was observed from apical meristem tips incubated in a medium supplemented with 1-naphthaleneacetic acid (NAA) and 6-benzylaminopurine (BAP) (Kartha et al., 1974). Similar observations were made by Cabral et al. (1993), who developed a regeneration system via organogenesis and somatic embryogenesis.
These findings led to the need to develop an in vitro SR production model which would serve as a tool for genetic and physiological approaches.
The aims of the present research were: (a) to develop an in vitro SR formation system for cassava; (b) to establish a tuberization criterion to compare roots differentiated in the soil vs. roots obtained in vitro; (c) to distinguish FRs from SRs regenerated in vitro; (d) to test a range of 29 cassava genotypes for their capacity to form SRs in vitro; and (e) to determine the influence of some intrinsic (segment primary position) and extrinsic (plant growth regulators applied of the culture media) factors on the formation of SRs in vitro.
MATERIALS AND METHODS
Plant material and culture conditions for SR formation
Cassava (M. esculenta Crantz) stem cuttings with approximately six buds were planted in pots with a 1 : 1 mixture of black soil and fine sand to improve drainage, and maintained under greenhouse conditions.
Uninodal segments (approx. 5–8 mm long) dissected from cassava plants obtained in vitro were used as the source of explants for all the experiments. Donor plants were cultured on Murashige and Skoog basal medium (MS) (Murashige and Skoog, 1962), containing 3 % sucrose, supplemented with 0·054 µm NAA, 0·044 µm BAP and 0·289 μm gibberellic acid, and solidified with 0·75 % agar (Sigma; A-1296). The SR formation medium was comprised of MS, with 5 % sucrose, 0·75 % agar (Sigma; A-1296), with the addition of 0·54 μm NAA plus 0·44 μm BAP. The pH of the culture media was adjusted to 5·8, and tubes (43 mL capacity) were autoclaved at 1·46 kg cm−2 for 20 min. The cultures were covered with Resinite AF-50® film (Casco S.A.C. Company, Buenos Aires) and incubated in a growth room at 27 ± 2°C under a 14 h photoperiod regime with an irradiance of 116 μmol m−2 s−1 provided by cool white fluorescent lamps.
Morphological and anatomical analysis
In order to identify and differentiate SRs and FRs, morphological and anatomical analyses were performed.
Histological examination was performed on the ‘EC 27’ cassava clone with a high capacity to regenerate SRs in vitro and the ‘1468’ cassava clone with low production of SRs in vitro.
The plants that had been grown under glasshouse conditions were carefully uprooted and gently cleaned with tap water 7 months after planting. Roots produced in vitro were sampled and analysed after cultivation (3, 6, 9, 12, 15, 18 and 21 d). All samples were fixed in FAA (formalin, acetic acid, 70 % ethanol, 5 : 5 : 90) and dehydrated with ‘Deshidratante histológico BIOPUR®’ S.R.L. (Gonzalez and Cristóbal, 1997). They were embedded in paraffin as described by Johansen (1940), and sectioned into 10 µm thick serial sections with a rotatory microtome. Sections were mounted on glass slides, stained with safranin (C.I. 50240)–astra blue (Luque et al., 1996) and observed under a light microscope. Starch was visualized using Lugol's reagent.
For scanning electron microscopy, proximal segments of the regenerated SRs and FRs were fixed in FAA, and then dehydrated through a graded acetone series. Subsequently, the samples were critical-point dried, using liquid CO2 as the transitional fluid, coated with gold–palladium in a sputter coater and finally viewed with a JEOL 5800 LV scanning electron microscope (SEM).
Effect of genotype on in vitro SR formation capacity
Twenty-nine clones were assayed. The plants were grown in vitro in the cassava germplasm bank at the Tissue Culture Laboratory of the IBONE. The clones evaluated were the following: ‘EC 55’, ‘6’, ‘Cg = Catiguá’, ‘EC 24’, ‘EC 27’, ‘EC 35’, ‘EC 1’, ‘MCol 1505’, ‘Amarilla’, ‘EC 11’, ‘EC 90’, ‘EC 78’, ‘Palomita’, ‘Carapé’, ‘EC 3’, ‘70’, ‘140’, ‘EC 5’, ‘EC 7’, ‘76’, ‘CM 3306-4’, ‘60’, ‘EC 64’, ‘9’, ‘MPar 75’, ‘CM’, ‘CM 3372-4’, ‘EM 2600-2’ and ‘1468’.
Effect of segment primary position on in vitro SR formation
To determine the effect of the segment primary position on SR production, plants with at least ten nodes were used as the explant source. Uninodal segments of the ‘EC 27’ clone were excised from the apex (position 10) to the base (position 1) of the donor plants. Clone ‘EC 27’ was selected due to its exceptional capacity for production of SRs in vitro.
Effect of cytokinins and auxins on in vitro SR formation
In the first experiment, the effect of the cytokinins BAP (0·44, 4·44 and 13·32 μm), zeatin (ZEA; 0·46, 4·56 and 13·69 μm), thidiazuron (TDZ; 0·045, 0·45 and 1·36 μm), 2-isopentenyladenine (2iP; 0·49, 4·92 and 14·76 μm) and kinetin (KIN; 0·46, 4·65 and 13·94 μm) or the auxins NAA (0·54, 5·38 and 16·13 μm), 3-indoleacetic acid (IAA; 0·57, 5·71 and 17·14 μm), indolebutyric acid (IBA; 0·49, 4·93 and 14·78 μm) and 2,4-dichlorophenoxyacetic acid (2,4-D; 0·45, 4·52 and 13·57 μm), applied alone to the culture induction media was evaluated. The control medium was composed of MS containing 5 % sucrose, without plant growth regulators.
In the second experiment, based on previous reports (Kartha et al., 1974; Cabral et al., 1993), the influence of the combined supply of NAA and BAP at different concentrations (BAP at 0·004, 0·022, 0·044, 0·22, 0·44, 2·22 and 4·44 μm; NAA at 0·005, 0·027, 0·054, 0·27, 0·54, 2·69 and 5·38 μm) on the in vitro SR formation was evaluated.
For these experiments, uninodal segments of the clone ‘EC 27’ were also used as the source of explants.
Experimental design
The experiments were conducted in a randomized complete block design (i.e. 29 clones × 1 culture condition; 10 explant primary positions × 1 culture condition; 1 clone × n different media) with three replications. Each replication consisted of ten test tubes with one nodal segment per tube, making 30 explants per treatment.
Data recording and statistical analysis
Explants were incubated on the SR formation medium and data recorded after 15 and 30 d of culture for genotype and segment primary position studies. Data on the evaluation of the effects of the culture media on SR formation were scored only after 15 d of culture.
The non-destructive measurements taken were: percentage of explants with SRs, number of SRs per explant, SR length and basal diameter, percentage of explants that formed shoots and the length of the regenerated shoots; and the destructive measurement was SR dry weight per explant. To compare more integrally the results of the different treatments, an adimensional relative storage root production index (RSRPI) was calculated.
RSRPI = [(% explants with SRs) × (no. of SRs × explant) × (SR dry weight × explant)]/100
This index was used for all experiments, except to study the effect of the segment primary position on the in vitro SR formation, for which only the percentage of explants with SRs and the number of SRs per explant were considered.
Means are given with standard errors indicated in the bar chart graphics. Data were subjected to one-way analysis of variance (ANOVA) and comparisons of means were made by Duncan's multiple comparison test (P ≤ 0·05).
Based on the percentage of explants with SRs, the number of SRs per explant and the SR dry weight per explant, distance phenograms were constructed using InfoStat software professional version 1 · 1 (InfoStat, 2002). The data consisted of the three quantitative characters scored for each of the 29 clones and 36 media analysed, respectively [each clone or medium represented one operational taxonomic unit (OTU)]. Cluster analysis was based on the average Euclidean distance coefficient. The method consisted of calculating a distance coefficient between each pair of the OTUs and linking the OTUs in a 2-D graph (phenogram). The resulting OTU × OTU distance matrix served as input in the calculation of a phenogram by the unweighted pair-group method, using arithmetic averages, UPGMA (Sneath and Sokal, 1973). The data that arise from the distance coefficient calculation using the three variables mentioned above were subjected to multivariate analysis of variance (MANOVA) according to Lawley–Hotelling (P < 0·0001) and comparisons of means performed by Hotelling's test (P ≤ 0·05).
RESULTS
Morphological analysis
The roots derived from stems growing in pots appeared in groups emerging from a callus proliferating at the distal end of the cutting, and originated an adventitious root system. Seven months later, three types of roots were found. In Fig. 1A, a light yellow root is observed, with uniform section and not more than 1 mm thick (from hereon called an in vivo primary FR). In Fig. 1B and C, two root types are shown that evidence a woody aspect and hard consistency. The root in Fig. 1B has a tortuous shape with a furrowed peridermis in some portions and a brown, brassy colour. This root type (in vivo secondary FR) is long and with a resistant cord aspect, as noticed when taken out of the substrate. In Fig. 1C a portion of an in vivo sessile SR is shown, that has a maximum thickness of around 6 cm, the diameter diminishing towards the distal end (radical meristem tips), also protected by a dark brown, scaly peridermis. Many in vivo SRs were inserted into the stem through a peduncle.
Fig. 1.
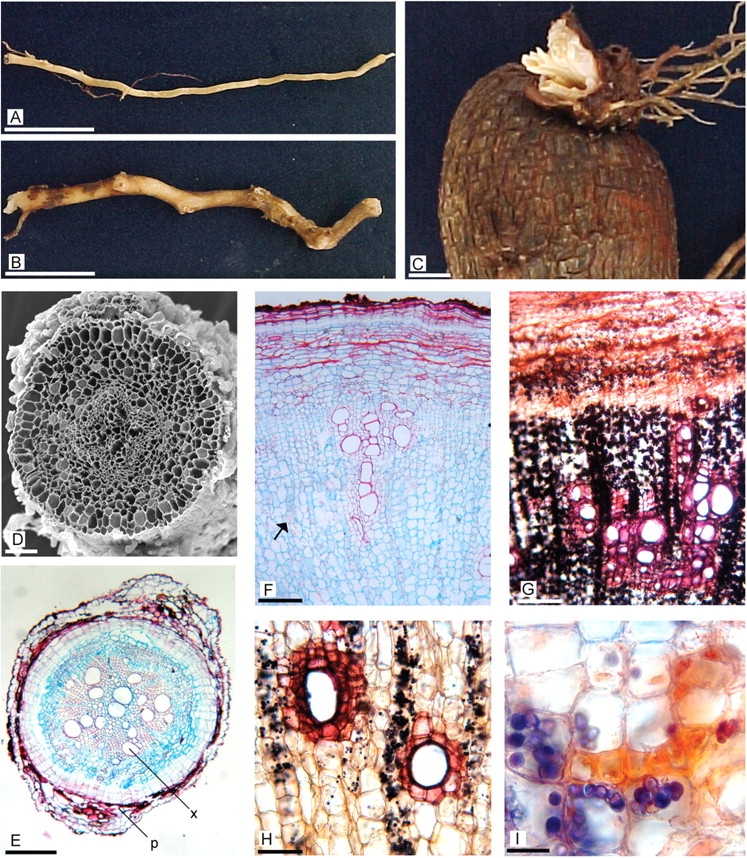
Morphological and anatomical characters of in vivo fibrous (FR) and storage roots (SR) of cassava stem cuttings cultured in pots. (A) Primary FR. (B) Secondary FR. (C) Sessile SR. (D) Transverse section of a primary FR. (E) Transverse section of a secondary FR. E, x, secondary xylem; p, peridermis. (F) Transverse section of an SR (arrow = xylem parenchyma). (G–I) Transverse section of an SR stained with safranin and Lugol's reagent. (H) Detail of the secondary xylem. (I) Detail of the secondary phloem. Scale bars: A–C = 1 cm; D–G = 50 µm; H = 100 µm. I = 20 µm.
Uninodal segments of cassava clones ‘EC 27’ and ‘1468’ cultured in vitro on the SR formation medium were analysed. After 3 d of culture, the explants sprouted and formed adventitious roots which originated from a direct or indirect process. After 15 d of culture, the roots were clearly distinguished as two types, fine FRs (Fig. 2A) and thick SRs, which could be sessile (Fig. 2B) or pedunculated (Fig. 2C, arrow: peduncle) according to the insertion. This terminology was adopted from Alves (2002) and El-Sharkawy (2003). Explants with both root types were often observed (Fig. 2D).
Fig. 2.
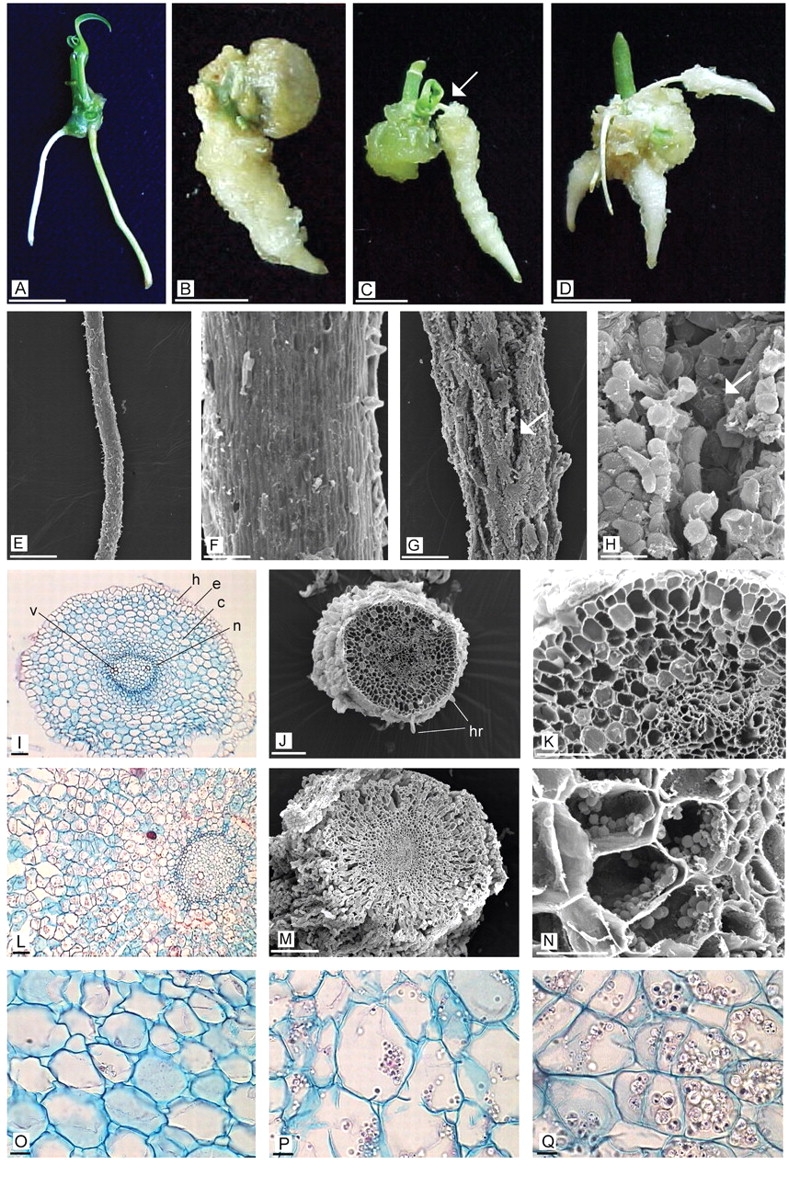
Morphological and anatomical differences between in vitro fibrous (FR) and storage roots (SR) of cassava regenerated in vitro from uninodal segment culture. (A) Explant with an FR. (B) Explant with a sessile SR. (C) Explant with a pedunculated SR (arrow: peduncle). (D) Explant with an FR and SR. (E, F) Scanning electron photomicrographs of an FR. (G and H) Scanning electron photomicrographs of an SR (arrow: longitudinal fissures). (I–K) Transverse sections of an FR. (I) e, epidermis; h, hypodermis; c, cortex; n, endodermis; v, vascular cylinder. (J) hr, root hairs. (L–N) Transverse sections of an SR. (O) Cortical cells of an FR after 21 d of culture. (P, Q) Cortical cells of an SR after 9 and 15 d of culture, respectively. Scale bars: A–D = 0·5 cm; E, G = 1 mm; F, H, J = 100 µm; I, K, L, N = 50 µm; M = 25 µm. O–Q, = 10 µm.
In vitro FRs were fine and had a zone with abundant root hairs (Fig. 2E). The root surface analysed by SEM presented a homogeneous and smooth epidermis composed of epidermal cells elongated longitudinally. These cells were closely packed, forming a compact layer without intercellular spaces (Fig. 2F).
The basal diameter of in vitro SRs was notably larger than that of FRs, in most cases it ranged between 2 and 3 mm (Fig. 2G). This type of root presented a furrowed surface with longitudinal fissures (Fig. 2G, H; arrow) caused by root thickening. In the zone of fissures, the epidermis was loose and the subepidermic layers belonging to the cortical parenchyma were seen. Where the epidermis was preserved, the epidermal cells were rounded and compactly joined together without intercellular spaces (Fig. 2H).
Anatomical analysis
The root transections showed peculiarities according to the root type, but not according to the genotypes analysed.
Transverse sections of the in vivo primary FRs (Fig. 1D) showed a continuous layer of small cells that form the epidermis, in which root hairs can be differentiated; the cortex consisted of 6–11 layers of small parenchymatous cells closely arranged with small intercellular spaces. No starch granules were observed in this tissue. Two layers of cortical cells stand out, an external layer beneath the epidermis (hypodermis) with cells lengthened in the radial direction and an internal layer (endodermis) with small cells that surround the central cylinder. Adjoining the endodermis was the pericycle, composed of 1–3 layers of parenchymatous cells, from which lateral roots arose. The vascular cylinder consisted of a centrally located exarch stele comprising xylem and phloem tissues. The xylem was either tetrarch or pentarch within the same clone ‘EC 27’ or ‘1468’. Xylem and phloem alternated with each other. Inside the vascular cylinder was the pith region formed by thin-walled parenchymatous cells.
The transections of the in vitro primary FRs (Fig. 2I–K) demonstrated that this root type was highly similar to those primary FRs grown in pots. In vitro (Fig. 2I–K) and in vivo (Fig. 1D) primary FRs lacked starch granules and had an identical tissue composition and organization.
The other two root types from plants growing in pots undergo secondary growth. In the anatomical analyses one showed a limited cambial activity forming an in vivo secondary FR with absorption and support functions, and the other showed a massive proliferation of the xylem parenchyma, termed the in vivo SR, whose function is dedicated to reserve storage compounds.
In transverse section, in vivo secondary FRs presented, from the centre to the edge (Fig. 1E): secondary xylem constituted by an axial system with solitary or multiple vessel members, sometimes filled with tylose; an axial parenchyma with thick-walled cells and fibres; and a radial system composed of rays (uniseriate, biseriate or multiseriate of up to three bands) of thin-walled parenchyma cells. It is necessary to highlight that, in some cases, plants that were cultivated for more than a cycle presented starch granules in the rays of the secondary xylem. Subsequently, the vascular cambium was observed, composed of small cells and, adjacent to this, secondary phloem and layers of thin-walled parenchyma cells were found with no evidence of starch-granule accumulation. Externally was the peridermis, formed by the phellogen-producing phelloderm (layer of cells without suberized walls) centripetally, and phellem (layer of cork cells that constitutes the external bark) centrifugally. The cell layers inside the functional phellogen up to the secondary phloem, comprise the internal bark.
In vivo SRs (Fig. 1F) and secondary FRs had, in general terms, a similar structure to that seen in the transverse sections; however, they clearly differ in the xylem parenchyma size which constitutes the principal reserve tissue (Fig. 1F, arrow; Fig. 1G, blue staining indicates the presence of starch). Up to a certain point (portion) of the secondary xylem, axial and radial systems can be differentiated, with an evident reserve accumulation with a radial pattern (Fig. 1H). Following this portion, such organization becomes less conspicuous because all parenchymatous cells become competent to store starch granules (lower part of Fig. 1G) forming a spongier tissue. In contrast to the secondary FR, the parenchyma layers external to the vascular cambium also accumulate starch granules. In the parenchymatous cells of secondary phloem (Fig. 1I) and adjacent cortical parenchymatous cells, the accumulation pattern spreads in bands or layers parallel to the surface (upper part of Fig. 1G).
The epidermis of in vitro SRs was composed of a uniseriate stratum of cells, which was fragmented mainly near the root base, due to growth and dilatation of the cortical tissue in the radial direction (Fig. 2L, M). The cortex was formed of >11 layers of cells which were relatively organized in radial files. The middle cortex was divided periclinally, increasing the layer number and generating a swelling in the radial direction. As a consequence of this radial thickening, fissures originated and reached up to the upper third of the cortical tissue. Moreover, this tissue presented large intercellular spaces (Fig. 2L). The most remarkable finding was the abundant deposition of starch granules (Fig. 2 N) either singly or as 2–4 elements. Single granules had a cylindrical shape, and the compound granules were oval and truncated. This morphology is coincident with that of starch granules synthesized in vivo. Delimitating the cortex was the endodermis composed of a single layer of cells. Around the vascular cylinder was the pericycle which consisted of one or three cell layers. Like the FR structure, the xylem alternated with the phloem and it was either tetrarch or pentarch within the same clone ‘EC 27’ or ‘1468’. The central region of the vascular cylinder was occupied by parenchymatous cells, forming the pith. In some cases, the pith was hollow.
After 21 d of culture, the in vitro FRs did not show starch granules (Fig. 2O) in any of the evaluated clones. However, after 6 d of culture, small starch granules were observed in the SRs, sometimes grouped together, similar to those found at 9 d (Fig. 2P). After 15 d of culture, some parenchymatous cells were almost filled with starch granules undergoing an extraordinary increase in size (Fig. 2Q), and compound granules were easily distinguishable. After 21 d of culture, the SR cortical tissue presented a large deposition of starch granules (data not shown). When serial slides of the transverse sections were analysed, centrifugal starch deposition was determined. On the other hand, longitudinal sections showed that the store decreased towards the root distal end.
Effect of genotype on in vitro SR formation
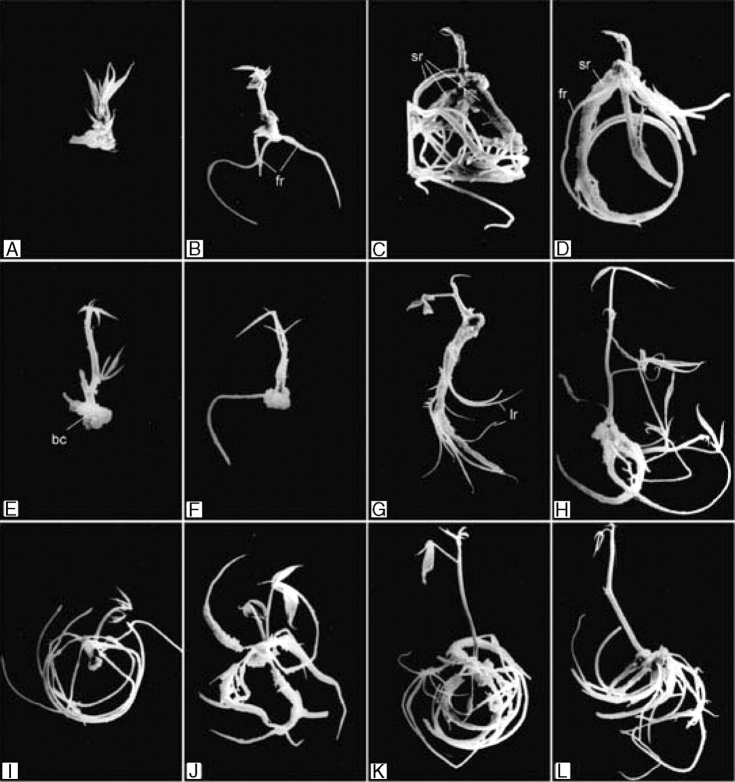
After 15 and 30 d of culture, the explants showed 12 different morphogenetic responses, which were organized into a dichotomic key (Table 1). Lateral roots were not considered to typify the responses obtained. Figure 3 shows each morphogenetic response described in Table 1.
Table 1.
Dichotomic key for the identification of the morphogenetic responses obtained in vitro
| 1. Uninodal segment without roots | |
| 2. Without basal callus | Type A (Fig. 3A) |
| 2′. With basal callus | Type B (Fig. 3E) |
| 1′. Uninodal segment with roots | |
| 3. With roots at the lower or the upper part of segment | |
| 4. With roots at the lower part of segment | |
| 5. With direct roots (FR or/ and SR) | |
| 6. With an only root type | |
| 7. With direct FR | Type C (Fig. 3B) |
| 7′. With direct SR | Type D (Fig. 3C) |
| 6′. With both root types | Type E (Fig. 3D) |
| 5′. With indirect roots (the roots were differentiated from basal callus of the segment) | |
| 8. With an only root type | |
| 9. With indirect FR | Type F (Fig. 3F) |
| 9′. With indirect SR | Type G (Fig. 3G) |
| 8′. With both root types | Type H (Fig. 3H) |
| 4′. With roots at the upper part of segment, but always with basal callus without roots | |
| 10. With an only direct root type | |
| 11. With FR | Type I (Fig. 3I) |
| 11′. With SR | Type J (Fig. 3J) |
| 10′. With both direct root types | Type K (Fig. 3K) |
| 3′. With FR and/or SR at both parts of segment | Type L (Fig. 3L) |
Fig. 3.
In vitro morphogenetic responses differentiated from uninodal segment culture of cassava in SR formation medium (MS 5 % sucrose + 0·54 μm NAA + 0·44 μm BAP). Bc, basal callus; fr, fibrous roots; lr, lateral roots; sr, storage roots.
After 15 and 30 d of culture, the most frequent responses were types D and G. To simplify the analysis, all the types relating to SR formation were considered explants with SRs.
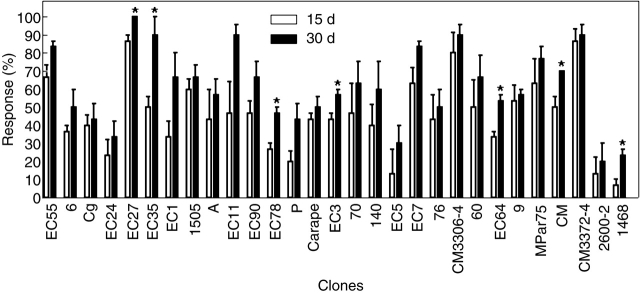
The increase of the percentage of explants with SRs between 15 and 30 d was only significant for seven clones: ‘1468’, ‘EC 78’, ‘EC 64’, ‘EC 3’, ‘CM’, ‘EC 35’ and ‘EC 27’ (Fig. 4) according to Duncan's multiple comparison test (P ≤ 0·05).
Fig. 4.
Effect of genotype on the capacity for in vitro SR formation: percentage of explants with an SR. Data are means ± s.e. of three replications. The asterisks indicate statistically significant differences between the percentage of explants with an SR scored at 15 and 30 d, according to Duncan's multiple comparison test (P ≤ 0·05).
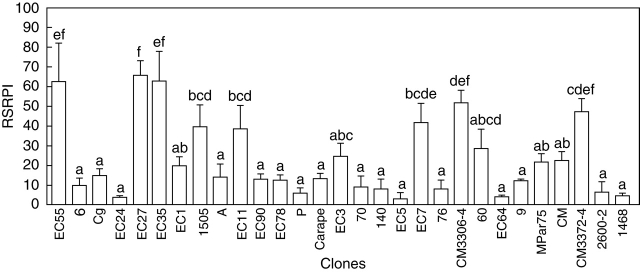
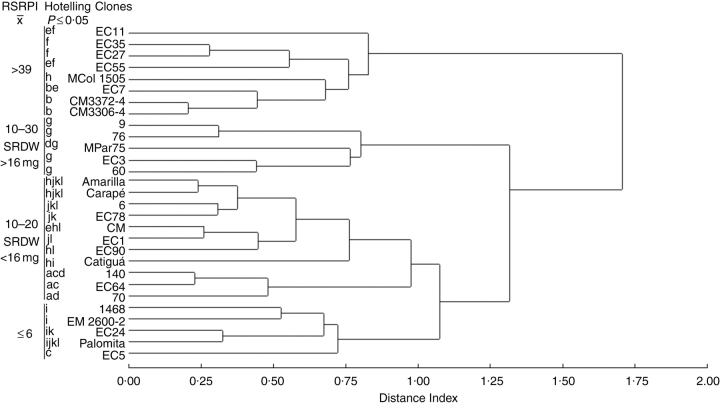
Although all evaluated genotypes produced SRs in vitro (Fig. 5), their capacity to do so varied significantly. According to the average linkage using the Euclidean distance of the 29 clones assayed, they were grouped into four clusters with a cut-off limit of 1. The cluster was correlated with the RSRPI values (Fig. 6). The groups with an RSRPI of 10–30 and 10–20 were distinguished by differences in the average dry weight of SRs (>16 mg and <16 mg, respectively). MANOVA indicated significant differences among genotypes according to Hotteling's test (P ≤ 0·05). Among the high yield clones (RSRPI > 39), clone ‘EC 27’ demonstrated high response uniformity.
Fig. 5.
Effect of genotype on the capacity for in vitro SR formation: RSRPI, relative storage root production index. Data are means ± s.e. of three replications. Different letters indicate statistically significant differences between clones scored at 30 d, according to Duncan's multiple comparison test (P ≤ 0·05).
Fig. 6.
Phenogram of 29 OTUs (29 clones) and three characters (the percentage of explants with an SR, the number of SRs per explant and the SR dry weight per explant) resulting from the UPGM cluster analysis of the OTU × OTU distance matrix. The coefficient used was the average Euclidean distance. Different letters indicate statistically significant differences between clones according to Hottelling's test (P ≤ 0·05). Clone clusters can be related to the RSRPI (relative storage root production index) and SRDW (SR dry weight).
With regard to SR length, the longest ones derived from the ‘EM 2600-2’ clone (10 cm long) and the shortest from the ‘EC 5’ clone (approx. 4 cm long). The basal diameter varied between 2 and 3 mm. The average percentage of explants that formed shoots ranged from 60 % (clone ‘60’) to 100 % (clones ‘EC 90’ and ‘EC 3’), showing a high variation of shoot length from 5 mm (clones ‘EC 5’, ‘Palomita’, ‘EC 24’ and ‘EC 55’) to 60 mm long (clone ‘70’) (data not shown).
Effect of segment primary position on in vitro SR formation
After 15 d of culture, the explants also demonstrated the 12 morphogenetic responses shown in the dichotomic key. The most frequent response was type G. The types relating to SR formation represented a greater percentage of response than those that only included FRs. The type L response was only observed from intermediate or basal segments.
From the first to sixth positions, the percentage of explants with SR reached 90–100 %. At 15 and 30 d of culture, this variable and the number of SRs per explant were significantly different depending on the segment primary position (Table 2). However, the differences in the percentage of explants with an SR produced between 15 and 30 d of culture were not statistically significant.
Table 2.
Effect of segment primary position on in vitro SR formation
| Nodes | Positions | Incubation time (d) | SRs per explant (%) | No. of SRs per explant | Hotelling's test (P ≤ 0·05) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apical | 10 | 15 | 33·33 | 1·07 | A | ||||||
| 30 | 46·67 | 1·67 | B | ||||||||
| 9 | 15 | 63·33 | 1·97 | B | C | ||||||
| 30 | 83·33 | 2·57 | D | E | F | ||||||
| 8 | 15 | 70 | 2·37 | C | D | ||||||
| 30 | 76·67 | 2·67 | D | F | |||||||
| Intermediate | 7 | 15 | 63·33 | 1·93 | B | C | |||||
| 30 | 66·67 | 1·93 | C | ||||||||
| 6 | 15 | 86·67 | 2·1 | D | F | ||||||
| 30 | 90 | 2·27 | D | E | F | ||||||
| 5 | 15 | 100 | 2·67 | E | G | ||||||
| 30 | 100 | 2·87 | G | ||||||||
| 4 | 15 | 96·67 | 2·87 | E | G | ||||||
| 30 | 96·67 | 3·27 | G | ||||||||
| Basal | 3 | 15 | 93·33 | 2·8 | E | F | G | ||||
| 30 | 93·33 | 3·03 | G | ||||||||
| 2 | 15 | 90 | 2·8 | E | F | G | |||||
| 30 | 93·33 | 3·2 | G | ||||||||
| 1 | 15 | 90 | 2·17 | D | E | F | |||||
| 30 | 90 | 2·67 | E | F | G | ||||||
RSRPI, relative storage root production index. Data are means ± s.e. of three replications. The percentage of explants with an SR and the number of SRs per explant obtained from each position after 15 and 30 d of culture were subjected to MANOVA. Different letters indicate statistically significant differences according to Hottelling's test (P ≤ 0·05).
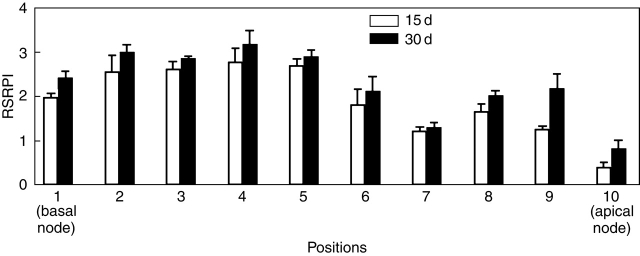
The RSRPI, independently of the incubation time, varied significantly with the segment primary position. RSRPI was higher in the explants coming from intermediate (fifth and forth position) and basal segments (third to the first position) than the apical explants (Fig. 7). The length of SR increased with the explant primary position; however, the basal diameter remained constant at 3 mm thick (data not shown).
Fig. 7.
Effect of segment primary position on in vitro SR formation. RSRPI, relative storage root production index. Data are means ± s.e. of three replications. Data were scored at 15 and 30 d of culture.
Effect of cytokinins and auxins on in vitro SR formation
Regarding the first experiment, in all media with cytokinins or auxins alone or those without them, the explants produced only FRs (data not shown). The lowest percentage of explants with an FR (30 %) was obtained with the medium supplemented with 13·32 μm BAP, followed by the media with 0·45 μm TDZ (60 %) and 1·36 μm TDZ (65 %), and the remaining media with the addition of the other cytokinins ranging from 70 to 90 %. Meanwhile, the addition of most auxins used at different concentrations produced 100 % of explants with an FR, except for the media with 4·93 μm IBA (90 %), 0·54 μm NAA (80 %), 0·57 μm IAA (75 %), 4·52 μm 2,4-D (70 %) or 13·57 μm 2,4-D (65 %), and the control medium (90 %). Therefore, the application of cytokinins or auxins alone to the culture media was completely ineffective in inducing SR formation at 15 d of culture. The number of FRs per explant derived from all media with cytokinins alone or without them ranged from three to six per explant, except for 13·32 μm BAP (yielding only one FR per explant). However, all auxins promoted the differentiation of 4–9 FRs per explant. On the other hand, the basal diameter of the roots regenerated in the medium supplemented with 13·32 μm BAP was 1 mm, while in the remaining media the roots were about 0·5 mm thick (data not shown).
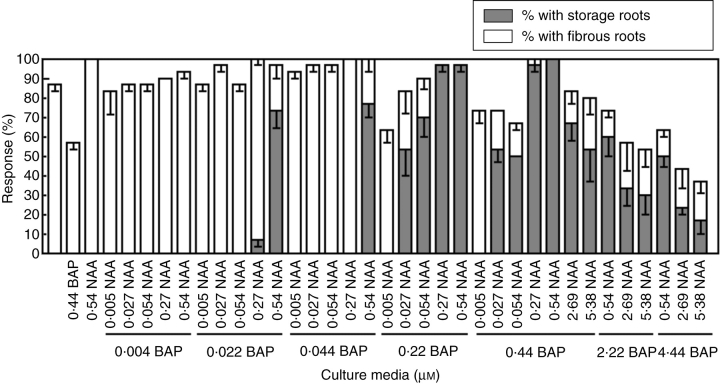
The results of the second experiment showed that the combination of the appropriate concentrations of NAA and BAP was essential for the in vitro SR formation (Fig. 8). After 15 d of culture, the explants also differentiated 12 morphogenetic responses as described in Table 1, but the frequency of the different types depended on the media. As in the first experiment, in the media with 0·44 μm BAP or 0·54 μm NAA alone or without additions, it was not possible to regenerate SRs. In the same way, when the media were supplemented with either 0·005 μm NAA or 0·004 μm BAP, each combined with other BAP or NAA concentrations, SRs were not differentiated. Similar results were obtained when the BAP concentrations were 0·022 or 0·044 μm combined with some NAA concentrations (i.e. 0·005, 0·027, 0·054 or 0·27 μm), except for 0·022 μm BAP + 0·27 μm NAA which yielded the lowest RSRPI. However, when the media contained 0·54 μm NAA combined with 0·022 or 0·044 μm BAP, the percentage of explants with SRs and the number of SRs per explant increased significantly although the SR dry weight per explant (data nor shown) and the RSRPI remained low (Fig. 9).
Fig. 8.
Effect of the combined supply of NAA and BAP on in vitro root formation. The percentage of explants with an SR and the percentage of explants with an FR. Data are means ± s.e. of three replications. Only data obtained after 15 d of culture are presented.
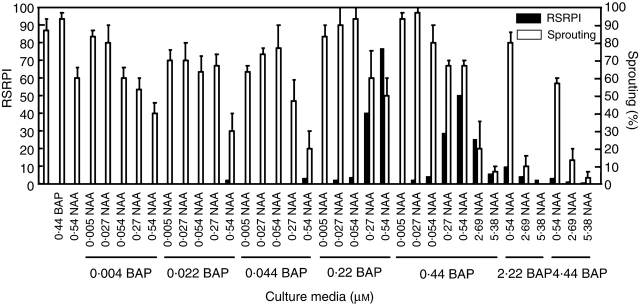
Fig. 9.
Effect of the combined supply of NAA and BAP on in vitro SR formation and bud sprouting. RSRPI, relative storage root production index. Percentage sprouting. Data are means ± s.e. of three replications. Only data obtained after 15 d of culture are presented.
The best responses were achieved when 0·54 μm NAA was combined with either 0·22 or 0·44 μm BAP (Fig. 9). It is important to recognize that the MS medium supplemented with 0·54 μm NAA + 0·44 μm BAP promoted SR formation in 100 % of the explants (Fig. 8) although the number of SRs per explant, the SR dry weight per explant (data not shown) and the RSRPI were lower than the other combinations. A considerable performance was also demonstrated by media supplied with 0·27 μm NAA + 0·22 or 0·44 μm BAP.
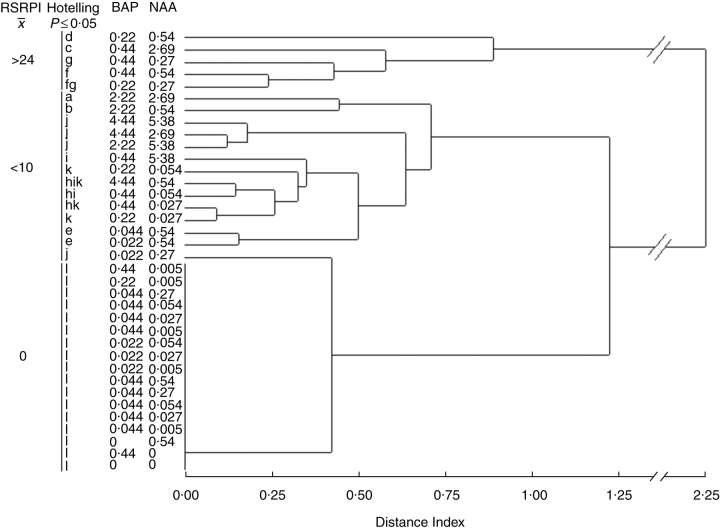
The distance phenogram constructed considering the three variables scored for the media evaluation at 15 d of culture discriminated three clusters with a cut-off limit of 1. These groups could be associated with the media's capacity to promote high, intermediate or low (or null) in vitro SR production (Fig. 10). MANOVA showed statistical differences between the results of the different media according to Hotteling's test (P ≤ 0·05).
Fig. 10.
Phenogram of 36 OTUs (36 media) and three characters (the percentage of explants with an SR, the number of SRs per explant and the SR dry weight per explant) resulting from the UPGM cluster analysis of the OTU × OTU distance matrix. The coefficient used was the average Euclidean distance. Different letters indicate statistically significant differences between the results of the different media according to Hottelling's test (P ≤ 0·05). Clusters can be related to RSRPI (relative storage root production index).
Higher concentrations of NAA and BAP combinations decreased the SR differentiation, even damaging the shoot regeneration (Fig. 9) and the rooting process (Fig. 8). The resultant RSRPI were similar to those scored for the lower concentrations of plant growth regulators. A remarkable fact was that the percentage of explants with shoots was significantly higher in the media that did not favour SR formation (Fig. 9).
DISCUSSION
Adventitious roots are present in many terrestrial plants and may help the species adapt to each life mode through the development of alternative forms. Radical dimorphism and trimorphism has been extensively documented (Samb and Kahlem, 1983; Barlow, 1986; Greig and Mauseth, 1991; Barlow, 1994) although little work has been done on the factor responsible for regulating the root type. It is believed that the different patterns are the result of genetic control strongly linked with environmental signals (Barlow, 1986). As has been seen here, cassava plants present a remarkable radical polymorphism that can also be influenced by external factors.
No specific research about in vitro cassava SR formation has been reported yet. Kartha et al. (1974) and Cabral et al. (1993), who worked on in vitro plant regeneration, mentioned that the explants could differentiate in vitro SRs under certain conditions. It is important to emphasize that this affirmation was based on the apparent morphological similarity of the in vitro and in vivo SRs.
The formation of storage organs comprises two different aspects: (a) the morphological development of the organ; and (b) the biochemical changes resulting in the biosynthesis and storage of reserve compounds (Xu et al., 1998). According to the root function classification described in Barlow (1994), this root type responds to the storage subsystem and represents a life strategy for capitalizing on the excess of assimilates. The facts that supported the comparison of the in vitro SRs with the in vivo SRs were not only the exomorphological similarity mentioned above, but also the development of the cortical parenchyma to function as a storage organ and the massive deposition of starch granules.
An SR is a root that has altered its polarity in growth and produces cells in a radial direction (Melis and van Staden, 1984). According to Domínguez et al. (1982), the essential difference between the in vivo SR and the FR of cassava lies in the growth polarity change from longitudinal to radial; however, this does not imply that the root ceased its longitudinal growth. For the development of a field-grown cassava SR, this change is accomplished through vascular secondary growth (Indira and Kurian, 1977). The primary root growth is associated with the longitudinal polarity characterized by an elongating root tip and a non-elongating basal portion. Secondary growth may develop within non-elongating primary root tissue, accompanied by the formation of secondary tissues, and resulting in a secondary polarity which is orthogonal (i.e. in the radial and circumferential planes) to the primary axial polarity upon which it is superimposed (Barlow et al., 2004). The anatomical analysis of the in vitro cassava SR allowed affirmation that the expansion in radial direction was carried out from a massive proliferation and enlargement of the parenchymatous cells in the middle cortex, but not from cambial activity. In the in vitro cassava SRs, the switch from longitudinal to radial growth might have been activated early as a consequence of inductive treatments (i.e. exogenous application of plant growth regulators), without undergoing secondary growth.
In early works about in vitro secondary growth differentiation, the general conclusions were that only under special culture conditions was this phenomenon possible. Torrey and Loomis (1967) reported that radish (Raphanus sativus) isolated roots formed cambium and secondary vascular tissue by using the basal feeding technique and hormone mixtures at the appropriate concentrations. In sweet potato, SR production under hydroponic culture and the in vitro regulation of secondary growth has been difficult. Nakatani (1994) demonstrated that only by improving the physical culture conditions was it possible to differentiate secondary growth from isolated roots 6–7 months after culture.
In other dicotyledonous plants such as primrose (Primula acaulis), the primary roots represent the storage organs. These roots increase the number of cortical cell layers mainly through secondary dilatation growth for the reactivation of primary middle cortex and a very weak cambial activity which only produced a limited thickening that remained confined to the narrow stele (Lux and Luxová, 2003/,4). Dilatation growth occurs in different tissues such as primary cortex (especially middle cortex), rhizodermis, axial parenchyma and the rays of bark, and it involves cell enlargement and/or renewed cell division (Lux et al., 2004). The peripheral tissue enlargement is produced by tangential and radial expansion of cells and is regulated by different factors, mainly plant growth regulators rather than physical forces (Lev-Yadun and Aloni, 1992). For example, Ludwigia peploides differentiate two root types as an adaptation to the aquatic environment, one of them forms a spongy cortex as a consequence of the radial expansion of the cells and the other has a more compact cortex, probably in order to absorb nutrients better (Ellmore, 1981; Barlow, 1986).
Apparently, the cortical tissue expansion observed in in vitro SRs of cassava could be related to a phenomenon of dilatation growth favoured by hormone treatments.
On the other hand, the in vitro SRs and FRs presented similarities with regard to the kind of root tissues, but there were physiological differences, as occurred among secondary roots in vivo. The in vitro and in vivo SRs exhibit physiological specialization for storage of reserve compounds. Although in vivo SRs could not to be strictly compared with the in vitro SRs, these presented features that could define them as storage organs.
The deposition of starch granules was taken as the criterion for differentiation of in vivo FR to SR initials (Indira and Kurian, 1977), although in later stages it is also possible to find some starch granules in the rays of in vivo FRs. In the in vitro SRs, an early massive accumulation of starch granules could also be seen, unlike the in vitro FRs.
It has been well established that primary roots can also store substances in the cortex, mainly starch (Fahn, 1990; Moore et al., 1995). However, Indira and Kurian (1977) and Moraes Dallaqua and Coral (2002), studying different cassava clones, reported that the starch deposition in roots grown in the field was always preceded by secondary growth. From the present findings, it can be concluded that an in vitro promoted specialized root, despite its primary structure, was able to store a massive quantity of starch granules. Therefore, it is considered appropriate to call them in vitro SRs. The starch granule deposition in the in vitro SRs was confined to cortical tissue and it occurred earlier than in SRs growing in vivo. After 6 d of culture, the in vitro SR, irrespective of the clone analysed, presented small starch granules; however, the in vivo SRs began to store reserve compounds only after secondary growth, at 26 d after planting (Indira and Kurian, 1977), or after 85–90 d (Lorenzi and Dias, 1993; Moraes Dallaqua and Coral, 2002).
In other species such as sweet potato, the starch deposition in field-grown SRs was confined to cortical tissue at 8 d, and at 20 d it extended to the vascular region which was increased by anomalous cambium (Indira and Kurian, 1977). In potato microtubers, the starch accumulation occurred in large cortical and pith parenchyma, which are thickened early (Ulloa et al., 1997), this being confirmed by Xu et al. (1998), who demonstrated that cortex and pith were involved in potato microtuberization, using a similar composition of media to that used in the present work. Xu et al. (1998) observed the same growth pattern in tubers growing in vivo, as did Vreugdenhil et al. (1999), who also reported that the starch accumulation was visible after 5 d of culture, in tubers of plants growing in pots and treated with short day conditions.
It was observed that the in vitro SR formation was strongly affected by the genotype in cassava. Likewise, the production of the plants grown in the field was significantly influenced by this factor (Montaldo et al., 1979), but it also varied with the year of evaluation (Henain and Cenoz, 1970) or planting site (Iglesias et al., 1998). Significant differences in the in vitro potato tuberization due to genotype have been extensively reported (Belletti et al., 1994; Leclerc et al., 1994; Gopal et al., 1998).
Information on how the stem position in the cassava plant affects the formation of the SR is scarce. Cabral and Carvalho (2000) observed that apical stem cuttings did not produce shoots or roots, while basal cuttings regenerated vigorous shoots and showed earlier SR formation. The present experiments were conducted under in vitro conditions and the results were similar to those obtained by Cabral and Carvalho (2000), since the explants coming from apical parts were less responsive than those from middle and basal parts. However, Mogilner et al. (1967a) reported that apical stem cuttings produced the highest SR fresh weight although the number of SRs per stem cuttings was lower. The effect of segment primary position may be related to several factors, such as stem age, bud maturity in different portions of the stem, and stem reserves (Cabral and Carvalho, 2000), which have been observed to increase from the top to the bottom of the plant stem (Mogilner et al., 1967a; Ramanujan and Indira, 1984).
The in vitro tuberization of radish was absolutely dependent on the requirements for an auxin and a cytokinin; in the absence of any one critical component, the response was markedly reduced or absent (Loomis and Torrey, 1964; Torrey and Loomis, 1967; Webster and Radin, 1972). Radish roots cultured under favourable conditions were able to undergo secondary growth although the cambial activity ceased after 28 d (Loomis and Torrey, 1964). Optimal concentrations of IAA (10 µm) and BAP (5 µm) stimulated an extraordinary increase in root diameter during the first 2–3 weeks of culture. IAA could be effectively substituted with NAA or 2,4-D in the presence of BAP, although the medium with NAA at 1 µm allowed the development of a better radial symmetrical structure. Peterson (1973) also found that an auxin and a cytokinin were necessary for maximum radial growth of the excised roots of turnip (Brassica rapa) behaving like radish. Neither Torrey and Loomis (1967) nor Peterson (1973) mentioned anything about starch deposition in differentiated SRs. In other species with a dimorphic root system, such as Jussiaea repens, the root type emerging at a node is controlled by the ratio of auxins and cytokinins (Samb and Kahlem, 1983).
Cassava in vitro SRs also required an auxin (NAA) and a cytokinin (BAP) to regenerate. They were not able to develop secondary growth, but formed a tissue competent to store reserve compounds, unlike in vitro FRs.
It has been postulated that hormones cause nutrient mobilization by creating new source–sink relationships, especially cytokinins. The metabolism of the treated area may be stimulated by the hormones so the nutrients move towards it (Salisbury and Ross, 1994; Taiz and Zeiger, 1998). Previous reports (Palmer and Smith, 1969; van Staden and Dimalla, 1976) suggested that cytokinins in the tubers act by establishing a metabolic storage sink which attracts metabolites to the organ in which they are present. This phenomenon could be related to the increase of the tuber growth when a cytokinin was exogenously applied (Gopal et al., 1998). According to Melis and van Staden (1984), cytokinins could attract metabolites together with auxins. Later Ehneß and Roitsh (1997) demonstrated that the sugar uptake was increased in response to cytokinin treatment in cells of Chenopodium rubrum. In a further review, the regulation of source–sink relationships by cytokinins and their interactions with other signals were dealt with extensively (Roitsh and Ehneß, 2000). These relationships (i.e. between auxins and cytokinins) may cause additive, synergic or inhibitory effects (Gifford and Evans, 1981). The NAA and BAP combinations possibly promoted, in some in vitro cassava roots, the establishment of a metabolic sink and induced them to become storage organs.
In tuber crops, there is an inverse relationship between vegetative and tuber growth. Factors that promote vegetative growth inhibit tuber growth. This was clearly observed in potato (Trippi, 1982) and dahlia plants (Halevy and Biran, 1975). In cassava plants, the shoot and SRs grow simultaneously, creating competition for the assimilates. This fact suggested that factors that favoured aerial growth may depress root growth (Ternes, 2002). In the last experiment, in which the influence of a combined supply of NAA and BAP was analysed, the combinations that promoted the better development of SRs did not favour shoot growth.
The SR formation system reported here is a first approach to development of a tuberization model although additional efforts are required to improve it. It was not possible to achieve root secondary growth, but after this work it will be feasible to advance in some aspects of in vitro cassava tuberization.
Two root types were differentiated in vitro and they could distinguished morphological and anatomically. Based on these criteria, 29 clones of cassava were able to form SRs in vitro although their capacity was significantly different. On the other hand, the SR formation was considerably affected by the segment primary position and it was strongly influenced by hormone treatment.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Ernestina Galdeano, PhD, for her valuable comments on the manuscript, and Aldo Goytia for the photographic plates. This paper is a part of the thesis of the senior author. The authors are grateful to CONICET and SGCyT (UNNE) for their financial support.
LITERATURE CITED
- Alves AAC. Cassava botany and physiology. In: Hillocks RJ, Thresh JM, Bellotti AC, editors. Cassava: biology, production and utilization. Wallingford, UK: CAB International; 2002. pp. 67–89. [Google Scholar]
- Barlow PW. Adventitious roots of whole plants: their forms, functions, and evolution. In: Jackson MB, editor. New root formation in plants and cuttings. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1986. pp. 67–110. [Google Scholar]
- Barlow PW. The origin, diversity and biology of shoot-borne roots. In: Davis TD, Haissig BE, editors. Biology of adventitious root formation. New York: Plenum Press; 1994. pp. 1–23. [Google Scholar]
- Barlow PW, Volkmann D, Baluška F. Polarity in roots. In: Lindsey K, editor. Polarity in plants. Oxford: Blackwell Publishing; 2004. pp. 192–241. [Google Scholar]
- Batista de Souza C, Carvalho L, Mattos Cascardo J. Comparative gene expression study to identify genes possibly related to storage root formation in cassava (Manihot esculenta Crantz). In: Zuñiga C, editor. Proceedings of the 6th International Scientific Meeting of the Cassava Biotechnology Network; Cali, Colombia: CIAT; 2004. p. 153. [Google Scholar]
- Belletti P, Lanteri S, Lotito S, Saracco F. Production of potato micro-tubers through in vitro culture. Acta Horticulturae. 1994;362:141–148. [Google Scholar]
- Cabral GB, Carvalho LJCB. The formation of storage root in cassava. In: Carvalho L, Thro A, Duarte Vilarinhos A, editors. Proceedings of the 4th International Scientific Meeting of the Cassava Biotechnology Network; Brasilia, Brazil: EMBRAPA Recursos Genéticos e Biotecnologia; 2000. pp. 345–356. [Google Scholar]
- Cabral GB, Carvalho LJCB. Analysis of proteins associated with storage root formation in cassava using two-dimensional gel electrophoresis. Revista Brasileira de Fisiologia Vegetal. 2001;13:41–48. [Google Scholar]
- Cabral GB, Aragao FJL, Matsumoto K, Monte-Neshich DC, Rech L. Cassava tissue culture: multiple shoots and somatic embryogenesis. In: Roca W, Thro A, editors. Proceedings of the 1st International Scientific Meeting of the Cassava Biotechnology Network; Cali, Colombia: CIAT; 1993. pp. 180–184. [Google Scholar]
- Carvalho LJCB, Mattos Cascardo J, Ferreira M, Loureiro M. Studies on proteins and enzymes related to tuberization and starch biosynthesis in cassava roots. In: Roca W, Thro A, editors. Proceedings of the 1st International Scientific Meeting of the Cassava Biotechnology Network; Cali, Colombia: CIAT; 1993. pp. 234–238. [Google Scholar]
- Cereda MP, Vilpoux O. Conservação de raízes. In: Cereda MP, Vilpoux O, editors. Tecnología, usos e potencialidades de tuberosas amiláceas Latinoamericanas. São Paulo, Brazil: Fundação Cargill; 2003. pp. 13–29. [Google Scholar]
- Domínguez CE, Ceballos LF, Fuentes C. Morfología de la planta de yuca. In: Domínguez CE, editor. Yuca: Investigación, Producción y Utilización. Cali, Colombia: CIAT/PNUD; 1982. pp. 28–49. [Google Scholar]
- Ehneß R, Roitsh T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. The Plant Journal. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy MA. Cassava biology and physiology. Plant Molecular Biology. 2003;53:621–641. doi: 10.1007/s11103-005-2270-7. [DOI] [PubMed] [Google Scholar]
- Ellmore GS. Root dimorphism in Ludwigia peploides (Onagraceae): structure and gas content of mature roots. American Journal of Botany. 1981;68:557–568. [Google Scholar]
- Fahn A. Plant anatomy. 4th edn. Oxford, UK: Pergamon Press; 1990. [Google Scholar]
- Gifford RM, Evans LT. Photosynthesis carbon portioning, and yield. Annual Review of Plant Physiology. 1981;32:485–509. [Google Scholar]
- Gonzalez AM, Cristóbal CL. Anatomía y ontogenia de semillas de Helicteres Lhotzkyana (Sterculiaceae) Bonplandia. 1997;9:287–294. [Google Scholar]
- Gopal J, Minocha JL, Dhaliwal HS. Microtuberization in potato (Solanum tuberosum L.) Plant Cell Reports. 1998;17:794–798. doi: 10.1007/s002990050485. [DOI] [PubMed] [Google Scholar]
- Greig N, Mauseth JD. Structure and function of dimorphic prop roots in Piper auritum L. Bulletin of the Torrey Botanical Club. 1991;118:176–183. [Google Scholar]
- Halevy AH, Biran I. Hormonal regulation of tuberization in Dahlia. Acta Horticulturae. 1975;47:319–330. [Google Scholar]
- Henain AE, Cenoz HM. Corrientes, Argentina: Facultad de Agronomía y Veterinaria, Universidad Nacional del Nordeste; 1970. Comportamiento de clones de mandioca (Manihot esculenta Crantz), cultivados en el Nordeste Argentino. [Google Scholar]
- Iglesias C, Lenis J, Calle F. Estructuración de un programa de mejoramiento y multiplicación de semilla de yuca en la Costa Atlántica Colombiana. In: Meek Muñoz E, Aldana Navarrete H, editors. Primer encuentro técnico nacional de producción y transformación de yuca. Tolú, Colombia: Lé Print Club Express; 1998. pp. 59–73. [Google Scholar]
- Indira P, Kurian T. A study on the comparative anatomical changes undergoing tuberization in the roots of cassava and sweet potato. Journal of Root Crops. 1977;3:29–32. [Google Scholar]
- InfoStat. InfoStat versión 1.1. Manual del Usuario. Grupo InfoStat. Córdoba, Argentina: Universidad Nacional de Córdoba; 2002. [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill Book Co; 1940. [Google Scholar]
- Kartha KK, Gamborg OL, Constabel F, Shyluk JP. Regeneration of cassava plants from apical meristems. Plant Science Letters. 1974;2:107–113. [Google Scholar]
- Leclerc Y, Donnelly DJ, Seabrook JEA. Microtuberization of layered shoots and nodal cuttings of potato: the influence of growth regulators and incubation periods. Plant Cell, Tissue and Organ Culture. 1994;37:113–120. [Google Scholar]
- Lev-Yadun S, Aloni R. Experimental induction of dilatation meristems in Melia azedarach L. Annals of Botany. 1992;70:379–386. [Google Scholar]
- Loomis RS, Torrey JG. Chemical control of vascular cambium initiation in isolated radish roots. Proceedings of the National Academy of Sciences of the USA. 1964;52:3–11. doi: 10.1073/pnas.52.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi JO, Dias CAC. Cultura da mandioca. Campinas, Brazil: Coordenadoria de Assistência Técnica Integral; 1993. [Google Scholar]
- Luque R, Sousa HC, Kraus JE. Métodos de coloração de Roeser (1972)-modificado- e Kropp (1972) visando a subtituição do azul de astra por azul de alcião 8 GS ou 8 GX. Acta Botanica Brasilica. 1996;10:199–212. [Google Scholar]
- Lux A, Luxová M. Growth and differentiation of root endodermis in Primula acaulis Jacq. Biologia Plantarum. 2003/4;47:91–97. [Google Scholar]
- Lux A, Luxová M, Abe J, Morita S. Root cortex: structural and functional variability and responses to environmental stress. Root Research. 2004;13:117–131. [Google Scholar]
- Melis RJM, van Staden J. Tuberization and hormones. Zeitschrift für Pflanzenphysiologie. 1984;113:271–283. [Google Scholar]
- Melis RJM, van Staden J. Tuberization in cassava (Manihot esculenta Crantz): cytokinin and abscisic acid activity in tuberous roots. Journal of Plant Physiology. 1985;118:357–366. doi: 10.1016/S0176-1617(85)80195-4. [DOI] [PubMed] [Google Scholar]
- Mogilner I, Orioli GA, Bletter CM. Ensayo de topófisis y fotoperiodismo en mandioca. Bonplandia. 1967a;2:266–272. [Google Scholar]
- Mogilner I, Portuguez Arias JD, Gotuzzo AD, Acosta JA. Influencia de la parte aérea de Manihot flabellifolia en la formación de raíces reservantes de Manihot esculenta utilizado como pie. Bonplandia. 1967b;2:137–142. [Google Scholar]
- Montaldo A, Gunz T, Montilla JJ, Pérez Alemán S, Reverón AE. San José, Costa Rica: Instituto Interamericano de Ciencias Agrícolas; 1979. La yuca o mandioca (Manihot esculenta) [Google Scholar]
- Moore R, Clark WD, Stern KR. Botany. Dubuque, IA: Wm. C. Brown Publishers; 1995. [Google Scholar]
- Moraes Dallaqua MA, Coral DJ. Morfo-anatomia. In: Cereda MP, editor. Agricultura: tuberosas amiláceas Latinoamericanas. São Paulo, Brazil: Fundação Cargill; 2002. pp. 48–65. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nakatani M. In vitro formation of tuberous roots in sweet potato. Japanese Journal of Crop Science. 1994;63:158–159. [Google Scholar]
- Palmer CE, Smith OE. Effect of abscicic-acid on elongation and kinetin induced tuberization of isolated stolons of Solanum tuberosum L. Plant and Cell Physiology. 1969;10:657–664. [Google Scholar]
- Peterson RL. Control of cambial activity in roots of turnips (Brassica rapa) Canadian Journal of Botany. 1973;51:475–480. [Google Scholar]
- Puonti-Kaerlas J. Cassava biotechnology. In: Tombs MP, editor. Biotechnology and genetic engineering reviews. Andover, UK: Intercept Ltd; 1998. pp. 329–364. [Google Scholar]
- Ramanujan T, Indira P. Effect of girdling on the distribution of total carbohydrates and hydrocyanic acid in cassava. Indian Journal of Plant Physiology. 1984;27:355–360. [Google Scholar]
- Rateaver B. Ann Arbor, MI: University of Michigan; 1951. Anatomy and regeneration in the stem and root of Manihot utilissima Pohl. PhD thesis. [Google Scholar]
- Roitsh T, Ehneß R. Regulation of source/sink relations by cytokinins. Plant Growth Regulation. 2000;32:359–367. [Google Scholar]
- Samb PI, Kahlem G. Déterminisme de l'organogénèse racinaire de Jussiaea repens L. Zeitschrift für Pflanzenphysiologie. 1983;109:279–284. [Google Scholar]
- Salisbury FB, Ross CW. Fisiología vegetal. México D.F, México: Grupo Editorial Iberoamérica S.A. de C.V; 1994. [Google Scholar]
- Scott GJ, Best R, Rosegrant M, Bokanga M. Roots and tubers in the global food system: a vision statement to the year 2020. Lima, Peru: Training and Communications Department of the International Potato Center; 2000. [Google Scholar]
- Sneath PH, Sokal RR. Numerical taxonomy – the principles and practice of numerical classification. San Francisco, CA: W.H. Freeman & Co; 1973. [Google Scholar]
- van Staden J, Dimalla GG. Endogenous cytokinins and tuberization in potato Solanum tuberosum. Annals of Botany. 1976;40:1117–1119. [Google Scholar]
- Struik PC, Vreugdenhil D, van Eck HJ, Bachem CW, Visser RGF. Physiological and genetic control of tuber formation. Potato Research. 1999;42:313–331. [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 2nd edn. Sunderland, MA: Sinauer Associates, Inc; 1998. [Google Scholar]
- Ternes M. Fisiologia da planta. In: Cereda MP, editor. Agricultura: tuberosas amiláceas Latinoamericanas. São Paulo, Brazil: Fundação Cargill; 2002. pp. 66–82. [Google Scholar]
- Torrey JG, Loomis RS. Auxin–cytokinin control of secondary vascular tissue formation in isolated roots of Raphanus. American Journal of Botany. 1967;54:1098–1106. [Google Scholar]
- Trippi VS. Ontogenia y senilidad en plantas. Córdoba, Argentina: Dirección General de Publicaciones, Universidad Nacional de Córdoba; 1982. [Google Scholar]
- Ulloa RM, Mac Intosh GC, Melchiorre M, Mentaberry AN, Dallari P, Moriconi DN, Téllez-Iñón MT. Protein kinase activity in different stages of potato (Solanum tuberosum L.) microtuberization. Plant Cell Reports. 1997;16:426–429. doi: 10.1007/BF01146787. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil D, Xu X, Jung ChJ, Lammeren AAM, Ewing EE. Initial anatomical changes associated with tuber formation on single-node potato (Solanum tuberosum L.) cuttings: a re-evaluation. Annals of Botany. 1999;84:675–680. [Google Scholar]
- Webster BD, Radin JW. Growth and development of cultured radish roots. American Journal of Botany. 1972;59:744–751. [Google Scholar]
- Xu X, Vreugdenhil D, van Lammeren AAM. Cell division and cell enlargement during potato tuber formation. Journal of Experimental Botany. 1998;49:573–582. [Google Scholar]