Abstract
Background and Aims
Oxalis pes-caprae is a widespread invasive weed in regions with a Mediterranean climate. In its native habitat (southern Africa) this species has been reported as heterostylous with trimorphic flowers and a self- and morph-incompatible reproductive system. In most of the areas invaded, only a pentaploid short-styled morphotype that reproduces mainly asexually by bulbils is reported, but this has only been confirmed empirically. This study aims to analyse the floral morph proportions in a wide distribution area, test the sexual female success, and explain the causes of low sexual reproduction of this species in the western area of the Mediterranean Basin.
Methods
Fifty-five populations of O. pes-caprae were sampled in the Iberian Peninsula and Morocco to evaluate the floral morph ratio and individual fruit set. In plants from a dimorphic population, hand-pollination experiments were performed to evaluate the effect of the pollen source on pollen tube growth through the style. The ploidy level and genome size of individuals of each floral morph were analysed using flow cytometry.
Key Results
From the populations studied 89·1 % were monomorphic, with most of them containing the short-styled (SS) floral morph, and 10·9 % were dimorphic containing long-styled (LS) and SS morphs. In some of these, isoplethy was verified but no fruit production was observed in any population. A sterile form was also recorded in several populations. Hand-pollination experiments revealed that pollen grains germinated over recipient stigmas. In intermorph crossings, pollen tubes were able to develop and fruit initiation was observed in some cases, while in intramorph pollinations, pollen tube development was sporadic and no fruit initiation was observed. All individuals within each floral form presented the same DNA ploidy level: SS plants were pentaploid and LS and the sterile form were tetraploid.
Conclusions
The low or null sexual reproduction success of this species in the area of invasion studied seems related with the high frequency of monomorphic populations, the unequal proportion of floral morphs in dimorphic populations and the presence of different ploidy levels between SS and LS morphs. The discovery of the occurrence of an LS floral morph and a sterile form, whose invading capacity in these areas is as yet unknown, will be valuable information for management programmes.
Key words: Flow cytometry, genome size, heterostyly, invasive plant, Oxalis pes-caprae, ploidy level, reproductive biology, weed
INTRODUCTION
The genus Oxalis (Oxalidaceae) is distributed worldwide and consists of approx. 800 species (Hussey et al., 1997). Oxalis pes-caprae, a native species from southern Africa, was introduced as an ornamental plant in several areas of the world (Baker, 1965), particularly in regions with a Mediterranean climate, where it is currently a troublesome and widespread invasive weed. This species arrived in the central Mediterranean Basin at the end of the eighteenth century (Rappa, 1911), and its rapid and vigorous vegetative reproduction and dispersal resulted in a high rate of colonization success. In the native region both wild populations and weedy races are found (Ornduff, 1987).
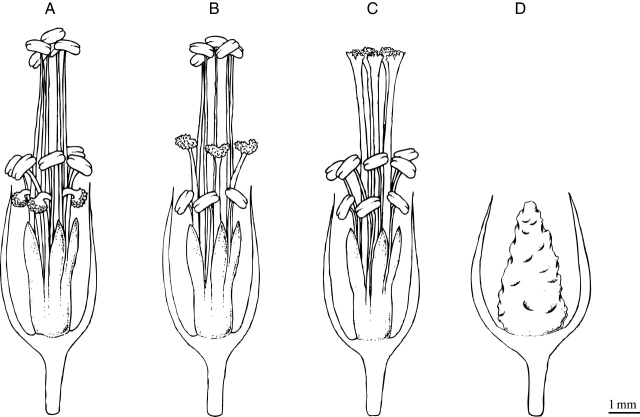
This species is heterostylic with tristylous flowers presenting short-, mid- and long-styled (SS, MS and LS, respectively) morphs (Fig. 1A–C) and a self- and morph-incompatibility reproductive system (Ornduff, 1987). In heterostylic species this usually implies that legitimate pollinations are only possible between opposite sex organs at the same height level (Lewis and Jones, 1992; Lloyd and Webb, 1992a). The outcome of this disassortative mating in heterostylic populations should be the presence of similar proportions of floral morphs (isoplethy; Lloyd and Webb, 1992b). In southern Africa, O. pes-caprae is represented by all floral morphs and reproduces both sexual and asexually (Ornduff, 1986). However, in most of the areas invaded, only the SS morphotype that reproduces mainly asexually by the production of bulbils has been reported (Ater, 2005; Vilà et al., 2006a). The ability to reproduce asexually, associated with founder events that this species experienced, may be responsible for the occurrence of the monomorphic populations reported. These patterns have been observed in other clonal heterostylous species such as Eichhornia crassipes (Barrett and Forno, 1982) and Nymphoides peltata (Wang et al., 2005), in which monomorphic populations and populations with highly biased morph ratios were found.
Fig. 1.
Illustration of the three floral morphs and sterile form present in Oxalis pes-caprae: (A) short-styled; (B) mid-styled; (C) long-styled; (D) sterile form. Legitimate pollinations are only possible between the same levels of individuals with different floral forms (sensu Ornduff, 1987).
The ability of O. pes-caprae to reproduce asexually, a shoot able to elongate rapidly and a root capable of contracting results in a highly successful vegetative spreading (Pütz, 1994). However, the first demographic study on this species revealed that bulbils were affected at different recruitment stages by several ecological factors such as post-dispersal predation, loss of viability and high intraspecific competition, and that the invasive success of O. pes-caprae seems to be more correlated with anthropogenic activities (Vilà et al., 2006a). In addition to the three floral morphs of this species, a new multipetal sterile (St) form lacking reproductive structures (Fig. 1D) was described for the Mediterranean Region (Valdés et al., 1987; Ater, 2000).
Additionally, in its native habitat, individuals of O. pes-caprae present different ploidy levels, with floral morphs being diploids (2n = 2x = 14), tetraploids (2n = 4x = 28) or pentaploids (2n = 5x = 35), with the latter individuals reproducing mainly asexually by bulbils (Ornduff, 1987). In most of the areas invaded, only the pentaploid SS morphotype has been reported (Baker, 1965). Moreover, for populations of the exotic range there are only eight records in the literature where other ploidy levels were found (for a review, see Ornduff, 1987).
Despite being a highly noxious weed (it can cause oxalate poisoning in livestock when consumed in large quantities; Gimeno et al., 2006) few efforts have been made to study this species in its exotic range and identify the causes of its low or null capacity to reproduce sexually. Therefore, the objectives of this study were to survey areas invaded by O. pes-caprae in the unexplored western Mediterranean region, to analyse if there are changes to population floral morph composition and DNA ploidy levels, and to examine if there is any correlation between morph and ploidy level. Moreover it is intended to evaluate the sexual reproduction capacity of this species in the invaded area. With this work it is hoped to contribute to a deeper knowledge of the reproduction system of this aggressive and invasive weed that can help management programmes in invaded areas.
MATERIALS AND METHODS
Plant material and study area
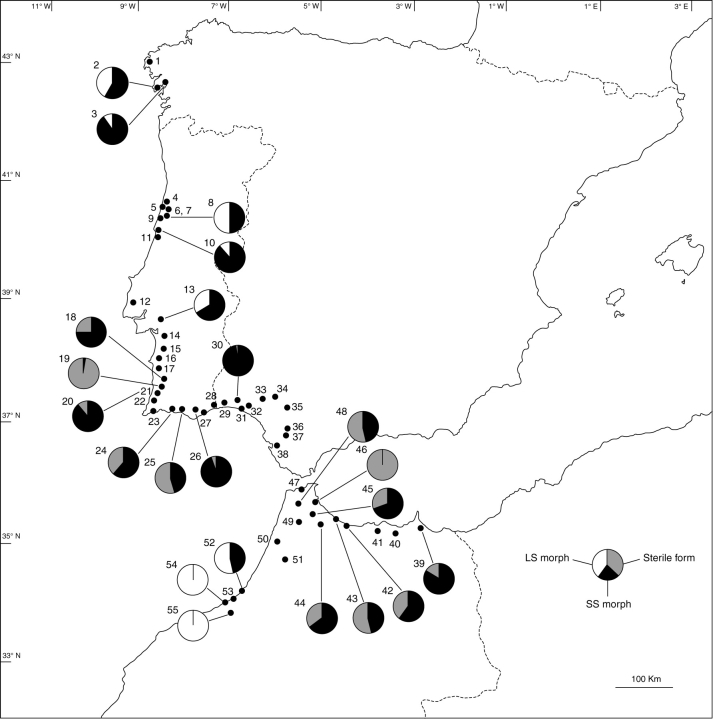
Oxalis pes-caprae L. is a perennial plant with a deeply buried bulb that sends out annual subterranean stems, bearing a high number of bulbils and a rosette of leaves. It produces inflorescences in umbellate cymes, with actinomorphic flowers (Young, 1968). The flowers present a yellow simpetalous corolla composed by five petals, and bear two rounds of five stamens and one round of five stigmas arranged in three levels according to the floral morphotype of each individual (SS, MS or LS, Fig. 1A–C; Ornduff, 1987). In the area studied, O. pes-caprae generally flowers in late winter to spring. This study was carried out during late winter of 2001, 2005 and 2006, and samples from 55 populations were collected throughout areas which had been highly invaded (mainly distributed along the coast) from La Coruña province (Spain) to Rabat province (Morocco) (Fig. 2 and Table 1). In the area studied, this species occurs in open habitats, generally in disturbed places such as cultivated ground, roadsides and abandoned fields, where it covers large areas.
Fig. 2.
Location and floral morph proportions of the Oxalis pes-caprae populations surveyed in Iberian Peninsula and Morocco. Population localities: 1, Muxía; 2, Boiro; 3, Rianxo; 4, Cacia; 5, Costa Nova; 6, Aveiro Sul; 7, Eirol; 8, Santo André; 9, Mira; 10, Figueira da Foz; 11, Lavos; 12, Enxara dos Cavaleiros; 13, Pegões; 14, Alcácer do Sal; 15, Grândola; 16, Santiago do Cacém; 17, Cercal; 18, Odemira; 19, São Teotónio; 20, Baiona; 21, Odeceixe; 22, Aljezur; 23, Marateca; 24, Alcantarilha; 25, Fonte de Boliqueime; 26, São Brás de Alportel; 27, Tavira; 28, Castro Marim; 29, Lepe; 30, Gibraleón; 31, Huelva; 32, San Juan del Puerto; 33, Manzanilla; 34, Gines; 35, Dos Hermanas; 36, El Cuervo de Sevilla; 37, between El Cuervo de Sevilla and Jerez de la Frontera; 38, Puerto Real; 39, Nador; 40, between Nador and Al Hoceïma; 41, Boudinar; 42, Amter; 43, Oued Laou (Uad Lau); 44, Nakhla; 45, Tétouan; 46, Marais Smir; 47, Ksar es Sghir; 48, Khemis Anjra; 49, Dar ben Saddok; 50, Aaouamra; 51, Arbaoua; 52, Rabat; 53, Harhoura; 54, Skhirate Côte; 55, Skhirate Centre. Populations 1–3 and 29–38 are located in Spain, 4–28 in Portugal and 39–55 in Morocco. Populations without a pie diagram are monomorphic containing the SS floral morph.
Table 1.
Results of floral morph proportion and relative nuclear DNA content analyses of the studied Oxalis pes-caprae populations
| Population | Floral morph proportion (%) | Relative nuclear DNA content analyses | ||||
|---|---|---|---|---|---|---|
| Floral morph | DNA index | CV (%) | Ploidy level | n | ||
| 1. Muxía | 100SS | – | – | – | – | – |
| 2. Boiro | 58SS : 42LS | SS | 0·185 ± 0·0017 | 3·12 | 5x | 5 |
| LS | 0·151 ± 0·0022 | 3·18 | 4x | 6 | ||
| 3. Rianxo | 91SS : 9LS | SS | 0·186 ± 0·0020 | 3·20 | 5x | 7 |
| LS | 0·153 ± 0·0024 | 3·78 | 4x | 5 | ||
| 4. Cacia | 100SS | SS | 0·182 ± 0·0006 | 2·91 | 5x | 3 |
| 5. Costa Nova | 100SS | SS | 0·182 ± 0·0026 | 2·79 | 5x | 5 |
| 6. Aveiro Sul | 100SS | SS | 0·181 ± 0·0029 | 3·04 | 5x | 5 |
| 7. Eirol | 100SS | SS | 0·181 ± 0·0024 | 2·85 | 5x | 5 |
| 8. Santo André | 51SS : 49LS | – | – | – | – | – |
| 9. Mira | 100SS | SS | 0·183 ± 0·0011 | 3·31 | 5x | 5 |
| 10. Figueira da Foz | 89SS : 11LS | SS | 0·182 ± 0·0020 | 2·84 | 5x | 10 |
| LS | 0·148 ± 0·0011 | 3·25 | 4x | 10 | ||
| 11. Lavos | 100SS | SS | 0·187 ± 0·0031 | 3·12 | 5x | 5 |
| 12. Enxara dos Cavaleiros | 100SS | – | – | – | – | – |
| 13. Pegões | 67SS : 33LS | SS | 0·183 ± 0·0012 | 2·70 | 5x | 5 |
| LS | 0·150 ± 0·0037 | 2·91 | 4x | 5 | ||
| 14. Alcácer do Sal | 100SS | SS | 0·183 ± 0·0029 | 3·51 | 5x | 5 |
| 15. Grândola | 100SS | SS | 0·182 ± 0·0027 | 3·50 | 5x | 5 |
| 16. Santiago do Cacém | 100SS | SS | 0·179 ± 0·0015 | 3·16 | 5x | 5 |
| 17. Cercal | 100SS | SS | 0·183 ± 0·0020 | 3·05 | 5x | 5 |
| 18. Odemira | 75SS : 25St | SS | 0·186 ± 0·0033 | 2·69 | 5x | 9 |
| St | 0·148 ± 0·0024 | 3·22 | 4x | 5 | ||
| 19. São Teotónio | 2SS : 98St | SS | 0·183 ± 0·0023 | 3·17 | 5x | 5 |
| St | 0·152 ± 0·0017 | 3·60 | 4x | 5 | ||
| 20. Baiona | 89SS : 11St | SS | 0·182 ± 0·0017 | 2·63 | 5x | 5 |
| St | 0·145 ± 0·0016 | 3·51 | 4x | 5 | ||
| 21. Odeceixe | 100SS | SS | 0·183 ± 0·0014 | 3·00 | 5x | 5 |
| 22. Aljezur | 100SS | SS | 0·180 ± 0·0009 | 3·41 | 5x | 5 |
| 23. Marateca | 100SS | SS | 0·178 ± 0·0010 | 3·51 | 5x | 5 |
| 24. Alcantarilha | 62SS : 38St | SS | 0·181 ± 0·0022 | 3·46 | 5x | 5 |
| St | 0·145 ± 0·0005 | 3·38 | 4x | 5 | ||
| 25. Fonte de Boliqueime | 60SS : 40St | SS | 0·183 ± 0·0023 | 3·92 | 5x | 5 |
| St | 0·149 ± 0·0022 | 4·19 | 4x | 5 | ||
| 26. São Brás de Alportel | 95SS : 5St | SS | 0·181 ± 0·0013 | 3·08 | 5x | 5 |
| St | 0·146 ± 0·0009 | 3·30 | 4x | 5 | ||
| 27. Tavira | 100SS | SS | 0·181 ± 0·0016 | 2·67 | 5x | 5 |
| 28. Castro Marim | 100SS | SS | 0·179 ± 0·0024 | 3·50 | 5x | 5 |
| 29. Lepe | 100SS | SS | 0·181 ± 0·0013 | 3·13 | 5x | 5 |
| 30. Gibraleón | 96SS : 2St | SS | 0·183 ± 0·0024 | 2·78 | 5x | 5 |
| St | 0·150 ± 0·0005 | 3·64 | 4x | 2 | ||
| 31. Huelva | 100SS | SS | 0·182 ± 0·0038 | 2·89 | 5x | 5 |
| 32. San Juan del Puerto | 100SS | SS | 0·181 ± 0·0016 | 3·15 | 5x | 5 |
| 33. Manzanilla | 100SS | SS | 0·181 ± 0·0018 | 3·45 | 5x | 5 |
| 34. Gines | 100SS | SS | 0·183 ± 0·0027 | 3·48 | 5x | 5 |
| 35. Dos Hermanas | 100SS | SS | 0·185 ± 0·0027 | 3·00 | 5x | 5 |
| 36. El Cuervo de Sevilla | 100SS | SS | 0·181 ± 0·0014 | 3·10 | 5x | 5 |
| 37. Between El Cuervo de Sevilla and Jerez de la Frontera | 100SS | SS | 0·184 ± 0·0018 | 2·91 | 5x | 5 |
| 38. Puerto Real | 100SS | SS | 0·182 ± 0·0031 | 3·67 | 5x | 5 |
| 39. Nador | 84SS : 16St | SS | 0·186 ± 0·0030 | 2·92 | 5x | 6 |
| St | 0·151 ± 0·0041 | 3·70 | 4x | 6 | ||
| 40. Between Nador and Al Hoceïma | 100SS | SS | 0·187 ± 0·0019 | 3·38 | 5x | 6 |
| 41. Boudinar | 100SS | SS | 0·189 ± 0·0024 | 2·94 | 5x | 2 |
| 42. Amter | 60SS : 40St | SS | 0·183 ± 0·0024 | 3·80 | 5x | 4 |
| St | 0·148 ± 0·0019 | 2·98 | 4x | 5 | ||
| 43. Oued Laou (Uad Lau) | 47SS : 53St | SS | 0·184 ± 0·0024 | 2·78 | 5x | 3 |
| St | 0·148 ± 0·0025 | 3·45 | 4x | 5 | ||
| 44. Nakhla | 65SS : 35St | SS | 0·181 ± 0·0011 | 2·83 | 5x | 5 |
| St | 0·150 ± 0·0047 | 4·38 | 4x | 5 | ||
| 45. Tétouan | 70SS : 30St | SS | 0·181 ± 0·0030 | 2·81 | 5x | 5 |
| St | 0·152 ± 0·0054 | 3·67 | 4x | 4 | ||
| 46. Marais Smir | 100St | St | 0·151 ± 0·0046 | 3·82 | 4x | 5 |
| 47. Ksar es Sghir | 100SS | SS | 0·182 ± 0·0008 | 2·69 | 5x | 3 |
| 48. Khemis Anjra | 47SS : 53St | SS | 0·186 ± 0·0044 | 3·49 | 5x | 5 |
| St | 0·146 ± 0·0021 | 3·08 | 4x | 4 | ||
| 49. Dar ben Saddok | 100SS | SS | 0·183 ± 0·0028 | 3·07 | 5x | 5 |
| 50. Aaouamra | 100SS | SS | 0·180 ± 0·0018 | 2·86 | 5x | 5 |
| 51. Arbaoua | 100SS | SS | 0·183 ± 0·0024 | 2·66 | 5x | 5 |
| 52. Rabat | 47SS : 53LS | SS | 0·181 ± 0·0011 | 2·72 | 5x | 5 |
| LS | 0·150 ± 0·0012 | 2·96 | 4x | 5 | ||
| 53. Harhoura | 100SS | SS | 0·181 ± 0·0013 | 2·73 | 5x | 5 |
| 54. Skhirate Côte | 100LS | LS | 0·150 ± 0·0018 | 3·02 | 4x | 5 |
| 55. Skhirate Centre | 100LS | LS | 0·148 ± 0·0012 | 2·70 | 4x | 3 |
Relative nuclear DNA values of each floral morph (SS, short-styled; LS, long-styled; St, sterile) are given as a mean and standard deviation of the DNA index relative to the internal reference standard Pisum sativum ‘Ctirad’.
The mean coefficient of variation (CV, %) of O. pes-caprae G0/G1 peak, ploidy level and the number of analysed plants (n) are also provided.
Morph ratio and fruit production
The floral morph ratio was estimated in all populations in the areas studied. To do this, longitudinal transects were made through each population and the floral morph of 100 individuals was recorded with a 5-m distance between samples (to avoid analysis of flowers from the same plant). Individual fruit set, i.e. the number of flowers that set fruits in each plant, was assessed in at least 20 plants following the same procedure.
Hand-pollination experiments
To evaluate the effect of the pollen source on pollen tube growth through the style, hand-pollination experiments were performed with the Rianxo (La Coruña, Spain) population (a population composed by SS and LS floral morphs). To do this, 32 plants from each morph were potted and transferred from the field to a greenhouse (22 ± 2 ºC, photoperiod of 16 h and light intensity of 530 ± 2 µmol m–2 s–1), where pollinator activities were excluded. Hand pollinations were performed within (illegitimate crossings) and between morphs (legitimate crossings) using pollen from each anther level. For each crossing 22–35 replicates were performed. Five days after pollination, the beginning of fruit development was assessed and pistils were collected and harvested in 70 % ethanol. After that, pistils were cleared and softened with 8 n sodium hydroxide for 6 h, rinsed in distilled water and stained overnight with 0·05 % (w/v) aniline blue prepared in 0·1 n potassium phosphate, according to Dafni et al. (2005). Pistils were then placed on a microscope slide with a drop of 50 % glycerine and were squashed beneath a coverslip. Samples were examined for the number of pollen grains adhering to the stigma, pollen-grain germination (%) and number of pollen tubes in the upper part of the style using a Nikon Eclipse 80i epifluorescence microscope (Nikon Instruments, Kanagawa, Japan) with the UV-2A filter cube.
Ploidy level analyses
DNA ploidy level and genome size of two to six plants belonging to each floral morph were estimated in 52 populations (Table 1) using flow cytometry (FCM). DNA ploidy levels of the individuals involved in the hand-pollination experiments were also determined. Potted plants were transferred from the field and maintained in a greenhouse under the same conditions as those described above. Nuclear suspensions were obtained from fresh young leaves of each plant following the protocol of Galbraith et al. (1983). Nuclei were released after chopping approx. 1–2 cm2 of leaf tissues of O. pes-caprae and Pisum sativum ‘Ctirad’ (reference standard with a 2C nuclear DNA content of 9·09 pg according to Doležel et al., 1998) with a razor blade in a glass Petri dish containing 0·5 mL of Otto I solution [100 mm citric acid, 0·5 % (v/v) Tween 20]. In some samples of LS morphotype, SS individuals of O. pes-caprae were used as an internal reference standard. Afterwards, the nuclear suspension was filtered using an 80-μm nylon filter into a cytometer sample tube and 1 mL of Otto II solution (400 mm Na2PO4.12H2O) was added to the isolate for staining at neutral pH. This two-step nucleus isolation procedure (Otto's buffer) was first described by Otto (1992) for animal tissues and further adapted to plant material by Doležel and Göhde (1995). The nuclear suspension was stained with 50 µg mL–1 of propidium iodide (Fluka, Buchs, Switzerland) and 50 µg mL–1 of RNase (Fluka) was added to avert staining of double-stranded RNA.
Samples were kept on ice and analysed within a 10- to 15-min period in a Coulter EPICS XL (Coulter Electronics, Hialeah, FL, USA) flow cytometer equipped with an air-cooled argon-ion blue laser tuned at 15 mW and operating at 488 nm. Doublets were removed from data analysis using a region defined in a fluorescence light pulse integral versus fluorescence light pulse height cytogram that included only the particles of interest. In each sample at least 3000 nuclei were analysed.
Absolute nuclear DNA estimations of O. pes-caprae in mass units (pg) were performed using the formula:
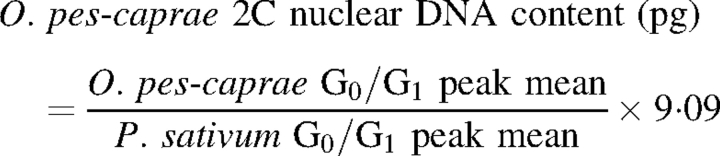 |
1 |
Conversion of mass units into number of base pairs (bp) was performed using the factor: 1 pg = 0·978 × 109 bp (Doležel et al., 2003).
Statistical analyses
In hand-pollination experiments a one-way ANOVA (SigmaStat for Windows Version 3·1; SPSS Inc.) was used to analyse differences in the percentage of germinated pollen grains according to the pollen source (floral morph and anther level). For data normalization, percentage values had to be transformed using an arcsine function. To evaluate if the pollen provided during hand-pollination experiments affected the number of pollen tubes which developed into the style, a Pearson correlation analysis between these variables was performed. A Kruskal–Wallis one-way ANOVA on ranks was used for analysing the number of pollen tubes in the style obtained in each crossing experiment. The multiple comparison Dunn's test was used for pair-wise comparison.
For relative DNA content analyses, an ANOVA with floral morphs nested within populations was applied. Differences in absolute genome size estimations among floral morhps were analysed using a one-way ANOVA. A Tukey–Kramer multiple comparison test was performed for pair-wise comparison.
RESULTS
Morph ratios analysis of the 55 populations studied is given in Table 1. From these, 49 populations (89·1 %) were monomorphic (regarding only the presence of sexual floral forms), containing either SS or LS morphs and/or the sterile form (St). Despite the occurrence of monomorphic populations of LS and St, SS accounts for 93·9 % of the cases (46 out of 49 populations). Indeed, only two populations were pure LS (4·1 %) and only one was pure St (2·0 %). The St form was also found in 13 of the SS populations with a variable degree of abundance: in two populations (numbers 43 and 48) similar proportions were observed, in one (number 19) the St form was dominant and, in the remaining populations, SS was the dominant form. Only six populations were found to be dimorphic (10·9 %) containing SS and LS floral morphs (Table 1). The analysis of morph ratio in mixed populations of SS and LS revealed that in three populations (5·5 %) the ratio between the two floral morphs was closer to 1 (isoplethic populations numbers 2, 8 and 52; Table 1). In the remaining populations (numbers 3, 10 and 13) SS individuals prevailed. No fruit and seed production was observed in any population from the areas studied.
A geographical analysis of the distribution of the floral morphs revealed that (a) monomorphic populations of SS form were arbitrarily distributed through the area studied, (b) populations with the St form were found in the south of the Iberian Peninsula and Morocco and (c) populations with the LS floral morph were restricted to some areas.
In hand-pollination experiments it was observed that pollen grains of LS and SS floral morphs germinated over recipient stigmas. Namely, pollen grains of mid-level anthers from SS germinated in 72·6 % of the cases, and from long-level anthers of the same morph in 67·1%. In the LS floral morph, pollen grains from short- and mid-level anthers presented a 60·4 % and 61·2 % germination success rate, respectively. Statistical analyses revealed significant differences (F = 12·97, P < 0·001) in germination values between pollen grains from mid-level anthers of SS and pollen grains from both levels of LS. The germination percentage of pollen grains from the long-level anthers of SS was not statistically different from any of the others. When considering the floral morph of the pollen donor and recipient stigma (Table 2), it was observed that pollen-grain germination was significantly higher (F = 25·23, P < 0·001) in intermorph pollinations.
Table 2.
Results of the hand pollination experiments in Oxalis pes-caprae from the Rianxo population
| Pollinations | No. of pollen grains on stigma | Germinated pollen grains (%) | No. of pollen tubes along the style | Fruit set (%) |
|---|---|---|---|---|
| Intramorph pollinations | ||||
| SS × SSmid | 269 ± 107·6 | 69·7 | 0·2 ± 0·72 (25)a | 0·0 |
| SS × SSlong | 165 ± 83·6 | 64·9 | 0·1 ± 0·24 (34)a | 0·0 |
| LS × LSshort | 546 ± 202·5 | 55·2 | 1·4 ± 2·87 (24)ab | 0·0 |
| LS × LSmid | 476 ± 177·5 | 50·2 | 0 (25)a | 0·0 |
| Intermorph pollinations | ||||
| SS × LSshort | 397 ± 151·3 | 66·9 | 30·1 ± 18·33 (24)cd | 0·0 |
| SS × LSmid | 188 ± 89·9 | 67·0 | 10·8 ± 9·78 (22)abc | 0·0 |
| LS × SSmid | 442 ± 77·6 | 75·4 | 81·0 ± 33·90 (35)d | 0·0 |
| LS × SSlong | 406 ± 106·3 | 69·3 | 58·6 ± 17·21 (30)cd | 27·3 |
The values are given as mean and standard deviation of the total number of pollen grains and pollen tubes and as the number of germinated pollen grains (%).
In crossings, the first floral morph represents the recipient stigma (female parent) and the second one the pollen donor (male parent); the anther level used for pollination is given in subscript.
Floral forms: SS, short-styled, LS, long-styled, St, sterile.
The number of replicates is given in parenthesis.
Values followed by the same superscript letters are not significantly different according to the multiple comparison test at P < 0·05.
The number of pollen tubes developed in the style was not correlated with the number of pollen grains provided in hand-pollination experiments (Pearson correlation, r2 = 0·223, P > 0·01). Pollen tube-development analyses showed that in intermorph crossings there was a variable growth of pollen tubes through the style, which usually reached the ovules. In contrast, in intramorph pollinations, the development of pollen tubes was sporadic, as most of the pollen tubes were unable to pass the stigmatic papillae. The analysis of intermorph crossings revealed statistically significant higher tube development in the reciprocal style from pollen grains of SS morph than from pollen grains of the LS morph (H = 197·46, P < 0·001). In addition, with the exception of the LS × SSlong crossing (recipient × pollen donor, respectively), where a low percentage of initial fruit set was obtained (27·3 %), no fruit initiation was observed in the remaining crosses (Table 2). The observed lack of pollen-tube development in intramorph pollinations was in agreement with the absence of fruit initiation. In intermorph crossings, despite the development of several pollen tubes along the style, a low percentage of fruit initiation was observed.
Flow cytometric analysis of the DNA ploidy level of O. pes-caprae provided histograms with well-defined peaks of both sample and internal reference standards. Nevertheless, some small differences in sample quality were detected according to the floral morph. Leaves of LS morph presented G0/G1 peaks with coefficients of variation (CV) ranging from 2·33 to 4·27 % (mean CV = 3·13 %), leaves of SS morph presented CV values ranging from 2·19 to 6·04 % (mean CV = 3·10 %) and peaks of leaves from the St form presented the highest CV values, which ranged from 2·56 to 5·55 % (mean CV = 3·59 %). The internal reference standard presented peaks with very good resolution (mean CV = 1·41 %).
The DNA ploidy level, given as a DNA index (DI = 2CO. pes./2CP. sat.), of two to ten individuals of each floral morph in every population analysed is presented in Table 1. All individuals within each floral morph presented the same DNA ploidy level. A mean DI of 0·151 ± 0·0062 was obtained for LS, a mean DI of 0·183 ± 0·0033 for SS and a mean DI of 0·149 ± 0·0036 for St. The low standard deviations that were obtained enabled a clear assignment of the ploidy level (Fig. 3). Statistically significant differences were detected among morphs while no statistically significant differences were found within morphs among populations (Table 3). The ratio between the DI of LS and SS morphs was 0·825, which shows that LS had approximately four-fifths of the nuclear DNA content of SS, corresponding to a tetraploid level for LS and a pentaploid level for SS. The LS morph and the St form presented a similar amount of nuclear DNA (no significant differences were obtained), which in this case reflects the same ploidy level. Also, nuclei from LS and SS morphs isolated, stained and analysed simultaneously, revealed a histogram with two close but distinct G0/G1 peaks that confirmed the ploidy level of both morphs (Fig. 3C). The ploidy level of the plants used in hand-pollination experiments showed that, as expected from the results above, SS individuals were pentaploid and LS individuals were tetraploid.
Fig. 3.
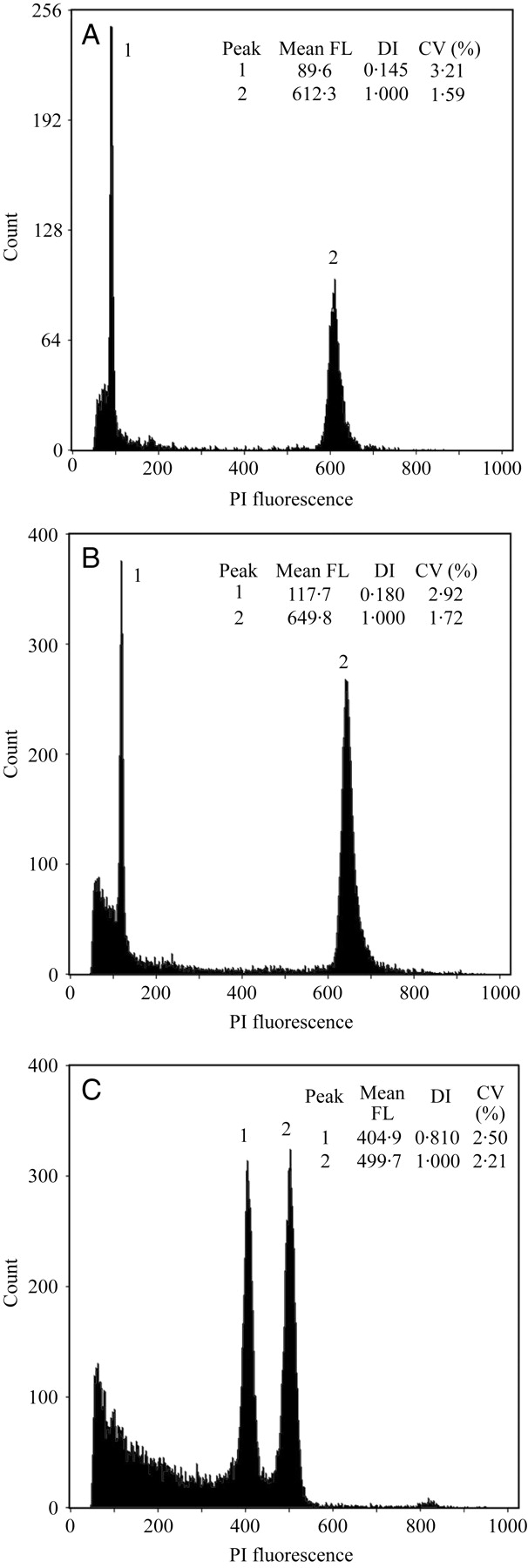
Histograms of relative fluorescence intensity obtained after simultaneous analysis of nuclei isolated from Pisum sativum ‘Ctirad’ (2C = 9·09 pg DNA, internal reference standard) and Oxalis pes-caprae from the Figueira da Foz population (number 10): (A) long-styled morph; (B) short-styled morph. In (C), a short-styled individual was used as an internal standard instead of P. sativum. The peaks marked 1 and 2 indicate nuclei at the G0/G1 phase of the sample and nuclei at the G0/G1 phase of the internal standard, respectively. The mean channel number (mean fluorescence light), DNA index (DI = mean channel number of sample/mean channel number of reference standard) and coefficient of variation value (CV, %) of each peak are also given.
Table 3.
ANOVA of DNA ploidy level with floral morphs nested within populations
| SS | d.f. | MS | F | P | Var. comp. (%) | |
|---|---|---|---|---|---|---|
| Morphotypes | 321·56 | 2 | 160·78 | 8·48 | <0·001 | 2·3 |
| Populations (morphotypes) | 1270·13 | 67 | 18·96 | 0·26 | 1·000 | –14·2 |
| Residual | 43907·36 | 602 | 72·93 | 112·0 |
SS, sum of squares; df, degrees of freedom; MS, mean square; Var. comp., variance component (%).
The estimation of the absolute nuclear DNA content of O. pes-caprae is given in Table 4. A mean 2C value of 1·66 ± 0·030 pg of DNA was obtained for the SS morph and mean 2C values of 1·37 ± 0·056 and 1·35 ± 0·033 pg of DNA were obtained for the LS morph and St form, respectively. These two latter values were statistically different (F = 2918·69, P < 0·001) from the estimation obtained in the SS morph and were highly reproducible among dates of analysis (no statistically significant differences were obtained). According to recent literature on this genus (Emshwiller, 2002; Loureiro et al., 2006) where Otto's was the only buffer capable of isolating nuclei from Oxalis leaves (supposedly due to the highly acid cell sap of leaves) and to the overall good quality of the results obtained in the present work, it seems that this is the most appropriate procedure for FCM analyses of O. pes-caprae.
Table 4.
Absolute genome size estimations of Oxalis pes-caprae floral morphs
| Floral morph | Ploidy level | Nuclear DNA content (pg/2C)* | 1C genome size (Mbp)† | CV (%) | n |
|---|---|---|---|---|---|
| Short-styled | 5x | 1·66 ± 0·030a | 325 | 3·10 | 39 |
| Long-styled | 4x | 1·37 ± 0·056b | 335 | 3·13 | 248 |
| Sterile | 4x | 1·35 ± 0·033b | 330 | 3·59 | 66 |
The values are given as mean and standard deviation of the nuclear DNA content in mass values (pg/2C) and as the mean of the 1C genome size in number of base pairs (Mbp).
The mean coefficient of variation (CV, %) of the G0/G1 peak for each morph and number of individuals analysed (n) are also provided.
* Values followed by the same letters are not significantly different according to the multiple comparison Tukey–Kramer test at P < 0·05.
† 1 pg of DNA = 978 Mbp (Doležel et al., 2003).
DISCUSSION
Heterostyly, a floral polymorphism that evolved to promote outcross pollinations and/or avoid self-interference between sex organs (Lloyd and Webb, 1992a), is usually genetically linked with a diallelic, sporophytic self-incompatibility system (Ganders, 1979). In this system, pollen rejection is controlled by the interaction of the self-incompatibility genotype of the pistil with the genotype of the pollen parent. Pollen grains present the products of two S alleles, and rejection occurs when either one of these alleles matches any of the S alleles expressed in the pistil (Matton et al., 1994). Therefore, in order to reproduce sexually, the presence of more than one floral morph and pollinator activity is of special importance (Lloyd and Webb, 1992b).
Ornduff (1987) described the heterostylic system of O. pes-caprae. This species is an invader of Mediterranean regions and it has been included in several lists of invasive weeds (Hussey et al., 1997; Bergmeier and Dimopoulos, 2001; Hadjikyriakou and Hadjisterkotis, 2002; Weber, 2003; Almeida et al., 2004). Nevertheless, only recently has the high impact of this species in the exotic range of distribution became a focus of attention (Rottenberg and Parker, 2004; Gimeno et al., 2006; Vilà and Gimeno, 2006; Vilà et al., 2006a, b), even though information on its sexual reproduction remains scarce (Ornduff, 1986, 1987; Rottenberg and Parker, 2004; Ater, 2005).
This is the first thorough study in natural conditions of O. pes-caprae sexual reproduction through its exotic range of distribution. The analysis of morph ratio proportion on the populations studied enabled the first observation of dimorphic populations of SS and LS in this region of the Mediterranean Basin, as until now only SS monomorphic populations had been reported for this area (Valdés et al., 1987; Ater, 2000). Within the mixed populations, three showed similar proportions of both floral morphs, as expected in heterostylous species (Dulberger, 1992). On the other hand, in the remaining populations, SS was the dominant floral morph. Oxalis pes-caprae seems to exhibit a classical pattern of founder events with a prevalence of monomorphic populations and dimorphic populations with biased morph ratios, both apparently maintained by asexual reproduction.
However, the occurrence of mixed populations of two reciprocal morphs opens the possibility of sexual reproduction. Nevertheless, field observations in both dimorphic and monomorphic populations revealed an absence of fruit production in O. pes-caprae individuals. Controlled pollinations (with all possible crossings) in a dimorphic population enabled the sexual reproduction system to be analysed in more detailed. First, it was possible to verify that pollen grains were able to germinate. This is not in total agreement with the results of Ornduff (1987), who found a high level of pollen sterility in the SS pentaploid floral morph. It was also possible to observe that, in inter-morph crossings, pollen tubes were able to develop and reach the ovary to apparently fertilize the ovules. This was the case of LS × SSlong, where fruit initiation was obtained. In all the other inter-morph crossings no fruit production was observed. Despite the low fruit production in inter-morph controlled crossings, these results are not in agreement with the total lack of fruit production recorded in natural populations, opening the possibility of occasional sexual reproduction. In intra-morph pollinations and, as expected from a diallelic sporophytic self-incompatibility system, pollen tubes were not able to pass the stigmatic papillae. Occasional development of pollen tubes throughout the style was observed in these pollinations but no fruits were observed. These results are in accordance with field observations on monomorphic populations, where the incompatibility system may be the main factor that blocks fruit production.
Ploidy level analysis of O. pes-caprae individuals using FCM revealed that all individuals with the SS floral morph were pentaploid. This is in accordance with what was previously known about this species that, in its exotic range of distribution, O. pes-caprae is mostly represented by a sterile pentaploid SS morph (Ornduff, 1987; Rottenberg and Parker, 2004). However, a different ploidy level was found for all LS and St individuals, i.e. tetraploidy. Whereas, tetraploid populations with all three style lengths are common in the native range of distribution (Ornduff, 1987), for the exotic range, the available information on O. pes-caprae ploidy levels is scarce. Symon (1961) and Michael (1964) reported the occurrence of spotty tetraploid populations with the three floral morphs in south and western Australia, respectively, with individuals reproducing sexually and asexually. Some tetraploid individuals were also found in other parts of the exotic range (Matthew, 1958; Borgen, 1974), but no information on the morph composition of those populations was provided (Ornduff, 1987). The occurrence of pentaploid and tetraploid individuals in the exotic range of distribution has been explained by some authors to be the result of several independent introduction episodes (Michael, 1964; Ornduff, 1986). It is believed that pentaploid individuals originated in southern Africa after a crossing between an unreduced gamete from a tetraploid individual and a haploid gamete from a diploid individual (until now no diploid individuals had been found outside the natural range of distribution of O. pes-caprae) and were further introduced in other territories. It was also suggested that tetraploid individuals in the exotic range of distribution could be the result of the fertilization of gametes with 14 chromosomes which originated from pentaploid plants (Ornduff, 1987).
In light of the results obtained in this work, together with previous knowledge of the reproductive system of this species, it is likely that the low or null sexual reproductive success in the exotic range of distribution could be a consequence of: (a) a high percentage of monomorphic populations (mostly of SS floral morph) and unequal proportion of floral morphs when dimorphic populations are found; and/or (b) the presence of different ploidy levels between LS and SS morphs in all the populations studied, which may limit the development of the fertilized ovule, as in inter-morph pollinations, pollen grains were apparently able to germinate, reach and fertilize the ovules. Problems in gametophyte formation or ovule abortion have been described in O. magnifica (Guth and Weller, 1986), and meiosis problems in the pollen mother cells after polyploidy or aneuploidy have been assumed for O. debilis (Luo et al., 2006) which presents the same ploidy level (2n = 5x = 35; Baker, 1965) as the SS floral morph of O. pes-caprae. Therefore, further information about the cytology of pollen grains and ovules of the pentaploid SS floral morph is necessary to understand the low sexual reproduction success in controlled pollinations, as sexual reproduction cannot be totally excluded from occurring in natural populations. Additionally, the occurrence of three populations with 1 : 1 morph ratios is usually the signature of sexual reproduction and the expected outcome of disassortative mating. Two hypotheses can be given as possible explanations for isoplethy in those populations: SS and LS morphs may have been introduced at the same time and present similar asexual propagation rates and/or, considering the present hand-pollination results, residual sexuality can occur. Genetic markers could be powerful tools to cope all these hypotheses.
Rottenberg and Parker (2004) in Israel detected genetic variability in several populations of O. pes-caprae composed of the functionally sterile SS floral morph. Given the absence of sexual reproduction, these results are surprising with authors proposing mutations and genome rearrangements as possible explanations. In the populations in the present study, the low but not totally excluded sexual reproduction could be a reasonable explanation for some of the genetic diversity detected in this species. Nevertheless, a thorough and large survey at this level that would enable the analysis of the causes and extent of genetic variability in this species is still needed in other areas of the regions invaded.
According to the information available in the literature, it is known that weedy races in the natural area of distribution are not necessarily pentaploid (Ornduff, 1987). Therefore, tetraploid individuals found throughout the area studied can also behave as invasive plants, despite the fact that in the few invaded places where this morphotype has been found previously it remained non-aggressive (Baker, 1965). The actual distribution pattern of populations containing LS floral morphs, associated with the predominance of SS populations, may be due to a very recent introduction of LS individuals by anthropogenic activities (the location of these populations are correlated with important areas of human activity), to a recent origin of LS in the populations studied and/or to a lower competitive capacity of LS individuals in comparison with SS ones. Also, the recent finding of frequent sterile individuals in several populations should be viewed with caution at a management level, as the competitive capacities of these plants are unknown. The absence of a sexual reproductive system in this form may result in a reduction in energy investment that could be used in bulbil production, and therefore improve its asexual capacities. Therefore, the major predominance of asexual reproduction in well-adapted genotypes of O. pes-caprae, together with its frequent dispersion and establishment by anthropogenic activities, confer this species with the capacity to be highly invasive.
This study has contributed to a better understanding of the biology of this invasive weed and has triggered other studies, which are necessary for understanding and controlling this species. Among these studies, it would be interesting to assess the asexual reproductive capacity of plants of different forms, the intra- and inter-population genetic variability on a large scale and the phylogeography of O. pes-caprae in invaded areas to understand its patterns and history of colonization.
ACKNOWLEDGEMENTS
The authors thank Dr Jorge Domínguez for allowing us to use the epifluorescence microscope and Sónia Rodrigues for her assistance on sample collection. We are also grateful to Dr Juan Arroyo and Dr Eleazar Rodriguez for critically reading the manuscript, to Prof. Spencer Barrett for his helpful comments at the Symposium ‘An Evolutionary Perspective of Biological Invasions’ held in Fribourg, Switzerland and to two anonymous reviewers for substantially improving the final version of this manuscript. The work was partially financed under grants PGIDT04PXIC31003PN from the Xunta de Galicia and BOS2003-07924-CO2-02 from the Spanish DGICYT to Luis Navarro. The work on flow cytometry was supported by the Portuguese Foundation for Science and Technology FCT/MCT project POCI/AGR/60672/2004. FCT also financed the work of Sílvia Castro (FCT/BD/10901/2002) and João Loureiro (FCT/BD/9003/2002).
LITERATURE CITED
- Almeida J, Marchante E, Marchante H, Freitas H. A brief report on the invasive flora of Portugal. Aliens. 2004;18:16–18. [Google Scholar]
- Ater M. Note sur la présence d'une forme stérile d'Oxalis pes-caprae L. au Maroc. Acta Botanica Malacitana. 2000;25:259–261. [Google Scholar]
- Ater M, et al. Biologie de la reproduction d'Oxalis pes-caprae au Maroc. In: Menéndez J, Bastida F, Fernández-Quintanilla C, Gonzáles JL, Recasens J, Royuela M, editors. Malherbologia Ibérica: soluciones comunes a problemas comunes. Huelva: Universidad de Huelva Publicaciones; 2005. [Google Scholar]
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York, NY: Academic Press; 1965. pp. 147–168. [Google Scholar]
- Barrett SCH, Forno IW. Style morph distribution in New World populations of Eichhornia crassipes (Mart.) Solms Laubach (water hyacinth) Aquatic Botany. 1982;13:299–306. [Google Scholar]
- Bergmeier E, Dimopoulos P. Chances and limits of floristic island inventories – the Dionysades group (South Aegean, Greece) re-visited. Phyton. 2001;41:277–293. [Google Scholar]
- Borgen L. Chromosome numbers of Macaronesian flowering plants. II. Norwegian Journal of Botany. 1974;21:195–210. [Google Scholar]
- Dafni A, Pacini E, Nepi M. Pollen and stigma biology. In: Dafni A, Kevan P, Husband B, editors. Practical pollination biology. Ontario: Enviroquest; 2005. pp. 83–142. [Google Scholar]
- Doležel J, Göhde W. Sex determination in dioecious plants Melandrium album and M. rubrum using high-resolution flow cytometry. Cytometry. 1995;19:103–106. doi: 10.1002/cyto.990190203. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák M, Nardi L, et al. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany. 1998;82:17–26. [Google Scholar]
- Doležel J, Bartoš J, Voglmayr H, Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytometry. 2003;51A:127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- Dulberger R. Floral polymorphism and their functional significance in the heterostylous syndrome. In: Barret SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 41–84. [Google Scholar]
- Emshwiller E. Ploidy levels among species in the ‘Oxalis tuberosa Alliance’ as inferred by flow cytometry. Annals of Botany. 2002;89:741–753. doi: 10.1093/aob/mcf135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. Rapid flow cytometric analysis of the cell-cycle in intact plant-tissues. Science. 1983;220:1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Gimeno I, Vilà M, Hulme P. Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae in the western Mediterranean. Journal of Biogeography. 2006;33:1559–1565. [Google Scholar]
- Guth CJ, Weller SG. Pollination, fertilization and ovule abortion in. Oxalis magnifica. American Journal of Botany. 1986;73:246–253. [Google Scholar]
- Hadjikyriakou G, Hadjisterkotis E. The adventive plants of Cyprus with new records of invasive species. Zeitschrift fur Jagdwissenschaft. 2002;48:59–71. [Google Scholar]
- Hussey B, Keighery G, Cousens R, Dodd J, Lloyd S. Western weeds – a guide to the weeds of Western Australia. Victoria Park: The Plant Protection Society of Western Australia; 1997. [Google Scholar]
- Lewis D, Jones D. The genetics of heterostyly. In: Barret SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 129–150. [Google Scholar]
- Lloyd D, Webb C. The evolution of heterostyly. In: Barret SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992a. pp. 151–178. [Google Scholar]
- Lloyd D, Webb C. The selection of heterostyly. In: Barret SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992b. pp. 179–207. [Google Scholar]
- Loureiro J, Rodriguez E, Doležel J, Santos C. Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Annals of Botany. 2006;98:679–689. doi: 10.1093/aob/mcl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SX, Zhang DX, Renner SS. Oxalis debilis in China: distribution of flower morphs, sterile pollen and polyploidy. Annals of Botany. 2006;98:459–464. doi: 10.1093/aob/mcl121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew P. Cytology of Oxalidaceae. Cytologia. 1958;23:200–210. [Google Scholar]
- Matton DP, Nass N, Clarke AE, Newbigin E. Self-incompatibility: how plants avoid illegitimate offspring. Proceedings of the National Academy of Sciences of the USA. 1994;91:1992–1997. doi: 10.1073/pnas.91.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael P. The identity and origin of varieties of Oxalis pes-caprae L. naturalized in Australia. Transactions of the Royal Society of South Australia. 1964;88:167–173. [Google Scholar]
- Ornduff R. The origin of weediness in. Oxalis pes-caprae. American Journal of Botany. 1986;73:779–780. [Google Scholar]
- Ornduff R. Reproductive systems and chromosome races of Oxalis pes-caprae L. and their bearing on the genesis of a noxious weed. Annals of the Missouri Botanical Garden. 1987;74:79–84. [Google Scholar]
- Otto F. Preparation and staining of cells for high-resolution DNA analysis. In: Radbruch A, editor. Flow cytometry and cell sorting. Berlin: Springer-Verlag; 1992. pp. 101–104. [Google Scholar]
- Pütz N. Vegetative spreading of Oxalis pes-caprae (Oxalidaceae) Plant Systematics and Evolution. 1994;191:57–67. [Google Scholar]
- Rappa F. Osservazioni sull Oxalis cernua Thunb. Bollettino del Orto Botanico Palermo. 1911;10:142–183. [Google Scholar]
- Rottenberg A, Parker JS. Asexual populations of the invasive weed Oxalis pes-caprae are genetically variable. Proceedings of the Royal Society of London Series B – Biological Sciences. 2004;271 doi: 10.1098/rsbl.2003.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon D. The species of Oxalis established in South Australia. Transactions of the Royal Society of South Australia. 1961;84:71–77. [Google Scholar]
- Valdés B, Talavera S, Fernández-Galiano E. Flora vascular de Andalucía Occidental. Barcelona: Ketres Edit; 1987. [Google Scholar]
- Vilà M, Gimeno I. Potential for higher invasiveness of the alien Oxalis pes-caprae on islands than on the mainland. Plant Ecology. 2006;183:47–53. [Google Scholar]
- Vilà M, Bartomeus I, Gimeno I, Traveset A, Moragues E. Demography of the invasive geophyte Oxalis pes-caprae across a Mediterranean island. Annals of Botany. 2006a;97:1055–1062. doi: 10.1093/aob/mcl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, et al. Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. Journal of Biogeography. 2006b;33:853–861. [Google Scholar]
- Wang Y, Wang QF, Guo YH, Barrett SCH. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltata: a distylous aquatic plant. New Phytologist. 2005;165:329–335. doi: 10.1111/j.1469-8137.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- Weber E. Invasive plant species of the world: a reference guide to environmental weeds. Wallingford, Oxon: CABI Publishing; 2003. [Google Scholar]
- Young D, et al. In: Flora Europaea. Tutin T, Heywood V, Burges N, Moore D, Valentine D, Walters S, editors. Cambridge: Cambridge University Press; 1968. pp. 192–193. Oxalis L. [Google Scholar]