Abstract
Background and Aims
Post-fire regeneration is a key process in Mediterranean shrubland dynamics, strongly determining the functional properties of the community. In this study, a test is carried out to deteremine whether there is co-variation between species regenerative types and functional attributes related to water use.
Methods
An analysis was made of the seasonal variations in leaf relative water content (RWC), leaf dry matter content (LDMC), leaf moisture (LM) and live fine fuel moisture (LFFM) in 30 woody species of a coastal shrubland, with different post-fire regenerative strategies (seeding, resprouting or both).
Key Results
RWC results suggest that the studied resprouters have more efficient mechanisms to reduce water losses and maintain water supply between seasons. In contrast, seeders are more drought tolerant. LDMC is higher in resprouters over the course of the year, suggesting a more efficient conservation of nutrients. The weight of the phylogenetic constraint to understand differences between regenerative strategies tends to be important for LDMC, while it is not the case for variables such as RWC.
Conclusions
Groups of species with different post-fire regenerative strategies (seeders and resprouters) have different functional traits related to water use. In addition to the role of phylogenetical constraints, these differences are also likely to be related to the respective life history characteristics. Therefore, the presence and abundance of species with different post-fire regenerative responses influence the functional properties of the communities.
Key words: Functional traits, leaf dry matter content, Mediterranean plants, post-fire, regenerative strategy, relative water content, resprouter, seeder, woody species
INTRODUCTION
Many studies have demonstrated that wildfires are an important disturbance in the evolution and dynamics of most Mediterranean-type ecosystems (Hanes, 1971; Whelan, 1995; Lloret et al., 2002). Accordingly, most Mediterranean woody species display post-fire regenerative mechanisms. Some species (resprouters) have organs that are protected from high temperatures to permit resprouting, and other species (seeders) can compensate for the loss of individuals immediately after wildfires with seeds that resist high temperatures and germinate by taking advantage of the increased space and resources available after a fire (Trabaud, 1987, 1991; Keeley, 1995). There is also a third group of species that use both post-fire regenerative strategies (seeder–resprouter group). Finally, in the Mediterranean ecosystems, there are a few species that cannot regenerate after a wildfire. Of course, significant variability can be found within these groups, according to the degree of vulnerability to fire intensity, and the type of structures promoting regrowth (such as lignotubers, crown roots, rhizomes or bulbs) (Lloret, 2004). However, seeders and resprouters are often considered the two main groups, since they represent the two basic types of post-fire regeneration (Zedler et al., 1983; Pausas et al., 2004; Pausas and Verdú, 2005).
Apart from their ecological features, species with resprouting or seeding capacity in the Mediterranean basin also have distinct evolutionary characteristics, due to their biogeographical history. While most of the seeder taxa evolved in the Quaternary (post-Pliocene), most of the resprouter taxa were already present in the Tertiary (pre-Pliocene) (Herrera, 1992) before the establishment of the typical Mediterranean climate (Suc, 1984; Jalut et al., 2000). Thus, some studies indicate that, in this region, post-fire regenerative attributes may be due to phylogenetic constraints and not necessarily to adaptation to environmental disturbances such as fires (Verdú, 2000; Verdú et al., 2003; Pausas and Verdú, 2005).
Fire might affect species composition by: (a) eliminating other species that cannot regenerate after fire (Vilà et al., 2001; Lloret and Vilà, 2003; Rodrigo et al., 2004); (b) changing the patterns of relative abundance (Eugenio and Lloret, 2004), according to the way their life history fits to a fire pattern; or (c) allowing the establishment of species new to the disturbed ecosystem. More specifically, a high rate of recurrence of fires might drive a community dominated by resprouters into a community with a high abundance of seeders (Bellingham and Sparrow, 2000; Pausas, 2001; Lloret et al., 2003). Functional ecosystem properties may differ according to the relationship between species composition and disturbance regime. Under similar climatic conditions, successive studies show that species composition largely determines the ecosystem functional properties, which in turn can be explained by the functional attributes of the species (Garnier et al., 2004; Polley et al., 2005). In this context, functional plant classification aims to group species according to functional similarities, thereby providing a more comprehensive description of community functions (Lavorel et al., 1999; Díaz and Cabido, 2001). Thus, models of ecosystem behaviour may be elaborated on species' functional properties under a variety of different circumstances, such as a fire (Keane et al., 2004).
In a Mediterranean context, functional attributes related to water economy or fire behaviour are a priori good candidates to explore the hypothesis that changes in species composition due to a fire may promote changes in the functional properties of the whole community. Leaf relative water content (RWC) is an indicator that is used to evaluate plant water status (Larcher, 1995; Teulat et al., 1997). Peñuelas et al. (2004) described how photosynthetic rates and stomatal conductances decreased as leaf RWC diminished in non-irrigated Phyllirea angustifolia plants. Leaf dry matter content (LDMC) has also been proposed as an indicator of plant resource use (Garnier et al., 2001a). This trait is related to leaf lifespan and it is involved in a fundamental trade-off between rapid production of biomass and an efficient conservation of nutrients (Grime et al., 1997; Poorter and Garnier, 1999; Ryser and Urbas, 2000). Finally, living fuel moisture content, which is determined by leaf loisture (LM) and live fine fuel moisture (LFFM), is used in various fire model systems (Andrews and Bevins, 2003; Piñol et al., 2005) as a determining factor for the ignition and propagation of fire (Chandler, 1983).
The purpose of this study is to characterize a set of co-existing woody species with different post-fire regenerative strategies (resprouter, seeder and seeder–resprouter), according to various attributes related to their resource use, particularly water economy, nutrient conservation and combustibility. To this end, the seasonal variation of the leaf RWC, the LDMC, the LM and the LFFM was analysed in 30 woody species from coastal shrublands of Catalonia.
The main aim of this study is to verify whether there is a co-variation between regenerative groups and functional groups. This hypothesis is based on the different biogeographical origins of the taxa co-existing in Mediterranean communities and the role that these species play during the course of the succession. Since both regenerative and functional attributes are constrained by phylogenetic history, phylogenetical distances between taxa were also considered in the analyses.
More specifically, the following questions are addressed: (a) does seasonality determine different leaf RWC and LDMC in plants with different post-fire regenerative strategies; and (b) do plants with different post-fire regenerative strategies have different moisture contents in live fine fuel (leaves and shoots)?
MATERIALS AND METHODS
Study site, species and general sampling procedure
The study area was located on the Massís del Montgrí, a Mediterranean protected coastal area located in the north-east of Catalonia, (north-east Iberian Peninsula, 42·16°N 3·24°W). Vegetation grows on limestone and is mainly dominated by open pine forests and also by Mediterranean shrublands with the dominant species Quercus coccifera, Cistus albidus, Cistus monspeliensis and Rosmarinus officinalis (Polo and Masip, 1987). Sampling was conducted in 1–2 m high mature shrublands that had been untouched by wildfire for > 10 years.
The area's climate is sub-humid Mediterranean, according to the Emberger classification (Emberger, 1942). The mean annual precipitation is 654·6 mm, with cool winters (mean minimum annual temperature: 4·1 °C) and warm summers (mean maximum annual temperature: 26·8 °C) (Ninyerola et al., 2000, 2003).
The study was carried out on a sub-set of 30 woody plant species growing in the study region and representative of the studied community and belonging to as many different families and regenerative strategies as possible in this type of community. They were classified into three groups depending on their post-fire regenerative strategies (Cucó, 1987; Papió, 1988; Lloret and Vilà, 1997; Verdú, 2000; Alberdi and Caverom, 2003; Lloret and Vilà, 2003), and after direct field observations in a nearby area that burned in September 2004: seeders (S), resprouters (R) and seeder–resprouters (SR). Seeders are species that germinate after fire but do not resprout (S + R–, sensu Pausas and Verdú, 2005); resprouters are considered to resprout but not to germinate (S– R+); and seeder–resprouters can germinate and resprout after fire (S + R+).
Seven species were considered as seeders, 14 as resprouters and nine as seeder–resprouters. Seeders belonged to two families, resprouters to 13 families and seeder–resprouters to four families (Table 1). Species that neither germinate nor resprout were not considered since there were none present in the studied community.
Table 1.
List of study species including their family, regenerative strategy and life form according to Raunkiaer classification (Raunkiaer, 1934)
| Study species | Family | Regenerative strategy | Life form* |
|---|---|---|---|
| Argyrolobium zanonii (Turra) P. W. Ball | Leguminosae | Seeder–resprouter | C |
| Calicotome spinosa (L.) LK | Leguminosae | Seeder–resprouter | NP |
| Cistus albidus L. | Cistaceae | Seeder | NP |
| Cistus monspeliensis L. | Cistaceae | Seeder | NP |
| Cistus salviifolius L. | Cistaceae | Seeder | NP |
| Clematis flammula L. | Ranunculaceae | Resprouter | PV |
| Coronilla minima (L.) | Leguminosae | Resprouter | C |
| Crataegus monogyna Jacq. | Rosaceae | Resprouter | MP |
| Daphne gnidium L. | Thymelaeaceae | Resprouter | NP |
| Dorycnium hirsutum (L.) ser. In DC. | Leguminosae | Seeder–resprouter | C |
| Dorycnium pentaphyllum Scop. | Leguminosae | Seeder–resprouter | C |
| Erica arborea L. | Ericaceae | Resprouter | MP |
| Fumana ericoides (Caav.) Gandg. | Cistaceae | Seeder | C |
| Fumana thymifolia (L.) | Cistaceae | Seeder | C |
| Globularia alypum L. | Globulariaceae | Seeder–resprouter | NP |
| Helianthemum nummularium (L.) Miller | Cistaceae | Seeder | C |
| Lavandula latifolia Med. | Labiatae | Seeder–resprouter | C |
| Lonicera implexa Aiton | Caprifoliaceae | Resprouter | PV |
| Olea europaea L. | Oleaceae | Resprouter | MP |
| Osyris alba L. | Santalaceae | Resprouter | NP |
| Phillyrea angustifolia L. | Oleaceae | Resprouter | NP |
| Pistacia lentiscus L. | Anacardiaceae | Resprouter | MP |
| Quercus coccifera L. | Fagaceae | Resprouter | NP |
| Quercus ilex L. | Fagaceae | Resprouter | MP |
| Rhamnus alaternus L. | Rhamnaceae | Resprouter | P |
| Rosmarinus officinalis L. | Labiatae | Seeder | NP |
| Smilax aspera L. | Liliaceae | Resprouter | PV |
| Staehelina dubia L. | Compositae | Seeder–resprouter | C |
| Teucrium polium L. | Labiatae | Seeder–resprouter | C |
| Thymus vulgaris L. | Labiatae | Seeder–resprouter | C |
* C, Chamaephyte; NP, Nano-Phanerophyte; MP, Macro-Phanerophyte; P, Phanerophyte; PV, Phanerophyte-Vine.
Sampling and measurements of traits
For each species, replicate samples were collected from ten different plants, on the following occasions: spring (May 2004), summer (August 2004), autumn (November 2004) and winter (February 2005). Individuals of the same species were collected throughout the year, on the same site and under similar conditions (on limestone without forest canopy).
Three leaf variables were estimated throughout the year: leaf relative water content (hereafter RWC in the text and W in the equations), leaf dry matter content (LDMC in the text and D in the equations) and leaf moisture (LM in the text and L in the equations). In addition, live fine fuel moisture (LFFM in the text and F in the equations) was also measured from shoots < 6 mm in diameter.
The RWC (%) was determined as
where Mf is the fresh mass, Mt is the turgid mass after rehydrating the leaves, and Md is the dry mass after drying the leaves in an oven. The leaf RWC takes into account the turgid mass of leaves, and so it is the proportion of the leaf water content related to the maximum water content that can potentially be achieved by the leaf.
The leaf dry matter content (D) (mg g−1) was determined as
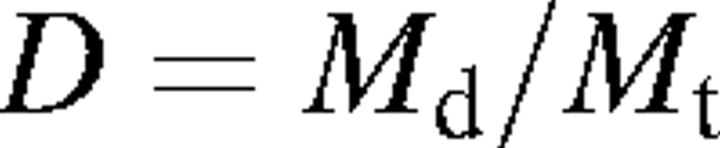 |
Thus, LDMC is the proportion of the leaf matter content without water related to the mass of the leaf with the maximum water content.
Leaf moisture (L) (%) and live fine fuel moisture (F) (%) of leaves and shoots, respectively, were determined as
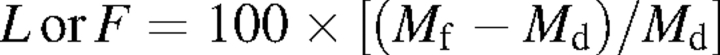 |
These parameters indicate the water content of leaves (L) and shoots (F) under field conditions in relation to its dry mass.
Leaf and shoot samples were collected from well-grown plants and taken from the part of the canopy exposed to direct sunlight at the time of sampling. Fully expanded leaves free from herbivore or pathogen damage were severed from a stem or twig, and the petioles were removed (Garnier et al., 2001b). The number of leaves sampled from each individual varied according to the size and the weight of the leaves of each species. In all species (except Calicotome spinosa in summer, autumn and winter; and Crataegus monogyna in winter, because there were no available leaves), the leaves were collected at noon (between noon and 2 pm). RWC was determined following an adaptation of the method used by Munné-Bosch and Peñuelas (2004). Leaves were stored in ice-box conditions, inside plastic jars filled with water to saturate the leaves (a previously weighed plastic jar filled with water was used for each individual). They were stored for 6–9 h, i.e. the period needed to reach water saturation (Espelta, 1996). After saturation was achieved, the fresh weight of leaves was obtained. Plastic jars were closed hermetically and were conserved in ice-box conditions so that there were no losses of water. Then the leaves were weighed outside the jar, in order to obtain their saturated weight (with a precision of 10−5 g). Finally, they were oven-dried for 48 h at 70 °C and weighed. LDMC and LM were obtained with the same procedure as RWC.
To determine LFFM, ten shoots (<6 mm diameter) from ten different individuals were collected for each species (except for Cistus salviifolius in spring). Each shoot was closed in a hermetically sealed plastic bag and stored in ice-box conditions so that the water lost during journeys between the field and the laboratory remained inside the plastic bag (Viegas et al., 2001). Then they were weighed. Finally, they were oven-dried for 48 h at 70 °C and weighed again (f. wt and d. wt, with a precision of 0·01 g).
Data analyses
The dependent variables for leaf data analyses were RWC, LM and LDMC. In the case of shoots, the dependent variable was LFFM. The differences between the species belonging to the three regenerative strategies during the seasons of the year were tested by using repeated-measures analyses of variance (ANOVAs), where the within-subject factor was season and the between-subject factor was regenerative strategy (S, SR and R). In these analyses, the replicates were the mean values of each species obtained from the ten sampled individuals. For shoot data analyses, the within-subject factor had four levels (spring, summer, autumn and winter), while for leaves there were three levels (summer, autumn and winter). RWC, LM and LDMC values for spring leaves could not be obtained in several study species. Consequently, spring was excluded from these repeated-measures ANOVAs of RWC, LM and LDMC to avoid too many missing values. All species, except C. spinosa and C. monogyna (because they lack leaves in some seasons), were included in the repeated-measures ANOVAs of leaf parameters. In the case of LFFM, all species, except C. salviifolius (no data from spring were available), were included in the analyses.
The relative seasonal variations of RWC, LM and LFFM were estimated as: (winter values – summer values)/(winter values). Accordingly, one-way ANOVAs were performed to test differences between regenerative strategies.
Since the spring season could not be included in the previous repeated-measures ANOVAs that considered the seasonal variation of some variables, a nested ANOVA was performed separately for each one of the four seasons, and for each of the dependent variables (RWC, LM, LDMC and LFFM). Species were considered a random factor nested within strategy.
Post hoc comparisons between the different regenerative strategies were carried out using Fisher's l.s.d. test for all situations with significant ANOVA results. To approximate normality in a better way, RWC and LDMC were transformed into their log-odds {i.e. log [LDMC/(1 − LDMC)]}, since they are proportions. LM and LFFM were not transformed as they fulfilled the requirements for parametric analyses.
As the set of studied species were not equally independent units and phylogenetic constraints influence species traits, the hypothesis was tested that differences between species in leaf and shoot variables are higher when the phylogenetic distance between species increases. First, following Pausas and Verdú (2005), a phylogenetic tree was assembled for the whole set of species by pruning the Hilu et al. (2003) angiosperm tree to the family level, where the respective species were grafted. Thus a phylogenetic tree was obtained, where the distances between the closest branches were assumed to be the unit (Fig. 1). The phylogenetic distance matrix between species was considered to be, for each pair of species, the sum of the respective number of steps until a common bifurcation. It was considered that the distance between species belonging to the same family was one, and the minimal distance between species belonging to different families was two. In this way it was considered that the distance between families was higher than between species of the same family, even when these species belong to different genera. Similarly, the values of the leaf or shoot variable distance matrix between species were calculated as (xi − xj)2, xi and xj being the values of a given variable x (RWC, LDMC, LM, LFFM) for the species i and j.
Fig. 1.
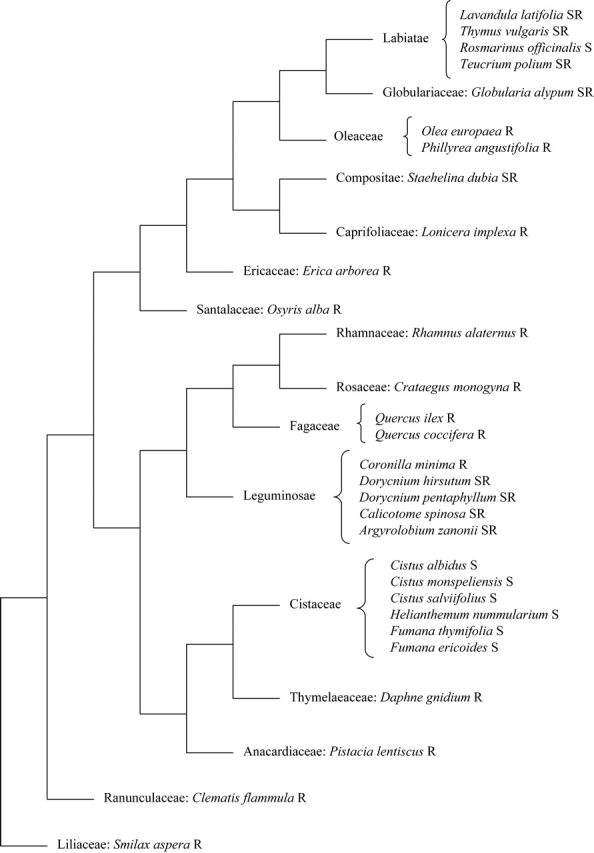
Phylogenetic tree assembled using information from Hilu et al. (2003). R, resprouter, S, seeder, SR, seeder–resprouter.
Three-way partial Mantel tests were then performed, in which the correlation between the variables and the regenerative type distances (A and B, respectively) were analysed when the effects of phylogenetic distance (C) are kept constant (Smouse et al., 1986; Fortin and Gurevitch, 1993). For these analyses, a ‘regeneration distance matrix’ was obtained from the absolute differences of the values given to each regenerative type: resprouters (0), seeder–resprouters (1) and seeders (2). This code was selected because seeder–resprouters share traits of both seeders and resprouters and there is not any a priori reason to assume that seeder–resprouters are closer to either of the two other categories. The statistics resulting from this partial test are regression coefficients (bAB·C) corresponding to the partial linear correlation of two distance matrices (A,B) after controlling for the linear effect of a third matrix (C). Significant differences from zero in these coefficients were assessed by comparing reference distributions obtained after 999 iterations that permuted the arrangement of the elements of one of the distance matrices. The test for each variable was performed for each one of the four seasons, and when data were unavailable for some species the species was dropped from the analysis and the matrices were modified accordingly.
The Statistica 6 (Statsoft) program was used for the statistical analyses. The Passage 1·0 program (Rosenberg, 2002) was used for Mantel tests to analyse the effects of phylogeny.
RESULTS
Leaf relative water content (RWC)
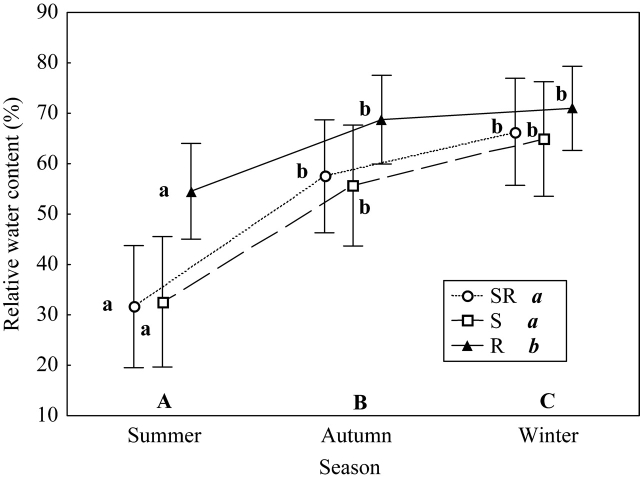
Overall, RWC was significantly higher in resprouters than in seeder–resprouters and seeder species (F2,25 = 5·73, P = 0·009). No significant differences between seeder–resprouters and seeders were found (Fig. 2). As expected, RWC significantly increased from summer to winter (F2,50 = 44·66, P < 0·001). Although this seasonal variation in RWC was less intense in resprouters than in the other two strategies, the interaction between season and strategy was only marginally significant (F4,50 = 2·47, P = 0·056) (Fig. 2). Accordingly, the relative seasonal variation was lower for resprouters than for the other two types (Table 2). In fact, when analysing each season separately, significant differences between regenerative strategies were observed only in spring (F2,149 = 6·22, P = 0·008) and summer (F2,233 = 12·04, P < 0·001). Post hoc analyses demonstrated that, in both spring and summer, resprouters had a significantly higher RWC than seeders and seeder–resprouters. The same pattern was observed when correlating differences in RWC between species and regenerative type, keeping the phylogenetic distance constant (Table 3).
Fig. 2.
Mean leaf relative water content (RWC) (%) of the three regenerative strategies [seeder (S), resprouter (R) and seeder–resprouter (SR)], in summer, autumn and winter. Vertical bars denote 0·95 confidence intervals. Post hoc Fisher's l.s.d. significant differences (P < 0·05) are indicated with different letters: upper case letters (A, B, C) indicate differences between seasons, italic lower case letters (a, b) in the key indicate differences between strategies, and roman lower case letters (a, b) indicate differences between seasons for a given strategy. All the species (except C. spinosa and C. monogyna) are included.
Table 2.
One-way ANOVA for the relative seasonal variation of the three water-related variables for the three different regenerative strategies
| n | F | P-value | Fisher's l.s.d. post hoc | |
|---|---|---|---|---|
| RWC | 28 | 9·35 | <0·001 | R < S, SR |
| LM | 28 | 8·48 | 0·002 | R < S, SR |
| LFFM | 30 | 4·43 | 0·021 | R, SR < S |
Values of the relative seasonal variation are the result of: [variable (winter) — variable (summer)]/variable (winter)]. Post hoc Fisher's l.s.d. significant differences (P < 0·05) are shown in the third column.
RWC, relative water content; LM, leaf moisture, LFFM, leaf fine fuel moisture; R, resprouter, S, seeder; SR, seeder–resprouter.
Table 3.
Summary of results of the partial Mantel test between the RWC, LDMC, LM and LFFM matrices and the regeneration distance matrix for each season, when the effect of phylogenetic distance was kept constant RWC and LDMC data were transformed into their log-odds (see text).
| Spring | Summer | Autumn | Winter | ||
|---|---|---|---|---|---|
| RWC | b | 0·251 | 0·173 | 0·027 | −0·067 |
| P-value | 0·017* | 0·010* | 0·728 | 0·214 | |
| LDMC | b | 0·045 | 0·001 | 0·118 | 0·063 |
| P-value | 0·7 | 0·978† | 0·052† | 0·246 | |
| LM | b | −0·099 | −0·01 | 0·064 | −0·007 |
| P-value | 0·339 | 0·881 | 0·327 | 0·902 | |
| LFFM | b | −0·1 | 0·016 | −0·077 | 0·141 |
| P-value | 0·171 | 0·874 | 0·235 | 0·033* |
*The partial Mantel test shows significant differences between regenerative strategies, which were also observed in the results of the nested ANOVA for each season, without taking into account phylogeny.
†The partial Mantel test does not show any significant differences between regenerative strategies, but significant differences were found in the results of the nested ANOVA for each season, without taking into account phylogeny.
RWC, relative water content; LDMC, leaf dry matter content; LM, leaf moisture; LFFM, leaf fine fuel moisture.
Leaf dry matter content (LDMC)
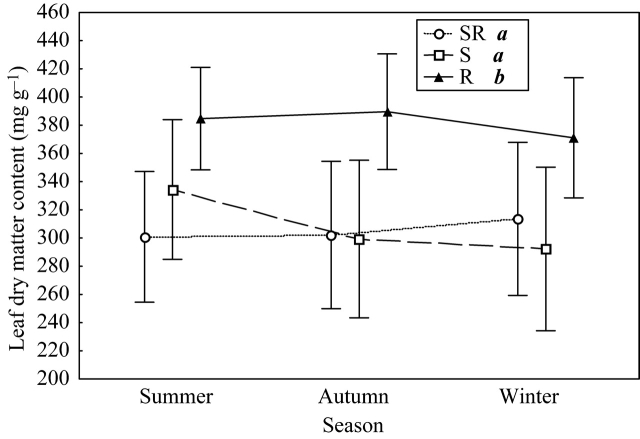
Overall, LDMC was significantly higher in resprouters than in the other two regenerative strategies (F2,25 = 4·50, P = 0·021) (Fig. 3), but no significant differences between seasons were found (F2,50 = 1·34, P = 0·269). The interaction between regenerative strategy and season was not significant (F2,50 = 1·82, P = 0·140). When analysing each season separately, significant differences between strategies were found in summer (F2,233 = 5·16, P = 0·013) and autumn (F2,248 = 5·65, P = 0·009). Post hoc analyses demonstrated that in summer, resprouters had a higher LDMC than seeders, and seeders had a higher LDMC than seeder–resprouters, while in autumn resprouters had a higher LDMC than the other two strategies that showed similar values. These differences between regenerative strategies can be attributed to phylogenetic constraints, since no significant correlation between differences in LDMC and regenerative type were found when keeping constant the effect of phylogenetic distance in partial Mantel tests (Table 3).
Fig. 3.
Mean leaf dry matter content (LDMC) (mg g−1) of the three regenerative strategies [seeder (S), resprouter (R) and seeder–resprouter (SR)], in summer, autumn and winter. Vertical bars denote 0·95 confidence intervals. Letters indicate post hoc Fisher's l.s.d. significant differences (P < 0·05) between strategies. All the species (except C. spinosa and C. monogyna) are included.
Leaf moisture (LM)
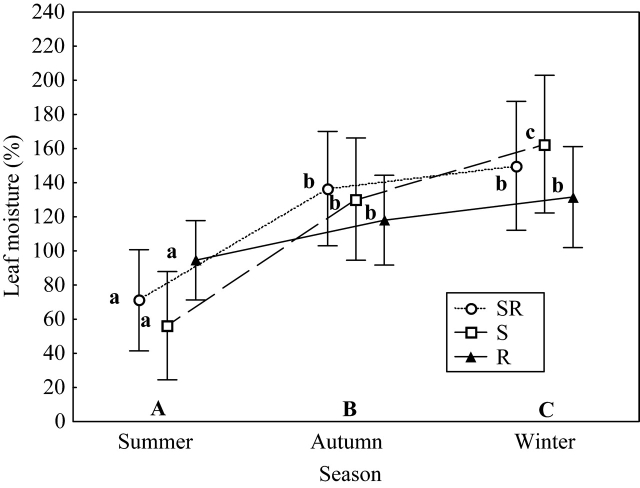
There was a significant increase in LM from summer to winter (F2,50 = 46·01, P < 0·001), but the seasonal pattern of variation changed according to the regenerative strategies (season × strategy interaction: F4,50 = 4·05, P = 0·006) (Fig. 4). Although there were no significant differences between regenerative strategies in a given season, seeders varied the most between summer and winter, while seeder–resprouters varied less and resprouters presented the most constant pattern across the seasons (Table 2). Thus, in winter, the LM of seeders was higher than in autumn, while the other two strategies did not display any significant differences between autumn and winter. As a result, of the three strategies, the seeders presented the lowest LM in summer, but their values were the highest in winter (Fig. 4). No significant correlations were observed between LM and regenerative type when keeping constant the effect of the phylogenetic distance (Table 3).
Fig. 4.
Leaf moisture (LM) (%) means of the three regenerative strategies [seeder (S), resprouter (R) and seeder–resprouter (SR)], in summer, autumn and winter. Vertical bars denote 0·95 confidence intervals. Post hoc Fisher's l.s.d. comparisons showing significant differences (P < 0·05) are indicated with different letters. Upper case letters (A, B, C) indicate significant differences between seasons, and lower case letters (a, b, c) indicate post hoc differences between seasons for a given strategy. All the species (except C. spinosa and C. monogyna) are included.
Live fine fuel moisture (LFFM)
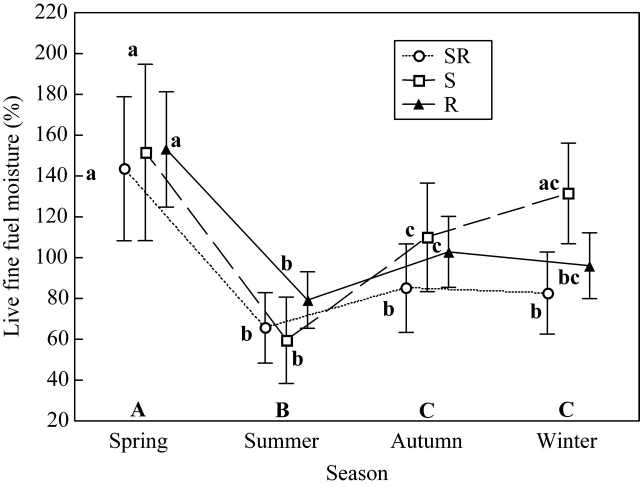
As expected, LFFM was significantly lower in summer than in autumn, winter and spring, the latter being the season with the highest values (F3,78 = 52·93, P < 0·001) (Fig. 5). Overall, differences between regenerative strategies were not significantly different (F2,26 = 0·88, P = 0·426), although seeders tended to attain higher LFFM values than the other regenerative strategies in autumn and winter (season×strategy interaction: F6,78 = 2·39, P = 0·036). Thus the seasonal pattern of seeders differed from the other regenerative strategies since the LFFM of seeders varied the most between summer and winter, while those of seeder–resprouters and resprouters varied the least over this period (Table 2). Accordingly, a nested ANOVA showed significant differences between strategies in winter (F2,259 = 5·71, P = 0·009), when seeders attained their highest values, followed by resprouters and seeder–resprouters (Fisher's l.s.d. post hoc test).
Fig. 5.
Mean live fine fuel moisture (LFFM) (%) of the three regenerative strategies [seeder (S), resprouter (R) and seeder–resprouter (SR)], in spring, summer, autumn and winter. Vertical bars denote 0·95 confidence intervals. Post hoc Fisher's l.s.d. significant differences (P < 0·05) are indicated with different letters. Upper case letters (A, B, C) indicate differences between seasons: lower case letters (a, b, c) indicate differences between seasons for a given strategy. All the species (except C. salviifolius) are included.
The same pattern was observed when correlating differences between species in LFFM (winter) and regenerative type when keeping the phylogenetic distance constant (Table 3).
DISCUSSION
Although in areas with a Mediterranean-type climate, all plant species are expected to endure Mediterranean summer drought, the present study demonstrates that the species with resprouting syndrome show different behaviour in the face of seasonal drought when compared with seeders. Thus, species with different post-fire regenerative strategies exhibit different functional properties within the ecosystem (Paula and Pausas, 2007). RWC results suggest that resprouters exhibit a higher and more stable water conservation strategy than do seeders from summer to winter. Accordingly, Correia and Catarino (1994) described very negative values of leaf water potential for the seeder Cistus species, with high variations throughout the year, while the resprouter Pistacia lentiscus sustained fewer variations and less water deficit over the course of the year. Therefore, morphological drought-avoiding traits are more common in resprouters, while non-resprouters should be physiologically more drought tolerant (Pausas et al., 2004), allowing them to survive on drier sites. The results are complementary to findings in similar communities (Paula and Pausas, 2007) and suggest that the resprouters under study have more mechanisms to reduce water losses and maintain water supply between seasons, so they tend to avoid dehydration. In contrast, seeders would tolerate lower leaf water contents in their tissues during drought periods. As a result, seeders tend to lose and gain water more easily than resprouters, and these differences may be due to traits such as cell characteristics that permit elasticity and osmotic adjustments (Medrano and Flexas, 2004).
In addition, plant water stress varies according to the depth and extension of root systems (Pereira and Chaves, 1993). Dawson and Pate (1996) described different root morphologies for Australian woody species with different resistance to fire: the most sensitive species had a single main root and a number of shallow lateral roots, while fire-resistant species had multiple lateral and main roots arising from a lignotuber. Similar descriptions are available of woody species from the Mediterranean basin: Cistus seedlings have shallow root systems while some resprouter species such as P. lentiscus have deeper rooted seedlings (Kummerow, 1981; Clemente et al., 2005). Thus it is highly feasible that species belonging to different post-fire regenerative strategies also differ with respect to the depth and extension of their root systems, and this factor could be involved in leaf water and nutrient conservation strategies. The extension of root systems is also related to above-ground plant size. In the present case, most of the resprouters are bigger than the seeders, and they could develop deeper root systems, allowing a more stable RWC throughout the year.
One important reason for the co-variation between post-fire regenerative traits and water use-related traits may be that these two groups of taxa (resprouters and seeders) originally evolved under different environments. Most of the resprouters under study are species that have evolved from ancient taxa present in the Tertiary (pre-Pliocene), before the establishment of the Mediterranean climate, while most seeders appeared later, contemporaneously with the Mediterranean climate, and they comprise fewer families. This difference in evolutionary context may, therefore, have contributed to the emergence of different strategies to tackle seasonal water deficit: relatively stable leaf water content and drought-avoiding traits in resprouters, and drough tolerance traits and poikilohydric tendencies in seeders, appearing under conditions of great variability in available water.
Seasonal variation is shown to be present in all the three variables related to water. The general pattern is that the lowest water content is found in summer, while it tends to increase between autumn and winter. As expected according to Garnieret al. (2001a), the present results suggest that LDMC is a parameter that does not vary so much between seasons, since this parameter relies on the dry mass of the leaf and the maximum water that can be stored. However, there are some interesting differences in LDMC in species with different regenerative strategies. It was found that resprouters have a consistently higher LDMC than seeders throughout the year. This means that the leaves of resprouters, when compared with those of seeders, have a structure with a greater proportion of dry matter in relation to saturated weight. This suggests a slower production of biomass, a longer leaf lifespan and a more efficient conservation of nutrients (Grime et al., 1997). Ryser and Urbas (2000) reported that differences in behaviour after disturbances are more closely related to the leaf lifespan and conservation of nutrients than to the availability of nutrients. In contrast, seeders, which often correspond to early successional species, would invest more resources in fast growth and reproduction, with a less efficient use of nutrients and water (Terradas, 1979; Nogueira et al., 2004).
LM and LFFM are often used as indicators of combustibility (Piñol et al., 1998; Viegas et al., 2001; Andrews and Bevins, 2003; Castro et al., 2003). The results for LM and LFFM indicate that seeders have more leaf and shoot moisture and that they rehydrate more in winter. Moreover, seasonal variation is higher in leaves than in shoots, in both strategies. Despite the tendencies observed in some species, the statistical analyses do not support the hypothesis that seeder species are at greater risk of fires in summer, at least with respect to this parameter related to combustibility. Further exploration of structural traits is needed to form a more complete picture of differences in combustibility and inflammability in the different regenerative strategies.
An important number of species in the Mediterranean basin display both post-fire regenerative strategies (seeding and resprouting). The present results suggest that this group tends to follow a similar strategy for water and nutrient use to that used by drought-tolerant seeders. This concurs with the evolutionary history of these species, which involves only four families (Leguminosae, Labiatae, Compositae and Globulariaceae) that mostly evolved in the Quaternary (post-Pliocene). However, the relatively stable seasonal pattern of LFFM in seeder–resprouters tends to be more similar to that of resprouters than that of seeders, indicating that they cannot rehydrate shoots to the same extent as seeders during autumn and winter.
It can be expected that species belonging to the same family should display more similar traits than distant taxa. This may be relevant for an evolutionary explanation of the occurrence of traits, but it is not particularly important when describing the pattern of changes of the functional attributes in the community in relation to the disturbance pattern. Thus the observed differences between regenerative types may be influenced by the fact that some of them are represented by a few families (seeders), and the phylogenetic and the regenerative effects are hardly distinguished. Accordingly, when the phylogenetic distance is kept constant, the differences between regenerative groups tend to disappear, suggesting that attributes related to leaf nutrient conservation and leaf structure are more likely to be determined by phylogenetic constraints. This is not the case with LFFM (in winter) and RWC, where differences between regenerative groups exist even when the weight of the phylogenetic distance is kept constant. RWC would appear as a plastic trait indicating leaf water status. It would quickly respond to water environmental conditions with a similar behaviour in phylogenetically distant taxa. Finally, LM and LFFM do not show any dependence on phylogenetic constraints, although, overall, there are no significant differences between the regenerative groups.
Overall, the present study reveals important differences in the leaf properties (water and dry matter content) of species that present distinct post-fire regeneration strategies throughout the various seasons. This supports the hypothesis that changes in the relative abundance of post-fire regenerative groups due to fires would promote changes in functional properties at the community level. These differences between regenerative types can be interpreted under the classical ‘r–K’ syndromes gradient. It is worth noting that this gradient of syndromes is determined by the evolutionary context. On the ‘r’ side we find species that emerged within the typical Mediterranean climate and that belong to a few families (Cistaceae, Labiatae), while on the ‘K’ side there are a number of Tertiary (pre-Pliocene) taxa belonging to a larger variety of families. This finding concurs with the observation in Mediterranean forests of the impact of drought episodes on these groups of species (Peñuelas et al., 2001). This suggests that the irregular water availability of the Mediterranean climate would have favoured the radiation of woody taxa with a relatively shorter lifespan, with a lesser ability to regulate water use (drought tolerance strategies) and produce a large number of seeds that can be stored in the soil seed bank until the appropriate conditions for establishment arise. Clearly, these traits closely correspond not only to the conditions found after wildfires, but also, generically, to the early stages of the succession that can be initiated by other disturbances (fire, clearing or drought episodes).
ACKNOWLEDGEMENTS
The authors would like to thank R. Ogaya, J. Piñol and J. Terradas, and two anonymous referees for fruitful suggestions and advice. Ll. Benejam, A. Burgas, M. Jané, M. Sallent, A. Saperas, A. Vilà, and M. Del Cacho helped in the field work. This study was funded by the Department of Universities, Research and Information Society of the Generalitat de Catalunya and the European social funds, and the Spanish MCYT project REN 2003-07198.
LITERATURE CITED
- Alberdi L, Cavero RY. Flora vascular post-incendio en un carrascal de Nazar (Navarra) Publicaciones de Biología, Universidad de Navarra, Serie Botánica. 2003;15:1–17. [Google Scholar]
- Andrews PL, Bevins CD. BehavePlus fire modeling system, version 2·0: overview. Proceedings of the Second International Wildland Fire Ecology and Fire Management Congress and Fifth Symposium on Fire and Forest Meteorology; Orlando, FL. American Meteorological Society; 2003. p. P5·11. [Google Scholar]
- Bellingham PJ, Sparrow AD. Resprouting as a life history strategy in woody plant communities. Oikos. 2000;89:409–416. [Google Scholar]
- Castro FX, Tudela A, Sebastia MT. Modeling moisture content in shrubs to predict fire risk in Catalonia (Spain) Agricultural and Forest Meteorology. 2003;116:49–59. [Google Scholar]
- Chandler C, Cheney P, Thomas P, Trabaud L, Williams D. Fire in forestry. Vol. 1. Forest fire behaviour and effects. New York: Wiley; 1983. [Google Scholar]
- Clemente AS, Rego FC, Correia OA. Growth, water relations and photosynthesis of seedlings and resprouts alter fire. Acta Oecologica. 2005;27:233–243. [Google Scholar]
- Correia OA, Catarino FM. Seasonal changes in soil-to-leaf resistance in Cistus sp. and Pistacea lentiscus. Acta Oecologica. 1996;15:189–300. [Google Scholar]
- Cucó ML. Mecanismes de regeneració. In: Terradas J, editor. Ecosistemes terrestres: la resposta als incendis i a d'altres pertorbacions. Diputació de Barcelona: Quaderns d'Ecologia Aplicada 10; 1987. pp. 45–62. [Google Scholar]
- Dawson ED, Pate JS. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: a stable isotope investigation. Oecologia. 1996;107:13–20. doi: 10.1007/BF00582230. [DOI] [PubMed] [Google Scholar]
- Díaz S, Cabido M. Vive la différence: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution. 2001;16:646–655. [Google Scholar]
- Emberger L. Un project de clasification des climats du point de vue phytosociologique. Bulletin de la Société Histoire Naturelle, Toulose. 1942;77:91–124. [Google Scholar]
- Espelta JM. Spain: Autonomous University of Barcelona; 1996. La regeneració de boscos d'alzina (Quercus ilex L.) i pi blanc (Pinus halepensis Mill.): estudi experimental de la resposta de les plàntules a la intensitat de llum i a la disponibilitat d'aigua. PhD Thesis. [Google Scholar]
- Eugenio M, Lloret F. Fire recurrence effects on the structure and composition of Mediterranean Pinus halepensis communities in Catalonia (northeast Iberian Peninsula) Écoscience. 2004;11:446–454. [Google Scholar]
- Fortin MJ, Gurevitch J. Mantel tests: spatial structure in field experiments. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York: Chapman and Hall; 1993. pp. 342–359. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001a;15:688–695. [Google Scholar]
- Garnier E, Laurent G, Bellmann A, Debain S, Berthelier P, Ducout B, et al. Consistency of species ranking based on functional leaf traits. New Phytologist. 2001b;152:69–83. doi: 10.1046/j.0028-646x.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Garnier E, Cortez J, Billes G, Navas ML, Roumet C, Debussche M, et al. Plant functional markers capture ecosystem properties duing secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JH, Rorison IH, et al. Integrated screening validates primary axes of specialization in plants. Oikos. 1997;79:259–281. [Google Scholar]
- Hanes T. Succession after fire in the chaparral of southern California. Ecological Monographs. 1971;41:27–42. [Google Scholar]
- Herrera C. Historical effects and sorting processes as explanations for contemporary ecological patterns: character syndromes in Mediterranean woody plants. American Naturalist. 1992;140:421–446. [Google Scholar]
- Hilu WK, Borsch T, Müller K, Soltis DE, Soltis PS, Savolainen V, et al. Angiosperm phylogeny based on MATK sequence information. American Journal of Botany. 2003;90:1758–1776. doi: 10.3732/ajb.90.12.1758. [DOI] [PubMed] [Google Scholar]
- Jalut G, Esteban-Amat A, Bonnet L, Gauquelin T, Fontugne M. Holocene climatic changes in the Western Mediterranean from south-east France to south-east Spain. Palaeogeography, Palaeoclimatology, Palaeoecology. 2000;160:255–290. [Google Scholar]
- Keane ER, Cary JC, Davies DI, Flannigan D, Gardner RH, Lavorel S, et al. A classification of landscape fire succession models: spatial simulations of fire and vegetation dynamics. Ecological Modelling. 2004;179:3–27. [Google Scholar]
- Keeley JE. Seed-germination patterns in fire-prone Mediterranean — climate regions. In: Arroyo MTK, Zedler PH, Fox MD, editors. Ecology and biogeography of Mediterranean ecosystems in Chile, California, and Australia. New York: Springer-Verlag; 1995. pp. 239–273. [Google Scholar]
- Kummerow J. Structure of rooths and root systems. In: Di Castri F, Goodall DW, Specht RL, editors. Mediterranean-type shrublands. Ecosystems of the world, 11. Amsterdam: Elsevier Scientific Publishers; 1981. pp. 269–288. [Google Scholar]
- Larcher W. Physiological plant ecology. 3rd edn. Berlin: Springer; 1995. [Google Scholar]
- Lavorel S, McIntyre S, Grigulis K. Plant response to disturbance in a Mediterranean grassland: how many functional groups? Journal of Vegetation Science. 1999;10:661–672. [Google Scholar]
- Lloret F. Régimen de incendios y regeneración. In: Valladares F, editor. Ecología del bosque mediterráneo en un mundo cambiante. Madrid: Ministerio de Ciencia y Tecnología; 2004. pp. 101–126. [Google Scholar]
- Lloret F, Vilà M. Clearing of vegetation in Mediterranean garrigue: response after a wildfire. Forest Ecology and Management. 1997;93:227–234. [Google Scholar]
- Lloret F, Vilà M. Diversity patterns of plant functional types in relation to fire regime and previous land use in Mediterranean woodlands. Journal of Vegetation Science. 2003;14:387–398. [Google Scholar]
- Lloret F, Calvo E, Pons X, Díaz-Delgado R. Wildfires and landscape patterns in the eastern Iberian Peninsula. Landscape Ecology. 2002;17:745–759. [Google Scholar]
- Lloret F, Pausas JG, Vilà M. Responses of Mediterranean plant species to different fire frequencies in Garraf Natural Park (Catalonia, Spain): field observations and modelling predictions. Plant Ecology. 2003;167:223–235. [Google Scholar]
- Medrano H, Flexas J. Respuesta de las plantas al estrés hídrico. In: Reigosa MJ, Pedrol N, Sánchez NA, editors. Ecofisiologia vegetal. Una ciencia de síntesis. Madrid: Thomson; 2004. pp. 253–286. [Google Scholar]
- Munné-Bosch S, Peñuelas J. Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Science. 2004;166:1105–1110. [Google Scholar]
- Ninyerola M, Pons X, Roure JM. A methodological approach of climatological modelling of air temperature and precipitation through GIS techniques. International Journal of Climatology. 2000;20:1823–1841. [Google Scholar]
- Ninyerola M, Pons X, Roure JM, Martín Vide J, Raso JM, Clavero P. Atles climàtic digital de Catalunya. 2003. CD-ROM. Servei Meteorològic de Catalunya y Dep. Medi Ambient Generalitat Catalunya. Available at http://opengis.uab.es/wms/iberis/index.htm .
- Nogueira A, Martinez CA, Ferreira LL, Prado CHBA. Photosynthesis and water use efficiency in twenty tropical tree species of differing succession status in a Brazilian reforestation. Photosynthetica. 2004;42:351–356. [Google Scholar]
- Papió CP. Respuesta al fuego de las principales especies de la vegetación de Garraf (Barcelona) Orsis. 1988;3:87–103. [Google Scholar]
- Pausas JG. Resprouting vs seeding – a Mediterranean perspective. Oikos. 2001;94:193–194. [Google Scholar]
- Pausas JG, Verdú M. Plant persistence traits in fire-prone ecosystems of the Mediterranean basin: a phylogenetic approach. Oikos. 2005;109:196–202. [Google Scholar]
- Pausas JG, Bradstoch RA, Keith DA, Keeley JE GCTE (Global Change of Terrestrial Ecosystems) Fire Network. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology. 2004;85:1085–1100. [Google Scholar]
- Paula P, Pausas JG. Leaf traits and resprouting ability in the Mediterranean basin. Functional Ecology. 2007 In press. [Google Scholar]
- Peñuelas J, Lloret F, Montoya R. Severe drought effects on Mediterranean woody flora in Spain. Forest Science. 2001;47:214–218. [Google Scholar]
- Peñuelas J, Munné-Bosch S, Llusià J, Filella I. Leaf reflectance and photo- and antioxidant protection in field-grown summer-stressed Phillyrea angustifolia. Optical signals of oxidative stress. New Phytologist. 2004;162:115–124. [Google Scholar]
- Pereira JS, Chaves MM. Plant water deficits in Mediterranean ecosystems. In: Smith JA, Griffiths H, editors. Water deficits: plant responses from cell to community. Oxford: BIOS Scientific Publishers Limited; 1993. pp. 21–236. [Google Scholar]
- Piñol J, Filella I, Ogaya R, Peñuelas J. Ground-based spectroradiometric estimation of live fine fuel moisture of Mediterranean plants. Agricultural and Forest Meteorology. 1998;90:173–186. [Google Scholar]
- Piñol J, Beven K, Viegas D. Modelling the effect of fire-exclusion and prescribed fire on wildfire size in Mediterranean ecosystems. Ecological Modelling. 2005;183:397–409. [Google Scholar]
- Polley HW, Derner JD, Wilsey BJ. Patterns of plant species diversity in remnant and restored tallgrass prairies. Restoration Ecology. 2005;13:480–487. [Google Scholar]
- Polo LA, Masip RS. Aproximación al conocimiento de la vegetación del macizo de Montgrí (Ampurdan, NE de la península ibérica) Ecología. 1987;1:121–132. [Google Scholar]
- Poorter H, Garnier E. Ecological significance of inherent variation in relative growth rate and its components. In: Pugnaire FI, Valladares F, editors. Handbook of functional plant ecology. New York: Marcel Dekker; 1999. pp. 81–120. [Google Scholar]
- Raunkiaer C. The life form of plants and statistical plant geography. Oxford: Oxford University Press; 1934. [Google Scholar]
- Rodrigo A, Retana J, Picó FX. Direct regeneration is not the only response of Mediterranean forests to large fires. Ecology. 2004;85:716–729. [Google Scholar]
- Rosenberg MS. PASSAGE. Pattern analysis, spatial statistics, and geographic exegesis. Department of Biology, Arizona State University; 2002. version 1·0. [Google Scholar]
- Ryser P, Urbas P. Ecological significance of leaf life span among Central European grass species. Oikos. 2000;91:41–50. [Google Scholar]
- Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology. 1986;35:627–632. [Google Scholar]
- Suc JP. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature. 1984;307:409–432. [Google Scholar]
- Terradas J. Ecologia d'avui. Barcelona: Teide editions; 1979. Collection Què Cal Saber. [Google Scholar]
- Teulat B, Monneveux P, Wery J, Borries C, Sourys I, Charrier A, This D. Relationships between relative water content and growth parameters under water stress in barley: a QTL study. New Phytologist. 1997;137:99–107. [Google Scholar]
- Trabaud L. Fire and survival traits of plants. In: Trabaud L, editor. The role of fire in ecological systems. The Hague: SPB Academic Publishing; 1987. pp. 65–89. [Google Scholar]
- Trabaud L. Fire regimes and phytomass growth dynamics in a Quercus coccifera garrigue. Journal of Vegetation Science. 1991;2:307–314. [Google Scholar]
- Verdú M. Ecological and evolutionary differences between Mediterranean seeders and resprouters. Journal of Vegetation Science. 2000;11:265–268. [Google Scholar]
- Verdú M, Dávila P, García-Fayos P, Flores-Hernández N, Valiente-Vanuet A. ‘Convergent’ traits of mediterranean woody plants belong to pre-mediterranean lineages. Biological Journal of the Linnean Society. 2003;78:415–427. [Google Scholar]
- Viegas DX, Piñol J, Viegas MT, Ogaya R. Estimating live fine fuels moisture content using meteorologically-based indices. International Journal of Wildland Fire. 2001;10:223–240. [Google Scholar]
- Vilà M, Lloret F, Ogheri E, Terradas E. Positive fire-grass feedback in Mediterranean basin woodlands. Forest Ecology and Management. 2001;147:3–14. [Google Scholar]
- Whelan RJ. The ecology of fire. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Zedler PH, Gautier CR, McMaster GS. Vegetation change in response to extreme events: the effect of a short interval between fires in California chaparral and coastal scrub. Ecology. 1983;64:809–818. [Google Scholar]