Abstract
Objective
African-Americans under age 75 have over twice the risk of stroke death than whites in the United States. Regardless of race, stroke death is ~50% higher in the “Stroke Belt” and “Stroke Buckle” states of the Southeastern United States. We assessed geographic and racial differences in estimated 10-year stroke risk.
Methods
The Reasons for Geographic and Racial Differences in Stroke study is a population-based cohort of men and women aged ≥45, recruited February 2003–September 2007 at this report, with oversampling of Stroke Belt / Buckle residents and African-Americans. Racial and regional differences in the Framingham Stroke Risk Score were studied in 23,940 participants without previous stroke or transient ischemic attack.
Results
The mean age, race and sex adjusted 10-year predicted stroke probability differed slightly across regions; 10.7% in the Belt, 10.4% in the Buckle, and 10.1% elsewhere (p <0.001). Geographic differences were largest for the score components of diabetes, and use of antihypertensive therapy. African-Americans had a higher age and sex-adjusted mean 10-year predicted stroke probability than whites; 11.3% versus 9.7%, respectively (p <0.001). Race differences were largest for the score components of hypertension, systolic blood pressure, diabetes, smoking, and left ventricular hypertrophy.
Interpretation
While African-Americans had a higher predicted stroke probability than whites, regional differences were small. Results suggest that interventions to reduce racial disparities in stroke risk factors hold promise to reduce the racial disparity in stroke mortality. The same may not be true regarding geographic disparities in stroke mortality.
Stroke is the third leading cause of death in the United States, accounting for 1 of every 16 deaths in 2004 1. Residents of the Southeastern United States (“stroke belt”) have approximately 50% higher rates of stroke mortality than the remainder of the United States 2–4. These geographic differences have existed since at least 1940 2, with excess deaths demonstrated for men, women, whites and African-Americans 4.
The cause of the excess stroke mortality in the stroke belt is unknown 5, 6. Differences in stroke incidence may contribute to the excess mortality, but little national data on regional differences in incidence are available to address this hypothesis. For white men and women, data from the first National Health and Nutrition Examination Survey (NHANES I) suggested that stroke incidence was higher in the Southeast than the Northeast, but the pattern was inconsistent between the Southeast and other regions 7. In addition, while the stroke belt is at least as pronounced for blacks as for whites 8–10 and the incidence of stroke was higher among blacks 11–13 in some studies, in NHANES I regional differences in stroke incidence among African-Americans were not as striking as those among whites 7. Lower rates of stroke hospitalization in the stroke belt could also explain underlying differences in mortality; however among Medicare recipients, those in Southeastern states were more likely to be hospitalized for stroke than in other regions 14. Another potential cause of the stroke belt is differences in case fatality by region; however little information is available to support this hypothesis 15. Similarly, differential case fatality according to race has not been clearly demonstrated 12, 13, 16. Hence, the available data suggest that geographic and racial variations in stroke mortality relate to differences in incidence, not case fatality. If this is the case, then disparities in stroke risk factors may underlie observed differences in stroke mortality.
The Framingham Heart Study investigators identified nine risk factors for stroke: age, sex, systolic blood pressure, antihypertensive therapy, diabetes, current smoking, prior cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy 17. The Framingham Stroke Risk Score (FSRS), developed and validated in a primarily white population, predicts the 10-year probability of stroke based on these nine risk factors. To address the role of stroke risk factors on regional and racial differences in stroke mortality, we studied geographic and racial differences in the FSRS in a national cohort.
Methods
Subjects
The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study is a national longitudinal study initiated in January of 2003 to elucidate the causes of geographic and racial disparities in stroke mortality 18. REGARDS was designed to recruit 30,000 community-dwelling African-American and white participants (50% from each group) aged 45 years or older from the continental United States. By design, 20% of the cohort resided in the “buckle” of the Stroke Belt (coastal plain region of NC, SC and GA), 30% from the Stroke Belt states (remainder of NC, SC, and GA, plus AL, MS, TN, AR, and LA), and 50% from the other 40 contiguous states (referred to as “rest of the nation” here). Participants were recruited from those randomly selected from a commercially available nationwide list purchased from Genesys. They were contacted by mail than telephone. Participants completed a standardized telephone interview including demographic information and medical history (including history of stroke or transient ischemic attack). An in-home examination was performed subsequently to obtain physical measures (blood pressure, height, weight), electrocardiogram, medication inventory, and fasting blood and urine samples. In the home, written informed consent was obtained using methods approved by the institutional review boards of all participating institutions. As of September 1, 2007, both the phone interview and in-home examination were completed for 29,185 participants. This analysis included 23,940 participants without a self-reported history of stroke or transient ischemic attack (n=3,182), and who had data for all components of the FSRS (≥1 component missing on 2,063). Those with missing data did not differ in sociodemographic characteristics from included participants.
Definitions
Race was defined by self-report requesting participants to select their race from a list (white, black or African-American, Asian, native Hawaiian or other Pacific Islander, American Indian, Alaska native or other). They were then asked whether they were also Hispanic or Latino. Only white or black non-Hispanic participants were eligible. Age, sex, use of antihypertensive therapy and history of heart disease (myocardial infarction or heart attack, coronary artery bypass surgery, coronary angioplasty or stenting) were defined based on self-report of a physician diagnosis. Systolic blood pressure was the average of two measurements taken by a trained technician after the participant was seated for 5 minutes, measured using a standard protocol and regularly tested aneroid sphygmomanometer. Diabetes was defined as fasting glucose above 6.99 mmol/L (126 mg/dL), non-fasting glucose above 11.1 mmol/L (200 mg/dL), or self-reported medication use for diabetes. Current smoking was classified for FSRS determination by the response to the question, “Do you smoke cigarettes now, even occasionally?” Atrial fibrillation was defined as either self-report of a diagnosis by a health care professional or by electrocardiogram. Left ventricular hypertrophy (LVH) was defined by electrocardiogram using the modified Cornell Index. While the Cornell Index might be favored 19, this requires a twelve-lead electrocardiogram and the first 6,490 participants enrolled had only a seven-lead electrocardiogram. The FSRS was calculated for each participant using age, sex, systolic blood pressure, antihypertensive therapy, diabetes, smoking, prior cardiovascular disease, atrial fibrillation, and LVH 17, 20. Among the first 15,017 participants with a 12-lead electrocardiogram, use of the Cornell Index to define LVH had minimal impact on the FSRS (correlation coefficient of scores with Cornell Index and modified Cornell Index = 0.99).
Data Analysis
Analyses were performed for both the mean FSRS and for each individual component of the FSRS. Because results were reported in sex-race strata, no weights were applied to the analysis. Analysis of variance was used to assess regional differences in factors contributing to the FSRS, after adjustment for age, sex, and race. All analyses were performed using SAS version 9.1.3.
Results
Levels of the risk factors that are part of the FSRS are shown for sex-race strata of the REGARDS population in Table 1. Table 2 provides the age-race-sex adjusted FSRS and its components by region. The mean 10-year age-race-sex adjusted predicted stroke risk in the stroke belt and buckle were 10.7% and 10.4%, respectively, only slightly higher than in the rest of the nation (10.1%). The difference in FSRS was driven primarily by regional differences in two risk factors: diabetes, and use of antihypertensive medications, with the largest regional difference in the prevalence of diabetes. The prevalence of diabetes in the stroke belt was 3.5 percentage points higher than in the rest of the nation, and 5.1 percentage points higher in the stroke buckle than the rest of the nation. For anti-hypertensive medication use these percentage differences were 4.5% and 5.2%, respectively.
Table 1.
Framingham Stroke Risk Score Components at Baseline in the REGARDS Cohort by Race and Sex, 2003 – 2007.
| Characteristic* | Men | Women | ||
|---|---|---|---|---|
| African- American |
White | African- American |
White | |
| (n=3744) | (n=7045) | (n=6112) | (n=7039) | |
| Age, years | 64.5 (9.1) | 66.3 (9.2) | 64.0 (9.2) | 64.2 (9.5) |
| Current Smoking | 725 (19) | 803 (11) | 943 (15) | 925 (13) |
| Anti-hypertensive medication use | 2108 (56) | 2933 (42) | 3911 (64) | 2841 (40) |
| Systolic Blood Pressure, mmHg | 132 (17) | 127 (16) | 130 (18) | 123 (16) |
| History of Heart disease | 835 (22) | 2007 (29) | 1026 (17) | 1139 (16) |
| Diabetes Mellitus | 1107 (30) | 1191 (17) | 1714 (28) | 868 (12) |
| Left Ventricular Hypertrophy | 272 (7) | 190 (3) | 859 (14) | 280 (4) |
| Atrial fibrillation | 236 (6) | 640 (9) | 445 (7) | 551 (8) |
Data shown are mean (SD) for age and blood pressure, and number (%) for other variables.
Table 2.
Mean Age, Race and Sex-adjusted Framingham Stroke Risk Score, and Percent or Mean Values for each Score component, by Geographic Region in the REGARDS cohort, 2003 – 2007.
| Framingham Stroke Risk Score |
Anti- hypertensive Medication Use |
Systolic Blood Pressure (mm Hg) |
History of Heart Disease |
Diabetes | Current Smoking |
Left Ventricular Hypertrophy |
Atrial Fibrillation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | N | Mean | p* | % | p | Mean | p | % | p | % | p | % | p | % | p | % | p |
| Rest of the | |||||||||||||||||
| Nation | 10,494 | 10.1 | - | 46.6 | - | 127.1 | - | 20.4 | - | 18.1 | - | 14.0 | - | 6.4 | - | 7.4 | - |
| Stroke Belt | 8,505 | 10.7 | <0.0001 | 51.1 | <0.0001 | 128.0 | 0.0002 | 21.3 | 0.12 | 21.6 | <0.0001 | 14.8 | 0.08 | 7.3 | 0.008 | 8.0 | 0.14 |
| Stroke Buckle | 4,941 | 10.4 | 0.03 | 51.8 | <0.0001 | 126.3 | 0.004 | 21.5 | 0.11 | 23.2 | <0.0001 | 13.6 | 0.55 | 6.3 | 0.90 | 8.4 | 0.01 |
p values are for comparison of the Stroke Belt or Buckle to the Rest of the Nation.
African-Americans had a higher age and sex-adjusted mean (SD) FSRS than whites, 11.3% (11.8) compared to 9.7% (10.1). This was true in all three regions (p <0.001) and reflected a substantially worse risk factor profile for all FSRS components (p <0.0001 for each) except history of heart disease and atrial fibrillation, which were less common in African-Americans. Table 3 shows geographic differences in FSRS and its components stratified according to race. Differences in the FSRS among regions were small but significant among whites. Among African-Americans the FSRS was only slightly higher in the stroke belt than the rest of the nation and it did not differ in the stroke buckle. Most regional differences in FSRS components comparing the stroke belt or buckle to the rest of the nation were similar in blacks and whites, except for higher prevalences of atrial fibrillation in the stroke buckle and heart disease in the stroke belt among whites but not blacks. Smoking was less common in the stroke buckle than other regions for blacks but not whites, and more common in the belt for whites, but not for blacks (p for interaction between race and region <0.0001). For both ethnicities, antihypertensive medication use was more common in the stroke belt or buckle compared to the rest of the nation; while systolic blood pressure was higher in the belt (but not buckle) among whites, and lower in the buckle (but not belt) among blacks (p for interaction between race and region =0.001). Diabetes was about twice as common among blacks than whites in all regions but the difference in prevalence of diabetes in the stroke belt compared to the rest of the nation was greater for whites (p for interaction between race and region 0.02). This was related to a similar prevalence of diabetes in the stroke belt compared to the rest of the nation among black men (figure 1). In other race/sex groups in figure 1 the prevalence of diabetes tended to increase from the rest of the nation to belt to buckle.
Table 3.
Mean Age and Sex-Adjusted Framingham Stroke Risk Score, and Percent or Mean Values for Each Score Component in the REGARDS Cohort by Geographic Region and Race, 2003 – 2007.
| Framingham Stroke Risk Score |
Anti- hypertensive Medication Use |
Systolic Blood Pressure |
History of Heart Disease |
Diabetes | Current Smoking |
Left Ventricular Hypertrophy |
Atrial Fibrillation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | Region | N | Mean | p* | % | p | Mean | p | % | p | % | p | % | P | % | p | % | p |
| White | Rest of the | |||||||||||||||||
| Nation | 5,678 | 9.3 | - | 38.2 | - | 124.5 | - | 21.3 | - | 12.4 | - | 11.1 | - | 3.1 | - | 7.8 | - | |
| Stroke Belt | 5,223 | 10.0 | <0.0001 | 42.4 | 0.0001 | 126.1 | <0.0001 | 23.1 | 0.02 | 16.0 | <0.0001 | 13.2 | 0.0008 | 3.9 | 0.03 | 8.4 | 0.25 | |
| Stroke Buckle | 3,183 | 9.8 | 0.003 | 43.7 | <0.0001 | 124.5 | 0.94 | 22.9 | 0.09 | 16.3 | <0.0001 | 12.7 | 0.03 | 2.8 | 0.41 | 9.6 | 0.003 | |
| African- American |
Rest of the | |||||||||||||||||
| Nation | 4,816 | 11.1 | - | 58.6 | - | 130.9 | - | 19.1 | - | 26.1 | - | 17.8 | - | 11.0 | - | 6.9 | - | |
| Stroke Belt | 3,282 | 11.6 | 0.04 | 63.5 | <0.0001 | 130.7 | 0.74 | 18.4 | 0.42 | 29.6 | 0.0006 | 17.0 | 0.31 | 12.2 | 0.08 | 7.1 | 0.64 | |
| Stroke Buckle | 1,758 | 11.2 | 0.69 | 63.2 | 0.0007 | 128.8 | <0.0001 | 19.3 | 0.86 | 33.6 | <0.0001 | 14.2 | 0.0005 | 11.4 | 0.64 | 6.6 | 0.76 | |
p values compare Stroke Belt or Buckle to the Rest of the Nation.
Figure 1.
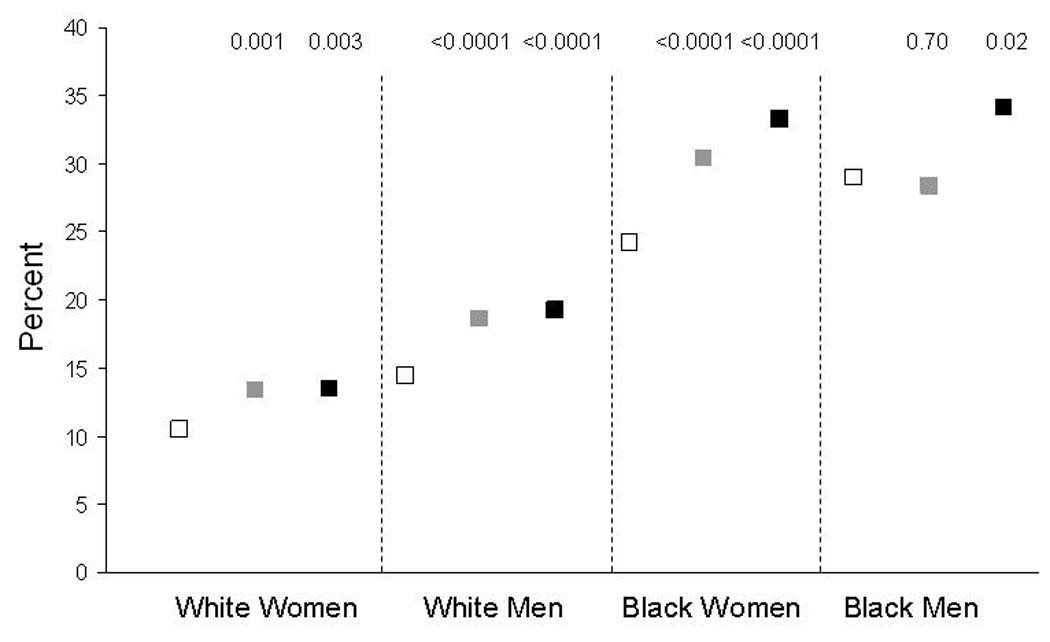
Mean age-Adjusted Prevalence of Diabetes by Sex, Race and Region. P values are for comparison to stratum-specific “rest of the nation” region. Open squares are the rest of the nation, gray squares are the stroke belt and black squares are the stroke buckle.
Figure 2 presents geographic differences in the age-adjusted FSRS stratified by race and sex. While differences between race and sex groups were clear and highly significant, differences by region were small. The average scores for those living in the stroke belt and buckle compared to the rest of the nation were significantly different at the p <0.05 level for only the belt and buckle region among white men, and the belt region among black and white women. There were no regional differences in scores among black men.
Figure 2.
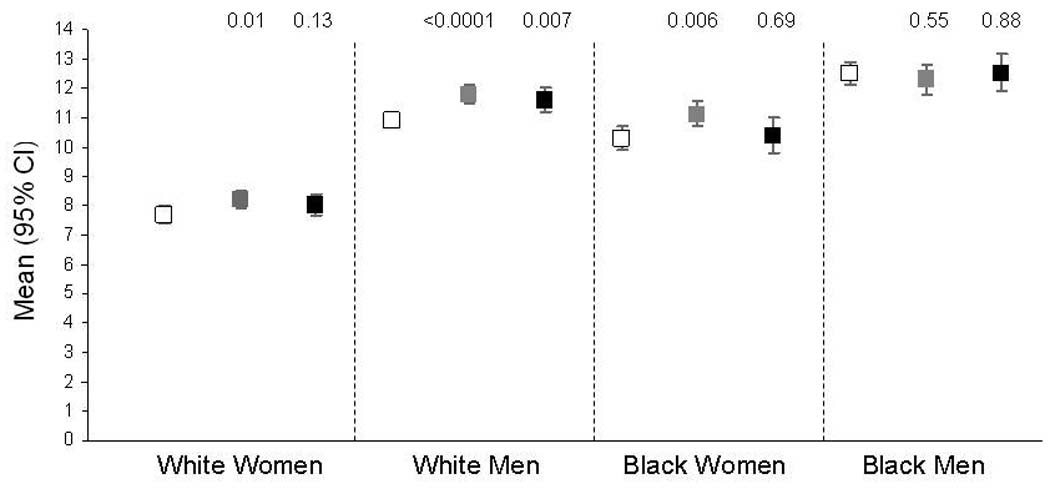
Mean Age-adjusted Framingham Stroke Risk Score by Sex, Race and Region. Error bars are 95% confidence interval of the mean. P values are for comparison to stratum-specific “rest of the nation” region. Open squares are the rest of the nation, gray squares are the stroke belt and black squares are the stroke buckle.
Discussion
Knowledge of racial and geographic patterns in stroke risk factors may provide useful information for public health interventions. Ultimately, the goal is to identify targets for interventions in specific population groups that may reduce the disparities in stroke incidence and consequently the burden of stroke in all populations. The FSRS is a summary index incorporating “traditional” stroke risk factors and estimates the predicted stroke incidence over 10 years. In this national study, geographic differences in the average FSRS across regions were small. This suggests that only a small portion of the 50% higher stroke mortality in these regions is driven by the “traditional” risk factors comprising the FSRS. Because hypertension is the strongest stroke risk factor we expected that hypertension would play a major role in differences in stroke fatality. However, geographic differences in diabetes were substantially larger than differences in hypertension (defined by medication use). Findings suggest that interventions to reduce geographic disparities in diabetes, including optimizing prevention, diagnosis and treatment, may hold promise for reducing geographic disparities in stroke mortality.
While geographic differences in diabetes prevalence were the largest differences seen among stroke risk factors, this association differed in race/sex strata, all of whom share the higher stroke mortality of the stroke belt and buckle. There were small differences in the prevalence of diabetes in white women, diabetes was not more common in the stroke belt than the rest of the nation among black men, and differences in diabetes prevalence by region were largest in black women. In the Behavioral Risk Factor Surveillance System, self-reported diabetes was highest in the southeastern US states in 2001, with the stroke belt states of Alabama ranking 1st (10.5%), Mississippi 2nd (10.3%), South Carolina 4th (9.4%), Arkansas 6th (8.9%), Louisiana 7th (8.5%), Tennessee 11th (8.3%), Georgia 22nd (7.7%), and North Carolina 23rd (7.6%)21.
Most papers on causes of the stroke belt have largely assumed that the cause is higher stroke incidence in this region 5, 6. Our findings of relatively small differences in FSRF by region, and of similar FSRS in the ‘higher risk’ stroke buckle than stroke belt raise a hypothesis that stroke case fatality might play a substantial role. While components of the FSRS might influence case fatality, other factors not addressed in our analysis, such as poverty, access to care and non-traditional risk factors also need to be considered. Studies such as REGARDS, which follow a large geographically dispersed cohort, will help to answer these questions.
While geographic differences in the FSRS were relatively small and influenced by selected component risk factors, differences in the FSRS between whites and blacks were substantially larger, with differences in many of the component risk factors, in particular diabetes and hypertension. These racial disparities have been previously described 22, 23 including higher systolic blood pressure in blacks than whites (by approximately 5 mmHg), even in the setting of higher prevalence of antihypertensive medication use 24. It has been suggested that higher prevalences of diabetes, hypertension and other risk factors contribute to racial disparities in stroke risk 22, 25 but few prospective studies are available with sufficient numbers of African-American and white participants to address this question. Follow-up in the REGARDS cohort will help clarify this.
Our findings extend previous reports on geographic variation in hypertension from the NHANES 26–28. In NHANES III 26, 27 there was a slightly higher prevalence of hypertension in the southeast for white men (27% versus 24%), white women (22% versus 21%), and black men (35% versus 33%), with a more substantial difference for black women (35% versus 28%). In further analysis assessing age groups (40–59 and 60–79 years), hypertension was more prevalent in the southeast in 7 of 8 age-race-gender strata; however, these differences were only statistically significant among black men aged 40–59 and white men aged 40–59 28. We are not aware of other studies providing a true regional comparison of hypertension prevalence. Because the focus of this paper was overall differences in the FSRS which considers use of antihypertensive medications and the average systolic blood pressure, we focused on these parameters rather than hypertension, per se. Anti-hypertensive medication use was more common in the stroke belt than the rest of the nation for both whites and African-Americans. However, the pattern of blood pressure was not consistent, with whites having a higher blood pressure in the stroke belt (but not buckle) than the rest of the nation, and African-Americans having lower systolic blood pressure in the stroke buckle (but not belt) than the rest of the nation. Thus, there is a mixed picture regarding geographic variations in hypertension and blood pressure, with NHANES showing trends for higher blood pressure in the Southeast, and REGARDS showing higher use of antihypertensive medications but an inconsistent pattern in measured blood pressure. The NHANES reports used a more broad definition of Southeast, which may explain some of these differences. We have reported detailed analyses of correlates of regional and racial differences in hypertension elsewhere 24.
Few population-based data are available on prevalence of atrial fibrillation and LVH by region and race. Case-control studies and hospital series suggest that atrial fibrillation is less prevalent and may be less strongly associated with stroke risk among blacks than whites in the U.S.29, 30. In one study of African-Americans (2.7% with previous stroke; 68% with hypertension), 49% of participants had LVH by echocardiogram, and this was associated with an increased risk of magnetic resonance imaging-documented stroke and white matter disease 31.
The strengths and weaknesses of this study merit discussion. Study examinations were conducted in participants’ homes by a large number of examiners rather than in a limited number of field sites as in most similar studies. While substantial training and standardization efforts were undertaken18, these cannot replicate the quality of field center-based epidemiologic studies. This shortcoming is offset by the advantages of a large nationally-based sample with oversampling of African-Americans and residents of the stroke belt and buckle, enabling optimal assessment of geographic and racial disparities. As with all epidemiologic studies, participation rates are a concern. We conservatively estimated a 40% participation rate in REGARDS, which compares favorably to other epidemiologic studies. The current analysis included 23,940 participants without stroke or TIA, with 41% African-Americans. Thus these results are more precise than other studies that included far fewer subjects 7, 28. Unlike the BRFSS 21, REGARDS measured most components of the FSRS rather than relying on self-reported data, providing better reliability for the FSRS. About 8% of participants were missing at least one component of the FSRS, leading to exclusion of these individuals. While we relied on self-reported cerebrovascular disease as a selection criterion for the current analysis, any impact of potential misclassification on findings would be small given the sample size and rarity of disease. As in other population-based studies, REGARDS is limited to non-institutionalized individuals who had a telephone, which may restrict generalizability. Finally, the FSRS has not been validated in African-Americans, therefore it may not be an optimal tool for assessing stroke mortality risk in this group.
In summary, we observed only modest differences in the FSRS between the stroke belt or stroke buckle compared to the rest of the US, and differences were mostly driven by geographic differences in diabetes and hypertension. Interventions to reduce geographic disparities in these factors may be promising for reducing geographic disparities in stroke mortality. However, given the small regional differences in the FSRS, it seems unlikely that the increased stroke mortality in the Southeast is due solely to differences in traditional risk factors that comprise the score. Follow up data from this study will examine these questions and the role of other risk factors. Conversely, we observed the expected racial differences in hypertension, diabetes, atrial fibrillation and LVH that could contribute to racial differences in stroke risk. These disparities offer hypotheses for interventions that might reduce racial disparities in stroke mortality.
Acknowledgments
The authors acknowledge the participating investigators and institutions: University of Alabama (Study PI, Data Coordination Center, Survey Research Unit): George Howard, Leslie McClure, Virginia Howard, Libby Wagner, Virginia Wadley, Rodney Go); University of Vermont (Central Laboratory): Mary Cushman; Wake Forest University (ECG Reading Center): Ron Prineas; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez, David Rhodes, Susanna Bowling, Sean Orr; University of Arkansas for Medical Sciences (Survey Research): LeaVonne Pulley; University of Cincinnati: Brett Kissela, Dawn Kleindorfer (Clinical Neuro-Epidemiology Unit) Examination Management Services Incorporated (In-Home Visits): Andra Graham; National Institute of Neurological Disorders and Stroke: Claudia Moy. This research is supported by U01 NS041588 from the National Institute of Neurological Disorders and Stroke, NIH, Department of Health and Human Services.
Footnotes
Conflict of interest disclosure: Dr. Moy is an employee of the funding agency, NIH.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Lanska DJ. Geographic distribution of stroke mortality in the United States: 1939–1941 to 1979–1981. Neurology. 1993;43:1839–1851. doi: 10.1212/wnl.43.9.1839. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Evans GW, Pearce K, et al. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke. 1995;26:1153–1158. doi: 10.1161/01.str.26.7.1153. [DOI] [PubMed] [Google Scholar]
- 4.Casper M, Barnett E, Williams G, Jr, et al. Atlas of stroke mortality: racial, ethnic and geographic disparities in the United States. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 5.Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci. 1993;317:160–167. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 7.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Followup Study. Am J Epidemiol. 1996;144:665–673. doi: 10.1093/oxfordjournals.aje.a008979. [DOI] [PubMed] [Google Scholar]
- 8.Howard G, Anderson R, Sorlie P, et al. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks. The National Longitudinal Mortality Study. Stroke. 1994;25:2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 9.Casper ML, Wing S, Anda RF, et al. The shifting stroke belt. Changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke. 1995;26:755–760. doi: 10.1161/01.str.26.5.755. [DOI] [PubMed] [Google Scholar]
- 10.Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997;28:1639–1647. doi: 10.1161/01.str.28.8.1639. [DOI] [PubMed] [Google Scholar]
- 11.Kittner SJ, White LR, Losonczy KG, et al. Black-white differences in stroke incidence in a national sample. The contribution of hypertension and diabetes mellitus. JAMA. 1990;264:1267–1270. [PubMed] [Google Scholar]
- 12.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 13.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 14.Public health and aging: hospitalizations for stroke among adults aged >/=65 years--United States, 2000. MMWR Morb Mortal Wkly Rep. 2003;52:586–589. [PubMed] [Google Scholar]
- 15.May DS, Casper ML, Croft JB, Giles WH. Trends in survival after stroke among Medicare beneficiaries. Stroke. 1994;25:1617–1622. doi: 10.1161/01.str.25.8.1617. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy BS, Kasl SV, Brass LM, Vaccarino V. Trends in hospitalized stroke for blacks and whites in the United States, 1980–1999. Neuroepidemiology. 2002;21:131–141. doi: 10.1159/000054810. [DOI] [PubMed] [Google Scholar]
- 17.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, et al. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Crow RS, Prineas RJ, Rautaharju P, et al. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension (results from Treatment of Mild Hypertension Study) Am J Cardiol. 1995;75:1233–1238. [PubMed] [Google Scholar]
- 20.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 21.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 22.Giles WH, Kittner SJ, Hebel JR, et al. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 1995;155:1319–1324. [PubMed] [Google Scholar]
- 23.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 24.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 25.Ohira T, Shahar E, Chambless LE, et al. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 26.Hall WD, Ferrario CM, Moore MA, et al. Hypertension-related morbidity and mortality in the southeastern United States. Am J Med Sci. 1997;313:195–209. doi: 10.1097/00000441-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Jones D, Basile J, Cushman W, et al. Managing hypertension in the southeastern United States: applying the guidelines from the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VI) Am J Med Sci. 1999;318:357–364. [PubMed] [Google Scholar]
- 28.Obisesan TO, Vargas CM, Gillum RF. Geographic variation in stroke risk in the United States. Region, urbanization, and hypertension in the Third National Health and Nutrition Examination Survey. Stroke. 2000;31:19–25. doi: 10.1161/01.str.31.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 30.Upshaw CB., Jr Reduced prevalence of atrial fibrillation in black patients compared with white patients attending an urban hospital: an electrocardiographic study. J Natl Med Assoc. 2002;94:204–208. [PMC free article] [PubMed] [Google Scholar]
- 31.Fox ER, Taylor HA, Jr, Benjamin EJ, et al. Left ventricular mass indexed to height and prevalent MRI cerebrovascular disease in an African American cohort: the Atherosclerotic Risk in Communities study. Stroke. 2005;36:546–550. doi: 10.1161/01.STR.0000154893.68957.55. [DOI] [PubMed] [Google Scholar]


