Abstract
Background and Aims
Increased levels of nitrogen (N) deposition lead to enhanced N contents and reduced productivity of many bryophyte species. This study aimed at elucidating the mechanisms by which enhanced N uptake may cause growth reduction of bryophytes, focusing on the effects of N addition on carbon (C) metabolism of bryophytes.
Methods
Plantlets of Thuidium tamariscinum and Hylocomium splendens were fertilized with NH4NO3 (N load equalling 30 kg ha−1 year−1) for 80 d, including a pulse labelling experiment with 13CO2 to dissect the partitioning of carbon in response to N addition.
Key Results
Growth of T. tamariscinum was not affected by N addition, while H. splendens showed a trend towards growth reduction. Total N concentration was significantly increased by N addition in H. splendens, a significant increase in amino acid-N was found in T. tamariscinum only. In both bryophyte species, a reduction in concentration of lipids, the greatest C storage pool, as well as markedly enhanced turnover rates of C storage pools in fertilized plants were observed.
Conclusions
The results suggest that growth reduction of H. splendens under high levels of N deposition may be caused by enhanced synthesis of N-containing organic compounds, most probably of cell wall proteins. Disturbance of cellular C metabolism, as indicated by enhanced C pool turnover, may further contribute to the decline in productivity of H. splendens.
Key words: Bryophytes, nitrogen deposition, growth, Thuidium tamariscinum, Hylocomium splendens, C metabolism, amino acids
INTRODUCTION
Atmospheric nitrogen (N) deposition increased dramatically in most parts of Europe during the second half of the 20th century (Galloway, 1995). Though currently emissions are not rising further, they still remain at a high level. Bryophytes have been shown to be highly sensitive to atmospheric deposition of pollutants since, due to the lack of a cuticle and a high surface area, they readily take up nutrients and pollutants from atmospheric sources (Bates, 2000; Zechmeister et al., 2003).
In addition to direct physiological effects on growth and N and carbon (C) metabolism, N deposition may also affect bryophytes indirectly by changing the competitiveness of higher plants and bryophytes (Bobbink et al., 1998; Pearce and van der Wal, 2002; Skrindo and Okland, 2002). With regard to direct effects of N deposition, bryophytes cannot be considered a homogenous functional group, as species differ considerably in their response to N fertilization (Dirkse and Martakis, 1992; Potter et al., 1995; Gordon et al., 2001; Paulissen et al., 2004). This at least partly reflects divergent nutrient acquisition strategies of different bryophyte species. Fast-growing bryophytes, which rely on nutrients released from litter, have been shown to react differently to nutrient addition than species predominantly depending on atmospheric nutrient supply (Bates, 1992, 1994). Generally, physiological properties and long-term adaptations determine individual responses of bryophyte species (Baxter et al., 1992; Paulissen et al., 2005).
Studies on the effects of increased N deposition on bryophytes published during the last 20 years revealed both decreased productivity (Potter et al., 1995; Pearce et al., 2003; Mitchell et al., 2004) and increased productivity of bryophytes (Aerts et al., 1992; Jonsdottir et al., 1995), even in the same bryophyte species. This may be due to the fact that levels of N deposition at the study sites as well as the amounts of experimentally applied N varied considerably. Moreover, results from short-term experiments are often different from long-term studies. Growth stimulation of bryophytes caused by N fertilization indicated that in oligotrophic habitats, productivity of bryophytes, usually considered to be adapted to low nutrient environments, was limited by N (Aerts et al., 1992; Jonsdottir et al., 1995). However, under current levels of N deposition in most parts of western and central Europe, the N supply of bryophytes may be super-optimal, exceeding bryophyte N demand for growth, and resulting in increased gametophyte N concentrations (Jauhiainen et al., 1998; Nordin and Gunnarsson, 2000). As a consequence of excess N uptake, reduced growth rates of bryophytes have been reported above a certain level of N content (Van Der Heijden et al., 2000; Pearce et al., 2003; Solga et al., 2005). However, the mechanisms behind this growth reduction are still unclear. It has been suggested that NH4+ toxicity causes membrane dysfunction (Limpens and Berendse, 2003; Paulissen et al., 2005) or that the accumulation of specific amino acids plays a role in this mechanism (Nordin and Gunnarsson, 2000; Paulissen et al., 2005). Evidence for either of these explanations is still missing since no comprehensive analysis of primary metabolites in N-treated bryophytes has been published yet.
This paper presents results from a fertilization experiment with NH4NO3 (N load equivalent to a deposition rate of 30 kg ha−1 year−1), with Hylocomium splendens and Thuidium tamariscinum, two large ectohydric feather mosses common on forest floors in temperate and boreal forests. In Austria the abundance of H. splendens has been observed to decrease at sites affected by high N deposition during the last 10 years, while that of T. tamariscinum has been reported to increase slightly (H. G. Zechmeister, unpubl. res.).
Any change in bryophyte abundance may exhibit strong effects on ecosystems, since bryophytes, although a minor element in terms of biomass, play an important role in nutrient cycling of many ecosystems, thus considerably influencing ecosystem processes (Oechel and Van Cleve, 1986; Chapin et al., 1987; Eckstein, 2000; Turetsky, 2003). This study therefore aimed at elucidating the mechanism by which enhanced N deposition may cause growth reduction of some bryophytes. The hypothesis was that excess N uptake stimulates the synthesis of organic N compounds, a process requiring energy and carbon which is thereby drained from growth processes. Moreover, when the N assimilation capacity of bryophytes is overloaded, inorganic N forms may accumulate, further impairing carbon metabolism of bryophytes. Thus it was assumed that the growth reduction of H. splendens observed in the field is caused by the accumulation of organic N compounds leading to a shortage of C, while T. tamariscinum is able to utilize additional N for growth. Therefore concentrations of C and N storage compounds were analysed in fertilized and unfertilized plants of both species, as well as C fluxes into different pools of extractable metabolites by a 13C-labelling experiment.
MATERIALS AND METHODS
Experimental design
Thuidium tamariscinum and Hylocomium splendens plants were collected in October 2002 at five sites near Steinhaus/Semmering located in the north-eastern Alps (47°37′N 15°47′E, 825 m a.s.l.). Vegetation was a montane forest dominated by Picea abies. Wet N deposition measured at the nearest ICP Forest Level II monitoring station at Mürzzuschlag (47°38′N 15°39′E, 715 m a.s.l.) was 3·9 kg N ha−1 year−1 in 2002 (Smidt, 2004). N deposition levels at the sampling sites are expected to be slightly higher than at Mürzzuschlag due to the greater altitude of the sampling location (approx. 200 m).
For each species plants from all sites were pooled. Plantlets comprising the two last annual segments were cut and 20 of each were put on filter paper in Petri dishes. Five replicate dishes were prepared for each species and treatment. The Petri dishes were placed in a climate chamber (50–80 µmol m−2 s−1 PAR at plant level, 90 % RH and 20 °C). After 2 weeks of acclimatization, fertilizer was applied as a solution of 0·5 mm NH4NO3 to a final N addition rate of 2·5 kg N ha−1 month−1 (equivalent to approx. 30 kg N ha−1 year−1). NH4NO3 solution or distilled water (control) was added as a fine mist using a spray bottle three times per week.
At the end of the 80-d treatment period plants were pulse labelled with 13CO2: The Petri dishes were placed in an acrylic glass box (6 L) together with a beaker containing 105 mg NaH13CO3. Lactic acid was added to the NaH13CO3 and the box was immediately closed for 2 h. In addition, controls were treated with NaH12CO3. After 13CO2 labelling plants were grown in a climate chamber under environmental conditions as given above for further 3 d. Plants were harvested, inactivated by shock-heating in a microwave oven and dried at 60 °C. After determination of dry weights, plant materials were finely ground in a ball mill.
Growth analysis
Relative growth rates were calculated from projection areas of shoots (‘leaf areas’) and dry weights using a software tool for plant growth analysis (Hunt et al., 2002). Final dry masses were determined by weighing. Initial dry weights were calculated from initial ‘leaf areas’ by an equation derived from correlation of final dry weights and the respective final ‘leaf areas’:
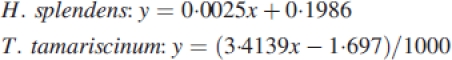 |
where x is the initial leaf area (cm2) and y the initial dry weight (g). Projection areas were determined by scanning and graphical analysis using Photoshop 7.
Determination of soluble carbohydrates, organic acids, nitrate and ammonium
Finely ground plant material (20 mg) was extracted with 0·5 mL of a mixture of methanol and chloroform (12 : 5) for 30 min at 60 °C. After adding 0.5 mL of water for phase separation, samples were centrifuged, and the aqueous phase (supernatant) and the chloroform (lipid) phase were stored separately at −20 °C. Aliquots of the aqueous phase were used for the following analyses. Soluble carbohydrates were determined by HPLC with pulsed amperometric detection (DX 500, Dionex) using an anion exchange column (Carbopac PA10, 250×2 mm, Dionex) and 100 mM NaOH at a flow rate of 0·25 mL min−1 (30 °C). Ammonium was determined by ion chromatography and conductivity detection after chemical suppression (DX 500, Dionex) using a cation exchange column (CS16, 5×250 mm, Dionex) and 48 mm methane sulfonic acid at a flow rate of 1 mL min−1 (40 °C).
For determination of anions, finely ground plant material (20 mg) was extracted in a shaking water bath with 1 ml water for 30 min at 80 °C. Samples were centrifuged after cooling and the supernatant was stored at −20 °C. Organic acids and nitrate were analysed by ion chromatography and conductivity detection after chemical suppression (DX 500, Dionex) using an anion exchange column (AS11, 250×4 mm, Dionex) and NaOH gradient elution (2·5 min isocratic at 0·05 mm, linear increase from 0·05 to 37·5 mm over 15·5 min) at a flow rate of 2 mL min−1.
Fractionation by ion exchange chromatography
Conditioning of cation and anion exchange resins was performed as follows. Cation exchange resin (DOWEX 50 W×8, 50–100 mesh; Sigma) was incubated with 1 m HCl at 60 °C, then rinsed thoroughly with water. Anion exchange resin (DOWEX 1×8, 50–100 mesh; Fluka) was suspended in 1 m NaOH at room temperature, rinsed with water, equilibrated with 1 m sodium-formate and again rinsed with excess water. SPE cartridges (Supelco filtration tubes, 3 mL) were filled with cation and anion exchange resins. Aliquots (500 µL) of the aqueous phase of methanol/chloroform/water (MCW) extracts were applied to coupled ion exchange columns (cation- above anion-exchange resin). Ion exchange columns were rinsed with 30 mL water and the eluate was collected. Cation exchange columns were eluted with 30 mL 3 m NH4OH, anion exchange columns with 20 ml 12 n formic acid. Eluates were taken to dryness using a rotary evaporator, redissolved in water and stored at −20 °C.
Amino acid analyses
Aliquots of the cationic fraction were analysed for amino acids by HPLC with UV absorbance detection (Dionex Summit) using a reversed phase column (Novapak C18, 3·9×300 mm; Waters) and a gradient of sodium acetate buffer (pH 6·4, containing 6 % acetonitrile, 0·05 % TEA, 0·02 % EDTA) and acetonitrile/water (3 : 2 (v/v), containing 0·02 % EDTA) at a flow rate of 1 mL min−1 (method modified from instructions given by the manufacturer, Waters). Before quantification, samples were dried under vacuum after adding a drying solution (methanol, 1 m sodium acetate and triethyl amine in a ratio of 1 : 1 : 0·5) and derivatized with phenyl isothiocyanate (derivatization solution consisting of methanol, TEA, water and phenylisothiocyanate at a ratio of 7 : 1 : 1 : 1). Norleucine, added to the samples before ion exchange, served as an internal standard. For peak identification and calibration an amino acid standard solution (No. 09428; Fluka) augmented with glutamine and asparagine was used.
Starch and protein preparation for quantification and carbon isotope analysis
Preparation of starch for carbon isotope analysis generally followed Wanek et al. (2001). Finely ground plant material (150 mg) was extracted with 4 mL of a mixture of methanol, chloroform and water (12 : 3 : 5, v/v/v) at 70 °C for 30 min, samples were centrifuged and the supernatant was decanted. This step was repeated twice, with incubation times of 10 min each. Samples were washed three times with water, each time shaken and centrifuged, then samples were dried. Purification of α-amylase solution was performed as described in Gottlicher et al. (2006). The pre-washed dried bryophyte samples were incubated with 750 µL of water at 100 °C for 15 min. After cooling, 250 µL of purified α-amylase solution were added and samples were incubated at 85 °C for 120 min. Samples were centrifuged, the supernatant was transferred to pre-cleaned centrifugal filter devices (Microcon YM-10, Regenerated Cellulose, 10000 MWCO, amicon Bioseperations, Millipore Corporation) and centrifuged. Aliquots of the filtrate were pipetted into tin capsules, dried and analysed by isotope ratio mass spectrometry (IRMS) (see below).
For determination of soluble proteins, finely ground plant material (10 mg) was extracted with 1·5 mL of 0·25 m NaOH at 95 °C for 60 min. After centrifugation, aliquots (1 mL) of the supernatant were mixed with 1 mL of 36 % trichloracetic acid to precipitate protein and centrifuged after 10 min. The protein precipitate was washed with 100 % ethanol, dried in vacuo and redissolved in 25 mm NaOH. Aliquots were pipetted into tin capsules, dried and analysed by IRMS.
Determination of carbon isotope ratios and carbon concentrations
For determination of isotope ratios and C contents of bulk material, plant powder (1·5–2 mg) was weighed into tin capsules and subjected to isotope ratio mass spectrometry (IRMS). Aliquots of neutral, cationic and anionic fractions of extracts (see ion exchange procedure), as well as aliquots of the lipid phases of MCW extracts (see soluble carbohydrates) were pipetted into tin capsules, dried and together with the starch hydrolysates and protein extracts analysed by IRMS. Samples were analysed by continuous-flow IRMS, consisting of an elemental analyser (EA 1100; CE instuments, Milan, Italy) connected to a gas isotope ratio mass spectrometer (DeltaPLUS; Finnigan MAT, Bremen, Germany). High purity CO2 (AGA, Vienna, Austria) was used as the reference gas.
13C incorporation was calculated according to the following equation:
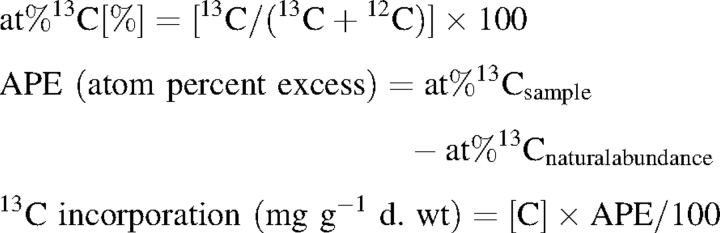 |
where [C] represents the C concentration of samples (mg C g−1 d. wt)
Turnover rates of C pools were calculated according to the following equation:
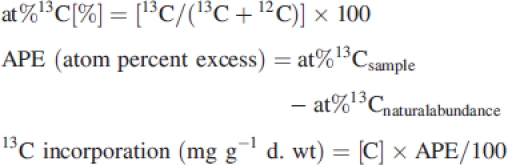 |
where T is the turnover rate (year−1), [13C] is the 13C incorporation into a C pool within 2 h (mg 13C g−1 d. wt) and [C] is the C concentration of the C pool (mg C g−1 d. wt). The factors 4 and 365 were used to calculate apparent daily and annual 13C incorporation rates. The authors are fully aware that the turnover rates calculated in this way represent rough estimates only, due to higher than ambient CO2 concentrations during labelling, the 2-h pulse–3-d chase period (daily incorporation was calculated by multiplying by 4, assuming 8 h to represent the daily period of maximum photosynthetic fixation) and seasonal fluctuations in bryophyte photosynthetic behaviour. However, they allow for C dynamics to be compared between treatments and species in this study.
Statistical analysis
Differences between treatments were analysed using the Mann–Whitney test. Comparison of species was performed by Kruskal–Wallis tests. These tests were chosen because the data generally did not meet the assumption of normality and equality of variance. All statistical analyses were performed using SPSS 10·0.
RESULTS
Growth
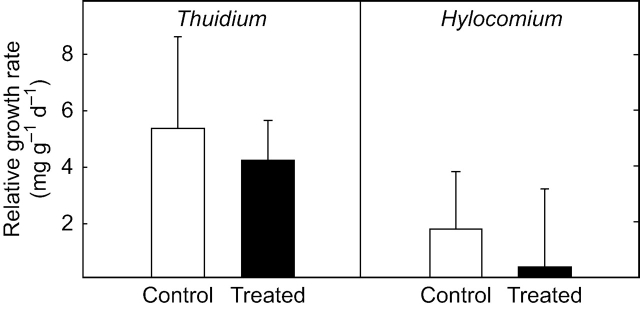
Hylocomium splendens generally showed lower relative growth rates than T. tamariscinum (Fig. 1). No statistically significant differences in growth rates were apparent between controls and N-treated plants of either bryophyte species. However, the relative growth rates of fertilized H. splendens plants declined by 75 % compared with controls.
Fig. 1.
Relative growth rates of control plants and plants treated with 0·5 mm NH4NO3 for 80 d of Thuidium tamariscinum and Hylocomium splendens. Values represent means +1 s.e.; n=5.
Carbon
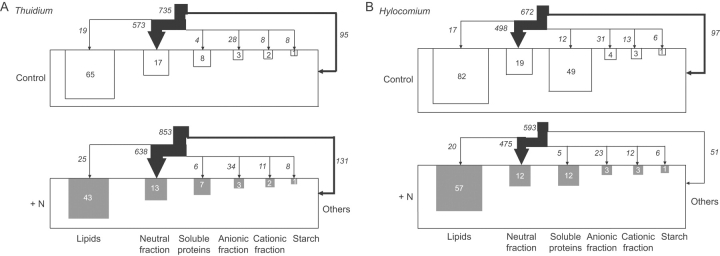
Analysis of C pools revealed that the greatest amount of C was stored in lipids, followed by neutral fraction and soluble proteins, with the exception of control plants of H. splendens, where the soluble protein pool was much larger than that of the neutral fraction (Fig. 2). In both bryophyte species, fertilized plants generally showed smaller C pools than controls, whereas carbon fluxes into fertilized plants tended to be increased in T. tamariscinum and to be slightly decreased in H. splendens (Fig. 2). The overall pattern of C fluxes was similar for all treatments, with the dominant C flux into neutral fraction.
Fig. 2.
13C fluxes into C pools of control plants and plants treated with NH4NO3 of (A) Thuidium tamariscinum and (B) Hylocomium splendens 3 d after pulse-chase-labelling with 13CO2. Values represent means (n=5) of C pools (mg C per 20 plantlets) and C fluxes (μg 13C per 20 plantlets). Dry weights per 20 plantlets at harvest were 0·38 g and 0·42 g in fertilized and control plants of T. tamariscinum, and 0·42 g and 0·48 g in fertilized and control plants of H. splendens.
Regarding C contents expressed on a dry weight basis, N treatment significantly decreased lipid concentrations in both species (Table 1A). Differences in C concentrations of other fractions were not significant. The sum of all extractable compounds made up a smaller portion of total C in fertilized plants than in controls, 42 % in fertilized versus 52 % in control plants in T. tamariscinum and 47 % versus 73 % in H. splendens. Thus, in fertilized plants a comparatively higher portion of total C was stored in substances which were not extracted by the procedure used in this study, i.e. cell wall components.
Table 1.
Carbon contents and 13C-incorporation of different fractions of extractable metabolites in control plants and plants fertilized with NH4NO3 of Thuidium tamariscinum and Hylocomium splendens
|
T. tamariscinum |
H. splendens |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Fertilized |
C×F | Control |
Fertilized |
C×F | |||||
| mg g−1 d. wt | (% of tot. C) | mg g−1 d. wt | (% of tot. C) | mg g−1 d. wt | (% of tot. C) | mg g−1 d. wt | (% of tot. C) | |||
| (A) Carbon contents | ||||||||||
| Lipids | 155·5 | (35·0) | 114·1 | (26·4) | * | 165·2 | (37·7) | 126·4 | (29·6) | + |
| Neutral fraction | 39·7 | (8·9) | 33·9 | (7·8) | n.s. | 37·6 | (8·6) | 28·9 | (6·8) | n.s. |
| Soluble proteins | 19·0 | (4·3) | 19·4 | (4·5) | n.s. | 101·9 | (23·3) | 28·0 | (6·6) | n.s. |
| Anionic fraction | 6·7 | (1·5) | 7·1 | (1·6) | n.s. | 7·4 | (1·7) | 6·8 | (1·6) | n.s. |
| Cationic fraction | 5·6 | (1·3) | 6·1 | (1·4) | n.s. | 6·1 | (1·4) | 6·1 | (1·4) | n.s. |
| Starch | 2·5 | (0·6) | 2·5 | (0·6) | n.s. | 2·6 | (0·6) | 2·9 | (0·7) | n.s. |
| Total carbon | 444·1 | 433·1 | n.s. | 437·6 | 426·5 | * | ||||
| (B) 13C-incorporation | ||||||||||
| Lipids | 0·044 | (2·6) | 0·064 | (2·8) | * | 0·033 | (2·4) | 0·045 | (3·2) | n.s. |
| Neutral fraction | 1·291 | (76·4) | 1·692 | (75·1) | n.s. | 1·023 | (73·5) | 1·108 | (79·7) | n.s. |
| Soluble proteins | 0·010 | (0·6) | 0·016 | (0·7) | * | 0·023 | (1·7) | 0·011 | (0·8) | + |
| Anionic fraction | 0·062 | (3·7) | 0·091 | (4·0) | n.s. | 0·063 | (4·5) | 0·055 | (4·0) | n.s. |
| Cationic fraction | 0·020 | (1·2) | 0·029 | (1·3) | n.s. | 0·026 | (1·9) | 0·029 | (2·1) | n.s. |
| Starch | 0·019 | (1·1) | 0·020 | (0·9) | n.s. | 0·012 | (0·9) | 0·015 | (1·1) | n.s. |
| Total carbon | 1·689 | 2·253 | n.s. | 1·391 | 1·389 | n.s. | ||||
Values are means of five. Significance levels: +, P<0·1; *, P<0·05.
Total 13C incorporation on a dry matter basis tended to increase with N fertilization in T. tamariscinum (Table 1B). This tendency was reflected in all fractions except for starch, with significant increases for lipids and soluble proteins. However, partitioning of the total 13C flux was nearly identical in both treatments and species. In H. splendens, 13C incorporation was not significantly influenced by N treatment, except for a decreased flux into soluble proteins. Hylocomium splendens generally showed lower 13C incorporation rates than T. tamariscinum. Calculation of C pool turnover rates from pool sizes and short-term 13C fluxes revealed a marked increase in turnover rates of C pools by N treatment in both bryophyte species. Turnover rates of lipids, the major C storage pool, increased from 0·4 year−1 in controls to 0·8 year−1 in fertilized plants of T. tamariscinum, and from 0·3 year−1 to 0·5 year−1 in H. splendens. Turnover rates of sugars (neutral fraction) increased from 47 to 72 in T. tamariscinum and from 40 to 56 in H. splendens.
The concentration of sucrose, the most abundant soluble carbohydrate, tended to increase with N treatment in T. tamariscinum, whereas in H. splendens the opposite was the case (Table 2). However, differences were not significant because of the high variation in values. In H. splendens decreased concentrations of glucose and fructose in fertilized plants were observed. Raffinose concentrations were elevated by N treatment in T. tamariscinum, and a similar trend was observed in H. splendens.
Table 2.
Concentrations of sugars, organic acids and amino acids in control plants and plants fertilized with NH4NO3 of Thuidium tamariscinum and Hylocomium splendens
|
T. tamariscinum |
H. splendens |
|||||
|---|---|---|---|---|---|---|
| Control | Fertilized | C×F | Control | Fertilized | C×F | |
| Sugars (μmol g−1 d. wt) | ||||||
| Glucose | 2·96 | 2·72 | n.s. | 7·74 | 3·19 | + |
| Fructose | 3·98 | 2·51 | n.s. | 7·61 | 3.52 | n.s. |
| Sucrose | 60·94 | 87·32 | n.s. | 105·21 | 74.79 | n.s. |
| Raffinose | 0·72 | 1·62 | + | 0·99 | 1·84 | n.s. |
| Organic acids (μmol g−1 d. wt) | ||||||
| Malic acid | 35·30 | 47·25 | n.s. | 45·23 | 35·79 | n.s. |
| Oxalic acid | 8·61 | 12·53 | * | 14·19 | 14·98 | n.s. |
| Citric acid | 4·74 | 4·8 | n.s. | 3·04 | 2·67 | n.s. |
| Amino acids (μmol g−1 d. wt) | ||||||
| Arg | 0·06 | 0·30 | n.s. | 1·26 | 2·44 | n.s. |
| Asn | 1·06 | 5·08 | * | 6·50 | 10·45 | n.s. |
| Gln | 0·62 | 3·15 | * | 0·64 | 1·09 | n.s. |
| Asp | 0·03 | 0·18 | * | 0·24 | 0·42 | n.s. |
| Glu | 1·93 | 2·56 | n.s. | 2·03 | 2·23 | n.s. |
| Ala | 0·49 | 1·26 | * | 1·14 | 1·48 | n.s. |
| Ser | 0·36 | 0·63 | n.s. | 0·76 | 1·34 | n.s. |
| Gly | 0·14 | 0·22 | n.s. | 0·48 | 0·79 | n.s. |
| Thr | 0·13 | 0·36 | n.s. | 0·35 | 0·87 | n.s. |
| Pro | 0·08 | 0·16 | n.s. | 0·54 | 0·81 | n.s. |
Values are means of five. Significance levels: +, P<0·1; *, P<0·05.
Malic acid, the dominant organic acid, showed a similar change in concentration as sucrose, with a positive effect of N addition in T. tamariscinum and a negative effect in H. splendens, although again differences were insignificant. Oxalic acid concentration increased significantly in N-fertilized plants of T. tamariscinum, whereas in H. splendens no effect of N treatment was evident.
Nitrogen
N fertilization resulted in a significantly greater N concentration in H. splendens (Fig. 3) and in T. tamariscinum a similar trend was found. If values were calculated on a plantlet basis (4·4 mg N per 20 plantlets in fertilized plants versus 3·9 mg N in controls in T. tamariscinum, 5·8 mg N versus 4·8 mg N in H. splendens), differences in H. splendens were no longer significant. Contents of amino acid-N in N-treated plants ranged from 340 µg g−1 in T. tamariscinum to 590 µg g−1 in H. splendens. A significant increase in amino acid-N was found in T. tamariscinum only. NH4+-N concentrations increased significantly by N addition in both species, whereas NO3−-N concentrations did not change significantly.
Fig. 3.
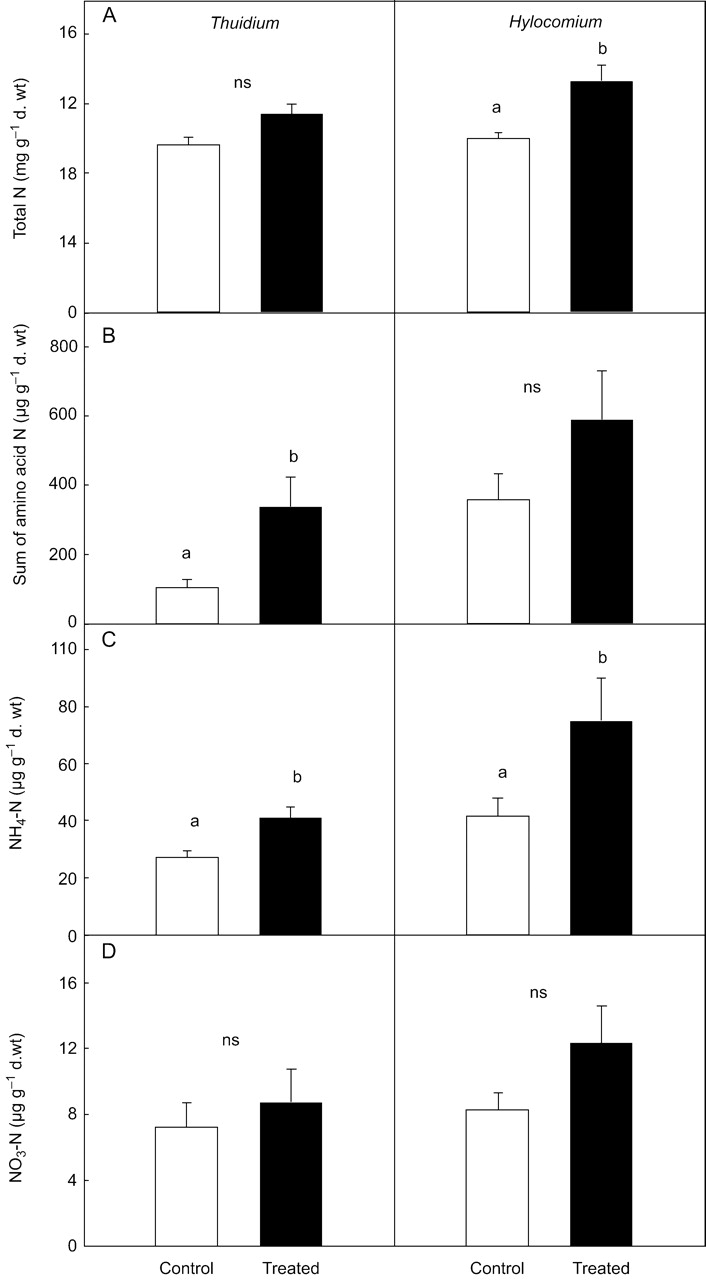
Concentrations of (A) total N, (B) amino acid-N, (C) NH4+-N and (D) NO3−-N of control plants and plants treated with NH4NO3 of Thuidium tamariscinum and Hylocomium splendens. Values represent means +1 s.e.; n=5. Significant differences (P<0·05) are indicated by different letters; n.s., not significant.
Dominant amino acids in T. tamariscinum were asparagine, glutamine, glutamic acid and alanine (Table 2). Concentrations of asparagine, glutamine, alanine and aspartic acid increased significantly by the N treatment. In T. tamariscinum, asparagine and glutamine comprised 42 % and 26 % of total amino acid-N in fertilized plants, compared with 28 % and 16 % in controls, respectively. In H. splendens, asparagine and arginine acted as major N storage compounds (containing 50 % and 20 % of total amino acid-N in both treatments). Despite a tendency to increased concentrations of most amino acids in fertilized plants, no significant differences between treatments were observed in H. splendens.
DISCUSSION
The strong tendency towards growth reduction in H. splendens (75 % decrease in RGR) (Fig. 1) is consistent with Swedish forest fertilization experiments showing a marked decrease in cover of H. splendens as a result of applying a N dose of 30 kg ha−1 year−1 (Dirkse and Martakis, 1992). In contrast to what was expected from observations of the increasing abundance of T. tamariscinum in Austria, growth rates of T. tamariscinum were not enhanced by N addition in this study. However, growth responses of bryophytes under laboratory conditions may differ from those observed in the field, since laboratory conditions are inevitably artificial, especially in terms of hydration regime and nutrient supply. Growth of feather mosses was shown to be closely related to microclimate (Busby et al., 1978), which could therefore explain lower growth rates of T. tamariscinum and H. splendens in the laboratory compared to field conditions (Busby et al., 1978; Rincón, 1993; Callaghan et al., 1997). Furthermore, aging of moss segments older than two years, which were not used for the experiment, may contribute to the nutrient supply of younger segments via reallocation of nutrients (Eckstein and Karlsson, 1999), thus also affecting growth.
Nevertheless, the present results demonstrate an increased metabolic activity of T. tamariscinum, as well as a considerably better physiological status of T. tamariscinum compared with H. splendens under enhanced N conditions, which may lead to enhanced productivity of T. tamariscinum in the longer term. First, carbon (13C) incorporation into fertilized T. tamariscinum plants increased (Fig. 2 and Table 1). Secondly, high C fixation rates of N-treated plants were reflected in increased concentrations of sucrose and organic acids (Table 2). In contrast, in H. splendens sucrose and organic acids tended to decrease despite a considerable reduction in plantlet growth. Generally, higher concentrations of all compounds analysed in H. splendens than in T. tamariscinum may be caused by lower growth of H. splendens. A negative correlation of soluble carbohydrates and total N has already been observed in Sphagnum species (Van Der Heijden et al., 2000), whereas results from a Swedish forest fertilization experiment showed no significant changes in content of soluble carbohydrates in H. splendens after 8 years of N addition (Forsum et al., 2006).
The increase in total N content observed (Fig. 3A) is consistent with results from comparable experiments (Soares and Pearson, 1997; Limpens and Berendse, 2003; Pearce et al., 2003). Similar to these studies, increased bryophyte N contents have been shown not to be the mere result of growth reduction but of enhanced (‘luxury’) N uptake. Concentrations of amino acid-N (Fig. 3B) were below values reported for Sphagnum species under similar N-deposition levels (Nordin and Gunnarsson, 2000; Limpens and Berendse, 2003) and below values described for H. splendens in a long-term fertilization experiment (Forsum et al., 2006). In Sphagnum species growth reduction was observed when amino acid-N concentrations exceeded 2 mg g−1 d. wt (Nordin and Gunnarsson, 2000). However, Limpens and Berendse (2003) did not find any correlation between amino acid-N and growth rates, even at amino acid-N concentrations well above 2 mg g−1. Accumulation of amino acids has been considered to be a possible cause of growth reduction in bryophytes under increased levels of N deposition (Nordin and Gunnarsson, 2000; Paulissen et al., 2005). However, the present results do not support this hypothesis. First, a marked increase in amino acid-N was only found in T. tamariscinum, which showed no growth reduction, whereas in H. splendens no significant differences in amino acid-N concentrations were observed, despite considerable reduction in growth rates. In T. tamariscinum predominantly the synthesis of amino acids with a low C : N ratio was enhanced by N addition, implying high C efficiency in N storage, as previously described for Sphagnum species (Baxter et al., 1992; Limpens and Berendse, 2003). Secondly, enhanced synthesis of free amino acids cannot have provoked C shortage, since the amount of C required for amino acid synthesis was very low in comparison to other C storage pools, nor did the C fluxes into cationic fraction significantly increase with N fertilization.
Regarding effects of N addition on C metabolism, the most striking changes were found in content of lipids in both species (Table 1A). Generally, the fraction of extractable C compounds declined. These observations were similar to those found for N, increasing total N not being paralleled by increasing soluble N. This suggests the accumulation of some unknown N-containing organic compounds, most probably of cell wall proteins, that are not extractable by common solvents but constitute a large fraction of total N in plants (Takashima et al., 2004). This may especially be the case in H. splendens, where the increase in total N content was higher than in T. tamariscinum. The need for energy and carbon for the synthesis of these organic compounds may have caused the observed growth reduction. Similar partitioning of C fluxes in both treatments is possibly due to the fact that the time span of 3 d between 13C-labelling and harvest was rather short, thus a great proportion of fixed C was still found in primary C fixation products, i.e. soluble sugars. Nevertheless, differences in subsequent metabolic conversion of sugars may become apparent in the longer term.
Carbon pool turnover rates greatly increased in response to N fertilization, particularly for lipids, sugars and proteins (all representing key storage components in bryophytes), pointing to N deposition as a stress factor. The acceleration of cellular C metabolism, implying enhanced respiratory losses, may also have contributed to the reduction of the major C storage pool (lipids) and a lower allocation of C to biomass production.
A hypothesis which has frequently been put forward as an explanation for reduced productivity of bryophytes by N deposition is that NH4+ toxicity might cause membrane dysfunction (Limpens and Berendse, 2003; Pearce et al., 2003; Paulissen et al., 2005). In this case, growth reduction of N-sensitive bryophyte species would not be caused by the accumulation of amino acids or other N-containing organic compounds but by the incapability of the N-assimilation apparatus to adapt to high NH4+ concentrations, i.e. the inefficiency in NH4+-assimilation. As indicators of severe membrane dysfunction, K+ leakage (Pearce et al., 2003; Paulissen et al., 2005) and even a decrease of N contents (Paulissen et al., 2005) have been observed. However, in the present study no evidence was found for NH4+ toxicity. K+ contents per replicate were similar in fertilized and control plants in both species (data not shown). Values for NH4+ were low (Fig. 3C) and may have comprised considerable amounts of extracellular NH4+ bound to cell walls. Similar values have recently been reported for H. splendens (Forsum et al., 2006).
Finally, down-regulation of growth can also occur via effects of dissolved organic or inorganic N compounds on regulation of C metabolism, such as modulation of gene expression by specific amino acids. This was, however, beyond the goals of the present study, and should be a next step forward in understanding the mechanisms involved in the regulation of metabolic pathways in bryophytes.
In summary, the present results support the hypothesis that growth reduction of bryophytes under high levels of N deposition was caused by the accumulation of N-containing organic compounds, most probably of cell wall proteins. The accumulation of free amino acids apparently was not responsible for the observed decrease in growth. Disturbance of cellular C metabolism as indicated by enhanced C pool turnover rates further seems to play an important role in growth reduction of bryophytes. No evidence for NH4+-toxicity was found as the cause of growth reduction. Other effects of increased N content on regulation of C metabolism may also contribute to the decrease in productivity of bryophytes.
ACKNOWLEDGEMENTS
We are grateful to Andreas Blöchl and Erich Inselsbacher for skilful help with HPLC analyses.
LITERATURE CITED
- Aerts R, Wallen B, Malmer N. Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. Journal of Ecology. 1992;80:131–140. [Google Scholar]
- Bates JW. Mineral nutrient acquisition and retention by bryophytes. Journal of Bryology. 1992;17:223–240. [Google Scholar]
- Bates JW. Responses of the mosses Brachythecium rutabulum and Pseudoscleropodium purum to a mineral nutrient pulse. Functional Ecology. 1994;8:686–693. [Google Scholar]
- Bates JW. Mineral nutrition, substratum ecology, and pollution. In: Shaw AJ, Goffinet B, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2000. pp. 248–311. [Google Scholar]
- Baxter R, Emes MJ, Lee JA. Effects of an experimentally applied increase in ammonium on growth and amino-acid-metabolism of Sphagnum cuspidatum Ehrh. ex Hoffm. from differently polluted areas. New Phytologist. 1992;120:265–274. [Google Scholar]
- Bobbink R, Hornung M, Roelofs JGM. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. Journal of Ecology. 1998;86:717–738. [Google Scholar]
- Busby JR, Bliss LC, Hamilton CD. Microclimate control of growth rates and habitats of boreal forest mosses, Tomenthypnum nitens and Hylocomium splendens. Ecological Monographs. 1978;48:95–110. [Google Scholar]
- Callaghan TV, Carlsson BA, Sonesson M, Temesvary A. Between-year variation in climate-related growth of circumarctic populations of the moss Hylocomium splendens. Functional Ecology. 1997;11:157–165. [Google Scholar]
- Chapin FS, Oechel WC, Vancleve K, Lawrence W. The role of mosses in the phosphorus cycling of an Alaskan black spruce forest. Oecologia. 1987;74:310–315. doi: 10.1007/BF00379375. [DOI] [PubMed] [Google Scholar]
- Dirkse GM, Martakis GFP. Effects of fertilizer on bryophytes in Swedish experiments on forest fertilization. Biological Conservation. 1992;59:155–161. [Google Scholar]
- Eckstein RL. Nitrogen retention by Hylocomium splendens in a subarctic birch woodland. Journal of Ecology. 2000;88:506–515. [Google Scholar]
- Eckstein RL, Karlsson PS. Recycling of nitrogen among segments of Hylocomium splendens as compared with Polytrichum commune: implications for clonal integration in an ectohydric bryophyte. Oikos. 1999;86:87–96. [Google Scholar]
- Forsum A, Dahlman L, Nasholm T, Nordin A. Nitrogen utilization by Hylocomium splendens in a boreal forest fertilization experiment. Functional Ecology. 2006;20:421–426. [Google Scholar]
- Galloway JN. Acid deposition: perspectives in time and space. Water, Air and Soil Pollution. 1995;85:15–24. [Google Scholar]
- Gordon C, Wynn JM, Woodin SJ. Impacts of increased nitrogen supply on high Arctic heath: the importance of bryophytes and phosphorus availability. New Phytologist. 2001;149:461–471. doi: 10.1046/j.1469-8137.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Göttlicher S, Knohl A, Wanek W, Buchmann N, Richter A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Communications in Mass Spectrometry. 2006;20:653–660. doi: 10.1002/rcm.2352. [DOI] [PubMed] [Google Scholar]
- Hunt R, Causton DR, Shipley B, Askew AP. A modern tool for classical plant growth analysis. Annals of Botany. 2002;90:485–488. doi: 10.1093/aob/mcf214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen J, Silvola J, Vasander H. Effects of increased carbon dioxide and nitrogen supply on mosses. In: Bates JW, Ashton NW, Duckett JG, editors. Bryology for the twenty-first century. Leeds: Maney Publishing and the British Bryological Society; 1998. pp. 343–360. [Google Scholar]
- Jonsdottir IS, Callaghan TV, Lee JA. Fate of added nitrogen in a moss sedge Arctic community and effects of increased nitrogen deposition. Science of the Total Environment. 1995;161:677–685. [Google Scholar]
- Limpens J, Berendse F. Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: the role of amino acid nitrogen concentration. Oecologia. 2003;135:339–345. doi: 10.1007/s00442-003-1224-5. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Sutton MA, Truscott AM, Leith ID, Cape JN, Pitcairn CER, et al. Growth and tissue nitrogen of epiphytic Atlantic bryophytes: effects of increased and decreased atmospheric N deposition. Functional Ecology. 2004;18:322–329. [Google Scholar]
- Nordin A, Gunnarsson U. Amino acid accumulation and growth of Sphagnum under different levels of N deposition. Ecoscience. 2000;7:474–480. [Google Scholar]
- Oechel WC, Van Cleve K. The role of bryophytes in nutrient cycling in the taiga. In: Van Cleve K, Chapin FSI, Dryness CT, Viereck LA, Flanagan PW, editors. Forest ecosystems in Alaskan taiga. A synthesis of structure and function. Berlin: Springer; 1986. pp. 121–137. [Google Scholar]
- Paulissen M, van der Ven PJM, Dees AJ, Bobbink R. Differential effects of nitrate and ammonium on three fen bryophyte species in relation to pollutant nitrogen input. New Phytologist. 2004;164:451–458. [Google Scholar]
- Paulissen M, Besalu LE, De Bruijn H, Van der Ven PJM, Bobbink R. Contrasting effects of ammonium enrichment on fen bryophytes. Journal of Bryology. 2005;27:109–117. [Google Scholar]
- Pearce ISK, van der Wal R. Effects of nitrogen deposition on growth and survival of montane Racomitrium lanuginosum heath. Biological Conservation. 2002;104:83–89. [Google Scholar]
- Pearce ISK, Woodin SJ, van der Wal R. Physiological and growth responses of the montane bryophyte Racomitrium lanuginosum to atmospheric nitrogen deposition. New Phytologist. 2003;160:145–155. doi: 10.1046/j.1469-8137.2003.00875.x. [DOI] [PubMed] [Google Scholar]
- Potter JA, Press MC, Callaghan TV, Lee JA. Growth responses of Polytrichum commune and Hylocomium splendens to simulated environmental change in the sub-arctic. New Phytologist. 1995;131:533–541. doi: 10.1111/j.1469-8137.1995.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Rincón E. Growth responses of six bryophyte species to different light intensities. Canadian Journal of Botany. 1993;71:661–665. [Google Scholar]
- Skrindo A, Okland RH. Effects of fertilization on understorey vegetation in a Norwegian Pinus sylvestris forest. Applied Vegetation Science. 2002;5:167–172. [Google Scholar]
- Smidt S. Ergebnisse der Level II Depositionsmessungen 1996–2003. Wien: Bundesamt und Forschungszentrum für Wald; 2004. [Google Scholar]
- Soares A, Pearson J. Short-term physiological responses of mosses to atmospheric ammonium and nitrate. Water, Air and Soil Pollution. 1997;93:225–242. [Google Scholar]
- Solga A, Burkhardt J, Zechmeister HG, Frahm JP. Nitrogen content, N-15 natural abundance and biomass of two pleurocarpous mosses Pleurozium schreberi (Brid.) Mitt. and Scleropodium purum (Hedw.) Limpr. in relation to atmospheric nitrogen deposition. Environmental Pollution. 2005;134:465–473. doi: 10.1016/j.envpol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Takashima T, Hikosaka K, Hirose T. Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell and Environment. 2004;27:1047–1054. [Google Scholar]
- Turetsky MR. The role of bryophytes in carbon and nitrogen cycling. Bryologist. 2003;106:395–409. [Google Scholar]
- Van Der Heijden E, Verbeek SK, Kuiper PJC. Elevated atmospheric CO2 and increased nitrogen deposition: effects on C and N metabolism and growth of the peat moss Sphagnum recurvum P. Beauv. var. mucronatum (Russ.) Warnst. Global Change Biology. 2000;6:201–212. [Google Scholar]
- Wanek W, Heintel S, Richter A. Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Communications in Mass Spectrometry. 2001;15:1136–1140. doi: 10.1002/rcm.353. [DOI] [PubMed] [Google Scholar]
- Zechmeister HG, Grodzinska K, Szarek-Lukaszewska G. Bryophytes. In: Markert BA, Breure AM, Zechmeister HG, editors. Bioindicators/biomonitors (principles, assessment, concepts) Amsterdam: Elsevier; 2003. pp. 329–375. [Google Scholar]