Abstract
Background and Aims
Plants exposed to solar ultraviolet-B radiation (UV-B, 280–315 nm) frequently suffer less insect herbivory than do plants that receive attenuated levels of UV-B. This anti-herbivore effect of solar UV-B exposure, which has been documented in several ecosystems, is in part mediated by changes in plant tissue quality. Exposure to UV-B can modify the abundance of a number of secondary metabolites, including phenolic compounds with potential impacts on insect herbivores. The aim of this study is to assess the potential anti-herbivore role of UV-B-induced phenolic compounds by comparing the phenolic profiles induced by UV-B and simulated insect herbivory in two wild species of the genus Nicotiana.
Methods
Plants grown under field and glasshouse conditions were exposed to contrasting levels of UV-B. Half of the plants of the attenuated UV-B treatment were given a simulated herbivory treatment, where leaves were mechanically damaged and immediately treated with oral secretions of Manduca sexta caterpillars. This treatment is known to mimic the impact of real herbivory on the expression of plant defences in Nicotiana. Phenolic profiles induced by UV-B and simulated herbivory were characterized using high-performance liquid chromatography–mass spectrometry (HPLC–MS).
Key Results
UV-B induced the accumulation of several UV-absorbing phenolic compounds that are known to play a significant role in UV-B screening. Interestingly, there was a significant convergence in the phenolic profiles induced by UV-B and simulated herbivory: chlorogenic acid and dicaffeoylspermidine isomers, in particular, displayed a similar pattern of response to these stimuli. In contrast, rutin, the only flavonoid that accumulated in significant quantities in the experiments, was only induced by UV-B.
Conclusions
The results suggest that the anti-herbivory effect induced by UV-B may be mediated at least in part by the accumulation of phenylpropanoid derivatives that are similar to those induced by the plant in response to insect herbivory.
Key words: Chlorogenic acid, flavonoids, phenolic compounds, rutin, UV-B, Nicotiana
INTRODUCTION
The effects of ultraviolet B radiation (UV-B) on the growth and ecological relationships of terrestrial plants have been studied intensively during the last three decades. This body of research has shown that solar UV-B is a significant modulator of the interactions between plants and phytophagous insects in terrestrial ecosystems. Exposure to solar UV-B frequently reduces the quality of plant tissues for herbivores (for reviews, see Ballaré et al., 2001; Caldwell et al., 2003; Roberts and Paul, 2006). This effect has been attributed to a variety of potential causes, including UV-induced variations in leaf nitrogen (Hatcher and Paul, 1994), specific phenolic derivatives (McCloud and Berenbaum, 1994), cyanogenic compounds (Lindroth et al., 2000) and defence-related proteins such as proteinase inhibitors (Stratmann et al., 2000, 2003).
One of the most consistent responses of plants to solar (Mazza et al., 2000) and enhanced (Searles et al., 2001) UV-B is the accumulation of increased levels of leaf phenolics (but see Rousseaux et al., 2001). These compounds act as selective sunscreens that reduce the penetration of UV-B through the epidermis and protect sensitive targets in the mesophyll cells. Besides this photo-protective function, several phenolic compounds are known to play a role in plant–herbivore interactions. Unfortunately, most of the field studies concerned with UV-induced photo-protection only report the change in the pool of phenolics that are extracted with a particular solvent, without identifying the individual compounds. This is a significant limitation if the goal is to assess the potential significance of UV-B-induced changes in phenolic chemistry on trophic relationships (Bassman, 2004; Roberts and Paul, 2006).
Detailed analytical information is available from studies carried out in growth-chamber and glasshouse conditions. Several of these studies were focused on woody perennials, and the majority of them show a significant impact of UV-B increasing the foliar content of compounds of the flavonoid fraction, which are effective UV-B filters. In one of the species that has been studied in greatest detail, Betula pendula, UV-B has been shown consistently to increase the contents of flavonoids with quercetin and kaempherol backbones (Lavola et al., 1998; de la Rosa et al., 2001; Tegelberg et al., 2004). Field studies with several species of trees confirmed a general tendency towards increased levels of flavonoids in response to UV-B (Tegelberg et al., 2001; Warren et al., 2002; Rousseaux et al., 2004). The impacts of UV-B on compounds belonging to the group of phenolic acids (such as chlorogenic acid; CHA), which may play important roles in plant–herbivore interactions, have received less attention. In B. pendula, there are results showing increased levels of CHA in response to UV-B (Tegelberg et al., 2004), and results showing no significant effects (Lavola et al., 1998; de la Rosa et al., 2001). In a multiyear field study, Tegelberg et al. (2001) found that the effects of UV-B supplementation on the various fractions of phenolic compounds in B. pendula varied between seasons.
The analytical information on changes in phenolic chemistry induced by solar UV-B (or ecologically meaningful UV-B treatments) is more limited in the case of herbaceous plants. Studies carried out under growth-chamber and glasshouse conditions have also focused on the flavonoid fraction and, in agreement with the results in woody perennials, most of the studies show a clear effect of UV-B increasing the levels of quercetin- and kaempferol-based flavonoids (e.g. Ballaré et al., 1995; Ryan et al., 1998; Wilson et al., 1998; Olsson et al., 1999; Hofmann et al., 2003).
In the experiments reported herein, the phenolic profile induced by solar and simulated solar UV-B was characterized in adult plants of two annual species of the genus Nicotiana, and this profile was compared with the changes induced in response to simulated insect herbivory. The purpose of this characterization was to assess the potential significance of phenolic compounds induced by solar UV-B on the interactions between plants and herbivorous insects.
MATERIALS AND METHODS
Two experiments were carried out, one in a glasshouse, using Nicotiana attenuata, and the other under field conditions, using Nicotiana longiflora. In each experiment, plants were exposed to two UV-B treatments (near-ambient and low UV-B) for 3 weeks, after which plants in the low UV-B treatment were either given a simulated herbivory treatment (see below) or left undamaged. This design allowed the effects of UV-B and insect herbivory on the abundance of phenolic compounds to be compared. The methods used for plant cultivation, UV-B manipulation and herbivory treatments have been described in detail by Izaguirre et al. (2003), and are only briefly outlined below.
Glasshouse experiment
Seedlings of N. attenuata were grown in individual 0·5 L pots in a glasshouse in Buenos Aires (34°S), without supplemental lighting. After 4 weeks of growth, the plants were transferred to the experimental UV-B treatments within the same glasshouse. There were two treatments: no-UV-B and simulated ambient UV-B, with three replicates per treatment. UV radiation was obtained with three UVB 313 fluorescent lamps (Q-Panel). The output of these lamps was filtered through a 0·1 mm clear polyester sheet [which filtered out both UV-C (<280 nm) and UV-B; no-UV-B treatment] or a 0·13 mm thick layer of cellulose diacetate (which filtered out the UV-C component; simulated ambient UV-B treatment). The cellulose diacetate film was replaced every 4 d. The plants were irradiated for 6 h each day, with the irradiation period centred at solar noon. The biologically effective daily UV doses, calculated using the generalized plant action spectrum (Caldwell, 1971) normalized at 300 nm, were 0 and 9·9 kJ m−2 for the no-UV-B and simulated ambient UV-B treatments, respectively (for details, see Izaguirre et al., 2003). The plants remained under the treatments until they were harvested to determine leaf phenolics.
Field experiment
The experiment was carried out during the summer in a field site in the Sierras Chicas (31°S, Córdoba, central Argentina). Nicotiana longiflora seedlings were grown for 1 week in a glasshouse, transferred to the field plots (1×1·4 m), and assigned to the experimental UV-B treatmets. There were two treatments: attenuated UV-B and near-ambient UV-B, with three replicates per treatment. The contrasting UV-B levels were obtained by covering the plots with clear plastic filters: clear polyester for the attenuated UV-B treatment (filtered out >90% of the UV-B component of solar radiation), and ‘Stretch’ film for the near-ambient UV-B treatment (>80% transmittance in the solar UV-B region). Both films had very high, similar transmittance in the UV-A (315–400 nm) and photosynthetically active radiation (PAR; 400–700 nm) regions of the spectrum (Fig. 1). The plants remained under the treatments until they were harvested to determine leaf phenolics.
Fig. 1.
Transmittance spectra of the films used for UV-B attenuation in the field study. Clear polyester (Oeste Aislante, Buenos Aires, 0·1 mm thick) and ‘Stretch’ polyethylene film (URFLEX S.A. Argentina, Buenos Aires, 0·025 mm thick) were used to create the ‘attenuated UV-B’ and ‘near-ambient UV-B’ treatments, respectively.
Simulated herbivory
Three weeks after the beginning of the UV-B manipulations, the plants of the no-UV-B and attenuated UV-B treatments were divided into two groups. In one of the groups, the plants were subjected to a simulated herbivory treatment; in the other group, the plants were left undisturbed and served as a control. The simulated herbivory treatment was applied to the two youngest fully expanded leaves of each plant. Three rows of puncture wounds were made with a fabric pattern wheel on each side of the mid-vein and 20 µL per plant of a 1 : 6 (v : v) diluted Manduca sexta regurgitate collected from fourth instar caterpillars were applied to the fresh wounds. This treatment induces defence-related gene expression responses that are equivalent to those elicited by actual insect herbivory (for details, see Halitschke et al., 2001 and references therein). Plants remained under the light treatments for an additional 72 h, until they were harvested for determinations of leaf phenolics.
Determination of leaf phenolics
Tissue samples were collected from wounded leaves and from leaves of the equivalent nodal position in the control plants of the attenuated UV-B and no UV-B treatments, 72 h after the simulated herbivory treatment. Undamaged plants from the near-ambient and simulated ambient UV-B treatments were also harvested at that time, collecting the same cohorts of leaves that were harvested in plants of the attenuated-UV-B and no-UV-B treatments. Leaf disks were taken at the time of harvest to determine the content of methanol-soluble, UV-absorbing phenolic compounds by measuring the absorbance of the extracts at 305 nm (A305), essentially as described in Izaguirre et al. (2006). The remaining leaf tissue was immediately frozen in liquid nitrogen.
Extraction of phenolic compounds for high-performance liquid chromatography (HPLC) analysis was performed following Keinänen et al. (2001). Briefly, green tissue (approx. 100–200 mg) without the mid-vein was ground frozen in liquid nitrogen and transferred to a centrifuge tube with 1·5 mL of 40 % aqueous methanol, containing 0·5 % acetic acid. After shaking for 2 h, samples were centrifuged (12 min, 13 000 rpm) and the supernatant was used for HPLC and mass spectrometry (HPLC-MS) analysis.
Details of the HPLC analysis have been described in Tan et al. (2004). In brief, samples (20 µL) were subjected to HPLC on a Nucleosil C-18 column (EC 250/4,120-5; Macherey and Nagel), with 0·1 % trifluoroacetic acid (TFA) as solvent A and 98 % acetonitrile/0·1 % TFA as solvent B, at a flow rate of 1 ml min−1 at 24 °C (gradient of solvent A: 100 % at 0, 94 % at 3, 80 % at 13, 76 % at 20, 37 % at 44, 0 % at 46 min), using a photodiode array detector 540 at 254 nm as part of the Biotech System (Solvent Delivery System 522, Autosampler 565, Jet-Stream plus, Degasy DG 1210, software CHROMA 2000; Biotech, Neufahrn, Germany).
Chemical structures were determined according to Tan et al. (2004) by electrospray ionization (ESI)-MS using a Hewlett-Packard (Avondale, PA, USA) HP 1100 HPLC coupled to a Micromass Quattro II (Waters, Micromass, Manchester, UK) tandem quadrupole mass spectrometer (geometry quadrupole–hexapole–quadrupole) equipped with an ESI source. The capillary and cone voltages in ESI mode were 3·3 kV and 18 V, respectively. Nitrogen for nebulization was applied at 15 L h−1, and drying gas at 250 L h−1 and 250 °C. The source and capillary were heated at 80 and 250 °C, respectively. The mass spectrometer was operated in conventional scanning mode using the first quadrupole. Positive-ion full-scan mass spectra were recorded from mass-to-charge ratio 90 to 650 (scanning time 1·5 s). Separation of compounds was achieved on a reverse phase column (5 µm Supelcosil C18 DB phase, 250×2·1 mm id, Supelco, Belafonte, PA, USA) equipped with a Supelguard pre-column (C18 DB phase, 20×2·1 id, Supelco). The solvent system was H2O with 0·1 % HCO2 H (A) or acetonitrile (B). The following gradient was used: 0–6 min, 2–4 % of B; 6–13 min 4–18 % of B; 13–17 min, 18–28 % of B; 17–22 min, 28–53 % of B; 22–24 min, 53–93 % of B and 24–29 min, hold 93 % of B. The flow was maintained at 0·4 mL min−1 and the column temperature was set at 30 °C. Effluent passed through a serially connected UV detector set at 254 or 320 nm before splitting to a waste and to the API source (flow aprox. 30 µL min−1).
Statistical analysis
Analysis of variance (ANOVA) was used, performing multiple comparisons on least-squares means (α=0·05) to detect significant treatment effects on phenolic compounds in each species (total soluble phenolics and individual phenolic peaks). When necessary, data were transformed to meet the assumptions of ANOVA. To assess the similarities in the phenolic response elicited by UV-B and simulated M. sexta herbivory, correlation analysis was used. This analysis was applied to the induction ratios calculated for each of the individual compounds identified by HPLC-MS [induction ratio=abundance of a compound in plants of the stress condition (UV-B or simulated herbivory) divided by its abundance in the control condition].
RESULTS
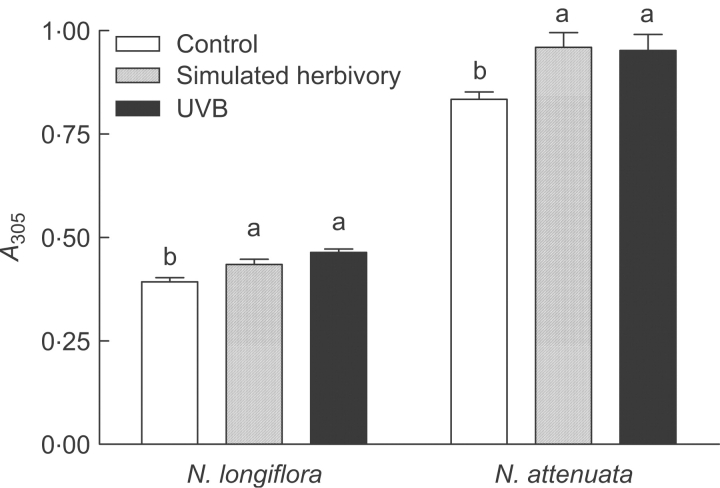
Plants of N. attenuata and N. longiflora responded to simulated herbivory and UV-B treatment with a significant increase in the pool of methanol-soluble UV-absorbing leaf phenolics (Fig. 2). Under the experimental conditions, the abundance of methanol-extractable leaf phenolics was lower in N. longiflora than in N. attenuata. However, the effects of simulated herbivory and UV-B were similarly expressed in both species (Fig. 2).
Fig. 2.
Comparison of the effects of UV-B and simulated herbivory on the abundance of methanol-extractable, UV-absorbing phenolic compounds (n=3). Nicotiana attenuata was grown in the glasshouse. The ‘Control’ and ‘UVB’ conditions correspond to the ‘no-UV-B’ and ‘simulated ambient UV-B’ treatments, respectively. Nicotiana longiflora was grown in the field. In this case, the ‘Control’ and ‘UVB’ conditions correspond to the ‘attenuated UV-B’ and ‘near-ambient UV-B treatments’, respectively. For a full description of the UV-B supplementation and attenuation treatments, see Materials and methods.
HPLC elution chromatograms indicated the presence of a variety of UV-absorbing compounds in the extracts of N. attenuata leaves. Nine of the peaks, which accounted for a large percentage (>90 %) of the total UV absorbance, displayed a significant response to UV-B and/or simulated herbivory (Table 1). MS analysis allowed these stress-responsive peaks to be identified as phenolic compounds belonging to the following categories: phenolic acids (three isomers of CHA); polyamines (three isomers of dicaffeoylspermidine and two isomers of an unknown compound closely related to dicaffeoylspermidine); and flavonoids (rutin) (Table 1). CHA and rutin were, by far, the most abundant compounds, accounting for >80 % of the total UV absorbance of the extracts.
Table 1.
Abundance of phenolic compounds in the control and the two stress conditions (TIC, total ion counts, per g of dry mass)
| Abundance (×104 cpm g−1) |
Relative abundance (%) |
|||||
|---|---|---|---|---|---|---|
| Isomer | Condition* | N. attenuata | N. longiflora | N. attenuata | N. longiflora | |
| Chlorogenic acid | A | Control | 15·4a | 14·0 | 12·9 | 16·6 |
| Simulated herbivory | 21·0a | 19·6 | ||||
| UVB | 28·4b | 16·6 | ||||
| B | Control | 34·0a | 42·8a | 28·5 | 50·7 | |
| Simulated herbivory | 58·0b | 64·4ab | ||||
| UVB | 71·2b | 65·3b | ||||
| C | Control | 3·0a | 4·4 | 2·5 | 5·2 | |
| Simulated herbivory | 4·9b | 7·0 | ||||
| UVB | 6·0b | 6·0 | ||||
| Dicaffeoylspermidine | A | Control | 1·0a | 0·5 | 0·8 | 0·6 |
| Simulated herbivory | 7·5b | 0·3 | ||||
| UVB | 5·1b | 0·8 | ||||
| B | Control | 4·1a | 0·8a | 3·4 | 1·0 | |
| Simulated herbivory | 25·0b | 1·2a | ||||
| UVB | 24·0b | 2·3b | ||||
| C | Control | 5·2a | 1·3a | 4·4 | 1·5 | |
| Simulated herbivory | 37·0b | 4·5b | ||||
| UVB | 25·9b | 2·9b | ||||
| Unknown | A | Control | 4·8a | 1·2a | 4·0 | 1·4 |
| Simulated herbivory | 25·0b | 2·7a | ||||
| UVB | 11·8b | 3·3b | ||||
| B | Control | 10·1a | 2·5 | 8·5 | 3·0 | |
| Simulated herbivory | 41·4b | 6·1 | ||||
| UVB | 29·0b | 4·5 | ||||
| Rutin | Control | 41·8a | 16·9a | 35·0 | 20·0 | |
| Simulated herbivory | 51·7a | 21·5a | ||||
| UVB | 106·0b | 37·4b | ||||
| Total | 100·0 | 100·0 |
The relative abundances of the various peaks were calculated based on ion count data for the control condition. Within each species and for each of the compounds, different letters indicate significant differences (P<0·05; n=3) between treatments.
* Nicotiana attenuata was grown in the glasshouse. The ‘Control’ and ‘UVB’ conditions correspond to the ‘no-UV-B’ and ‘simulated ambient UV-B’ treatments, respectively. Nicotiana longiflora was grown in the field. In this case, the ‘Control’ and ‘UVB’ conditions correspond to the ‘attenuated UV-B’ and ‘near-ambient UV-B treatments’, respectively. For a full description of the UV-B supplementation and attenuation treatments, see Materials and Methods.
Both the basal levels of leaf phenolics and the magnitude of the phenolic response induced by UV-B and simulated herbivory were higher in N. attenuata than in N. longiflora. Comparisons between species are difficult to interpret, however, since irradiation treatments and growing conditions differed between experiments. Fewer peaks in N. longiflora showed statistically significant changes compared with N. attenuata; however, the pattern of response of the two species was similar for the flavonoid rutin and the most abundant isomers of CHA and dicaffeoylspermidine (Table 1).
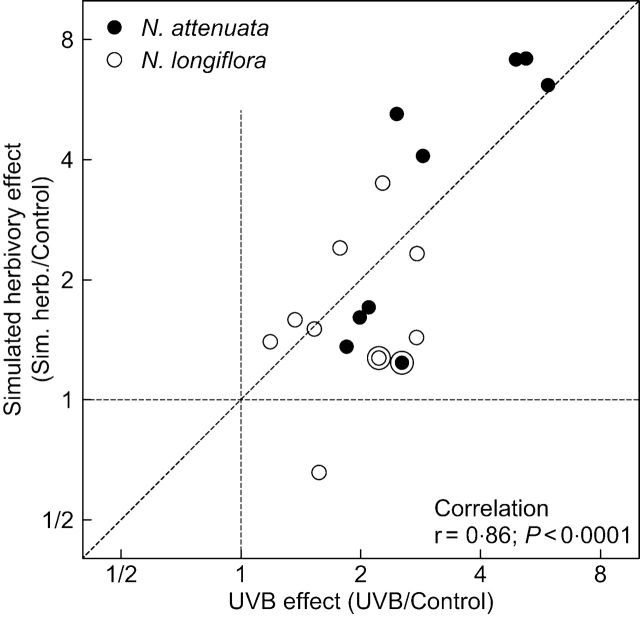
The effects of simulated herbivory and UV-B on the abundance of UV-absorbing phenolic compounds were compared using correlation analysis. The induction ratios for many of the compounds showed similar responses to UV-B and simulated herbivory; all but one of the data points were found in the North-east (positive) quadrant of the simulated herbivory vs. UV-B plot, and the correlation coefficient was highly significant (Fig. 3). This convergent pattern of induction was particularly clear for phenylpropanoid derivatives, such as the most abundant isomers of CHA and the polyamines (dicaffeoylspermidine-related compounds). In contrast, for rutin, the only flavonoid that accumulated in significant quantities in the experiments, there was a clear response to UV-B radiation, but little or no response to simulated herbivory (Fig. 3, circled data points).
Fig. 3.
Comparison of the effects of UV-B and simulated herbivory on the accumulation of UV-absorbing phenolic compounds. Each data point indicates the induction ratio for each of the nine individual compounds identified in Table 1 (i.e. the abundance of the compound in plants of the stress treatment divided by its abundance in the control condition). Circled data points correspond to rutin (a flavonoid); all others correspond to phenylpropanoid derivatives. All data (n=2 species×9 peaks=18) were used in the correlation analysis (r=0·86 for N. attenuata, and r=0·36 for N. longiflora). The diagonal line represents a 1 : 1 relationship.
DISCUSSION
The effects of UV-B on flavonoid levels in N. attenuata were similar to the effects described in previous work with woody and herbaceous species. The increase in the flavonoid rutin was consistent with previous observations showing increased levels of quercetin glucosides (Lavola et al., 1998; Ryan et al., 1998; Wilson et al., 1998; Olsson et al., 1999; de la Rosa et al., 2001; Hofmann et al., 2003; Tegelberg et al., 2004). Rutin is not typically induced by insect herbivory in N. attenuata (Kessler and Baldwin, 2004), which is also consistent with the lack of effect of the simulated-herbivory treatment in the present experiments.
Interestingly, UV-B caused significant increases in the levels of the most abundant isomer of CHA (Table 1). CHA is a potent cellular antioxidant (Grace and Logan, 2000; Niggeweg et al., 2004). Previous work has shown that natural doses of UV-B increase the foliar content of other non-enzymatic (Giordano et al., 2004) and enzymatic antioxidants (Mazza et al., 1999a), and this antioxidant response is thought to be an important component of the acclimation mechanisms that prevent (or minimize) UV-B-induced oxidative damage under natural conditions (Giordano et al., 2004).
In addition to its antioxidant function, CHA appears to play a significant role as a defence molecule in plants that are under attack from chewing insects (Stamp and Osier, 1998). In Nicotiana spp, CHA is strongly induced in response to natural herbivory (Kranthi et al., 2003; Kessler and Baldwin, 2004), simulated herbivory (Izaguirre et al., 2006; Table 1 and Fig. 3) and treatment with the defence-related hormone jasmonic acid (JA) (Keinänen et al., 2001). Both in N. attenuata (Kessler and Baldwin, 2004) and in N. longiflora (Izaguirre et al., 2006), increased levels of CHA correlate with a decrease in the performance of M. sexta caterpillars. Therefore, the present results (Table 1, Fig. 3) suggest that elevated levels of CHA and related compounds may explain, at least in part, the negative effect of plant exposure to solar UV-B on the performance of chewing insects that have been documented in Nicotiana (Izaguirre et al., 2003) and other species of the Solanaceae (Ballaré et al., 1996). The available evidence from manipulative field experiments suggests a negative, continuous relationship between UV-B irradiance and intensity of insect herbivory (Ballaré et al., 1996; Mazza et al., 1999b). Under natural conditions, exposure to solar UV-B varies with a number of factors, including shading by neighbouring plants. In this context, it is interesting to note that reduced levels of defences (including CHA) have been reported in response to shading and competition (Stamp et al., 2004; see also Roberts and Paul, 2006).
Previous work in the genus Nicotiana has indicated that solar UV-B and herbivory induce convergent responses at the gene expression level. In particular, solar UV-B and herbivory appear to have similar effects in downregulating a number of genes encoding proteins of the photosynthetic apparatus, and upregulating genes encoding enzymes of the octadecanoid pathway, which leads to the formation of JA and other oxylipins (Izaguirre et al., 2003). JA is known to regulate many of the defence responses that plants mount in response to insect attack (Schilmiller and Howe, 2005). The role of JA in UV-B-induced responses is not clear, however. In Arabidopsis, UV-B induces increased levels of JA (at least in plants grown under low levels of PAR) (A.-H.-Mackerness et al., 1999). In tomato, Stratmann et al. (2000) failed to detect any effects of UV-B on JA levels, but they found that UV-B treatments enhanced certain JA-dependent anti-herbivore responses induced by wounding. These observations have lent support to the idea that UV-B and JA activate signalling cascades that may share one or more functional elements.
The function of JA in the regulation of the biosynthesis of phenolic compounds has not been investigated in detail. It is known that JA can regulate the expression of some of the enzymes of this biosynthetic pathway. For example, in several plant species, treatment with JA or methyl-JA (MeJA) increases the expression of phenylalanine ammonia-lyase (PAL; Gundlach et al., 1992; McConn et al., 1997; Thoma et al., 2003; Wang and Wu, 2005) and chalcone synthase (CHS; Creelman et al., 1992; Rojo et al., 1998; Richard et al., 2000; Ali et al., 2003). It has also been shown that jar-1, an Arabidopsis mutant impaired in JA signalling, has reduced levels of UV-B-absorbing leaf phenolics (Caputo et al., 2006).
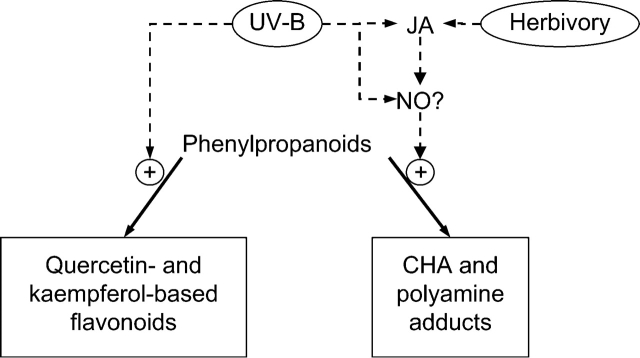
A possible explanation for the partially convergent phenolic responses elicited by UV-B and herbivory in the present experiments is that UV-B upregulates genes of the JA pathway, which in turn controls the expression of genes involved in the regulation of phenylpropanoid and CHA (but not flavonoid) biosynthesis (Fig. 4). JA has been reported to cause increased levels of CHA in N. attenuata, but without affecting rutin accumulation (Keinänen et al., 2001; Lou and Baldwin, 2003). Alternatively, UV-B could activate signalling components downstream of JA, such as nitric oxide (NO). NO accumulation is induced by MeJA in Taxus cell cultures (Wang and Wu, 2005) and by MeJA and wounding in Arabidopsis (Huang et al., 2004). UV-B has also been shown to increase NO levels in broad bean (Zhang et al., 2003; He et al., 2005). The role of NO in the control of phenolic responses is not well understood, but it has been shown that NO can increase PAL activity (Durner et al., 1998). Further testing of the model outlined in Fig. 4 will be required. The use of transgenic lines of N. attenuata with reduced expression of key enzymes of the JA pathway (Halitschke et al., 2004) should allow a direct evaluation of the involvement of JA in the UV-induced regulation of leaf phenolics.
Fig. 4.
Schematic diagram showing possible interactions between herbivory and UV-B in the induction of UV-absorbing phenolic compounds in leaves of Nicotiana spp. Both stimuli result in increased accumulation of phenylpropanoids, CHA and polyamine adducts, presumably using JA and/or NO as intermediate signalling molecules. UV-absorbing flavonoids (such as rutin) are clearly induced by UV-B, but not by insect herbivory.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from ANPCyT, UBACyT and the Max Planck Gesellschaft.
LITERATURE CITED
- A.-H.-Mackerness S, Surplus SL, Blake P, John CF, Buchannan-Wollaston V, Jordan BR, Thomas B. Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant, Cell and Environment. 1999;22:1413–1423. [Google Scholar]
- Ali GS, Reddy VS, Lindgren PB, Jakobek JL, Reddy ASN. Differential expression of genes encoding calmodulin-binding proteins in response to bacterial pathogens and inducers of defense responses. Plant Molecular Biology. 2003;51:803–815. doi: 10.1023/a:1023001403794. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Barnes PW, Flint SD, Price S. Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings. II Time-course, comparison with flavonoid responses and adaptive significance. Physiologia Plantarum. 1995;93:593–601. [Google Scholar]
- Ballaré CL, Scopel AL, Stapleton AE, Yanovsky MJ. Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiology. 1996;112:161–170. doi: 10.1104/pp.112.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Rousseaux MC, Searles PS, Zaller JG, Giordano CV, Robson TM, et al. Impacts of solar ultraviolet-B radiation on terrestrial ecosystems of Tierra del Fuego (southern Argentina). An overview of recent progress. Journal of Photochemistry and Photobiology B: Biology. 2001;62:67–77. doi: 10.1016/s1011-1344(01)00152-x. [DOI] [PubMed] [Google Scholar]
- Bassman JH. Ecosystem consequences of enhanced solar ultraviolet radiation: secondary plant metabolites as mediators of multiple trophic interactions in terrestrial plant communities. Photochemistry and Photobiology. 2004;79:382–398. doi: 10.1562/si-03-24.1. [DOI] [PubMed] [Google Scholar]
- Caldwell MM. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, editor. Photophysiology. Vol. 6. New York: Academic Press; 1971. pp. 131–177. [Google Scholar]
- Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, et al. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochemical and Photobiological Sciences. 2003;2:29–38. doi: 10.1039/b211159b. [DOI] [PubMed] [Google Scholar]
- Caputo CV, Rutitzky M, Ballaré CL. Solar UV-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.). Impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia. 2006;149:81–90. doi: 10.1007/s00442-006-0422-3. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proceedings of the National Academy of Sciences of the USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proceedings of the National Academy of Sciences of the USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano CV, Galatro A, Puntarulo S, Ballaré CL. The inhibitory effects of UV-B radiation (280–315 nm) on Gunnera magellanica growth correlate with increased DNA damage but not with oxidative damage to lipids. Plant, Cell and Environment. 2004;27:1415–1423. [Google Scholar]
- Grace SC, Logan BA. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:1499–1510. doi: 10.1098/rstb.2000.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proceedings of the National Academy of Sciences of the USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (lepidoptera, sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid–amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiology. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Ziegler J, Keinänen M, Baldwin IT. Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant Journal. 2004;40:35–46. doi: 10.1111/j.1365-313X.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Hatcher PE, Paul ND. The effect of elevated UV-B radiation on herbivory of pea by Autographa gamma. Entomologia Experimentalis et Applicata. 1994;71:227–233. [Google Scholar]
- He J-M, Xu H, She X-P, Song X-G, Zhao W-M. The role and the interrelationship of hydrogen peroxide and nitric oxide in the UV-B-induced stomatal closure in broad bean. Functional Plant Biology. 2005;32:237–247. doi: 10.1071/FP04185. [DOI] [PubMed] [Google Scholar]
- Hofmann RW, Campbell BD, Bloor SJ, Swinny EE, Markham KR, Ryan KG, Fountain DF. Responses to UV-B radiation in Trifolium repens L. – physiological links to plant productivity and water availability. Plant, Cell and Environment. 2003;26:603–612. [Google Scholar]
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218:938–946. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL. Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiology. 2003;132:1755–1767. doi: 10.1104/pp.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Mazza CA, Biondini M, Baldwin IT, Ballaré CL. Remote sensing of future competitors: impacts on plant defenses. Proceedings of the National Academy of Sciences of the USA. 2006;103:7170–7174. doi: 10.1073/pnas.0509805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. Journal of Agricultural and Food Chemistry. 2001;49:3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant Journal. 2004;38:639–649. doi: 10.1111/j.1365-313X.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- Kranthi S, Kranthi KR, Wanjari RR. Influence of semilooper damage on cotton host-plant resistance to Helicoverpa armigera (Hub) Plant Science. 2003;164:157–163. [Google Scholar]
- Lavola A, Julkunen-Tiitto R, Roininen H, Aphalo PJ. Host-plant preference of an insect herbivore mediated by UV-B and CO2 in relation to plant secondary metabolites. Biochemical Systematics and Ecology. 1998;26:1–12. [Google Scholar]
- Lindroth RL, Hofmann RW, Campbell BD, McNabb WC, Hunt DY. Population differences in Trifolium repens L. response to ultraviolet-B radiation: foliar chemistry and consequences for two lepidopteran herbivores. Oecologia. 2000;122:20–28. doi: 10.1007/PL00008831. [DOI] [PubMed] [Google Scholar]
- Lou Y, Baldwin IT. Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proceedings of the National Academy of Sciences of the USA. 2003;100:14581–14586. doi: 10.1073/pnas.2135348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Battista D, Zima A, Szwarcberg-Bracchitta M, Giordano CV, Acevedo A, Scopel AL, Ballaré CL. The effects of solar UV-B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses. Plant, Cell and Environment. 1999;22:61–67. [Google Scholar]
- Mazza CA, Zavala J, Scopel AL, Ballaré CL. Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proceedings of the National Academy of Sciences of the USA. 1999;96:980–985. doi: 10.1073/pnas.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology. 2000;122:117–125. doi: 10.1104/pp.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud ES, Berenbaum MR. Stratospheric ozone depletion and plant–insect interactions: effects of UVB radiation on foliage quality of Citrus jambhiri for Trichoplusia ni. Journal of Chemical Ecology. 1994;20:525–539. doi: 10.1007/BF02059595. [DOI] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggeweg R, Michael AJ, Martin C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nature Biotechnology. 2004;22:746–754. doi: 10.1038/nbt966. [DOI] [PubMed] [Google Scholar]
- Olsson LC, Veit M, Bornman JF. Epidermal transmittance and phenolic composition in leaves of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napus grown under enhanced UV-B radiation. Physiologia Plantarum. 1999;107:259–266. [Google Scholar]
- Richard S, Lapointe G, Rutledge RG, Séguin A. Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant and Cell Physiology. 2000;41:982–987. doi: 10.1093/pcp/pcd017. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Paul ND. Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytologist. 2006;170:677–699. doi: 10.1111/j.1469-8137.2006.01707.x. [DOI] [PubMed] [Google Scholar]
- Rojo E, Titarenko E, León J, Berger S, Vancanneyt G, Serrano JJS. Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in Arabidopsis thaliana. Plant Journal. 1998;13:153–165. doi: 10.1046/j.1365-313x.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- de la Rosa TM, Julkunen-Tiitto R, Lehto T, Aphalo PJ. Secondary metabolites and nutrient concentrations in silver birch seedlings under five levels of daily UV-B exposure and two relative nutrient addition rates. New Phytologist. 2001;150:121–131. [Google Scholar]
- Rousseaux MC, Scopel AL, Searles PS, Caldwell MM, Sala OE, Ballaré CL. Responses to solar ultraviolet-B radiation in a shrub-dominated natural ecosystem of Tierra del Fuego (southern Argentina) Global Change Biology. 2001;7:467–478. [Google Scholar]
- Rousseaux MC, Julkunen-Tiitto R, Searles PS, Scopel AL, Aphalo PJ, Ballaré CL. Solar UV-B radiation affects leaf quality and insect herbivory in the southern beech tree Notofagus antarctica. Oecologia. 2004;138:505–512. doi: 10.1007/s00442-003-1471-5. [DOI] [PubMed] [Google Scholar]
- Ryan KG, Markham KR, Bloor SJ, Bradley JM, Mitchell KA, Jordan BR. UVB radiation induced increase in quercetin: kaempferol ratio in wild-type and transgenic lines of Petunia. Photochemistry and Photobiology. 1998;68:323–330. [Google Scholar]
- Schilmiller AL, Howe GA. Systemic signaling in the wound response. Current Opinion in Plant Biology. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Searles PS, Flint SD, Caldwell MM. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia. 2001;127:1–10. doi: 10.1007/s004420000592. [DOI] [PubMed] [Google Scholar]
- Stamp NE, Osier TL. Response of five insect herbivores to multiple allelochemicals under fluctuating temperatures. Entomologia Experimentalis et Applicata. 1998;88:81–96. [Google Scholar]
- Stamp NE, Bradfield M, Li S, Alexander B. Effect of competition on plant allometry and defense. American Midland Naturalist. 2004;151:50–64. [Google Scholar]
- Stratmann JW. Ultraviolet-B radiation co-opts defense signaling pathways. Trends in Plant Science. 2003;8:526–533. doi: 10.1016/j.tplants.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Stratmann JW, Stelmach BA, Weiler EW, Ryan CA. UVB/UVA radiation activates a 48 kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochemistry and Photobiology. 2000;71:116–23. doi: 10.1562/0031-8655(2000)071<0116:sipuur>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tan J, Bednarek P, Liu J, Schneider B, Svatoš A, Hahlbrock K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry. 2004;65:691–699. doi: 10.1016/j.phytochem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ. The effects of long-term elevated UV-B on the growth and phenolics of field-grown silver birch (Betula pendula) Global Change Biology. 2001;7:839–848. [Google Scholar]
- Tegelberg R, Julkunen-Tiitto R, Aphalo PJ. Red: far-red light ratio and UV-B radiation: their effects on leaf phenolics and growth of silver birch seedlings. Plant, Cell and Environment. 2004;27:1005–1013. [Google Scholar]
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant Journal. 2003;34:363–375. doi: 10.1046/j.1365-313x.2003.01730.x. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wu JY. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant and Cell Physiology. 2005;46:923–930. doi: 10.1093/pcp/pci098. [DOI] [PubMed] [Google Scholar]
- Warren JM, Bassman JH, Eigenbrode S. Leaf chemical changes induced in Populus trichocarpa by enhanced UV-B radiation and concomitant effects on herbivory by Chrysomela scripta (Coleoptera: Chrysomelidae) Tree Physiology. 2002;22:1137–1146. doi: 10.1093/treephys/22.15-16.1137. [DOI] [PubMed] [Google Scholar]
- Wilson KE, Wilson MI, Greenberg BM. Identification of the flavonoid glycosides that accumulate in Brassica napus L. cv. Topas specifically in response to ultraviolet B radiation. Photochemistry and Photobiology. 1998;67:547–553. [Google Scholar]
- Zhang M, An L, Feng H, Chen T, Chen K, Liu Y, Tang H, Chang J, Wang X. The cascade mechanisms of nitric oxide as a second messenger of ultraviolet B in inhibiting mesocotyl elongations. Photochemistry and Photobiology. 2003;77:219–225. doi: 10.1562/0031-8655(2003)077<0219:tcmono>2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.