Abstract
Background and Aims
This study explores basic physiological features and time relations of recovery of photosynthetic activity and CO2 uptake following rehydration of a desiccation-tolerant moss in relation to the full temporal sequence of cytological changes associated with recovery to the normal hydrated state. It seeks reconciliation of the apparently conflicting published physiological and cytological evidence on recovery from desiccation in bryophytes.
Methods
Observations were made of water-stress responses and recovery using infrared gas analysis and modulated chlorophyll fluorescence, and of structural and ultrastructural changes by light and transmission electron microscopy.
Key Results
Net CO2 uptake fell to zero at approx. 40 % RWC, paralleling the fluorescence parameter ΦPSII at 200 µmol m–2 s–1 PPFD. On re-wetting the moss after 9–18 d desiccation, the initially negative net CO2 uptake became positive 10–30 min after re-wetting, restoring a net carbon balance after approx. 0·3–1 h. The parameter Fv/Fm reached approx. 80 % of its pre-desiccation value within approx. 10 min of re-wetting. In the presence of the protein-synthesis inhibitors chloramphenicol and cycloheximide, recovery of Fv/Fm (and CO2 exchange) proceeded normally in the dark, but declined rapidly in the light. Though initial recovery was rapid, both net CO2 uptake and Fv/Fm required approx. 24 h to recover completely to pre-desiccation values. The fixation protocols produced neither swelling of tissues nor plasmolysis. Thylakoids, grana and mitochondrial cristae remained intact throughout the drying–re-wetting cycle, but there were striking changes in the form of the organelles, especially the chloroplasts, which had prominent lobes and lamellar extensions in the normally hydrated state, but rounded off when desiccated, returning slowly to their normal state within approx. 24 h of re-wetting. Sub-cellular events during desiccation and re-wetting were generally similar to those seen in published data from the pteridophyte Selaginella lepidophylla.
Conclusions
Initial recovery of respiration and photosynthesis (as of protein synthesis) is very rapid, and independent of protein synthesis, suggesting physical reactivation of systems conserved intact through desiccation and rehydration, but full recovery takes approx. 24 h. This is consistent with the cytological evidence, which shows the thylakoids and cristae remaining intact through the whole course of dehydration and rehydration. Substantial and co-ordinated changes in other cell components, which must affect spatial relationships of organelles and metabolic systems, return to normal on a time span similar to full recovery of photosynthesis. Comparison of the present data with recently published results suggests a significant role for the cytoskeleton in desiccation responses.
Key words: Bryophyta, chlorophyll fluorescence, chloroplasts, CO2 exchange desiccation tolerance, electron microscopy, metabolic inhibitors, mosses, Polytrichum formosum
INTRODUCTION
The phenomenon of desiccation tolerance in bryophtes is well known: many species can withstand drying to water contents of 5–10 % of their dry weight, in which state effectively no liquid phase remains in the cells, and return to normal metabolism and growth following rehydration (Alpert, 2006). The essential facts of bryophyte desiccation tolerance are well documented (Irmscher, 1912; Höfler, 1946; Clausen, 1952; Abel, 1956; Hosokawa and Kubota, 1957; Hinshiri and Proctor, 1971; Bewley, 1973; Dilks and Proctor, 1974, 1976, 1979; Schonbeck and Bewley, 1981a, b). However, we are still far from understanding fully the mechanisms of desiccation tolerance in either bryophytes or vascular ‘resurrection’ plants (Bewley, 1979; Bewley and Krochko, 1982; Stewart, 1990; Ingram and Bartels, 1996; Oliver and Bewley, 1997; Hartung et al., 1998; Kappen and Valladares, 1999; Scott, 2000; Proctor and Pence, 2002; Proctor and Tuba, 2002; Oliver et al., 2005). In particular, there has been disagreement about whether desiccation tolerance in such mosses as Tortula (Syntrichia) ruralis is constitutive, with recovery essentially a matter of reassembly and reactivation of components conserved intact through a drying–rehydration cycle, or non-constitutive, the latter involving processes variously described as acclimation, hardening or ‘repair’ (Mayaba et al., 2001). The idea of a repair-based mechanism of desiccation tolerance in bryophytes was introduced by Bewley in the context of the recovery of normal function by cell membranes, which are leaky to ions and metabolites immediately following rehydration (Bewley, 1979; Bewley and Krochko, 1982). It was extended by Oliver and Bewley (1984b) to take account of the fine-structural changes (including swelling of chloroplasts and mitochondria, and varying degrees of apparent disruption of the thylakoids and mitochondrial cristae) seen in electron micrographs of cells of freshly rehydrated bryophytes, and has since been reiterated in a succession of reviews (Bewley and Oliver, 1992; Oliver, 1996; Oliver and Bewley, 1997; Oliver et al., 1998). However, these reviews include no new cytological data, and what ‘repair’ might embrace remained largely undefined. Alpert and Oliver (2002) discuss evidence from recent physiological work, and Oliver et al. (2005) envisage a substantial role for constitutive tolerance in bryophytes. An ultrastructural study using freeze-fractured preparations (Platt et al., 1994) showed that organelles and membranes in Tortula ruralis and the ‘resurrection’ plant Selaginella lepidophylla are not disorganized or disrupted in the desiccated state. Further work on dry, partially and fully hydrated S. lepidophylla, using freeze-substitution and conventional fixations with buffers of different osmotic strengths, showed that, in this pteridophyte at least, what has been described as desiccation-induced damage is almost certainly an artefact of inadequate fixation (Platt et al., 1997). These authors indicated a pressing need to re-examine the cytological changes in de- and rehydrating mosses using improved fixation protocols. A recent study of the effects of desiccation and rehydration on the food-conducting leptoids and meristematic cells in Polytrichum formosum using both conventional and anhydrous fixations gave no evidence of damage to organelles but showed major changes in the endomembrane domains, microtubular cytoskeleton and in the shapes of the plastids and mitochondria (Pressel et al., 2006).
Physiological data show that the rates of recovery on remoistening vary significantly between different systems, but that recovery of some major functions of bryophyte cells may be very rapid. Infrared gas analysis shows almost instant reactivation of respiration, and return to substantially normal rates of net photosynthesis within 30–60 min in such species as Tortula (Syntrichia) ruralis, Grimmia pulvinata and Andreaea rothii (Tuba et al., 1996; Proctor and Pence, 2002), and 14CO2 uptake experiments show that photosynthesis begins within a few minutes of rehydration (Proctor and Smirnoff, 2000). Recovery of the photosystems is also very rapid (Proctor and Smirnoff, 2000; Proctor, 2001); in the initial stages, half-recovery times for Fv/Fm [a measure of the maximum quantum efficiency of photosystem II (PSII)] may be as short as 20–40 s. This suggests that the integrity of the thylakoids is preserved throughout the events of desiccation and re-moistening. On the other hand, the fact that complete recovery of photosynthesis clearly takes much longer (up to 24 h or more) suggests the involvement in recovery of other features of cell biology, as yet unknown. Also unexplained is why excessively rapid drying is often fatal.
The objectives of the work described in this paper were (a) to explore further the basic physiological features and time relations of the recovery of photosynthesis and photosynthetic CO2 uptake, (b) to provide a comprehensive account of the full temporal sequence of the cytological changes associated with recovery to the normal hydrated state, (c) to seek a reconciliation of the apparently conflicting published physiological and cytological evidence on recovery, and (d) to seek explanations for the slow return of metabolic activities to full pre-desiccation values, and for death following excessively rapid dehydration.
MATERIALS AND METHODS
The desiccation-tolerant moss Polytrichum formosum Hedw. was chosen for ease of handling in physiological experiments, and because optimal fixation and sectioning protocols for ultrastructural studies had been worked out in earlier studies (Ligrone and Duckett, 1994; Pressel et al., 2006).
Sources of material
Material for the physiological experiments was collected from Quercus petraea (Mattuschka) Liebl. coppice in Stoke Woods, Exeter, UK (50°45′N, 3°31′W, OSGB grid reference SX 925961) on 21 February 2002. Material for electron microscopy was from mixed Quercus/Fagus woodland, Box Hill, Surrey, UK (51°15′N, 0°18′W, OSGB grid reference TQ 182513)
Desiccation treatments
Unless otherwise stated, batches of eight cut shoots (2-cm apical lengths) were allowed to dry naturally in the laboratory atmosphere (40–50 % RH, 20–22 °C); water loss from a representative sample of eight shoots closely fitted an exponential decay curve, with a half-desiccation time of 17 min.
Cytological protocols
The controls were freshly collected fully hydrated shoots, while the desiccated sample consisted of shoots dried out naturally and kept in this condition for 18 d.
For light microscopy, hydrated and rehydrated samples were mounted in water, dehydrated samples in immersion oil. Both were examined with a Leitz Ortholux microscope using differential interference-contrast optics.
For electron microscopy, leaves from control, and shoots allowed to rehydrate for 0·5, 2·0, 12, 24 and 48 h, were cut into 2-mm lengths and fixed at room temperature for 3 h at pH 7 in 3 % (v/v) glutaraldehyde, 1 % formaldehyde (freshly prepared from paraformaldehyde) and 0·75 % tannic acid in 0·05 m Na-phosphate buffer. Leaf samples from dry shoots and shoots rehydrated for 0·5 h were fixed with that same protocol detailed above but in 0·1 m Na-phosphate buffer. Preliminary experiments using buffers of lower osmolarity produced extensive swelling of the thylakoids in dehydrated and freshly rehydrated samples, as described previously in Selaginella (Platt et al., 1997), and were discontinued. After rinsing in buffer the samples were post-fixed overnight with 1 % osmium tetroxide in 0·1 m buffer, pH 6·8, dehydrated and embedded in Spurr's resin via propylene oxide, as described by Ligrone and Duckett (1994). Thin sections, cut with a diamond knife, were sequentially stained with methanolic uranyl acetate and basic lead citrate and examined with a JEOL 1200EX2 electron microscope.
CO2-exchange measurements
Measurements of net CO2 exchange were made in the laboratory with a portable infrared gas analyser (IRGA) (LCA-2; ADC Ltd, Hoddesdon, Herts, UK), with a Parkinson leaf chamber (broad-leaf type), in the differential mode. Eight 2-cm apical cut lengths of moss shoot were used in the leaf chamber for each measurement. The light source was a quartz-halogen fibre-optic illuminator (Schott), set to give a photosynthetic photon flux density (PPFD) of 500 µmol m–2 s–1.
Chlorophyll-fluorescence measurements
Chlorophyll fluorescence was measured with a modulated chlorophyll fluorometer (FMS1; Hansatech Ltd, King's Lynn, UK). Definitions and calculation of fluorescence parameters follow Maxwell and Johnson (2000). Measurements of ΦPSII (effective quantum yield of PSII), qP (a measure of the oxidation state of the first electron acceptor QA) and NPQ (non-photochemical quenching) were made using the internal actinic light source of the instrument. The measuring sequence for these (starting with dark-relaxed material) was: saturating flash (0·8 s) to measure Fv/Fm, actinic light on for 6 min, saturating flash, actinic light off followed by far-red pulse and measurement of F0′. Unless otherwise stated, the actinic light source was set to give approx. 150 µmol m–2 s–1 PPFD.
Metabolic inhibitors
Chloramphenicol (CMP, here used at 3 mmol L–1), inhibits synthesis of chloroplast-encoded proteins; cycloheximide (CHX, here used at 0·3 mmol L–1) is an inhibitor of nuclear-encoded protein synthesis (Bottomley and Bohnert, 1982; Galling, 1982). The concentrations used were determined from previous experiments to give substantially complete inhibition without significant side effects (Proctor and Smirnoff, 2000).
Desiccation experiments
Batches of eight cut shoots (approx. 2 cm) were maintained at different intensities of desiccation in glass desiccators, over salts in equilibrium with their saturated solutions: sodium acetate, CH3COONa·3H2O (74 % RH, –41 MPa); potassium carbonate, K2CO3·2H2O (43 % RH, –114 MPa); potassium acetate, CH3COOK (20 % RH, –218 MPa), or a 75 % w/w sulfuric acid/water mixture (5 % RH, –412 MPa).
RESULTS
Physiological changes
Effects of drying
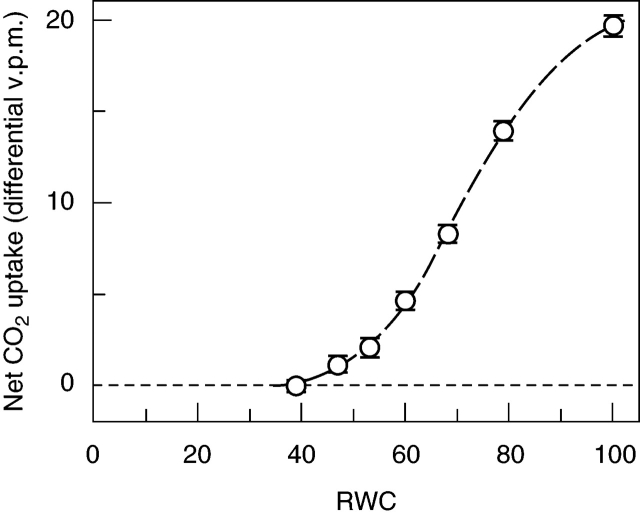
IRGA measurements showed that photosynthesis declined as water content dropped below full turgor, falling to zero at approx. 40 % relative water content (RWC; probably corresponding to a true cell RWC of 30–35 %, because apoplast water is likely to account for approx. 10 % of the total) (Fig. 1). Of the chlorophyll-fluorescence parameters, Fv/Fm was little changed down to about 60 % RWC, but then declined progressively to about 0·2 at 20 % RWC. ΦPSII (measured at approx. 200 µmol m–2 s–1 PPFD) closely paralleled net photosynthesis, falling to approx. 10 % of its full-turgor value at 40 % RWC and zero at 20 % RWC. The photochemical quenching parameter qP dropped steeply between 70 % and 50 % RWC, from approx. 0·8 at full turgor to approx. 0·2 at 20–40 % RWC. NPQ was variable, but showed a marked peak between 80 % and 50 % RWC, where it averaged about twice full-turgor values; it fell very steeply with further water loss, to near-zero at or below 40 % RWC (Fig. 2A–D).
Fig. 1.
Response of net CO2 uptake to relative water content during drying of leafy shoots of Polytrichum formosum, measured by IRGA. All measurements were made on the same sample of eight 2-cm apical shoot lengths. Following initial weighing of the shoots at full turgor but free of superincumbent water, a measurement was made in presence of excess water on filter paper in the IRGA chamber. The shoots were blotted dry, exposed to air briefly, and then alternately weighed and returned to the IRGA chamber for measurement. Finally the shoots were oven dried at 70 °C to determine dry weight for calculation of RWC. To allow for manipulation and equilibration time at the beginning of each reading period, RWC for each reading was taken as the lower third of the interval between successive weighings. Fitted logistic curve. Error bars represent a combined estimate of the standard deviation of the sets of five individual IRGA readings on which each point is based.
Fig. 2.
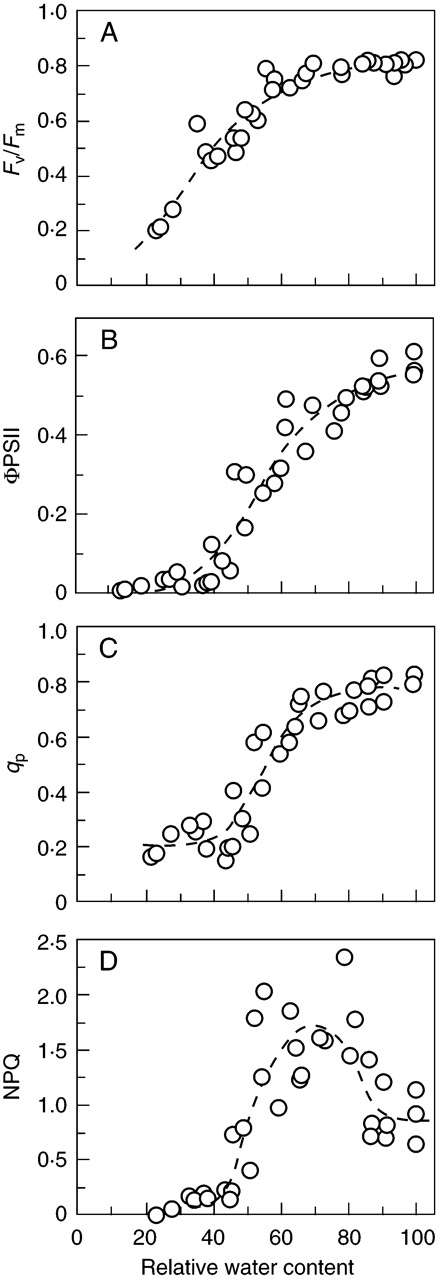
Response of chlorophyll-fluorescence parameters of leafy shoots of Polytrichum formosum during drying. All points on the graphs are from different individual shoots. Shoots were weighed at full turgor, and allowed to dry for various periods of time. They were then weighed before and after the measuring sequence. First Fv/Fm was measured; then continuous light (approx. 200 µmol m–2 s–1 PPFD) was turned on for 5 min, after which a saturating flash followed by a longer far-red pulse allowed calculation of ΦPSII, qP and NPQ. (A) Fv/Fm, with fitted logistic curve; (B) ΦPSII, with fitted logistic curve (compare with Fig. 1); (C) qP, with fitted logistic curve; (D) NPQ, with portions of two logistic curves and a quadratic fitted to overlapping lower, upper and middle sections of the data.
Recovery of gas exchange on rehydration
IRGA measurements consistently showed high rates of respiration within 1 min of remoistening, typically reaching a maximum after 4–10 min, before returning gradually to more normal dark-respiration values. Photosynthesis recovered more slowly, so that it was generally 8–15 min before the ‘dark’ and ‘light’ gas-exchange curves diverged measurably. Compensation (zero net CO2 exchange) was attained in 15–20 min after up to 9 d of desiccation, and the initial net carbon loss recouped after about 30 min. After 60 min, net photosynthesis had settled at a rather steady level, about 70–80 % of its pre-desiccation value. After 18 d of desiccation recovery was slower. Compensation was reached after approx. 28 min, and just over 1 h was required to recoup the initial net carbon loss. The rate of net photosynthesis after 60 min was about one-third of the pre-desiccation value, which was slowly regained over a period of 2–3 d. (Fig. 3A, B)
Fig. 3.
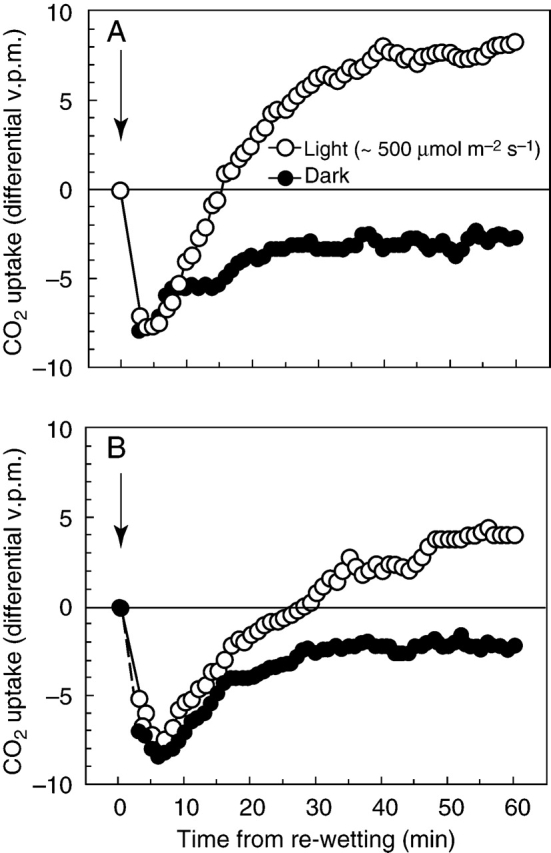
CO2 exchange of Polytrichum formosum following re-wetting in light (approx. 500 µmol m–2 s–1 PPFD) and dark; arrows show time of re-wetting. The curves are from approximately matched samples of eight 2-cm apical shoot lengths; the plotted points are running means of five readings taken at 30-s intervals. The data are not precise enough to allow interpretation of small differences between the dark and light curves during the first 10–15 min of recovery. (A) After 9 d of desiccation. The mean of the last 15 points was 7·93 (s.d.=0·59); the mean after 24 h was 8·48 (s.d.=0·65). (B) After 18 d of desiccation. The mean of the last 15 points of the light curve was 4·13 (s.d.=0·64); the mean after 3 d of recovery was 11·02 (s.d.=0·76). Data from two IRGA light-response curves (not shown), at 500 µmol m–2 s–1 PPFD (approx. –10·5 to –11·5 differential v.p.m.), is consistent with recovery to near pre-desiccation values within 12–24(–48) h.
Recovery of chlorophyll fluorescence on rehydration
After a few days' desiccation, Fv/Fm recovered quickly on remoistening (though less quickly than in Tortula ruralis or Grimmia pulvinata), reaching approx. 80 % of the unstressed value after 10 min and approx. 95 % of the unstressed value within 1 h. Further recovery over the ensuing 12–24 h was much slower (Fig. 4A). After longer desiccation initial recovery was slower, reaching a rather steady (but lower) level from which further recovery over the ensuing 1–3 d was slow, but could still be complete provided desiccation was not too prolonged. The parameter ΦPSII, measuring the effective quantum yield in light (here approx. 500 µmol m–2 s–1 PPFD), recovers more slowly than Fv/Fm, and rather closely parallels the recovery of photosynthesis as measured by CO2 exchange (Fig. 4B).
Fig. 4.
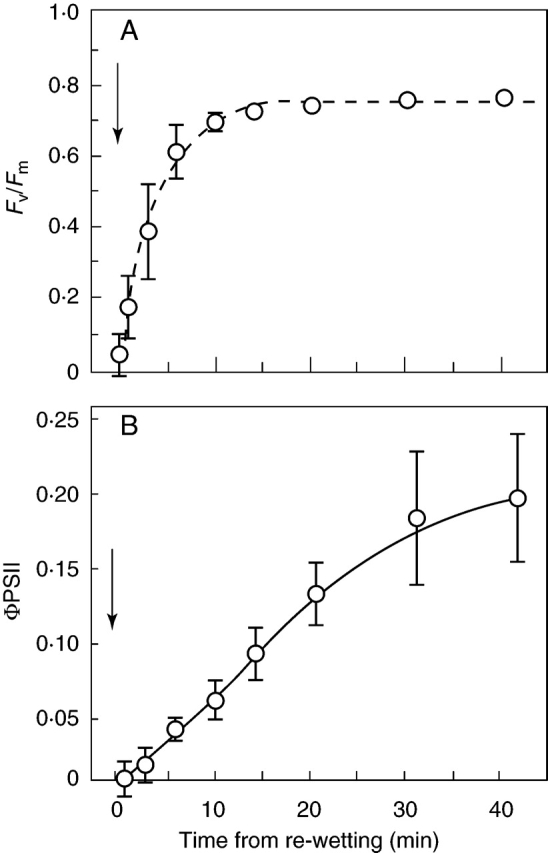
Recovery of chlorophyll-fluorescence parameters in leafy shoots of Polytrichum formosum following re-wetting (arrows). (A) Fv/Fm, with fitted exponential curve; the half-recovery time is 160 s. This parameter shows much faster recovery than photosynthetic CO2 fixation, reflecting the very rapid recovery of maximum quantum efficiency of photosystem II. (B) ΦPSII at 500 µmol m–2 s–1. This is a measure of effective quantum yield and thus of electron flow through PSII, and much more nearly parallels the recovery of photosynthesis.
Desiccation tolerance of P. formosum: the response space
The results of the experiment shown in Fig. 5 illustrate the relationship of recovery to desiccation time and desiccation intensity. For short or moderate desiccation times (<14 d), desiccation intensity made little difference to ultimate survival, but did have some effect on recovery rates. After prolonged desiccation, survival and recovery were best at approx. 40 % RH (–125 MPa at 20 °C), and recovery was conspicuously poor at the highest humidity (approx. 74 %, –41 MPa).
Fig. 5.
Polytrichum formosum. The chlorophyll-fluorescence parameter Fv/Fm measured 20 min and 24 (–48) h after re-moistening, following desiccation for different periods of time at relative humidities of 5 % (approx. –405 MPa), 20 % (approx. –218 MPa), 43 % (approx. –114 MPa) and 74 % (approx. –37 MPa), with fitted quadratic curves for each recovery time. For short dry periods (up to 2 weeks), recovery was most rapid after desiccation in the middle of the range shown here, but ultimate recovery was little influenced by desiccation intensity. After more prolonged desiccation P. formosum recovered best after the 43 % RH treatment, and performed poorly at both the highest and the lowest humidities.
The effect of protein-synthesis inhibitors on recovery
Recovery of P. formosum in the presence of chloramphenicol (CMP; inhibiting chloroplast-encoded protein synthesis) and cycloheximide (CHX; inhibiting nuclear-encoded protein synthesis) showed essentially the same pattern as in Racomitrium lanuginosum, Grimmia pulvinata, Rhytidiadelphus loreus (Proctor and Smirnoff, 2000), Tortula ruralis (Proctor, 2001) and Porella platyphylla (M. C. F. Proctor, unpubl. res.). In the dark, Fv/Fm recovered equally in water and in the two inhibitors over a period of 48 h. In the light (approx. 150 µmol m–2 s–1 PPFD), Fv/Fm recovered to a steady level of about 0·7 in water, but in CMP there was an immediate and progressive decline, reaching very low levels within 40–50 h, and in CHX a less immediate but still strongly marked decline over the same period (Fig. 6A, B).
Fig. 6.
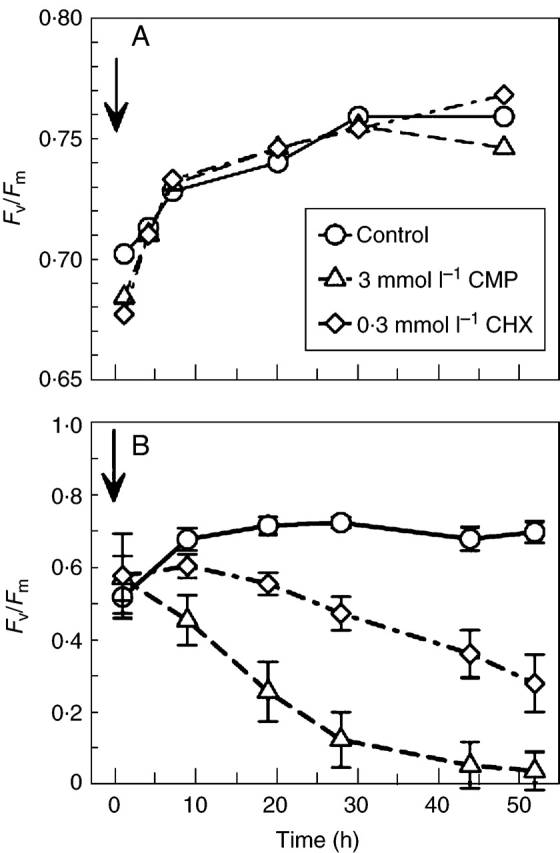
Polytrichum formosum. Recovery of the chlorophyll-fluorescence parameter Fv/Fm after desiccation at 43 % RH in the presence of protein-synthesis inhibitors. Arrows show time of re-wetting. (A) Recovery in dark after 9 d of desiccation; circles, control; triangles, 3·0 mmol l–1 chloramphenicol (CMP); diamonds, 0·3 mmol l–1 cycloheximide (CHX). Points are means of three replicates: error bars are omitted for clarity, the sample standard deviation from a pooled estimate is 0·035 (treatment differences not significant). (B) Recovery in light (approx. 150 µmol m–2 s–1 PPFD), showing rapid decline in CMP-treated samples, and slower decline in CHX. Means of five replicates, with standard deviation.
The results of an essentially similar experiment using IRGA measurements of CO2 exchange are shown in Table 1. The pre-desiccation rate of CO2 uptake was first measured for all the experimental treatments. The samples were then air-dried for 3 d, remoistened with either deionized water (control) or inhibitor solution. Half the samples were kept in the dark for 42 h, when CO2 exchange was measured at 500 µmol m–2 s–1 PPFD. A parallel set of samples was kept in the light (150 µmol m–2 s–1), and CO2 exchange measured after 24 and 48 h. In Table 1, the effects of the treatments on the mean rate of CO2 uptake are expressed relative to the pre-desiccation values. There was no significant difference between the control and experimental (inhibitor) treatments after the dark recovery period. In the light, the control samples showed a slow progressive increase towards the pre-desiccation rate of CO2 uptake. Carbon dioxide uptake declined steeply in both of the inhibitor treatments, especially with CMP, where it fell to zero 24 h after exposure to light.
Table 1.
Polytrichum formosum. IRGA measurements of net photosynthesis (as CO2 exchange) following 3 d air dry, and subsequent remoistening and recovery in either water (control), 3 mmol l−1 chloramphenicol (CMP) or 0·3 mmol l−1 cycloheximide (CHX), in dark and light (mean±s.d., n=2)
| Treatment | Recovery in dark (42 h) | Recovery in light (24 h) (approx. 150 µmol m−2 s−1) | Recovery in light (48 h) (approx. 150 µmol m−2 s−1) |
|---|---|---|---|
| Control | 0·63±0·01 | 0·72±0·11 | 0·81±0·02 |
| CMP (3 mmol l−1) | 0·58±0·11 | 0·00±0·10 | −0·10±0·19 |
| CHX (0·3 mmol l−1) | 0·59±0·16 | 0·27±0·03 | 0·19±0·00 |
Entries in the table are relative to the pre-desiccation measurement for each sample of shoots.
ANOVA of the raw means for the dark-recovery data shows a highly significant effect of desiccation, but no significant effect of the inhibitor treatments. There is a significant positive effect of light on recovery of photosynthesis in the controls (P<0·01); the effects of the two protein-synthesis inhibitors in the light are both highly significant (P<0·001).
Cytological changes: light-microscope observations
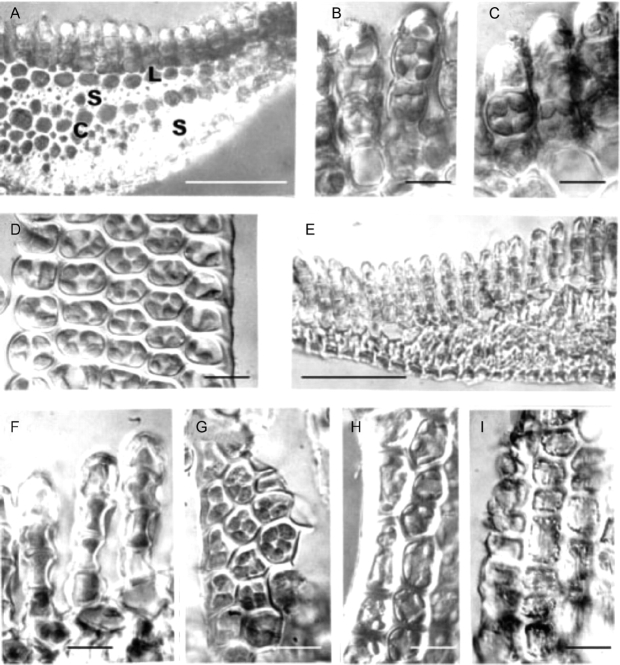
The appearance of hydrated and desiccated leaves of Polytrichum formosum is shown in Fig. 7. Sections through fully hydrated leaves (Fig. 7A) clearly show the central conducting strand and stereid bands in the midrib region overlain by a single layer of larger thin-walled cells; this layer underlies the closely packed longitudinal lamellae that make up the main photosynthetic tissue of the plant, and forms the pale unistratose lamina at the leaf margins (Proctor, 2005). Both in sections (Fig. 7B and C) and in side views (Fig. 7D) the cells of the lamellae contain two to six chloroplasts, (4–)5–7 µm in diameter, virtually filling the lumina. The chloroplasts are generally rounded or ovoid, but a few have cusp-like extremities lying along the cell walls. The cells of the lamina contain more numerous disc-shaped chloroplasts (up to ten per cell, visible by through-focusing) mainly around the periphery (Fig. 7H).
Fig. 7.
Light microscopy of Polytrichum formosum leaves. (A–D, H) Fully hydrated specimens mounted in water. (E–G, I) Completely desiccated specimens mounted in immersion oil. (A) Transverse section of a leaf midrib showing the dorsal lamellae, larger thin-walled lamina cells, stereid bands and conducting strand. (B, C) Hand-cut sections showing chloroplasts virtually filling the leaf lamella cells. No differences are apparent between the freshly collected fully hydrated specimens (B) and those hydrated from 12 h (C). (D) Surface view of a lamella. (E) In desiccated leaves the entire midrib region is highly shrunken and individual cells are difficult to distinguish. (F, G) Leaf lamella sections and surface views. Note the concave outer walls and chloroplasts the same size and shape as in fully hydrated cells. (H) Leaf lamina containing disc-shaped chloroplasts. (I) In these dehydrated lamina cells note the peripheral lipid droplets and the longitudinal striations caused by buckling of the cell walls. Scale bars: A, E=0·5 mm; B–D, F–I=10 µm.
Sections through desiccated leaves reveal a highly shrunken midrib region (Fig. 7E) while the lamella cells (Fig. 7F) have highly concave outer walls. Both in sections (Fig. 7F) and in surface views (Fig. 7G) chloroplasts with the same dimensions as in hydrated specimens fill the lumina. Chloroplasts are less in evidence in dehydrated lamina cells at the leaf margins (Fig. 7I) whose most prominent features are peripheral lipid droplets and longitudinal striations caused by buckling of the outer walls.
Changes in cell size and shape through de- and rehydration
During dehydration the shapes of the lumina of the leaf lamella cells change from approximately spherical (Fig. 7B, C) to concave discs (Fig. 7F) with volumes only 65 % of those in the fully hydrated condition (Table 2). Dehydrated lamella cells remain in their collapsed condition both when visualized directly in immersion oil and after fixing and embedding for transmission electron microscopy. After 0·5 h rehydration (see Fig. 11A) the lumina still have a reduced volume and a central concavity, and do not fully regain their original volume and shape until 12 h after re-wetting (Table 2).
Table 2.
Dimensions and volumes of leaf lamella-cell lumina of Polytrichum formosum at different states of hydration
| State of hydration | Lumen shape | Lumen dimensions (μm) | Lumen volume (μm3) | Volume reduction (%) |
|---|---|---|---|---|
| Fully hydrated (control) | Approx. spherical | 8·5 diam. | 320 | – |
| Dehydrated | Concave disc | 8·0 diam., 6·0 edge depth,2·8 centre depth | 215 | 35 |
| 0·5 h rehydration | Concave disc | 8·0 diam., 6·0 edge depth,3·8 centre depth | 245 | 23 |
| 2·0 h rehydration | Approx. spherical with slightly concave outer faces | 8·5 µm diam. | 310 | 5 |
| 12 h, 24 h, 48 h rehydration | Approx. spherical | 8·5 µm diam. | 320 | – |
Data for the rehydrating samples are based on measurements from 30 cells under TEM, for fully hydrated samples a combination of 30 cells under the TEM and 50 cells observed directly by light microscopy, and for the dehydrated samples a combination of 30 cells under the TEM prepared by conventional and anhydrous fixation and 50 cells mounted in immersion oil.
Fig. 11.
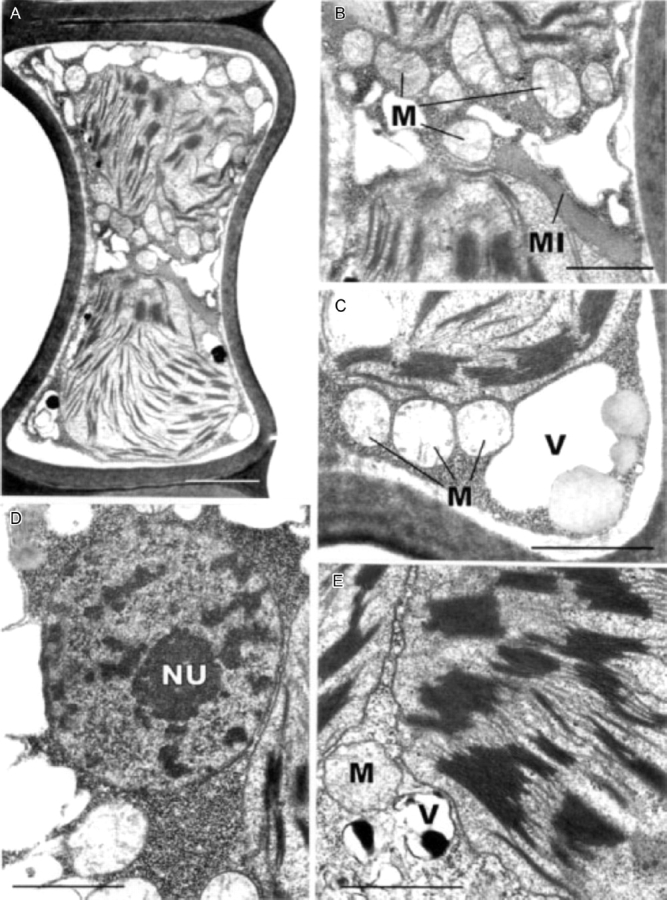
Transmission electron microscopy of leaf-lamella cells of Polytrichum formosum 0·5 h after rehydration. (A) General aspect showing the concave outer walls, rounded chloroplasts and small vacuoles. (B) Detail of (A) showing mitochondria and an elongate microbody closely appressed to the chloroplasts. (C) Detail showing grana in a chloroplasts, mitochondria, a vacuole and spherical peripheral lipid droplets. (D) Nucleus with blocks of condensed chromatin, with the adjacent cytoplasm packed with ribosomes. (E) Detail of grana, and vacuoles with dense contents. M, mitochondrion; MI, microbody; NU, nucleolus; V, vacuole. Scale bars: A=2 µm; B–E=1 µm.
These data clearly demonstrate three things: (1) during desiccation of P. formosum, shrinkage of the protoplasts is followed by a corresponding collapse of the cell walls, or cytorrhysis (Oparka, 1994); (2) the fixation protocol used produced neither significant swelling of the cell contents nor plasmolysis; (3) complete rehydration of the protoplasts takes at least 2 h with clear implications for the full resumption of metabolic activity.
Cytological changes: electron microscopy
Hydrated leaves
Fully hydrated leaf-lamella cells contain a centrally located nucleus, 3–4 µm in diameter, and peripherally arranged chloroplasts (Fig. 8A, B). The former has an undulate envelope, a single nucleolus with a prominent nuclolar organizer and finely granular nucleoplasm lacking aggregations of chromatin.
Fig. 8.
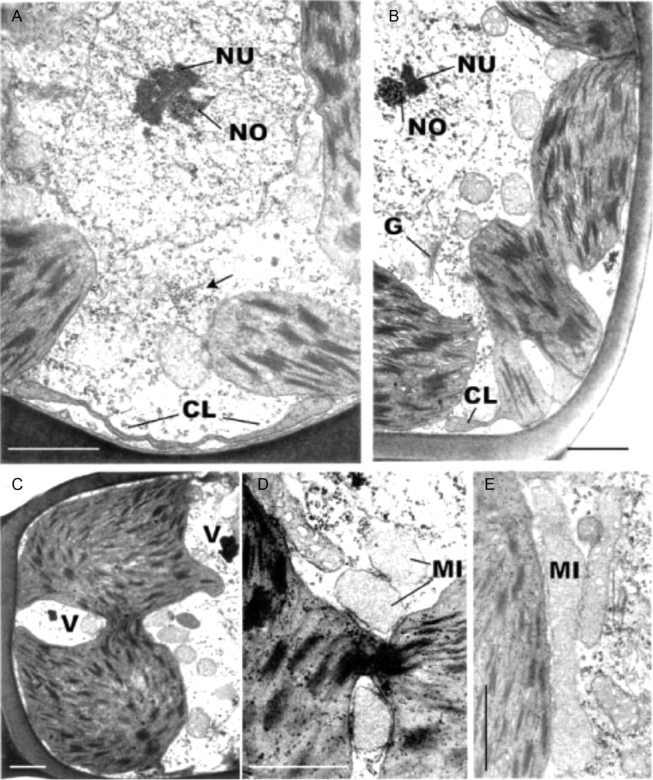
Transmission electron microscopy of fully hydrated leaf-lamella cells of Polytrichum formosum. (A, B) General views showing the central nucleus and peripheral variously lobed chloroplasts. The cytoplasm contains prominent mitochondria, occasional Golgi bodies and scattered polysomes, and a ribosome aggregation (arrowed). (C) Bilobed chloroplasts with a broad thylakoid-containing the isthmus. (D, E) Microbodies lying close to chloroplasts envelopes particularly in the vicinity of isthmus regions. Note the dense stroma and scattered ribosomes in the chloroplasts. CL, Chloroplast lobes, G, Golgi body, MI, microbody; NO, nucleolar organizer; NU, nucleolus; V, vacuole. Scale bars=1 µm.
The chloroplasts display pleomorphic profiles, very different from the apparently simple spheres and ovoids seen under the light microscope, each consisting of two or three main lobes packed with thylakoids and interconnected by thylakoid-containing isthmuses of various dimensions (Fig. 8B–D). Adjacent to the plasmalemma the extremities of the plastids extend as thin sheets measuring up to 3–5 µm in length but scarcely 50 nm in thickness (Figs 8A and 9A). Serial sectioning and the absence of circular plastid profiles, 50 nm in diameter, confirms these extensions as sheet-like rather than tubular. The sheets lack internal membranes and their swollen edges sometimes contain small grana (Fig. 9A). The main body of each chloroplast is packed with grana containing up to 20 thylakoids interconnected by numerous parallel intergranal thylakoids (Fig. 9B). Scattered through the dense stroma are plastid ribosomes and small plastoglobuli.
Fig. 9.
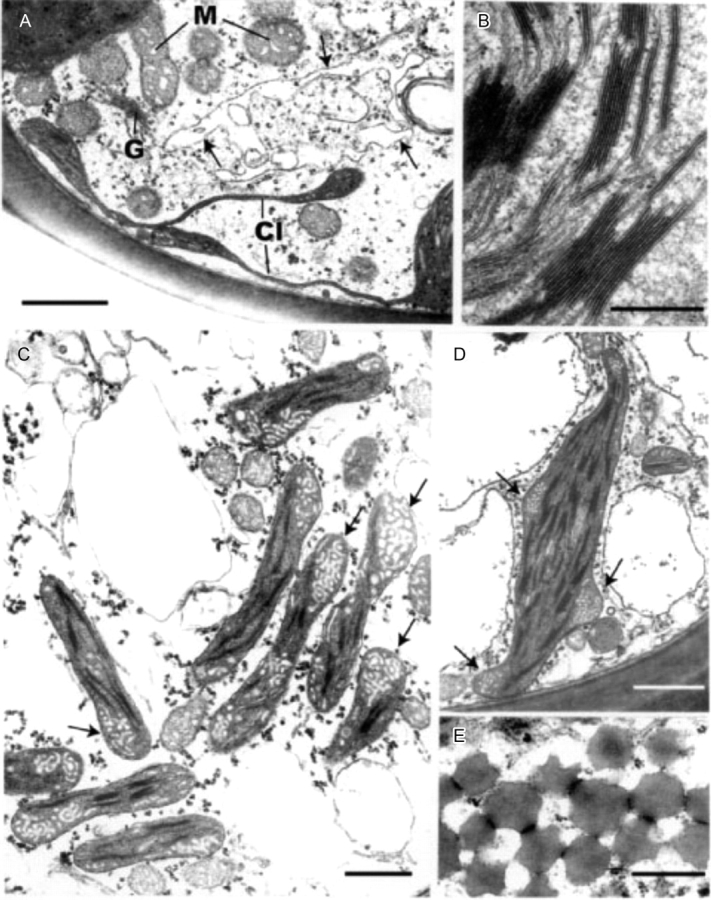
Transmission electron microscopy of fully hydrated leaf lamella (A) and lamina cells (C–E) of Polytrichum formosum. (A) Lamellate chloroplast extensions with thylakoids in their swollen ends. Note the highly irregular vacuolar profile (arrowed). (B) Detail of grana. (C, D) Discoidal chloroplasts with small grana and prominent areas of peripheral reticulum (arrowed). Note the numerous small vacuolar profiles and scattered polysomes in the cytoplasm. (E) Peripheral lipid droplets. Cl, chloroplast lobes, G, Golgi body; M, mitochondrion. Scale bars: A, C, D=1 µm; B, E=0·5 µm.
The bulk of the cytoplasm, located in the centre of the cells along with the nucleus, contains highly pleomorphic vacuoles with electron-opaque deposits, scattered polysomes, occasional aggregations of ribosomes and sparse Golgi bodies with small peripheral vesicles plus short single ER profiles (Figs 8A, B and 9A). The vacuole system occupies between 5 % and 10 % of the volume of the hydrated cells. Spherical and elongate microbodies tend to be clustered around the plastid isthmuses, together with mitochondria with dense matrix and prominent saccate cristae (Figs 8B, D, E and 9A).
The ultrastructure of the leaf lamina cells (Fig. 9C–E) is rather different from that of the lamellae. The nuclei have the same morphology (not shown) and the electron-lucent cytoplasm contains scattered polysomes, The lamina cells have numerous spheroidal vacuoles of various sizes. Their discoidal chloroplasts have small grana of usually less than ten thylakoids and conspicuous regions of peripheral reticulum. The chloroplasts are associated with mitochondria of similar size and substructure to those in the lamellae but microbodies are rarely present. A final notable feature of the lamina cells are the abundant lipid droplets 300–500 nm in diameter, sometimes forming an almost continuous sheet at the cell periphery (Fig. 9E). When in contact with membrane-bound vesicles the lipid bodies often present concave faces (Fig. 9E). Similar lipid bodies occur sporadically in the lamella cells.
Dehydrated leaves
Dehydrated lamella cells contain numerous small vacuoles up to 2–3 µm in diameter with transparent contents and mostly located at the cell periphery (Fig. 10A, B). The nuclei, usually also displaced to the cell periphery, are slightly smaller than when fully hydrated (2·8–3·2 µm in diameter) and contain conspicuous blocks of condensed chromatin in addition to the nucleolus (Fig. 10A). The chloroplasts, spherical or oval in outline, are located in the inside of the cells and frequently contain two sets of thylakoids lying side by side and forming well-organized grana (Fig. 10A, B). The cytoplasm contains aggregates of ribosomes particularly around the nucleus (Fig. 10E). Golgi bodies (not shown) are similar in appearance to those in hydrated material. The mitochondria have a loosely fibrillar matrix and the cristae appear as tubules or plates (Fig. 10C, G). They tend to lie in rows closely appressed to the chloroplasts (Fig. 10C, G) with the microbodies occupying a similar location (Fig. 10C). The cytoplasmic lipid droplets in both the lamella and lamina cells are usually spherical (Fig. 10D) and the ribosomes in the lamina cells less densely packed (Fig. 10F). Lamina-cell mitochondria have the same organization as those in the lamellae; the chloroplasts are ovoid or rounded, and contain small grana (Fig. 10F) but no peripheral reticulum.
Fig. 10.
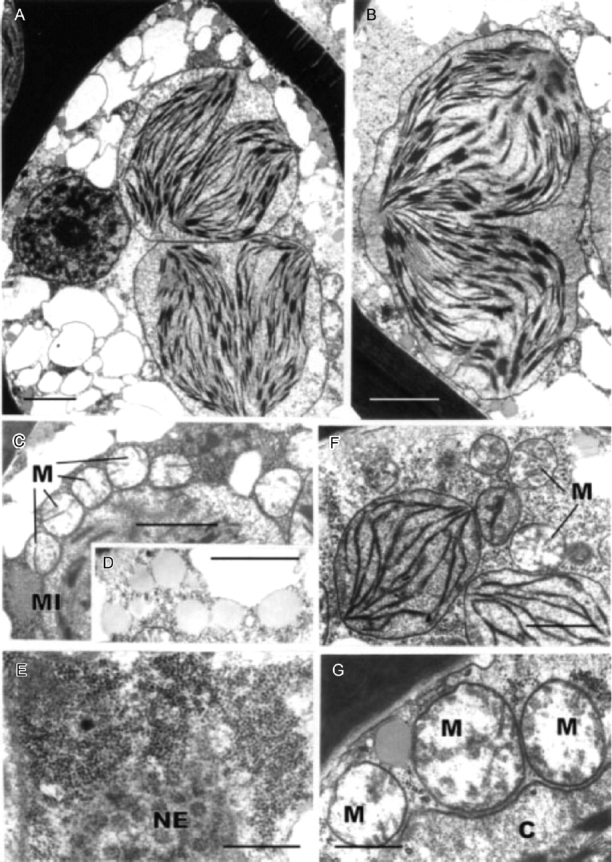
Transmission electron microscopy of dehydrated leaf-lamella (A–E) and lamina (F) cells of Polytrichum formosum. (A) General aspect. Note the condensed chromatin in the nucleus, numerous small vacuoles and two ovoid chloroplasts each containing two groups of thylakoids closely juxtaposed. (B) Detail of ovoid chloroplast containing two groups of thylakoids. (C) Mitochondria with clear stroma and tubular cristae lying close to a chloroplast. (D) Spherical lipid droplets. (E) Aggregation of ribosomes adjacent to the nuclear envelope containing numerous pores. (F) Ovoid chloroplasts in a leaf lamina cell lacking peripheral reticulum. (G) Detail of mitochondria lying adjacent to a chloroplast. C, Chloroplast; M, mitochondrion; MI, microbody; NE, nuclear envelope. Scale bars: A, B=2 µm; C, D, F=1 µm; E, G=0·5 µm.
Changes during rehydration
After rehydration for 0·5 h the lamella cells at first sight look much the same as in dehydrated leaves (Fig. 11). They contain numerous small vacuoles, some with dense contents, scattered both at the periphery and in the internal cytoplasm (Fig. 11A, E). The chloroplasts, still ovoid to spherical in shape and associated with mitochondria and microbodies closely appressed to their envelopes, have started moving to a peripheral position (Fig. 11B, E). The thylakoid system (Fig. 11C, E) comprises well-organized grana and parallel intergranal thylakoids. The mitochondrial matrix remains electron lucent (Fig. 11B, C) and the cristae tubular. Blocks of condensed chromatin remain conspicuous in the nucleus whilst the aggregations of ribosomes in the surrounding, cytoplasm show a slightly wider centre-to-centre spacing; 50–60 nm compared with 30–40 nm in dehydrated cells (Fig. 11D).
After 2 h of rehydration (Fig. 12A–C) the lamella cells show a dramatic reduction in the size of the vacuoles. These are now <1 µm in diameter and nearly all contain dense material (Fig. 12A). The cytoplasm is less dense than at 0·5 h and the ribosomes are dispersed. The chloroplasts retain a spherical to ovoid shape but their envelopes have become highly undulate (Fig. 12B). Microbodies and mitochondria no longer lie closely appressed to the chloroplasts. The mitochondrial matrix is denser and the cristae mostly saccate (Fig. 12B), features even more pronounced in the lamina cells (Fig. 12C) whose chloroplasts are now discoidal. Blocks of condensed chromatin still remain in the nuclei but are far less conspicuous (not shown).
Fig. 12.
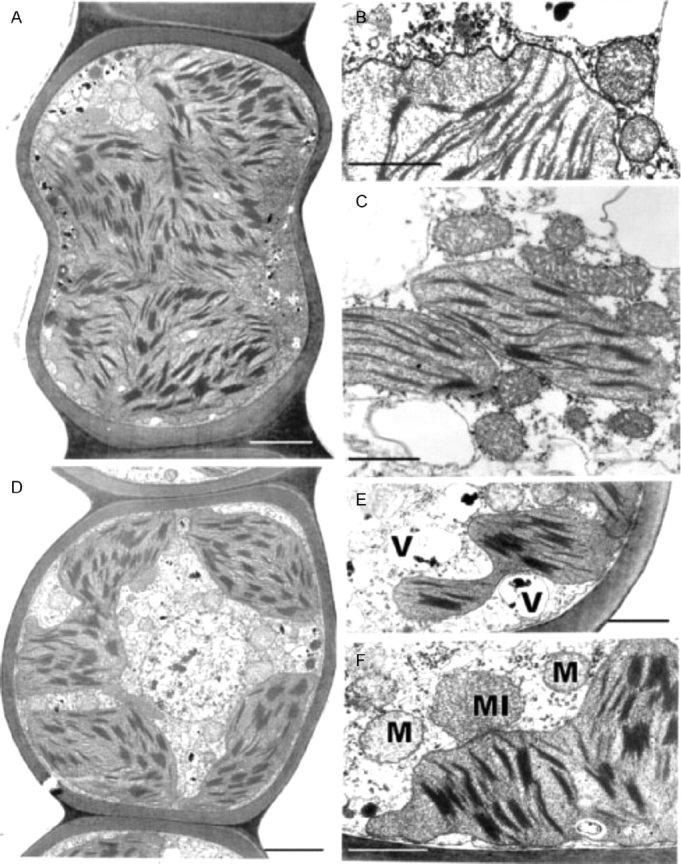
Transmission electron microscopy of Polytrichum formosum leaf cells 2 h (A–C) and 12 h (D–F) after rehydration. (A) General aspect of a lamella cell showing ovoid and spherical chloroplasts with undulate envelopes, and small peripheral vacuoles with dense contents. (B) Detail of a lamella cell showing an undulate chloroplast envelope and mitochondria. (C) Discoidal chloroplasts, mitochondria with dense matrix and saccate cristae, and clear cytoplasm with scattered ribosomes in a lamina cell. (D) General aspect of a lamella cell showing a central nucleus lacking condensed chromatin, and lobed chloroplasts. (E, F) Details of lamina cells showing the chloroplast lobes, vacuoles, a microbody and mitochondria, and polysomes scattered in the electron-transparent cytoplasm. M, Mitochondrion; MI, microbody; V, vacuole. Scale bars: A, D=2 µm; B, C, E, F=1 µm.
After 12 h of rehydration the cytology of the lamina cells has returned to that seen in the hydrated leaves (not shown) and many features of the lamella cells have also returned to ‘normal’. The nucleus is again in a central position and no longer contains masses of condensed chromatin (Fig. 12D); the now largely electron-transparent cytoplasm contains single profiles of RER, scattered polysomes and mitochondria with dense matrix and saccate cristae (Fig. 12E, F). The vacuoles, however, remain small and mostly spherical with dense contents (Fig. 12E). The chloroplasts are now extensively lobed with their margins along the plasmalemma frequently forming short blunt protuberances lacking in thylakoids (Fig. 12F).
After 24 h of rehydration the lamella cells have virtually the same appearance as in the original hydrated specimens, with variously lobed peripheral chloroplasts (Fig. 13A), and small irregularly shaped vacuoles (Fig. 13B). The lamellar extensions from the chloroplasts (Fig. 13C) are longer and narrower than after 12 h but not until after 48 h (Fig. 13D) do they become flat plates with swollen margins like those in the original hydrated specimens. Peripheral reticulum reappears in the lamina-cell chloroplasts 24 h after rehydration.
Fig. 13.
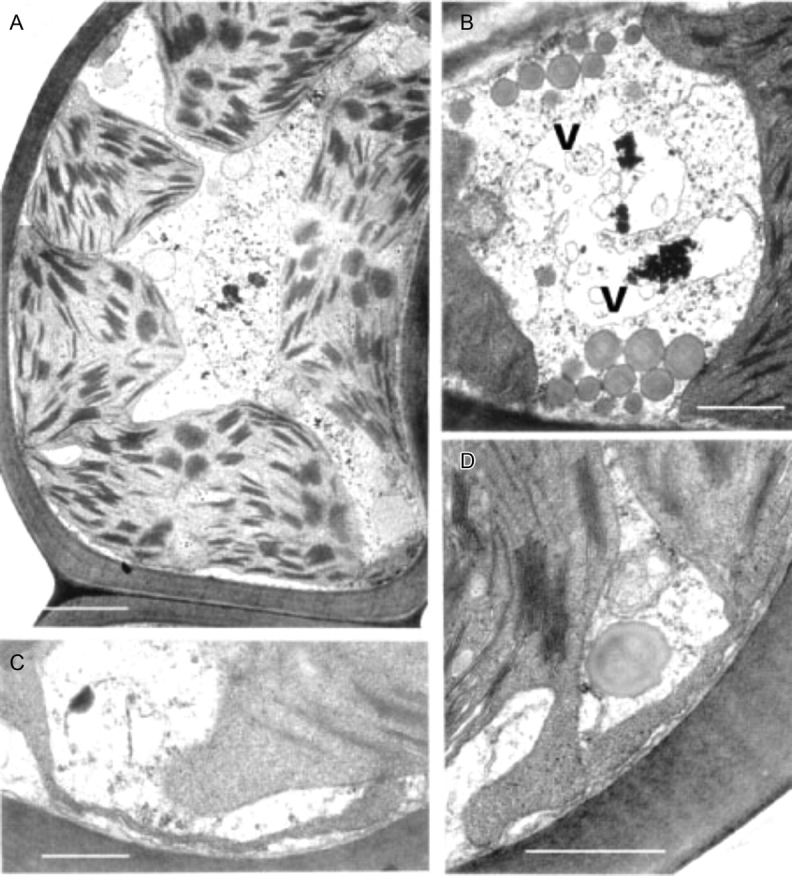
Transmission electron microscopy of Polytrichum formosum leaf-lamella cells 24 h (A–C) and 48 h (D) after rehydration. (A) General aspect; the cell has almost completely returned to its original condition with variously lobed peripheral chloroplasts. (B) Pleomorphic vacuole. (C) Relatively coarse chloroplast extension after 24 h. (D) Lamellar chloroplast extensions like those in the original hydrated specimens. V, Vacuole. Scale bars: A, B=2 µm; C, D=0·5 µm.
DISCUSSION
The physiological and cytological results set out above present a nicely consistent picture of events through a drying and re-wetting cycle in Polytrichum formosum. There is no indication of damage to membranes or organelles in the course of either desiccation or rehydration, but there are major morphological changes on a time scale that is reflected in physiological events. Certain basic processes (respiration, photosynthetic charge separation, protein synthesis) start very rapidly on rehydration. Others, including photosynthetic carbon fixation recover more slowly in a manner that suggests dependence on progressive re-establishment of the spatial relationships of the fully hydrated cell. The physiological and electron microscope data both suggest that recovery on re-wetting is primarily a matter of reassembly and reactivation of components which have been conserved undamaged through the desiccation–rehydration cycle. In particular, it seems clear from physiological experiments that recovery in itself involves little or no de novo protein synthesis; in the dark, recovery appears indifferent to the presence of protein-synthesis inhibitors. In the light, protein synthesis, especially of chloroplast-encoded proteins, is clearly essential for the repair of ongoing photo-damage (Proctor and Smirnoff, 2000; Proctor, 2001).
The present study shows that desiccation and rehydration involve remarkable cytological changes in the photosynthetic cells in the leaves of Polytrichum formosum. The changes recorded here are closely consistent with those described previously in the leptoids of P. formosum (Pressel et al., 2006), in the leaves of other mosses and in Selaginella lepidophylla (Platt et al., 1997), and provide a ready explanation for the rapid resumption of respiration and photosynthesis following rehydration and the much slower return of these processes to pre-desiccation rates. A few cytological features are unchanged through the drying and re-wetting cycle, some recover within minutes, but others take many hours to regain their normal morphology.
Unfortunately, Polytrichum formosum has proved unsuitable for high pressure freeze-substitution (Schmid, 1998), which would have avoided any question of possible artefacts from conventional aqueous chemical fixation. Dehydrated specimens fixed anhydrously using hexane produce results wholly consistent with standard aqueous fixation (Pressel et al., 2006). The cell volumes recorded in dehydrated and rehydrating specimens (Table 1) show that these do not swell as a result of imbibition of excess water during aqueous fixation; this was also the case in dry leaves of Selaginella lepidophylla when freeze-substitution images were compared with those from conventional fixation (Platt et al., 1997). Indeed the latter study revealed remarkably few ultrastructural differences between freeze-substitution and conventional fixation apart from the higher electron-opacity of the mitochondrial matrix and chloroplast stroma using the former protocol. The statement by Platt et al. (1997) that conventional fixation produced significant swelling of the mitochondria and chloroplasts is not borne out by direct measurements of their published micrographs. It therefore seems reasonable to conclude that the present observations on changes in the ultrastructure of Polytrichum leaves are not fixation artefacts.
The finding by Platt et al. (1997), borne out by preliminary work on P. formosum by ourselves, that newly rehydrated cells are much more sensitive to the osmolarity of the fixative than fully hydrated controls, now provides an explanation for the damaged organelles described in earlier studies that lent weight to the concept of damage and repair. These results indicate that, immediately following rehydration, bryophyte cells appear to be extremely fragile. The recent discovery that dehydration causes depolymerization of the microtubule cytoskeleton in both leptoids and meristematic cells of P. formosum (Pressel et al., 2006) provides a likely explanation. Until rehydrated cells have reassembled the cytoskeletal network that forms the framework supporting the cytoplasmic organization they may well be particularly prone to damage from osmotic stress.
Two features of the leaf cells of Polytrichum that remain virtually unchanged through the drying and re-wetting cycle are the grana–stroma thylakoid networks in the chloroplasts of both the lamella and lamina cells and the mitochondrial cristae, although the latter change from tubular to swollen within the first 2 h after re-wetting. These cytological features are consistent with the rapid resumption of photosynthesis and respiration during rehydration. The microbodies closely associated with the chloroplasts of Polytrichum, and remaining unchanged throughout drying and re-wetting, have a key role in removing the superoxide radicals produced in response to desiccation stress in bryophytes (Smirnoff, 1993; Minibayeva and Beckett, 2001; Mayaba et al., 2002). Similar microbodies have frequently been noted in the vicinity of the choroplasts in a variey of bryophyte tissues (Duckett and Renzaglia, 1988) but their particular prominence in the leaves of Polytrichum and Tortula (Syntrichia) ruralis (Robertson, 1991) may well be associated with desiccation tolerance in both these species. It would interesting to explore relationships between the ‘oxidative bursts’ and microbodies in a range of taxa from contrasting habitats.
Features of the desiccated leaf lamina cells that can be readily explained as simply due to the physical loss of essentially free water, and which are closely in line with the reduced volume of the lumina (Table 1), include the replacement of the central pleomorphic vacuole system with numerous small vacuoles located at the cell periphery, clustering of the ribosomes, chromatin condensation, and close juxtaposition of the mitochondria and chloroplasts. Some of these features return to the predesiccated condition within 1–2 h of rehydration (dispersal of the ribosomes, separation of chloroplasts and mitochondria) others take much longer to recover (chromatin dispersal, reappearance of pleomorphic vacuoles). The close packing of the ribosomes in the desiccated cells and their separation following rehydration is wholly consistent with biochemical data that the almost immediate resumption of RNA and protein synthesis during rehydration involves existing ribosomes (Oliver and Bewley, 1984a). Similarly the finding that chromatin decondensation may take several hours explains the delayed resumption of transcription following rehydration. There is no evidence from the present and previous studies that desiccation in mosses leads to the formation of crystalline arrays of ribosomes like those described in various animal, vascular plant and prokaryote cells under conditions of stress (Duckett, 1975; Barbieri et al., 1995; Nissen et al., 2000). In the same context, crystals of RUBISCO (the so-called stromacentres) that sometimes appear in the stroma of plastids in stressed homoiohydric plants, were not seen in dehydrated Polytrichum nor are these apparent in micrographs in previous studies. Similarly, crystals are absent from the matrix of dehydrated microbodies in Polytrichum leaves.
Other cytological changes where recovery is more protracted require further explanation. A notable feature of desiccation in the leaf cells of Polytrichum formosum, also seen previously in the food-conducting cells of the same species (Pressel et al., 2006) and in the leaves of Selaginella lepidophylla is the development of numerous small vacuoles replacing the vacuolar system typical of the hydrated state. In every case these take several hours to disappear following rehydration. The origins and possible roles of the small vacuoles are unknown. However, one possibility is that they accumulate soluble carbohydrate moieties, as shown in food-conducting cells (Schmid, 1998), that increase the osmoticum and thus protect the cells against damage from water stress. In Selaginella these vacuoles may be the sites of accumulation of trehalose associated with desiccation (Zantella et al., 1999). The relatively large sizes of the vacuoles occurring in the leaf lamina cells of Polytrichum, both in the dry state and in the first phase of rehydration, might reflect water retention/uptake promoted by the accumulation of osmotically active solutes. In the samples fixed 2 h after rehydration the vacuoles are much smaller, although the overall volume of lamella cells has increased remarkably, which suggests a redistribution of water and osmotically active components between the vacuoles and cytoplasm. The central pleomorphic vacuolar system typical of the hydrated state, however, is fully re-established only after 24–48 h. Such a wide delay suggests that the two vacuolar systems might be ontogenetically unrelated.
Probably the most striking and unexpected cytological feature associated with de- and rehydration in the leaf cells of Polytrichum is the change in shape of the chloroplasts. Although the overall volume of the chloroplasts hardly changes between dehydrated and fully hydrated cells, desiccation sees the complete disappearance of all the isthmuses between lobed choroplasts and the lamellar extensions in the cells of the leaf lamellae and peripheral reticulum from the chloroplasts of the leaf lamina. The images of two or even three closely apposed thylakoid systems within the same chloroplast can be interpreted as resulting from the coming together of two thylakoid systems that, prior to desiccation, lay separately within different lobes of the same chloroplast (Fig. 7B and C). Similar close juxtaposition of separate thylakoid systems is also visible in micrographs of dry Selaginella leaves (Platt et al., 1997; Thomson and Platt, 1997). Although multilobed plastids have been described from a variety of tissues in bryophytes, and lamellate plastids are a characteristic feature of spermatogenesis and sporogenesis in mosses (Duckett and Renzaglia, 1988), lamellar extensions from fully differentiated chloroplasts do not appear to have been noted previously and are not apparent in an earlier ultrastructural study of Polytrichum leaves (Paolillo and Reighard, 1967). The disappearance and reappearance of the chloroplast isthmuses and lamellar extensions during drying and re-wetting may be highly significant in the desiccation biology of Polytrichum. In simple physical terms their disappearance clearly minimizes the surface area of the chloroplasts, and the same applies to the mitochondria which are invariably spherical in the dry state (Fig. 9) but are often elongated in the hydrated state (Figs 7E and 11C). In this condition the chloroplasts are likely to be more resistant to stresses associated with dehydration as well as with the rapid osmotic uptake of water immediately following rehydration when the cytoskeleton is absent (see below).
Whereas the unchanged thylakoid systems account for the rapid resumption of photosynthesis following rehydration, the gradual re-formation of the lamellar extensions over a period of up to 48 h provides an explanation for the similarly gradual return of photosythesis to pre-desiccation rates. The increased surface area of the lamellar extensions must certainly facilitate exchanges of metabolites between the chloroplasts and cytoplasm. The disappearance during desiccation of peripheral reticulum, and its slow reappearance following rehydration may also be significant in this context; in vascular plants, peripheral reticulum is thought to be involved in metabolite exchanges (Laetch and Price, 1969).
The lamellar extensions of the chloroplasts invite comparison with the highly dynamic extensions of the plastid envelope known as stromules that have been described from various higher plant tissues. Little is known about stromule function; they are tubular rather than lamellate, and their most likely role is in enhancing the exchange of metabolites with the cytoplasm (Kohler and Hanson, 2000; Kohler et al., 2000; Kwok and Hanson 2004a, b; Waters et al., 2004; Gielwanowska et al., 2005). Experimental data suggest that the shape and motility of stromules are controlled by microfilaments and microtubules, findings closely in line with the report of Pressel et al. (2006) that a key feature of desiccation in Polytrichum is the depolymerization of the microtubule cytoskeleton. This suggests as a working hypothesis that rounding off of the chloroplasts (and mitochondria) during desiccation in Polytrichum leaves may be associated with the loss of the cytoskeleton, and that re-formation of the lamellar extensions depends on its repolymerization.
Neither the physiological nor the fine-structural results of the present work support the kind of simple damage-repair hypothesis of bryophyte desiccation tolerance that seemed reasonable 20 years ago, and was consistent with the evidence available at that time. There seems in fact now to be little evidence that desiccation tolerance in bryophytes is ‘repair based’ in the sense originally envisaged, and a good deal to indicate that the suite of ‘constitutive’ protective substances (including sugars and LEA-like proteins) in bryophytes and the ‘inducible’ desiccation protectants of vascular resurrection plants have important features in common. Oliver et al. (2005) see constitutive protection as playing an important role in the desiccation tolerance of bryophytes. Repair of the essential systems like respiration, light-capture and CO2 fixation, and protein synthesis, now looks to be largely physical, and probably not metabolically costly in terms of either energy or materials. The ‘cost’ may well be mainly in producing (inter alia) LEA-like proteins and high concentrations of sugars beyond the normal needs of metabolism – and maybe in having to run metabolism in a cramped and viscous cell. As Alpert and Oliver (2002) and Alpert (2006) suggest, the difference between bryophytes and vascular plants may essentially reflect their difference in physical size. A bryophyte shoot exposed to dry air will dry to the point where metabolism ceases within a few minutes, while a drying shoot of a vascular resurrection plant may remain metabolically active for many hours. If the bryophyte is to survive at all in a drought-prone habitat, its desiccation tolerance must be constitutive; the vascular plant has time for tolerance to be induced when drought threatens. In this scenario, ‘repair’ in the bryophyte may be not so much repair of damaged structures as repair and maintenance of its ‘constitutive’ tolerance mechanism. There is certainly a less sharp boundary between desiccation tolerance in bryophytes and vascular plants than might appear from contrasting Tortula (Syntrichia) ruralis (or Polytrichum formosum) with Craterostigma plantagineum, or even Selaginella lepidophylla. Some small ferns and Selaginella species withstand similar drying rates to Polytrichum, and many bryophytes show varying degrees of inducibility in their desiccation tolerance (Abel, 1956; Mayaba et al., 2001). The common factors are likely to be more important than the differences.
The findings of the present and previous studies, that de- and rehydration are not simply a matter of physical removal and addition of water but involve a highly organized series of cytological changes taking place over several hours, open the way to exciting advances in understanding the biology of desiccation. Particularly inviting are experiments using drugs that affect the cytoskeleton to discover whether reformation of chloroplast extensions depends on microtubules and/or microfilaments and whether, in the absence of these extensions, photosynthesis fails to return to predesiccation values. Is the absence of a cytoskeleton one reason why newly rehydrated cells remain highly sensitive to the osmolarity of fixatives? Does unnaturally rapid drying lead to death because there is insufficient time for the cytological adjustments (including the formation of a new vacuole system and the retraction of chloroplast extensions) that are critical to survival through desiccation? From the standpoint of functional genomics we are now in a better position to elucidate relationships between de- and rehydrins, cytoskeletal dynamics and membrane stabilization.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Keith Pell (QMUL) for technical assistance.
LITERATURE CITED
- Abel WO. Die Austrocknungsresistenz der Laubmoose. Sitzungsberichte. Österreichische Akademie der Wissenschaften. Mathematisch-naturwissenschaftliche Klasse, Abt. 1956;165:619–707. I. [Google Scholar]
- Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology. 2006;209:1575–1584. doi: 10.1242/jeb.02179. [DOI] [PubMed] [Google Scholar]
- Alpert P, Oliver MJ. Drying without dying. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford: CABI Publishing; 2002. pp. 3–43. [Google Scholar]
- Barbieri M, Vittone A, Maraldi NM. Cell stress and ribosome crystallization. Journal of Submicroscopic Cytology and Pathology. 1995;27:199–207. [PubMed] [Google Scholar]
- Bewley JD. Polyribosomes conserved during desiccation of the moss Tortula ruralis are active. Plant Physiology. 1973;51:285–288. doi: 10.1104/pp.51.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Physiological aspects of desiccation tolerance. Annual Review of Plant Physiology. 1979;30:195–238. [Google Scholar]
- Bewley JD, Krochko JE. Desiccation-tolerance. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopaedia of plant physiology. 12B. Berlin: Springer-Verlag; 1982. pp. 325–378. New Series. [Google Scholar]
- Bewley JD, Oliver MJ. Desiccation tolerance in vegetative plant tissues and seeds. Protein synthesis in relation to desiccation and a potential role for protection and repair mechanisms. In: Somero GN, Osmond CB, Bolis CL, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Heidelberg: Springer-Verlag; 1992. pp. 141–160. [Google Scholar]
- Bottomley W, Bohnert HJ. The biosynthesis of chloroplast proteins. In: Parthier B, Boulter D, editors. Encyclopedia of plant physiology. 14B. Berlin: Springer-Verlag; 1982. pp. 531–596. New Series. [Google Scholar]
- Clausen E. Hepatics and humidity: a study on the occurrence of hepatics in a Danish tract and the influence of relative humidity on their distribution. Dansk Botanisk Arkiv. 1952;15:1–81. [Google Scholar]
- Dilks TJK, Proctor MCF. The pattern of recovery of bryophytes after desiccation. Journal of Bryology. 1974;8:97–115. [Google Scholar]
- Dilks TJK, Proctor MCF. Effects of intermittent desiccation on bryophytes. Journal of Bryology. 1976;9:249–264. [Google Scholar]
- Dilks TJK, Proctor MCF. Photosynthesis, respiration and water content in bryophytes. New Phytologist. 1979;82:97–114. [Google Scholar]
- Duckett JG. Pentagonal arrays of ribosomes in fertilised eggs of Pteridium aquilinum (L.) Kutin. Journal of Ultrastructure Research. 1975;30:390–397. doi: 10.1016/s0022-5320(72)90013-5. [DOI] [PubMed] [Google Scholar]
- Duckett JG, Renzaglia KS. Ultrastructure and development of plastids in bryophytes. Advances in Bryology. 1988;3:33–93. [Google Scholar]
- Galling G. Use (and misuse) of inhibitors in gene expression. In: Parthier B, Boulter D, editors. Encyclopedia of plant physiology. 14B. Berlin: Springer-Verlag; 1982. pp. 663–677. New Series. [Google Scholar]
- Gielwanowska I, Szczuka E, Bednara J, Gorecki R. Anatomical features and ultrastructure of Deschampsia antarctica (Poaceae) leaves from different growing habitats. Annals of Botany. 2005;96:1109–1119. doi: 10.1093/aob/mci262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung W, Schiller P, Dietz K-J. Physiology of poikilohydric plants. Progress in Botany. 1998;59:299–327. [Google Scholar]
- Hinshiri HM, Proctor MCF. The effect of desiccation on subsequent assimilation and respiration of the bryophytes Anomodon viticulosus and Porella platyphylla. New Phytologist. 1971;70:527–538. [Google Scholar]
- Höfler K. Über Trockenhärtung und Härtungsgrenzen einiger Lebermoose. Anzeiger der Akademie der Wissenschaften in Wien. Mathematische-naturwissenschaftliche Klasse. 1946;1945:5–8. [Google Scholar]
- Hosokawa T, Kubota H. On the osmotic pressure and resistance to desiccation of epiphytic mosses from a beech forest, south-west Japan. Journal of Ecology. 1957;45:579–591. [Google Scholar]
- Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Irmscher E. Über die Resistenz der Laubmoose gegen Austrocknung und Kälte. Jahrbücher für wissenschaftliche Botanik. 1912;50:387–449. [Google Scholar]
- Kappen L, Valladares F. Opportunistic growth and desiccation tolerance: the ecological success of poikilohydrous autotrophs. In: Pugnaire FI, Valladares F, editors. Handbook of functional plant ecology. New York, NY: Martin Dekker; 1999. pp. 9–80. [Google Scholar]
- Kohler RH, Hanson MR. Plastid tubules are tissue-specific and developmentally regulated. Journal of Cell Science. 2000;113:81–89. doi: 10.1242/jcs.113.1.81. [DOI] [PubMed] [Google Scholar]
- Kohler RH, Schwille P, Webb WW, Hanson MR. Active protein transport through plastid tubules: velocity quantified by fluorescence correlation spectroscopy. Journal of Cell Science. 2000;113:3921–3930. doi: 10.1242/jcs.113.22.3921. [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. Stromules and the dynamic nature of plastid morphology. Journal of Microscopy. 2004a;214:124–137. doi: 10.1111/j.0022-2720.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- Kwok EY, Hanson MR. In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. BMC Plant Biology. 2004b;4:1–9. doi: 10.1186/1471-2229-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch WM, Price I. Development of dimorphic chloroplasts of sugar cane. American Journal of Botany. 1969;56:77–87. [Google Scholar]
- Ligrone R, Duckett JG. Cytoplasmic polarity and endoplasmic microtubules associated with the nucleus and organelles are ubiquitous features of food-conducting cells in bryalean mosses (Bryophyta) New Phytologist. 1994;127:601–604. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Minibayeva F, Beckett RP. High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytologist. 2001;152:333–341. [Google Scholar]
- Mayaba N, Beckett RP, Csintalan Z, Tuba Z. ABA increases the desiccation tolerance of photosynthesis in the Afromontane understory moss. Atrichum androgynum. Annals of Botany. 2001;86:1093–1100. [Google Scholar]
- Mayaba N, Minibayeva F, Beckett RP. An oxidative burst of hydrogen peroxide during rehydration following desiccation in the moss Atrichum androgynum. New Phytologist. 2002;155:275–283. [Google Scholar]
- Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000;289:920–930. doi: 10.1126/science.289.5481.920. [DOI] [PubMed] [Google Scholar]
- Oliver MJ. Desiccation tolerance in vegetative plant cells. Physiologia Plantarum. 1996;97:779–787. [Google Scholar]
- Oliver MJ, Bewley JD. Plant desiccation and protein synthesis. V. Stability of poly (A)– and poly (A)+ RNA during desiccation and their synthesis upon rehydration in the desiccation-tolerant moss Tortula ruralis and the intolerant moss Crataneuron filicinum. Plant Physiology. 1984a;74:917–922. doi: 10.1104/pp.74.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MJ, Bewley JD. Desiccation and ultrastructure in bryophytes. Advances in Bryology. 1984b;2:91–131. [Google Scholar]
- Oliver MJ, Bewley JD. Desiccation-tolerance of plant tissues: a mechanistic overview. Horticultural Reviews. 1997;18:171–213. [Google Scholar]
- Oliver MJ, Wood AJ, O'Mahony P. ‘To dryness and beyond’ – preparation for the dried state and rehydration in vegetative desiccation-tolerant plants. Plant Growth Regulation. 1998;24:193–201. [Google Scholar]
- Oliver MJ, Velten J, Mischler BD. Desiccation tolerance in bryophytes: a reflection of a primitive strategy for plant survival in dehydrating habitats. Integrative and Comparative Biology. 2005;45:788–799. doi: 10.1093/icb/45.5.788. [DOI] [PubMed] [Google Scholar]
- Oparka KJ. Plasmolysis: new insights into an old process. New Phytologist. 1994;126:571–591. Tansley Review No. 67. [Google Scholar]
- Paolillo DJ, Jr, Reighard JA. Ultrastructural features of some polytrichaceous leaves. Bryologist. 1967;70:61–69. [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW. Membranes and organelles of dehydrated Selaginella and Tortula retain their normal configuration and structural integrity. Protoplasma. 1994;178:57–65. [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW. Importance of the fixative for reliable ultrastructural preservation of poikilohydric plant tissues: observations on dry, partially, and fully hydrated tissues of. Selaginella lepidophylla. Annals of Botany. 1997;80:599–610. [Google Scholar]
- Pressel S, Ligrone R, Duckett JG. The effects of de- and rehydration on food-conducting cells in the moss Polytrichum formosum Hedw.: a cytological study. Annals of Botany. 2006;98:67–76. doi: 10.1093/aob/mcl092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF. Patterns of desiccation tolerance and recovery in bryophytes. Plant Growth Regulation. 2001;35:147–156. [Google Scholar]
- Proctor MCF. Why do Polytrichaceae have lamellae? Journal of Bryology. 2005;27:219–227. [Google Scholar]
- Proctor MCF, Pence VC. Vegetative tissues: bryophytes, vascular resurrection plants and vegetative propagules. In: Black M, Pritchard H, editors. Desiccation and survival in plants: drying without dying. Wallingford: CABI Publishing; 2002. pp. 207–237. [Google Scholar]
- Proctor MCF, Smirnoff N. Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. Journal of Experimental Botany. 2000;51:1695–1704. doi: 10.1093/jexbot/51.351.1695. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. Poikilohydry and homiohydry: antithesis or spectrum of possibilities? New Phytologist. 2002;156:327–349. doi: 10.1046/j.1469-8137.2002.00526.x. Tansley Review No. 141. [DOI] [PubMed] [Google Scholar]
- Robertson EJ. A cytological study of the Musci. University of London; 1991. PhD Dissertation. [Google Scholar]
- Schmid A. A new photoassimilate translocation mechanism in the Polytrichales? University of London; 1998. PhD Dissertation. [Google Scholar]
- Schonbeck MW, Bewley JD. Responses of the moss Tortula ruralis to desiccation treatments. I. Effects of minimum water content and rates of dehydration and rehydration. Canadian Journal of Botany. 1981a;59:2698–2706. [Google Scholar]
- Schonbeck MW, Bewley JD. Responses of the moss Tortula ruralis to desiccation treatments. II. Variations in desiccation tolerance. Canadian Journal of Botany. 1981b;59:2707–2712. [Google Scholar]
- Scott P. Resurrection plants and the secrets of eternal leaf. Annals of Botany. 2000;85:159–166. [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Stewart GR. Desiccation injury, anhydrobiosis and survival. In: Jones HG, Flowers TJ, Jones MB, editors. Plants under stress: biochemistry, physiology and ecology and their application to plant improvement. Cambridge: Cambridge University Press; 1990. pp. 115–130. [Google Scholar]
- Thomson WW, Platt KA. Conservation of cell order in desiccated mesophyll of Selaginella lepidophylla (Hook. and Grev.) Spring. Annals of Botany. 1997;79:439–447. [Google Scholar]
- Tuba Z, Csintalan Zs, Proctor MCF. Photosynthetic responses of a moss, Tortula ruralis ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation, a baseline study at present-day CO2 concentration. New Phytologist. 1996;133:353–361. doi: 10.1111/j.1469-8137.1996.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Waters MT, Fray RG, Pyke KA. Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. The Plant Journal. 2004;39:655–667. doi: 10.1111/j.1365-313X.2004.02164.x. [DOI] [PubMed] [Google Scholar]
- Zantella R, Mascorro-Gallardo JO, Van Kijck P, Folch-Mallol J, Bonini B, Van Vaeck C, et al. A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiology. 1999;119:1473–1482. doi: 10.1104/pp.119.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.