Abstract
Background and Aims
The knotweed complex, Fallopia spp. (Polygonaceae), belongs to the most troublesome invasive species in Europe and North America. Vegetative regeneration is widely recognized as the main mode of reproduction in the adventive regions. However, the contribution of sexual reproduction to the success of these invasive species has only been detailed for the British Isles. An examination was made as to how hybridization may influence the sexual reproduction of the complex in Belgium and to determine how it may contribute to the dispersal of the species.
Methods
Studies were made of floral biology, reproductive success, seed rain, seed bank, germination capacity, seedling survival and dispersal capacity in order to characterize the reproductive biology of the species. Moreover, chromosome counts and flow cytometry were used to assess the hybrid status of seedlings produced by sexual reproduction.
Key Results
In the area investigated, extensive sexual reproduction by hybridization within the complex, including one horticultural species, was demonstrated. A small percentage of seeds may be dispersed outside the maternal clone (>16 m) allowing the formation of genetically differentiated individuals. Seed germination was possible even after a winter cold period.
Conclusions
The extensive sexual reproduction by hybridization could further contribute to the dramatic invasive success of knotweeds in Belgium and should not be underestimated when considering control and management measures.
Key words: Chromosome counts, dispersal capacity, Fallopia aubertii, Fallopia×bohemica, Fallopia japonica, Fallopia sachalinensis, flow cytometry, germination, hybridization, invasive plant, sexual reproduction
INTRODUCTION
Reproductive strategy is a central feature of the ecology of invasive species as it determines the potential for range expansion (Daehler and Strong, 1994, 1996; Rejmánek and Richardson, 1996; Pyšek, 1997; Grotkopp et al., 2002). Sexual reproduction, high levels of fecundity and strong vegetative regeneration are some of the characteristics most frequently cited when compiling criteria promoting plant invasion (Baker, 1974; Noble, 1989; Rejmánek, 1995; Crawley et al., 1996). As mostly perennial plants, invasive species are able to reproduce sexually through seed production and/or clonally through vegetative propagation. The advantages of the clonal propagation are a rapid and almost unlimited propagation of combinations of favourable genes, a possibility for sterile genotypes to increase, a potential to colonize rapidly favourable habitats, a lifespan potentially unlimited, and a repartition of clone extinction risks (Silander, 1985). Sexual reproduction has the advantage of generating genotypic diversity, increasing the ability of species to adapt to adventive environments. Moreover, thanks to various dispersal mechanisms, seed production will extensively contribute to long-distance dispersal, which will also determine the expansion of the species (Levin, 2000). The trade-off between ‘reproductive’ vs. ‘vegetative’ strategies is thus a crucial determinant of successful invasion (Eckert et al., 2003; Brown and Eckert, 2005; Lui et al., 2005). Furthermore, interspecific hybridization with aliens has often stimulated the evolution of new and sometimes invasive species (Abbott, 1992) and the increase in human activities has provided new occasions for interspecific hybridization and the formation of new niches that better suit the hybrids than the parents (Vilà et al., 2000). Because of the swiftness of the evolutionary processes involved, invasions are excellent opportunities to study short-term evolution of species (Allendorf and Lundquist, 2003).
Belgium and its neighbours have suffered from invasion by exotic plants that have dramatically increased their range in the last 30 years (Muller, 2000; Verloove, 2002). Knotweeds, Fallopia spp., are among the most troublesome invaders in this area (Godefroid, 1996; Verloove, 2002) and more generally in Europe and Northern America (Weber, 2003), enhancing nutrient cycling rates and topsoil fertility and decreasing the plant species diversity of invaded sites (Vanderhoeven et al., 2005). Originally from Asia (China, Japan, parts of Korea and Taiwan) and introduced into Europe in mid 1800s as ornamental plants and fodder (Bailey and Conolly, 2000), knotweeds are now widely naturalized (Godefroid, 1996; Fojcik and Tokarska-Guzik, 2000; Hollingsworth and Bailey, 2000a, b; Forman and Kesseli, 2003; Mandák et al., 2003). These plants thrive in various habitats including riparian ecosystems, and disturbed areas such as roadsides and waste places (Weber, 2003).
Hybridization processes have often been described within Fallopia spp. A basic scheme of the relationship between the different taxa of the genus has been drawn in the British Isles based on molecular markers (RAPDs and ISSR) and chromosome counts (Bailey and Stace, 1992; Bailey et al., 1995; Hollingsworth et al., 1998; Hollingsworth and Bailey, 2000a, b; Pashley et al., 2003), and in the Czech Republic, based on isozymes and flow cytometry analyses (Mandák et al., 2003; Pyšek et al., 2003). The following species and hybrids have been described: F. japonica (octoploid, 2n=88); F. sachalinensis (tetraploid, 2n=44), less widespread than F. japonica; F.×bohemica, with varying ploidy levels from tetraploid to octoploid (Bailey, 2003; Bímová et al., 2003), an apparent result of hybridization between the first two taxa and of the resulting backcrosses and which seems to be more invasive compared with its parents; F. japonica var. compacta, tetraploid and rare; and F. aubertii (syn. F. baldschuanica), diploid, rare in nature but nowadays commonly cultivated. The hybrid between F. japonica and F. aubertii has been reported as F.×conollyana but up to now, only a minute proportion has ever germinated and become established in nature (Bailey, 2001).
In their native range where they occur in open habitats, for instance as pioneer species of volcanic ash (Schnitzler and Muller, 1998; Bailey, 2003), F. japonica and F. sachalinensis both reproduce by sexual reproduction, with hermaphrodite and male-sterile stands (Tanaka, 1966), and by vegetative regeneration (Maruta, 1976). On the other hand, in the British Isles, every plant of F. japonica (referred to as var. japonica) examined by Bailey (1994) was male-sterile, characterized by small, included anthers, implying that all reproduction is by vegetative spread (Hollingsworth and Bailey, 2000a). No pure-bred seed has been found on British plants of F. japonica (Beerling et al., 1994). Because F. sachalinensis occurs as both hermaphrodite and male-sterile plants, pure-bred seeds are formed and reproduction can both occur sexually and asexually (Bailey, 1994). The hybrid F.×bohemica has both male-sterile and hermaphrodite individuals with partial to full fertility (Bailey et al., 1996). Since only one female clone of Japanese knotweed is found in Britain, it is unable to reproduce itself, and any seed found on these plants is the result of pollination by a related species. It has also been found (Bailey, 1989) that the hermaphrodite plants of F. sachalinensis and F.×bohemica are self-incompatible, i.e. they are unable to form seed without an additional source of pollen. Up to now, the reproductive biology and the fertility of the invasive Fallopia spp. have only been assessed from a quantitative point of view in the UK (Bailey, 1994) and through germination experiments and seedling monitoring in Northern America (Forman and Kesseli, 2003). In west continental Europe, particularly, data concerning the sexual status of the species and their ability to hybridize are lacking (Lambinon et al., 2004).
Invasive Fallopia species are extremely difficult to control by both manual and chemical methods (Weber, 2003). The combination of highly successful vegetative propagation (Bímová et al., 2003), hybridization and polyploidization, high competitive capacity, and potential outcrossing greatly contribute to the worrying success of these invasive species (Child and Wade, 2000; Hathaway, 2000; Child et al., 2001; Mandák et al., 2004).
The aim of the present study was to examine how hybridization may influence the sexual reproduction of Fallopia species in Belgium and to determine how it may contribute to their invasive success. Different components of the reproductive biology including (a) floral biology, (b) reproductive success, (c) seed rain, (d) seed bank, (e) germination capacity, (f) seedling survival, and (g) dispersal capacity, were followed. Moreover, the hybrid status of seedlings produced by sexual reproduction was assessed by mitotic root tip chromosome counts and flow cytometry.
MATERIALS AND METHODS
Floral structure
Fallopia spp. are characterized by a five-tepaled perianth with a trigonous gynoecium, short style, and trifid fimbriate stigma surrounded by eight anthers. In male sterile plants, the anthers are small, flattened, empty, and included within the perianth, whereas the male-fertile flowers have large full anthers well exerted from the perianth, displaying varying degrees of stigmatic development (Bailey, 1989).
Study area
In Belgium, the four following exotic species have been described within the genus: three erect rhizomatous perennials F. japonica (Houtt.) Ronse Decraene, F. sachalinensis (F. Schmidt Petrop.) Ronse Decraene and F.×bohemica (Chrtek et Chrtková) J.P. Bailey; and one climbing perennial, F. aubertii (L. Henry) [syn. F. baldschuanica (Regel) Holub] (Holub, 1970; Haraldson, 1978; Bailey and Stace, 1992; Bailey, 1994, 2001; Beerling et al., 1994; Lambinon et al., 2004). Fallopia×bohemica, as mentioned above, is a hybrid between F. japonica and F. sachalinensis and of the resulting backcrosses with the parent species. Fallopia japonica var. compacta (Hook. F.) J.P. Bailey is much rarer.
Four sites were selected in Belgium. The different species were distinguished in the field according to morphological vegetative characters reported in the literature (Barral, 1994; Beerling et al., 1994; Jager, 1994; Anonyme, 2001; Lambinon et al., 2004). The main study site was a semi-rural landscape (1300 ha) in the vicinity of Gembloux (50°33′N; 4°41′E) where a complete landscape survey was performed in 2002 and 2003 to identify all clones of F. japonica, F. sachalinensis, F.×bohemica and F. aubertii. Additional clones of F. japonica and F.×bohemica were also selected in the neighbourhood of Namur (50°27′N; 4°51′E). As F. sachalinensis is not widespread in Belgium, the study area was extended to the region of Brussels (50°50′N; 4°21′E). Additionally, one hermaphrodite clone of F. sachalinensis was selected in Kelmis (50°43′N; 6°00′E).
Floral biology
Flower buds and flowers were collected from Fallopia clones, stored in ethyl alcohol (70 %) until examination. The morphology of two flowers per clone (development of stamen, stigma and ovary) was observed using a binocular microscope (Nikon Japan SMZ-10A) on 20 clones of F. japonica, five of F.×bohemica and six of F. sachalinensis in the summer of 2002, and seven F. aubertii clones in the summer of 2004. The diameter of the corolla was measured with a ×10 lens. The ovary was dissected and the length and width of the ovule measured at ×25. The number of pollen grains per flower was assessed. Buds were acetolysed in 50 µL of a mixture of 8 : 1 acetic anhydride–H2SO4 and the number of pollen grains counted under a microscope (Nikon Japan Labophot-2) at ×100. Mature stamens were crushed in a drop of lactophenol cotton blue on a microscope slide and the pollen examined at ×100. Pollen was considered viable and potentially fertile if it appeared normal in shape and if the cytoplasm was stained dark blue (Jacquemart and Thompson, 1996). The percentage of viable pollen grains was calculated on the basis of 100 observations when sufficient pollen grains were present.
Pollination and reproductive success
To assess pollination success, the number of pollen grains deposited on stigmas of three open-pollinated flowers per clone was estimated in the autumn of 2002 on 29 clones of F. japonica, three of F.×bohemica, six of F. sachalinensis and seven of F. aubertii. Stigmas were acetolysed in 25 µL of a mixture of 8 : 1 acetic anhydride–H2SO4 and pollen was counted under a microscope at ×100. Fruit set was estimated from summer to autumn 2002 on 19 clones of F. japonica, five of F.×bohemica, six of F. sachalinensis, and from summer to autumn 2004 on ten of F. aubertii plants. The fruit set was estimated as the ratio (number of ripe fruits)/(number of developed flowers produced per inflorescence) for three inflorescences per clone. As there is only one ovule in each flower, fruit set was equivalent to seed set. It was not possible to estimate the fruit set of the hermaphrodite F. sachalinensis as it was unfortunately destroyed during the experiment.
Seed rain and seed bank
Fallopia japonica clones were used to assess the potential seed rain, the survival of seeds in the seed bank, and the total seed production per clone. The potential seed rain and the total seed production per clone were estimated for nine clones of F. japonica in autumn 2002 by recording the mean number of seeds (seedstem) on three randomly selected stems and the mean number of stems (stemunit) in a 3 m2 area per clone. The surface of each clone (surf) was estimated from its two longest axes. The mean number of seeds produced per square metre (potential seed rain) was estimated as seedstem×stemunit. The total number of seeds produced per clone was estimated as seedstem×stemunit×surf.
To assess the survival of seeds in the seed bank, soil samples were collected under the canopy of 14 clones of F. japonica in the spring and in autumn 2003. For each clone, a sample of five bulked soil cores (4 cm diameter, 5 cm depth) was taken. The cores were divided into two layers, according to the soil depth: litter (the fibrous horizon with a high organic matter content) and 0–5 cm. Samples were air-dried at 20 °C, sieved through a 2-mm-mesh sieve and subsequently poured into plastic containers filled with expanded clay (argex), covered with a sterilized garden compost–sand mix (75 : 25 %). Trays were placed in the greenhouse at a temperature of 20 °C and a light period of 16 h. Soil was kept moist by a regular supply of distilled water. Emerging seedlings were counted and removed as soon as they could be identified.
Seed viability
Seed viability was tested with a tetrazolium test (Kearns and Inouye, 1993). Before treatment, seeds were saturated with distilled water at room temperature for 1 d, bisected through the embryo, incubated at 35 °C for 2 h in a 1 % solution of 2,3,5-triphenyl-2H-tetrazolium chloride and examined under a microscope. Twenty-five fresh seeds per clone were tested from ten clones of F. japonica and 50 fresh seeds per clone on two clones of male sterile F. sachalinensis. The tetrazolium test was based on the visual reduction (development of red staining) of 2,3,5-triphenyl-2H-tetrazolium chloride.
Seed germination
Three bulked seed lots were collected on (a) five clones of F. japonica in the autumn of 2002, (b) ten clones of F. japonica in the autumn of 2003, and (c) two male sterile clones of F. sachalinensis in the autumn of 2003. Due to the extremely low seed production, it was not possible to assess germination the capacity of F.×bohemica. Seeds from the different lots were dried at 25 °C for 2 d and stored at room temperature until required. The main objective was to test for the effect of a cold, humid period on the germination rate of seeds. Two stratification treatments were then applied to each seed lot: (1) in Petri dishes and (2) in compost. (1) Fifty seeds were sown per dish on wet filter paper. Five replicates per lot (ten for F. japonica in 2003) were set to germinate immediately without stratification after seed collection in a growth chamber at 22 °C, 16 h light (‘Petri fresh’ treatment). Five replicates (ten for F. japonica in 2003) were stored for 3 months at 4 °C in the dark before being transferred to the immediate conditions (‘Petri stratified’ treatment). Germinating seeds were recorded every day for 30 d after sowing. (2) Fifty seeds were sown in plastic containers filled with a commercial organic culture soil (expanded clay, argex, covered with a sterilized garden compost–sand mix, 75 : 25 %). Five replicates per seed lot (ten for F. japonica in 2003) were placed immediately in a glasshouse at 22 °C and 16 h light (‘compost fresh’ treatment). Five replicates per seed lot (ten for F. japonica in 2003) were placed outside from December 2003 to the end of spring 2004 in order to simulate natural germination conditions (‘compost stratified’ treatment). The number of seedlings emerging was recorded every day during the period of emergence. Moreover, survival was recorded for each seedling from the ‘compost stratified’ treatment in 2002 for 135 d. Seed viability was tested on non-germinating and stratified seeds in Petri dishes using the tetrazolium test.
As the importance of F. aubertii in the landscapes studied appeared only during the course of the study, germination tests were not included in the initial protocol for this species. Then, seeds were collected from two clones in autumn 2004 and the compost treatment was used on this seed lot as described for the other species.
Field observations of germination and seedling establishment were performed during the spring and the summer of 2003 inside and around all the Fallopia clones in the vicinity of Gembloux. Sites were checked weekly for 5 months for evidence of new germinations or seedling development in the field.
Cytology
To gain more insight into the parental origin of seeds, chromosomes were counted in autumn 2004 on 12 seedlings resulting from the germination of 11 seeds collected on F. japonica and one seed on F. sachalinensis. Chromosomes were counted on fresh roots tips according to the method developed by Bailey and Stace (1992).
Flow cytometry
In order to gain more information on the hybridization pattern, ploidy level was measured by flow cytometry on 55 seedlings collected from four clones of F. japonica in the vicinity of Gembloux in autumn 2005. This technique allows the rapid determination of the relative DNA content of nuclei by measuring the fluorescence of a fluorochrome that specifically binds to DNA (Galbraith et al., 1983). Small leaf discs were chopped with a razor blade in Petri dishes, after addition of 500 µL 100 mm sodium hydrogen phosphate at pH 7, and containing 0·5 % Tween 20. After filtration through a 30-μm nylon filter, 500 µL of a solution of 5 g L−1 DAPI (fluorochrome) in 100 mm sodium hydrogen phosphate was added. Flow cytometry measurements were performed with a Partec machine (CA3 software 1995) equipped with a UV lamp. The fluorochrome was excited at 340 nm and emitted at 470 nm. A tetraploid individual (Fallopia sachalinensis) whose chromosome number was known was used as an internal standard for each measurement. We used the ratio (mean fluorescence intensity of seedling)/(mean fluorescence intensity of internal standard) to assess the ploidy level of seedlings. To interpret the observations more accurately, flow cytometry assessment of adult hybrids, for which chromosome numbers had previously been counted, was also used (M.-S. Tiébré, unpubl. res.).
Seed dispersal
In autumn 2003, the dispersal capacity of F. japonica seeds was measured in situ on two clones. According to the method of Bullock and Clarke (2000), seed traps were placed at doubling increasing distances from the edge of the clone (0·2, 0·5, 1, 2, 4, 8 and 16 m) along two transects per clone (SSE and SW: clone 1) (SSW and WSW: clone 2). Seed traps consisted of a 10-cm-diameter by 12-cm-long section of PVC tubing closed by a 0·1-mm net at the bottom and a 2-cm grid at the top to limit seed predation. Seed traps were dug in so as to be level with the soil surface. The number of traps was doubled for each doubling distance to keep sampling effort constant, with two traps at the first three sampling distances (up to 1 m) (Bullock and Clarke, 2000). Each seed trap was collected weekly for 2 months and the number of filled seeds was counted.
Statistical analyses
Concerning floral biology, comparisons between taxa were performed using one-way ANOVA followed by pairwise comparisons with Tukey–HSD post hoc tests for mean pollen/flower (square root transformation) and mean ovule length (logarithmic transformation). The non-parametric Kruskal–Wallis test was used followed by pairwise comparisons between taxa with the Mann–Whitney test for mean pollen viability, mean ovule width, mean corolla diameter, mean number of pollen per stigma and mean fruit set, as they could not be successfully transformed.
In the germination experiment, the effect of the stratification treatment was assessed independently in Petri dishes and compost. Within each seed lot (F. japonica 2002, F. japonica 2003 and F. sachalinensis 2003), mean germination percentages were compared between stratified and non-stratified treatments in Petri dishes and between stratified and non-stratified treatments in compost by t-tests. Secondly, to contrast germination success between years, mean germination percentages were compared between F. japonica 2002 and 2003 for each individual combination of stratification and substrate treatments independently (compost-fresh, compost-stratified, Petri-fresh, Petri-stratified), using t-tests. Thirdly, to contrast germination success between taxa, mean germination percentages were compared between F. japonica 2003 and F. sachalinensis 2003 for each individual combination of stratification and substrate treatments independently (compost-fresh, compost-stratified, Petri-fresh, Petri-stratified), using t-tests. All germination percentages were arcsine-transformed before analysis. Means are given with their standard deviation. Analyses were performed using XLSTAT (version 6·0; Addinsoft 2002) and Statistica (version 7·1; Statsoft 2005).
RESULTS
Floral biology
All Fallopia japonica clones and one F. sachalinensis clone showed high levels of male infertility with 0–4 and 0–40 pollen grains per flower, respectively (Table 1). None of the pollen grains observed were viable. Those clones were removed from statistical comparisons of means for those two characters. Other F. sachalinensis, F.×bohemica and F. aubertii clones had higher male fertility, with pollen grains per flower ranging, respectively, from 7306 to 8072, from 492 to 7280, and from 3718 to 12 502. The highest values of pollen viability were observed for the hermaphrodite F. sachalinensis (63–97 %) followed by F.×bohemica (3–48 %). Fallopia aubertii showed lower pollen viability (1–13 %). A significant difference was found for mean number of pollen grains per flower among taxa (Kruskal–Wallis test H=78·99, P=0·000) with F.×bohemica significantly differing from F. aubertii, whereas F. sachalinensis was not significantly different from the two other taxa. Significant differences were also found for mean pollen viability among the three taxa (Kruskal–Wallis test H=15·23, P=0·000), with all pairwise comparisons of means being significant.
Table 1.
Mean number of pollen grains per flower, pollen viability, ovule length and width, and diameter of the corolla of F. japonica, F. sachalinensis, F.×bohemica and F. aubertii
| Mean pollen per flower | Mean pollen viability (%) | Mean ovule length (mm) | Mean ovule width (mm) | Mean corolla diameter (mm) | ||
|---|---|---|---|---|---|---|
| F. japonica | F | 0·10 (0·63) | 0·00 (0·00) | 0·58 (0·16)a | 0·31 (0·06)a | 3·76 (0·17)a |
| F. sachalinensis | F | 8·00 (13·73) | 0·00 (0·00) | 0·72 (0·23)a | 0·38 (0·16)a | 3·98 (0·43)ab |
| F. sachalinensis | H | 7689 (541·60)ab | 79·87 (24·22)a | 0·42 (0·08)a | 0·21 (0·01)b | 4·58 (0·11)bc |
| F.×bohemica | H | 3643 (2432)a | 21·34 (13·04) b | 0·46 (0·10)a | 0·23 (0·03)b | 4·44 (0·39)c |
| F. aubertii | H | 8318 (2616)b | 7·43 (2·98) c | 0.53 (0·10)a | 0·24 (0·04)b | 6·30 (0·62)d |
F, Male sterile flowers; H, hermaphrodite flowers.
Standard deviations are given in brackets. The same superscript letter within a column indicates no significant difference between species. Comparisons between taxa were performed using one-way ANOVA followed by the Tukey–HSD test for mean pollen/flower (square root transformation) and mean ovule length (logarithmic transformation), and using the Kruskal–Wallis test followed by the non-parametric Mann–Whitney test for mean pollen viability, mean ovule width and mean corolla diameter.
A significant difference was found for mean ovule width among taxa (Kruskal–Wallis test H=34·51, P=0·000) with male sterile F. japonica and F. sachalinensis producing significantly larger ovules than hermaphrodite clones (Table 1). Mean corolla diameter was significantly different among taxa (Kruskal–Wallis test H=45·54, P<0·0001) with hermaphrodite clones producing significantly larger corollas than male sterile clones (except for the comparisons between male-sterile and hermaphrodite F. sachalinensis) (Table 1).
The mean number of pollen grains deposited per stigma varied significantly among taxa (Kruskal–Wallis test: H=78·99, P<0·001) with a higher pollen load (8–5048 pollen grains) for F. aubertii compared with other taxa, and with a very low pollen load (0–14 pollen grains) for F. japonica being significantly different from all other taxa (Table 2).
Table 2.
Mean number of pollen grains per stigma and fruit set after free pollination of F. japonica, F. sachalinensis, F.×bohemica and F. aubertii
| Mean no. of pollen per stigma | Mean fruit set (%) | ||
|---|---|---|---|
| F. japonica | F | 0·68 (1·91)a | 0·44 (1·04)a |
| F. sachalinensis | F | 7·73 (9·35)b | 5·06 (7·27)b |
| F. sachalinensis | H | 4·67 (4·62)b | n.d. |
| F.×bohemica | H | 26·33 (37·09)b | 0·01 (0·01)c |
| F. aubertii | H | 1566 (1439)c | 19·55 (24·91)b |
F, Male sterile flowers; H, hermaphrodite flowers; n.d. not determined.
Standard deviations are given in brackets. The same superscript letter within a column indicates no significant difference between species. The Kruskal–Wallis test followed by the non-parametric Mann–Whitney test was used to test differences between species.
Mean fruit set differed significantly among taxa (Kruskal–Wallis test: H=19·06, P=0·000) with extremely low fruit set for the hybrid F.×bohemica (0–0·03 %, significantly different from all other taxa), and the highest values observed for F. aubertii (0–35 %, significantly different from all other taxa). Only an average of 0·4 % of fruit set was observed for F. japonica (0–1·75 %).
Seed rain and seed bank
Despite the very low fruit set observed for F. japonica, the estimated number of seeds produced per clone was very large due to the numerous flowers borne by each clone (range=125 348–1207 217; mean±s.d.=424 597±410 789). This corresponded to a mean density of 1974±1133 seeds m−2 (range=361–3680). However, only three seedlings emerged from the seed bank in spring and two seedlings in autumn, respectively, leading to a mean seed bank density of 34·12±92·19 seeds m−2 in the spring and 22·75±57·82 seeds m−2 in autumn. These five seedlings emerged from seeds found in the litter (two in the spring and one in the autumn).
Seed germination and viability
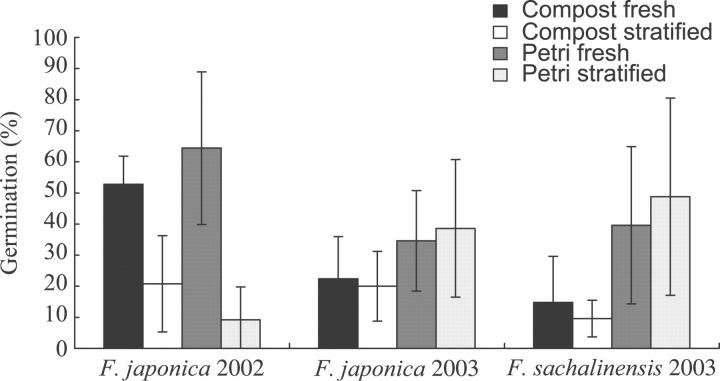
Germination percentages varied from 9·2±10·5 % to 64·4±24·5 % depending on seed lots and treatment (Fig. 1). For germinations performed on seeds collected in 2002 from F. japonica clones, germination percentages differed significantly among fresh and stratified seeds both on compost (t-test: t=4·0, P=0·004) and in Petri dishes (t-test: t=4·48, P=0·002). Germination was lowered by 60·6 % and 85·7 % after stratification on compost and Petri dishes, respectively. In contrast, for germination performed on seeds collected in 2003 from F. japonica and F. sachalinensis, no significant difference was observed among fresh and stratified seeds on either compost or in Petri dishes. When comparing mean germination percentages for each treatment among seeds collected in 2002 and 2003 from F. japonica, a significant decrease was found from 2002 to 2003 for fresh seeds both on compost (t-test: t=4·02, P=0·001) and in Petri dishes (t-test: t=2·85, P=0·014), and a significant increase for stratified seeds in Petri dishes (t-test: t=–3·19, P=0·007). When comparing germination of seeds collected in 2003, no significant difference was found for the four treatments between F. japonica and F. sachalinensis. No germination was observed for seeds collected on F. aubertii.
Fig. 1.
Mean germination percentage of seeds collected on F. japonica in 2002 and 2003 and F. sachalinensis in 2003 with and without stratification. Bars indicate the s.d. All data were arcsine-transformed.
The tetrazolium test revealed that 70·4±19·5 % of fresh seeds (mean±s.d.) collected from F. japonica and 69·0±29·7 % from the male-sterile F. sachalinensis were viable. The tetrazolium test performed on non-germinating and stratified seeds indicated that those seeds were not viable in both species. A microbiological analysis showed that germination of seeds was inhibited by the presence of Penicillium mould. The extensive monitoring of seedling survival, in the field, indicated no evidence of seedling establishment. However, survival of seedlings from the ‘compost stratified’ treatment in 2002 was (mean±s.d.) 20·8±15·5 % (range=10·0–48·0) at the end of the germination experiment and decreased to 11·2±6·1 % (range=2·0–16·0) after 135 d of growth.
Cytology
The most common observation (seven out of 11) for seeds collected from F. japonica (2n=88) consisted of seedlings with 54 chromosomes, for which putative paternal species should be F. aubertii (2n=20). Four observations consisted of seedlings for which putative paternal species should be F.×bohemica (probable chromosome number 2n=66). These seedlings were characterized by different chromosome numbers from one nucleus to the other within the individual (Table 3). These seedlings gave aneuploid ranges between 78 and 79, 77 and 86, 103 and 105, and 104 and 110. The chromosome count of one seed collected from F. sachalinensis (2n=44) showed 32 chromosomes, indicating F. aubertii (2n=20) as the putative male parent.
Table 3.
Seedling chromosome number, putative male parent and number of seedlings from seeds collected fro F. japonica and F. sachalinensis species
| Maternal species | Seedling chromosome no. | Putative male parent | Probable chromosome no. ofmale parent | Male gamete | No. of seedlings |
|---|---|---|---|---|---|
| F. japonica | 2n=54 | F. aubertii | 2n=20 | x=10 | 7/11 |
| F. japonica | 2n=103–105 | F.×bohemica | 2n=66 | x=59–61 | 1/11 |
| F. japonica | 2n=104–110 | F.×bohemica | 2n=66 | x=60–66 | 1/11 |
| F. japonica | 2n=77–86 | F.×bohemica | 2n=66 | x=33–42 | 1/11 |
| F. japonica | 2n=78–79 | F.×bohemica | 2n=66 | x=34–35 | 1/11 |
| F. sachalinensis | 2n=32 | F. aubertii | 2n=20 | x=10 | 1/1 |
Flow cytometry
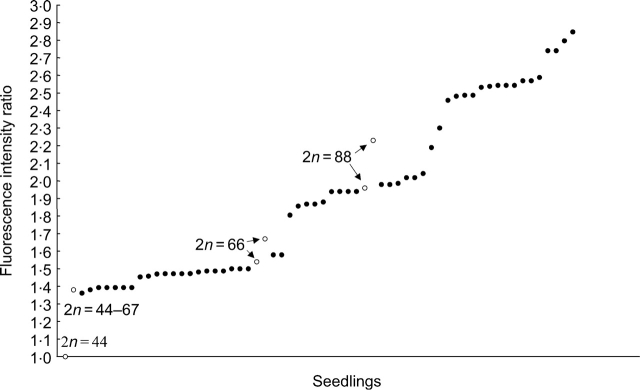
Clear discontinuities in the frequency distribution of fluorescence intensities allowed the ploidy level of the seedlings sampled to be estimated by comparison to fluorescence intensities of adult hybrids with known chromosome numbers (Fig. 2). Seven (12·7 %) seedlings exhibited peak ratios ranging from 1·36 to 1·39. This corresponded to the peak ratio of an adult hybrid with 2n varying from 44 to 66. These seedlings were most probably hexaploid hybrids with F. sachalinensis that, in addition to the normal chromosome number (2n=66) suffered from mitotic alterations. Fourteen (25·4 %) seedlings displayed peak ratios ranging from 1·46 to 1·50. This range did not fit any observation on an adult hybrid but the intermediate position of those seedlings between hybrid adults with 2n=44 and 2n=66 suggest the probability of crosses with F. aubertii (2n=20) leading to hybrids that should exhibit 2n=54. Because of the larger size of F. aubertii chromosomes (Bailey, 1989), it is expected that the fluorescence peak ratio was not directly proportional to the number of chromosomes when compared with hybrids with other parental species. A third group consisted of two observations (3·6 %) with a peak ratio of 1·58 included in the observed range for adult hexaploid hybrids (2n=66) with F. sachalinensis as paternal parent. Eventually, a fourth group with 32 observations (58·3 %) displayed peak ratios ranging from 1·81 to 2·85. These seedlings may result from different situations in which crossing with unreduced gametes are likely to play a major role: octoploid hybrids resulting from crossing with unreduced pollen of F. sachalinensis, backcross with totally to partially reduced pollen of F.×bohemica leading to chromosome numbers varying around 77, and backcross with totally to partially unreduced gametes of F.×bohemica leading to chromosome numbers varying around 110. Those last two situations were found in the present cytological observations on seedlings (see above).
Fig. 2.
Fluorescence intensity ratios for seedlings collected from F. japonica and reference adult hybrids. Closed circles are peak ratios for the different observed seedlings arranged in increasing order; open circles are minimal and maximal peak ratios observed for adult hybrids with a known chromosome number.
Seed dispersal
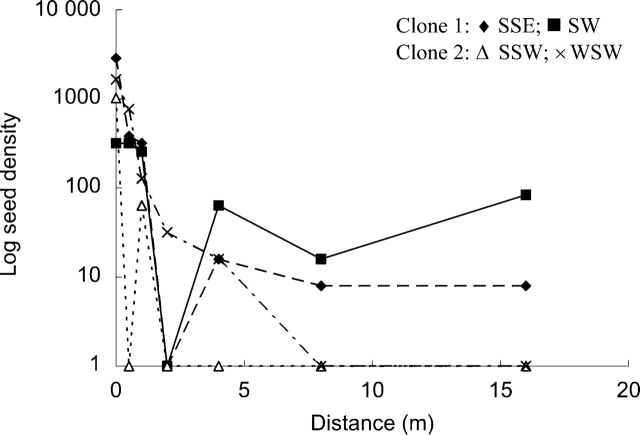
In total, 160 seeds were trapped over the four transects with 88 % at the first two positions near the clone (≤0·5 m). These results indicated that the majority of seeds fell near the maternal clone (Fig. 3). When data were pooled across transects, seed density followed a declining inverse exponential function (R2=0·478, P=0·000, slope=–1·29). However, the tail of the curve indicated a capacity for dispersal beyond 16 m with a small proportion of seeds (1·10 %; 0–21 seeds) moved at 16 m.
Fig. 3.
Observed dispersal capacity for F. japonica in a seed-trap experiment along two transects per clone.
DISCUSSION
Extensive sexual reproduction by hybridization
Vegetative regeneration is recognized as the main mode of reproduction of knotweeds outside their native range (Beerling et al., 1994; Child and Wade, 2000; Weber, 2003). However, the possibility of sexual reproduction is a crucial feature, not only because of the possibility for seed production but also because producing new genotypes can further enhance the invasive ability of knotweeds. Although extensive studies have already been conducted by Bailey et al. (1995) in the UK, no data were available until now concerning the potential for sexual reproduction in western continental Europe.
In the present study, the male sterile status of F. japonica was confirmed and, in close agreement with observations previously conducted in the British Isles (Bailey, 1994), the possibility of the restoration of sexual reproduction of F. japonica through hybridization processes in the adventive range was demonstrated. Other taxa from the Fallopia complex present in Belgium (F. sachalinensis, F. aubertii, F.×bohemica) displayed clones producing viable pollen and constituted a potential source for hybridization with F. japonica. In vitro pollen germination experiments performed by Bailey (1994) confirmed that F. japonica is capable of fertilization when presented with the pollen of other species or hybrids in the section naturalized in the British countryside. The present results, based on chromosome counts and polyploidy level assessment by flow cytometry, demonstrated that in Belgium as well, three taxa participated in hybridization with F. japonica. The most commonly adopted position in Belgium is that F. sachalinensis is the paternal species hybridizing with F. japonica (Lambinon et al., 2004). However, the present observations revealed that the majority of seeds collected from F. japonica turned out to have resulted from backcrosses with F.×bohemica and crosses with F. aubertii. In addition, it was found that two other taxa, F. sachalinensis and F. aubertii, produced seeds, a feature that may promote the invasive success of the complex in the study zone. Nevertheless, the significance of seed production in regard to invasive success may vary among taxa as germination success turned out to be different. Interspecific hybridization associated with seed production results in an intricate situation in the Fallopia complex where the role of each taxon in the invasive success of the complex may be different.
Despite its hybridization with F. japonica and its production of seed, the role of F. aubertii in the invasive success of Fallopia in the study zone is probably currently still limited. Fallopia aubertii exhibited the highest seed set but none of these seeds germinated in the germination experiment on compost. Further analyses are needed to assess the paternal origin of these seeds. A set of arguments suggest that they should also result mainly from hybridization with other taxa: (a) only a small proportion of the pollen produced by the species was viable; (b) this species has been shown to exhibit very strong self-incompatible reaction (Bailey, 1994); and (c) it was found that all the clones of the species share the same genotype (M.-S. Tiébré, unpubl. res.). Currently, seed production in F. aubertii is probably of low importance for the invasive success of the Fallopia complex. Additionally, it was found that a large proportion of seeds borne by F. japonica originated from hybridization with F. aubertii, and at least some of those seeds were viable as they germinated. This points to the potentially important role of F. aubertii in restoring sexual reproduction in F. japonica. However, these hybrids are still of low importance in the study zone as the resulting hybrid, F.×conollyana, was not detected in a large survey of adult Fallopia clones across Belgium (M.-S. Tiébré, unpubl. res.). This suggests that field conditions may be inadequate for the development of those hybrids in the study zone: climate might be a major constraint. In a study focusing on F.×conollyana, the hybrid between F. japonica and F. aubertii (F. baldschuanica), Bailey (2001, 2003) argued that seeds which give rise to F.×conollyana often contain enough endosperm for germination (leading to good viability tests) but there is frequently a gap between the pericarp and the endosperm which could enable the development of soil fungi in damp winter conditions.
The presence of F. sachalinensis was more important than generally reported for the territory (Lambinon et al., 2004). Both male-sterile and hermaphrodite stands of F. sachalinensis were detected but the hermaphrodite seemed to be much more rare (only one single male-fertile clone found). However, since male-fertile individuals seemed to be quite rare in Belgium, the potential for recurrent hybrid formation with F. japonica (or other Fallopia taxa) should not be widespread but more restricted to sympatric areas. Unfortunately, it was not possible to assess the fruit set of the hermaphrodite F. sachalinensis as the clone was destroyed before the end of the experiment. The fruit set of 5 % observed for the male-sterile clones reached the same order of magnitude as the British clones (Bailey, 1989), this species being rarely capable of self-fertilization (Bailey, 1994). It is stressed that despite a more discrete occurrence in Belgium, the influence of F. sachalinensis within the invasive knotweeds complex should not be underestimated. Due to the huge number of inflorescences per clone, F. sachalinensis is capable of producing numerous seeds. It is a donor of pollen for F. japonica generating the hybrid taxon F.×bohemica and, moreover, it is capable of vigorous vegetative regeneration mainly from stems (Bímová et al., 2003).
Fallopia×bohemica has also been considered as rare in Belgium and was only described as a hybrid of horticultural origin (Lambinon et al., 2004). However, preliminary investigations on adult clones in the study area, based on flow cytometry analysis and molecular markers, indicate that the occurrence of the hybrids has been underestimated: F.×bohemica is actually widely distributed across Belgium though its frequency varies between regions (M.-S. Tiébré, unpubl. res.). The widespread distribution of F.×bohemica in the study landscapes, together with the demonstrated production of large amount of partially viable pollen is consistent with the finding that the majority of seeds produced by F. japonica originated from backcrosses with F.×bohemica. The resulting seedlings showed a variety of chromosome numbers, most probably as a consequence of fertilization with unreduced or partially reduced pollen, due to the very irregular meiosis of the paternal hexaploid F.×bohemica with the formation of univalents, bivalents, trivalents and rare quadrivalents (Bailey and Stace, 1992; Bailey, 1994, 2003). Obviously, F.×bohemica has become a major component of the invasive success of exotic Fallopia in the study zone as it promotes the sexual reproduction of F. japonica and may restore genotypic diversity and adaptation potential in the adventive area (Hollingsworth and Bailey, 2000a). In addition, F.×bohemica has been shown to exhibit higher vegetative regeneration potential than all relating taxa in the Czech Republic (Bímová et al., 2003). In contrast, seed set of F.×bohemica was almost nil, a common feature of hybrid plants.
The fate of seeds produced by F. japonica
Seed set of F. japonica was very low, from 0 % to <2 %, a feature that should be related to the low pollination success observed (on average less than one pollen grain per stigma). Nevertheless, the very low fruit set observed in F. japonica must be balanced against the huge amount of flowers borne by each clone, leading to a very important total production of seed and potential seed rain per clone. In addition, controlled germination experiments revealed that the germination of seeds is possible even after stratification treatment. Considering the high potential for regeneration by seeds, a puzzling observation was that field observations did not indicate any successful germination or seedling establishment in the study area. Furthermore, although the majority of seeds fell near the maternal clone, a very small seed bank in spring and autumn was observed despite the extensive potential seed rain and density: only five seedlings emerged from the seed bank. As a result, the seed bank of the Japanese knotweed may be considered as inconsequential or transient (Bakker et al., 1996; Thompson et al., 1997). One hypothesis may be that seeds suffered from a high predation rate. Certainly, some seeds were eaten by birds. Bailey et al. (1995) reported that some plants in Leicester had been completely stripped of their seeds by house sparrows (Passer domesticus). Further experiments are needed to explore fully pre- and post-dispersal seed predation and the way it may affect seed bank formation. A second hypothesis may be that experimental germination conditions on compost were more favourable than in the field. In germination experiments conducted in North America, Forman and Kesseli (2003) also showed that F. japonica produced large quantities of seeds that had a high germination capacity, but in contrast to the present study, they observed many seedlings in the field. The authors argued that seedling survival is likely to be more dependent on the availability of adequate resources such as light and water, than on temperature. In the present study sites, uninvaded adjacent vegetation was dense, perennial herbaceous vegetation, which might have exerted heavy competition preventing Fallopia seedlings from growing. Bailey et al. (1995) also pointed out that edaphic and climatic factors are critical for seed germination in the first place and survival after the winter. As in 2002, it was found that stratification induced a significant reduction in germination success. It is suggested that a cold, humid winter has a negative effect on seedling survival, at least in some years. Under the assumption of global warming (Dukes and Mooney, 1999), milder winters might result in greater germination percentages. It should also be considered that germination events in the field might be rare or site-specific, and could have been undetected in the survey. The high occurrence of adult hybrids in the study area and the observation that there is high genotypic diversity in these hybrids (M.-S. Tiébré, unpubl. res.), similar to that observed in the UK (Hollingsworth et al., 1998), indicated that seedling establishment does occur in the field, albeit probably at a low percentage in comparison to the total seed rain. Further experiments are needed to assess the best conditions for hybrid seedling establishment.
The seed trap experiment showed that the majority of seed produced by F. japonica fell near the maternal clone, a general pattern of dispersion in plant species (Bullock and Clarke, 2000). However, a small proportion of seed was collected in the furthest traps indicating that seeds may be dispersed beyond 16 m, which leads to the possibility of founding a new and genetically different clone. As far as is known, this is the first assessment of dispersal capacity of hybrid seeds. Moreover, in the case of plants growing next to riverbanks, which are often observed in Belgium (Lambinon et al., 2004), there is a good chance of hybrid seeds being dispersed downstream for much greater distances, increasing the invasive potential of the complex.
Conclusions
Hybridization processes combined with polyploidy can lead to genotypic and genomic alterations, resulting in an increase in genetic diversity (Bretagnolle et al., 1998; Soltis and Soltis, 2000; Wendel, 2000; Liu and Wendel, 2003; Doyle et al., 2004; Soltis et al., 2004) and stimulating invasiveness (Ellstrand and Schierenbeck, 2000). The intensification of human activities has provided new opportunities for interspecific hybridization and the setting of new niches that suit hybrids better than their parents (Vilà et al., 2000). The restoration of sexual reproduction in F. japonica by hybridization with related taxa could generate considerable genotypic diversity, which was lacking in the adventive range (Hollingsworth et al., 1998). This may increase the ability of the Fallopia complex to adapt to new environments. Moreover, the newly formed genotypes would be fixed by extensive vegetative regeneration. The extensive sexual reproduction by hybridization observed in the present study could further contribute to the dramatic invasive success of knotweeds in Belgium and should not be underestimated, particularly in the face of global change. It adds a new dimension to the control and management measures required for these plants (Child and Wade, 2000; Pashley et al., 2003). Although extensive efforts must be devoted to control vegetative spread, attention should also be paid to preventing the formation of new clones of hybrid origin by acting before the flowering period. Moreover, it has been demonstrated that the ornamental F. aubertii, widely available for sale in Belgium, significantly outcrosses with wild clones of knotweeds. Considering the potential for hybrid adaptation to modifications in environmental conditions, it is suggested that this species should no longer be commercialized.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Belgian Science Policy and is part of the project ‘INPLANBEL: Invasive Plants in Belgium: Patterns, Processes and Monitoring’ (contract EV/11/27C). We would like to thank John Bailey for his help concerning cytological identification of our reference samples, Béatrice Lagrange for technical assistance in the field, and two anonymous reviewers for helpful suggestions. We would also like to thank the Ministry of Education and Scientific Research of the Republic of Ivory Coast for funding M. -S. Tiébré.
LITERATURE CITED
- Abbott RJ. Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends in Ecology and Evolution. 1992;7:401–405. doi: 10.1016/0169-5347(92)90020-C. [DOI] [PubMed] [Google Scholar]
- Allendorf FW, Lundquist LL. Introduction, population biology, evolution and control of invasive species. Conservation Biology. 2003;17:24–30. [Google Scholar]
- Anonyme . Etude bibliographique sur la renouée du Japan: écologie, biologie et modalité de gestion. Val d'Authie, France: C.P.I.E. Val d'Authie; 2001. [Google Scholar]
- Bailey JP. Cytology and breeding behaviour of giant alien Polygonum species in Britain. UK: University of Leicester; 1989. PhD Thesis. [Google Scholar]
- Bailey JP. Reproductive biology and fertility of Fallopia japonica (Japanese knotweed) and its hybrids in the British Isles. In: De Waal LC, Child LE, Wade PM, Brock JH, editors. Ecology and management of invasive riverside plants. Chichester: John Wiley and Sons; 1994. pp. 141–158. [Google Scholar]
- Bailey JP. Fallopia×conollyana the railway-yard knotweed. Watsonia. 2001;23:539–541. [Google Scholar]
- Bailey JP. Japanese knotweed s. l. at home and abroad. In: Child L, Brock JH, Prach K, Pyšek P, Wade PM, Williamson M, editors. Plant invasions – ecological threats and management solutions. Leiden: Backhuys Publishers; 2003. pp. 183–196. [Google Scholar]
- Bailey JP, Conolly AP. Prize-winners to pariahs – a history of Japanese knotweed s. l. (Polygonaceae) in the British Isles. Watsonia. 2000;23:93–110. [Google Scholar]
- Bailey JP, Stace CA. Chromosome number, morphology, pairing, and DNA values of species and hybrids in the genus Fallopia (Polygonaceae) Plant Systematics and Evolution. 1992;180:29–52. [Google Scholar]
- Bailey JP, Child LE, Wade M. Assessment of the genetic variation and spread of British populations of Fallopia japonica and its hybrid Fallopia×bohemica. In: Pyšek P, Prach K, Rejmánek M, Wade M, editors. Plant invasions: general aspects and special problems. Amsterdam: SPB Academic Publishing; 1995. pp. 141–150. [Google Scholar]
- Bailey JP, Child LE, Conolly AP. A survey of the distribution of Fallopia×bohemica (Chrtek and Chrtkova) J. Bailey (Polygonaceae) in the British Isles. Watsonia. 1996;21:187–198. [Google Scholar]
- Baker HG. The evolution of weeds. Annual Review of Ecology and Systematics. 1974;7:1–24. [Google Scholar]
- Bakker JP, Poschlod P, Strykstra RJ, Bekker RM, Thompson K. Seed banks and seed dispersal: important topics in restoration ecology. Acta Botanica Neerlandica. 1996;45:461–490. [Google Scholar]
- Barral V. France: Université Joseph Fourier; 1994. Biologie et biogéographie de Polygonum cuspidatum. Master Thesis. [Google Scholar]
- Beerling DJ, Bailey JP, Conolly AP. Biological flora of the British Isles. Fallopia japonica (Houtt.) Ronse Decraene. Journal of Ecology. 1994;82:959–979. [Google Scholar]
- Bímová K, Mandák B, Pyšek P. Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae) Plant Ecology. 2003;166:1–11. [Google Scholar]
- Bretagnolle F, Felber F, Calame FG, Küpfer P. La polyploïdie chez les plantes. Botanica Helvetica. 1998;108:5–37. [Google Scholar]
- Brown JS, Eckert CG. Evolutionary increase in sexual and clonal reproductive capacity during biological invasion in an aquatic plant Butomus umbellatus (Butomaceae) American Journal of Botany. 2005;92:495–502. doi: 10.3732/ajb.92.3.495. [DOI] [PubMed] [Google Scholar]
- Bullock JM, Clarke RT. Long distance seed dispersal by wind: measuring and modelling the tail of the curve. Oecologia. 2000;124:506–521. doi: 10.1007/PL00008876. [DOI] [PubMed] [Google Scholar]
- Child L, Wade M. The Japanese knotweed manual. Chichester: Packard Publishing; 2000. [Google Scholar]
- Child L, Wade M, Hathaway S. Strategic invasive plant management, linking Policy and practice: a case study of Fallopia japonica in Swansea, South Wales (U.K.) In: Brundu JBG, Camarda I, Child L, Wade M, editors. Plant invasions: species ecology and ecosystem management. Leiden: Backhuys Publishers; 2001. pp. 291–302. [Google Scholar]
- Crawley MJP, Harvey PH, Purvis A. Comparative ecology of the native and alien floras of the British Isles. Philosophical Transactions of the Royal Society of London, B. 1996;351:1251–1259. [Google Scholar]
- Daehler CC, Strong DR. Variable reproductive output among clones of Spartina alterniflora (Poaceae) including San Francisco Bay, California: the influence of herbivory, pollination and establishment rate. American Journal of Botany. 1994;81:307–313. [Google Scholar]
- Daehler CC, Strong DR. Status, prediction and prevention of introduced cordgrass Spartina spp. invasions in pacific estuaries, USA. Biological Conservation. 1996;78:51–58. [Google Scholar]
- Doyle JJ, Doyle JL, Rauscher JT, Brown AHD. Diploid and polyploid reticulate evolution throughout the history of the perennial soybeans (Glycine subgenus Glycine) New Phytologist. 2004;161:121–132. [Google Scholar]
- Dukes JS, Mooney HA. Does global change increase the success of biological invaders. Trends in Ecology and Evolution. 1999;14:135–139. doi: 10.1016/s0169-5347(98)01554-7. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Lui K, Bronson K, Corradini P, Bruneau A. Population genetic consequences of extreme variation in sexual and clonal reproduction in an aquatic plant. Molecular Ecology. 2003;12:331–334. doi: 10.1046/j.1365-294x.2003.01737.x. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridisation as a stimulus for the evolution of invasiveness in plants. Proceedings of the National Academy of Sciences of the USA. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojcik B, Tokarska-Guzik B. Reynoutria×bohemica (Polygonaceae) – nowy takson we florze Polski. Fragmenta Floristica et Geobotanica Polonica. 2000;7:63–71. [Google Scholar]
- Forman J, Kesseli RV. Sexual reproduction in the invasive species Fallopia japonica (Polygonaceae) American Journal of Botany. 2003;90:586–592. doi: 10.3732/ajb.90.4.586. [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabadi E. Rapid flow cytometry analysis of the cell cycle in plant tissue. Science. 1983;220:1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Godefroid S. A propos de l'extension spectaculaire de Fallopia japonica, F. sachalinensis, Buddleja davidii et Senecio inaequidens en Région bruxelloise. Dumotiera. 1996;63:9–16. [Google Scholar]
- Grotkopp E, Rejmánek M, Rost T. Towards a causal explanation of plant invasiveness: seedling growth and life history strategies of 29 pine (Pinus) species. American Naturalist. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- Haraldson K. Anatomy and taxonomy in Polygonaceae subfam. Polygonoideae Meisn. emend. Jaretzky. Symbolae Botanicae Upsalienses. 1978;XXII [Google Scholar]
- Hathaway S. Surveys on the spread of Japanese knotweed Fallopia japonica in Swansea and strategies for its control. Aspects of Applied Biology. 2000;28:55–62. [Google Scholar]
- Hollingsworth ML, Bailey JP. Hybridisation and clonal diversity in some introduced Fallopia species (Polygonaceae) Watsonia. 2000a;23:111–121. [Google Scholar]
- Hollingsworth ML, Bailey JP. Evidence for massive clonal growth in the invasive weed Fallopia japonica (Japanese knotweed) Botanical Journal of the Linnean Society. 2000b;133:463–472. [Google Scholar]
- Hollingsworth ML, Hollingsworth PM, Jenkins GI, Bailey JP, Ferris C. The use of molecular markers to study patterns of genotypic diversity in some invasive alien Fallopia sp. (Polygonaceae) Molecular Ecology. 1998;7:1681–1691. [Google Scholar]
- Holub J. Fallopia Adans. 1763 instead of bilderdykia Dum. Folia Geobotanica and Phytotaxonomica. 1970;6:171–177. [Google Scholar]
- Jacquemart AL, Thompson JD. Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the Upper Ardennes, Belgium. Canadian Journal of Botany. 1996;74:210–221. [Google Scholar]
- Jager C. Répartition, écologie et possibilités de contrôle de l'expansion de la renouée du Japon en Lorraine. France: Mémoire de Maîtrise, Université de Metz; 1994. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Boulder, CO: University Press of Colorado; 1993. [Google Scholar]
- Lambinon J, Delvosalle L, Duvigneaud J. Nouvelle flore de la Belgique, du grand-duché de Luxembourg, du nord de la France et des régions voisines (Ptéridophytes et Spermaphytes) Meise: Jardin Botanique National de Belgique; 2004. [Google Scholar]
- Levin DA. The origin, expansion, and demise of plant species. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Liu B, Wendel JF. Epigenetic phenomena and the evolution of plant allopolyploids. Molecular Phylogenetics and Evolution. 2003;29:365–379. doi: 10.1016/s1055-7903(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Lui K, Thompson FL, Eckert CG. Causes and consequences of extreme variation in reproductive strategy and vegetative growth among invasive populations of a clonal aquatic plant, Butomus umbellatus L. (Butomaceae) Biological Invasions. 2005;7:427–444. [Google Scholar]
- Mandák B, Pyšek P, Lysak M, Suda J, Krahulcova A, Bímová K. Variation in DNA-ploidy levels of Reynoutria taxa in the Czech Republic. Annals of Botany. 2003;92:265–272. doi: 10.1093/aob/mcg141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandák B, Pyšek P, Bímová K. History of the invasion and distribution of Reynoutria taxa in the Czech Republic: a hybrid spreading faster than its parents. Preslia Praha. 2004;76:15–64. [Google Scholar]
- Maruta E. Seedling establishment of Polygonum cuspidatum on Mount Fuji. Japanese Journal of Ecology. 1976;26:101–105. [Google Scholar]
- Muller S. Les espèces végétales invasives en France: bilan des connaissances et propositions d'action. Revue d'Ecologie-la Terre et la Vie. 2000;(Supplément 7) [Google Scholar]
- Noble IR. Attributes of invaders and the invading process: terrestrial and vascular plants. In: Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FK, Rejmánek M, Williamson M, editors. Biological invasions: a global perspective. Chichester: John Wiley and Son; 1989. pp. 301–313. [Google Scholar]
- Pashley CH, Bailey JP, Ferris C. Further evidence of the role of Dolgellau, Wales, in the production and dispersal of Japanese knotweed s. l. In: Child L, Brock JH, Brundu G, Prach K, Pyšek P, Wade MP, Williamson M, editors. Plant invasions: ecological threats and management solutions. Leiden: Backhuys Publishers; 2003. pp. 197–211. [Google Scholar]
- Pyšek P. Clonality and plant invasions: can a trait make a difference? In: de Kroon H, van Groenendael J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 405–427. [Google Scholar]
- Pyšek P, Brock JH, Bímová K, Mandák B, Jarošík V, Koukolíková I, et al. Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: the determinant of invasibility at the genotype level. American Journal of Botany. 2003;90:1487–1495. doi: 10.3732/ajb.90.10.1487. [DOI] [PubMed] [Google Scholar]
- Rejmánek M. What makes a species invasive? In: Pyšek P, Prach K, Wade M,, editors. Plant invasions: general aspects and special problems. Amsterdam: SPB Academic Publishing; 1995. pp. 3–13. [Google Scholar]
- Rejmánek M, Richardson DM. What attributes make some plant species more invasive. Ecology. 1996;77:1655–1661. [Google Scholar]
- Schnitzler A, Muller S. Ecologie et biogéographie de plantes hautement invasives en Europe: les Renouées géantes du Japon (Fallopia japonica et F. sachalinensis) Revue d'Ecologie-la Terre et la Vie. 1998;53:3–38. [Google Scholar]
- Silander JA. Microevolution in clonal plants. In: Jackson JBC, Buss LW, Cook RE, editors. Population biology and evolution of clonal organisms. New Haven, Connecticut: Yale University Press; 1985. pp. 107–152. [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Pires JC, Kovarik A, Tate JA, Mavrodiev E. Recent and recurrent polyploidy in Tragopogon (Asteraceae): cytogenetic, genomic and genetic comparisons. Biological Journal of the Linnean Society. 2004;82:485–501. [Google Scholar]
- Tanaka H. The insect visitors of Polygonum cuspidatum Sieb. and Zucc. Collecting and Breeding. 1966;28:141–143. [Google Scholar]
- Thompson K, Bakker J, Bekker R. The soil seed banks of North West Europe: methodology, density and longevity. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Vanderhoeven S, Dassonville N, Meerts P. Increased topsoil mineral nutrient concentrations under exotic invasive plants in Belgium. Plant and Soil. 2005;275:169–179. [Google Scholar]
- Verloove F. Ingeburgerde plantensoorten in Vlaanderen. Brussels. Mededeling van het Instituut voor Natuurbehoud. 2002;20 [Google Scholar]
- Vilà M, Weber E, D'Antonio CM. Conservation implications of invasion by plant hybridization. Biological Invasions. 2000;2:207–217. [Google Scholar]
- Weber E. Invasive plants in the world: a reference guide to environmental weeds. London: CABI-Publishing; 2003. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.