Abstract
Background and Aims
Plants from gypsum habitats are classified as gypsophiles and gypsovags. The former include both narrow endemics limited to small gypsum areas and regionally dominant gypsophiles growing in gypsum areas of large regions, whereas gypsovags are plants that can grow both in gypsum and non-gypsum soils. Factors controlling the distribution of gypsum plants are still not fully understood.
Methods
To assess how the different types of gypsum plants deal with the stressful conditions of gypsum substrates, comparisons were made of the leaf chemical composition of four gypsovags, five regionally dominant gypsophiles and four narrow gypsum endemics growing in two massive gypsum areas of the Iberian Peninsula.
Key Results
The chemical composition of gypsovags was clearly different from regionally dominant gypsophiles, while the chemical composition of narrow-gypsophile endemics was more similar to the chemical composition of gypsovags than to that of regionally dominant gypsophiles. Regionally dominant gypsophiles showed higher concentrations of ash, Ca, S, N, Mg P and Na, whereas gypsovags and local gypsophile endemics displayed higher concentrations of C and greater C : N ratios.
Conclusions
Such differences suggest that the three groups of gypsum plants follow diverse ecological strategies. It is suggested that regionally dominant gypsophiles might fit the ‘specialist’ model, being species specifically adapted to gypsum, whereas both gypsovags and narrow-gypsophile endemics might fit the ‘refuge’ model, being stress-tolerant species that find refuge on gypsum soils from competition. The analysis of the leaf chemical composition could be a good predictor of the degree of plants specialization to gypsum soils.
Key words: Gypsophily, gypsum-rich soils, leaf chemical composition, narrow-endemic gypsophytes, Mediterranean semi-arid environments, plant conservation, edaphic endemism
INTRODUCTION
Gypsum soils spread over 100 million ha around the world (Verheye and Boyadgiev, 1997). They are confined to arid and semi-arid climates where low precipitation prevents gypsum from being removed by leaching (Parsons, 1976). Together with the arid conditions, gypsum soils have particularly stressful physical and chemical properties for plant life. Among the adverse physical features are the presence of a hard soil surface crust, which can restrict seedling establishment (Meyer, 1986; Escudero et al., 1999, 2000b); the mechanical instability of the soil material due to its lack of plasticity, cohesion and aggregation (Bridges and Burnham, 1980); and, in certain areas, its low porosity, which might limit the penetration of plant roots (Guerrero Campo et al., 1999b). In semi-arid regions, the low water retention of massive gypsum soils leads to a high infiltration of rainwater, which increases water deficit during drought periods (Guerrero Campo et al., 1999b), although in some arid regions gypsum soils have been shown to display higher water availability during drought than adjacent soils (Meyer and García-Moya, 1989). Chemically adverse features of gypsum soils are mainly related to the intense nutritional impoverishment of the soil caused by the exchange of calcium for other ions retained in the soil complex (Meyer et al., 1992; Guerrero Campo et al., 1999b), and by the high concentration of sulfate ions, which can be toxic for plants (Duvigneaud, 1968; Ruiz et al., 2003). Such stressful conditions make gypsum soils largely unsuitable for the growth of trees (Rivas-Martínez and Costa, 1970), and thus vegetation is composed mainly of stress-tolerant sub-shrubs, some scattered shrubs, herbaceous perennials and annual plants (Parsons, 1976; Hodgson et al., 1994).
Despite gypsum soils constituting extremely adverse habitats for plant life, they give rise to one of the most conspicuous and diversified set of endemic and rare plants in arid and semi-arid regions (Johnston, 1941; Parsons, 1976; Powell and Turner, 1977; Meyer, 1986; Meyer and García-Moya, 1989; Cerrillo et al., 2002; Mota et al., 2003). Most of these plants are seriously threatened, constituting a global biodiversity conservation priority (Meyer, 1986). In spite of being among the most threatened habitats in Europe and, specifically, in the Mediterranean Basin (Gómez-Campo, 1987; European Community, 1992; Mota et al., 2003), gypsum environments have received remarkably little study (Meyer, 1986; Meyer et al., 1992; Escudero et al., 1999, 2000a, b).
Two different models have been proposed to explain the occurrence of edaphic endemics. In the ‘refuge’ model, edaphic endemics are stress-tolerant species that are not specifically adapted to the atypical soils in which they grow, but are able to tolerate the adverse and stressful conditions they impose. These species are out-competed from normal adjacent soils by dominant species and take refuge in marginal and unfertile soils, where interspecific competition is weaker (Gankin and Major, 1964). In the ‘specialist’ model, edaphic endemics are fit for the atypical soils in which they live, being more competitive on them, while becoming less competitive in normal and widely distributed habitats (Meyer, 1986).
Plants from gypsum habitats are classified as gypsophiles when they occur only in gypsum soils, and gypsovags when they can grow in gypsum soils but also in other non-gypsum soils (Duvigneaud, 1968; Meyer, 1986). Factors controlling the distribution and performance of gypsophiles and gypsovags are still not fully understood (Duvigneaud, 1968; Boukhris and Lossaint, 1970; Meyer, 1986; Escudero et al., 1999, 2000b; Romao and Escudero, 2005). We hypothesize that gypsovags might be stress-tolerant plants that can occur in gypsum soils because competition with trees and shrubs is lower and their physiological abilities enable them to counteract the pernicious limitations of these soils. Therefore, our expectation is that they might fit the ‘refuge’ model. In contrast, gypsophiles, which include two groups of plants – those extremely narrow endemics limited to small gypsum areas, and those widely distributed in most gypsum areas of large regions, i.e. regionally dominant gypsophiles (Mota et al., 2003) – might fit both models.
Some early studies on gypsum plants have reported that gypsophiles have higher concentrations of sulfur, calcium and total ash than gypsovags (Duvigneaud and Denaeyer-De Smet, 1966; Duvigneaud, 1968). Differences in leaf chemical composition may result from differences in the physiology and, ultimately, in the adaptation of plants to atypical substrates. In a recent study on congeneric serpentine and non-serpentine shrubs, serpentine species exhibited selective root-to-shoot Ca translocation and Mg exclusion mechanisms, which resulted in a greater tolerance of Ca deficiency and Mg toxicity, and higher leaf Ca : Mg ratios than their non-serpentine counterparts (O'Dell et al., 2006). Consequently, high leaf Ca : Mg ratio was linked to the ability of plants to survive on serpentine soils and served as a classificatory trait for serpentine and non-serpentine species (O'Dell et al., 2006). Similarly, many specialists of gypsum substrates have the ability to tolerate the accumulation of those elements found in excess in the soil, showing a distinctive leaf chemical composition (Duvigneaud and Denaeyer-De Smet, 1973). In the present study, use is made of those early findings to assess: (1) whether the chemical composition of gypsovags and regionally dominant gypsophiles differs; (2) whether the chemical composition of narrow-gypsophile endemics is more similar to that of gypsum specialists (i.e. regionally dominant gypsophiles) or to that of species that take refuge in gypsum soils (i.e. gypsovags); and (3) whether the variability in the chemical composition of plants growing in different gypsum outcrops may obscure the differences between gypsophiles and gypsovags.
MATERIALS AND METHODS
Species and study area
Thirteen species were selected for analysis: four gypsovags and nine gypsophiles. The latter comprised five widely distributed gypsophiles and four narrow gypsum endemics (Table 1). The distinction between the two types of gypsophiles was made according to the extent of their distribution area. Accordingly, widely distributed gypsophiles were species that showed a wide distribution range, growing in most gypsum outcrops of a large territory (the Iberian Peninsula), whereas narrow gypsum endemics were species that showed a limited number of populations, growing only in one gypsum area of the Iberian Peninsula. All study species were shrubs or sub-shrubs, which are the prevalent growth forms in gypsum outcrops (Parsons, 1976), and had a similar branch morphology and architecture.
Table 1.
Location and characteristics of study species and sites
| Species | Species typea | nb | Sampling sitesc | UTMd | Altitude (m a.s.l.) | Date of sampling | Substratume |
|---|---|---|---|---|---|---|---|
| Centaurea hyssopifolia Vahl. | LE | 4 | Chinchón (M) | VK6247 | 640 | 6-Feb-03 | G |
| Gypsophila struthium L. subsp. hispanica (Wilk.) G. López | G | 5 | Villamayor 1 (Z) | XM8820 | 300 | 3-Feb-03 | G |
| Helianthemum marifolium (L.) Mill. subsp. conquense Borja & Rivas Goday | LE | 7 | Tendilla (Cu) | WK4214 | 780 | 3-Mar-03 | G |
| Helianthemum squamatum (L.) Pers. | G | 5 | Villamayor 1 (Z) | XM8718 | 290 | 21-Jan-03 | G |
| Chinchón (M) | VK6247 | 640 | 27-Jan-03 | G | |||
| Herniaria fruticosa L. | G | C | Villamayor 1 (Z) | XM8820 | 300 | 20-Jan-03 | G |
| XM8718 | 290 | 21-Jan-03 | G | ||||
| Chinchón (M) | VK6247 | 640 | 27-Jan-03 | G | |||
| Lepidium subulatum L. | G | 5 | Villamayor 1 (Z) | XM8820 | 320 | 13-Jan-03 | G |
| 4 | Chinchón (M) | VK6247 | 640 | 27-Jan-03 | G | ||
| Linum suffruticosum L. | GV | 5 | Villamayor 1 (Z) | XM8820 | 320 | 7-Jan-03 | G |
| Santos de la Humosa (M) | VK8451 | 850 | 20-Apr-03 | Ca | |||
| Chinchón (M) | VK6247 | 640 | 10-Apr-03 | G | |||
| Ononis tridentata L. | G | 5 | Villamayor 1 (Z) | XM8820 | 300 | 20-Jan-03 | G |
| 4 | Chinchón (M) | VK6247 | 640 | 27-Jan-03 | G | ||
| Rosmarinus officinalis L. | GV | 5 | Peñaflor (Z) | XM8626 | 300 | 24-Jan-03 | G |
| Murillo Gallego (Z) | XM8582 | 460 | 23-Jan-03 | Ca | |||
| Villarejo (M) | VK7541 | 700 | 6-Feb-03 | Ca | |||
| Valdarachas (M) | VK8223 | 760 | 9-Feb-03 | G | |||
| Salvia lavandulifolia Vahl. | GV | 5 | Villamayor 1 (Z) | XM8820 | 320 | 21-Jan-03 | G |
| Villamayor 2 (Z) | XM8920 | 340 | 21-Jan-03 | G + Ca | |||
| Orés (Z) | XM6783 | 760 | 23-Jan-03 | Ca | |||
| C | Alcubierre (Hu) | YM0732 | 560 | 22-Jan-03 | G + Ca | ||
| 5 | Villarejo (M) | VK7541 | 700 | 6-Feb-03 | Ca | ||
| Chinchón (M) | VK8223 | 760 | 27-Jan-03 | G | |||
| Teucrium polium L. subsp. capitatum (L.) Arcangelli | GV | 3 | Villarejo (M) | VK7541 | 700 | 6-Feb-03 | Ca |
| 5 | Chinchón (M) | VK6247 | 640 | 6-Feb-03 | G | ||
| Teucrium pumilum L. | LE | 6 | Chinchón (M) | VK6247 | 640 | G | |
| Thymus lacaitae Pau | LE. | 5 | Alcalá de Henares (M) | VK8282 | 710 | 28-Jan-03 | G |
aLE = Local endemism; G = gypsophile; GV = gypsovag.
bn = number of sampled individuals; C = composite sample.
cM = Madrid (central Spain, Middle Tajo Basin); Cu = Cuenca (central Spain, Middle Tajo Basin); Z = Zaragoza (north-east Spain, Middle Ebro Basin) Hu = Huesca (north-east Spain, Middle Ebro Basin).
dUTM = Universal Transverse Mercator coordinates.
eType of substratum: G = gypsum soil; Ca = calcareous soil; G + Ca = calcareous soils with small amounts of gypsum.
Two different areas located in different biogeographical provinces of the Iberian Peninsula and more than 350 km apart were selected for study: central Spain (Middle Tajo Basin, near Madrid) and north-east Spain (Middle Ebro Basin, near Zaragoza). In both regions, samples were taken of gypsophiles growing in massive gypsum soils and gypsovags growing both in gypsum soils and calcareous soils. Populations were located as close as possible within each region to minimize environmental variability. The gypsophile Gypsophila struthium subsp. hispanica was collected only near Zaragoza, and the gypsovag Teucrium polium subsp. capitatum was sampled only near Madrid. Narrow gypsum endemics were collected exclusively in central Spain, as such types of endemics are much more frequent in this area than in the Middle Ebro Basin (Mota et al., 1998). Study species and sampling sites are shown in Table 1, while climatic and edaphic features of each sampling site are given in Table 2.
Table 2.
Climatic and edaphic characteristics of study sites
| Study sites | Areaa | Climate |
Soil |
|||||
|---|---|---|---|---|---|---|---|---|
| T (° C) | P (mm) | pH | SOM (%) | N (%) | CaCO3 (%) | Gypsum (%) | ||
| Villamayor 1 (Z) | Z | 14·2 | 370 | 7·7 | 1·9 | 0·14 | 20·0 | 55·5 |
| Villamayor 2 (Z) | Z | 14·2 | 370 | 7·7 | 4·7 | 0·25 | 44·8 | 2·0 |
| Murillo de Gállego (Z) | Z | 12·8 | 680 | 7·6 | 3·9 | 0·13 | 38·4 | 0·0 |
| Orés (Z) | Z | 12·4 | 780 | 8·5 | 3·2 | 0·13 | 32·2 | 0·0 |
| Alcalá de Henares (M) | M | 13·2 | 350 | 7·8 | 1·8 | 0·15 | 20·6 | 32·0 |
| Chinchón (M) | M | 13·8 | 422 | 7·7 | 2·1 | 0·12 | 9·7 | 59·3 |
| Santos de la Humosa (M) | M | 13·0 | 450 | 8·3 | 5·2 | 0·22 | 38·8 | 0·0 |
| Villarejo (M) | M | 13·5 | 445 | 8·2 | 4·7 | 0·18 | 35·4 | 0·0 |
| Valdarachas (M) | M | 13·3 | 440 | 7·9 | 2·8 | 0·11 | 19·3 | 40·4 |
| Tendilla (Cu) | M | 13·0 | 410 | 7·8 | 2·4 | 0·17 | 22·3 | 29·3 |
T = Mean annual temperature; P = total annual rainfall; SOM = soil organic matter; N = nitrogen.
aM = Madrid (central Spain, Middle Tajo Basin); Z = Zaragoza (north-east Spain, Middle Ebro Basin).
Sampling of plant material
The species considered here bear two types of shoots, long and short, i.e. dolichoblasts and brachyblasts, respectively, which have different types of leaves (Orshan, 1972; Margaris, 1981). To obtain leaf samples that are comparable between different species it is important to avoid such leaf heterogeneity, which may affect leaf nutrient concentrations (Alonso and Herrera, 2001). To overcome such variability, only a single leaf type common to all selected species was sampled, namely the mature leaves of brachyblasts. Leaf phenological stage is also an important factor affecting the chemical composition of leaves (Aerts and Chapin, 2000); hence to obtain comparable samples it is necessary to collect leaves in similar phenological stages. In plants from Mediterranean semi-arid environments, brachyblasts start developing in spring and grow steadily until summer, when they arrest their elongation. Brachyblast growth resumes in autumn and stops again with the arrival of winter cold (Orshan, 1989; Palacio and Montserrat-Martí, 2005). In these species, brachyblast nutrient concentrations increase during autumn, but remain quite stable during winter (Palacio et al., 2006). Thus, both the growth and the chemical composition of brachyblast are steady during winter. For this reason, all plant material was collected between January and early February. In order to minimize other known sources of variation, such as topography (Guerrero Campo et al., 1999a) and age (Aerts and Chapin, 2000), only mature individuals in similar positions within gypsum landscapes were collected, excluding piedmonds, summits and other atypical sites where the study species are rarer.
Three to five adult individuals in each studied population were harvested (Table 1). Once in the laboratory, a 15-g sample of the brachyblast leaves of each individual was collected and oven-dried to a constant weight at 60 °C. Dry and damaged leaves were excluded from the analyses. For Herniaria fruticosa and Salvia lavandulifolia (in the Sierra de Alcubierre, Middle Ebro Basin) we could only obtain a single composite sample per population, as the small leaf biomass of the plant individuals prevented the collection of one 15-g leaf sample per individual (Table 1).
Chemical analyses
Samples were ground in a mill (IKA MF10, IKA-Werke, Staufen, Denmark) to a fine powder. N and C concentrations were analysed with an elemental analyzer (Elementar VarioMax N/CN, Hanau, Germany). Subsamples were burnt at 550 °C for 4 h and ash was dissolved in HNO3–HCl–H2O (1 : 3 : 9) and filtered. Concentrations of Na and K were measured in the soluble (silica-free) ash by flame photometry, Ca and Mg concentrations were determined by complexometry (Allen, 1989) and P concentration was assessed by vanado-molybdate colorimetry (Becker, 1961). Total sulfur was analysed using a turbidimetric method with barium chloride (Allen, 1989).
Statistical analyses
Constrained ordinations were used to examine the multivariate relationships between the nutrient content of each individual and a complete set of classificatory dummy variables (Legendre and Anderson, 1999). The evaluation of the effect of a set of classificatory variables on this multivariate data set can be viewed as a problem of covariation. This type of covariation can efficiently be approached by means of constrained ordinations considering the dummy variables as variables upon which statistical analyses are performed. As suggested by McCune (1997), these techniques (ter Braak, 1986; ter Braak and Prentice, 1988) can be used as tools for hypothesis testing (Borcard et al., 1992). Classificatory predictors were the type of soil (gysum/calcareous), the region of origin (Madrid/Zaragoza), the type of gypsum plant (gypsophile/gypsovag) and, finally, the distribution range (wide/narrow). A nutrient-content data matrix was constructed with all individual plants and their chemical content (see Appendix). The null hypothesis was that the influence of these classificatory variables on the multivariate nutrient-content data matrix was not significantly different from random. This means that variables are not able to explain significant fractions of the total variation in the nutrient matrix. With this in mind, a Detrended Correspondence Analysis (Hill and Gauch, 1980) was conducted with the complete nutrient-content data set by detrending by segments and non-linear rescaling of the axes, which has the property that the extracted axes are scaled in units of average standard deviation (Gauch, 1982). This technique is exclusively used for measuring the length of the extracted axes. Values of the extracted gradients above 3 s.d. units suggest the use of techniques assuming unimodal responses, such as Canonical Correspondence Analysis (CCA) or other related techniques (ter Braak, 1986; Legendre and Anderson, 1999). Since the extracted gradients of this data set were relatively short (s.d.<2), we conducted a Redundancy Analysis (hereafter RDA), which is a constraining ordination technique that assumes linear responses of the species with the extracted axes. Total variation explained (TVE) by the classificatory variables was calculated as the sum of all extracted canonical axes (Borcard et al., 1992). A Monte Carlo permutation test (1000 randomizations) was performed to determine the accuracy of the relationship between the two data sets, using the sum of all canonical eigenvalues or trace to build the F-ratio statistic (ter Braak, 1990; Verdonschot and ter Braak, 1994; Legendre and Anderson, 1999).
If the RDA model with all the dummy predictors and selected interactions was significant, a forward stepwise procedure was carried out to select a reduced model including only significant variables. Explanatory variables were incorporated one at a time and step by step in the order of their decreasing eigenvalues after eliminating the variation accounted for by the already-included variables. The process stopped when the new variable was not significant (P > 0·01 after being adjusted for multiple comparison with the Holm's method; Legendre and Legendre, 1998). Improvement of the reduced model with each new selected variable was determined by a Monte Carlo permutation test with 1000 randomizations.
Up to four independent ordinations were conducted with every group of dummy variables in order to know whether each group of variables was able to explain significant fractions of variation. When the RDA model with the gypsum plant type was conducted, the interaction between wide distribution and gypsophile was included to account for the variability within gypsophiles. A variance partitioning was performed with RDA to evaluate the relative importance of some predictors after adjusting the variability of other variables. This last data set was considered as a covariable data set (Borcard et al., 1992). This procedure has been called partial RDA because it determines the variation explained by the explanatory variables after removing the variation accounted for by the covariable data set. More specifically, we wanted to know if the remaining information after adjusting our main data set to the covariable data set could be significantly explained by the resulting matrix. If not, both explanatory data sets were concomitant. All multivariate analyses were conducted with CANOCO for Windows v. 4·5 (ter Braak and Smilauer, 1997).
Generalized linear models (McCullagh and Nelder, 1989) were constructed for nutrient concentration of gypsovags using S-PLUS. GLMs allow the handling of larger distribution types for the response variable than standard linear regressions. Type of soil (1. d.f.), site location (1 d.f.) and the corresponding interaction were included as fixed variables. Chi-square tests were conducted to evaluate whether or not selected predictors explained a significant fraction of the total variance (Guisan et al., 2002).
Some populations of Salvia lavandulifolia occured in soils that were calcareous but slightly covered with gypsum materials. Individuals from these sites were included in our RDA models as ‘1’ for both dummy soil variables, ‘Calcareous soil’ and ‘Gypsum’. In the corresponding GLMs, these plants were not included. Only five plants per site and soil (genuine gypsum and calcareous soils) were considered.
RESULTS
All the classificatory variables considered, i.e. type of soil (gysum/calcareous), region of origin (Madrid/Zaragoza), type of gypsum plant (gypsophile/gypsovag), distribution range (wide/narrow) and the interaction between type of gypsum plant and distribution range (gypsophile wide/gypsophile narrow), had a significant effect on the multivariate nutrient-content data matrix when analysed together (F = 26·051, P = 0·002) and accounted for 51·2 % of the total variance explained (TVE). Nevertheless, only ‘gypsophile wide’ and ‘calcareous soil’ were selected as significant explanatory variables (P = 0·002 and 0·03, respectively, after the F-ratio test) when a forward stepwise procedure was conducted (Fig. 1). Altogether, these two variables explained 49 % of the TVE, which corresponds to 98 % of the variance explained by the saturated RDA model. However, of the two significant variables, ‘gypsophile wide’ explained most of the variability whereas ‘calcareous soil’ explained a small but significant fraction of the TVE.
Fig. 1.
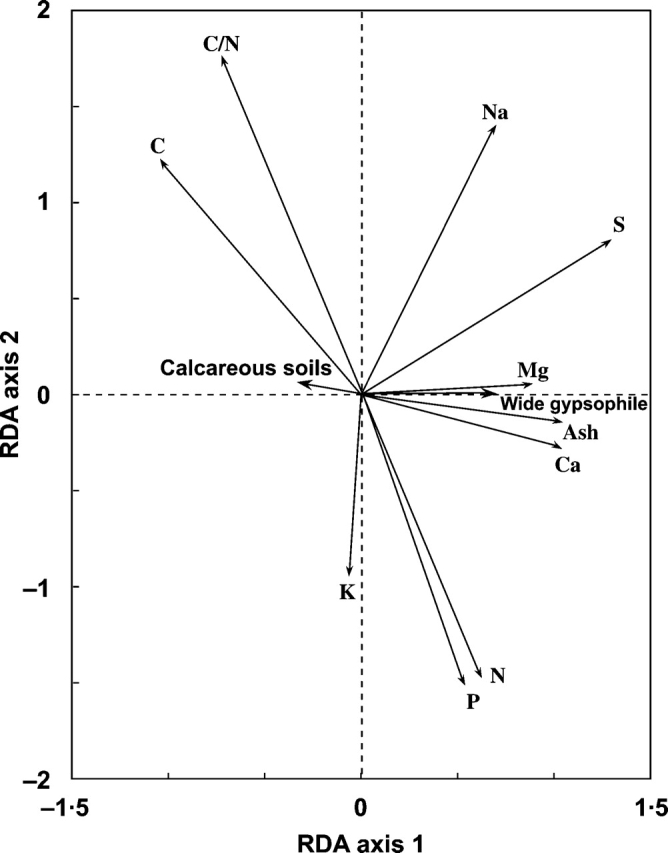
Biplot for the RDA model with all the dummy predictors and selected interactions. Only ‘gypsophile wide’ and ‘calcareous soils’ are represented as they were the only factors selected as significant explanatory variables after a forward stepwise procedure. P = 0·002 and 0·03, for ‘gypsophile wide’ and ‘calcareous soils’, respectively after an F-ratio test. See text for further details of calculations.
The analysis of the effect of the gypsum plant type (i.e. gypsovags, wide gypsophiles and narrow gypsophiles) separately from the rest of classificatory variables, showed that: (1) the chemical composition of gypsovags was clearly different from that of regionally dominant gypsophiles (wide gypsophiles; Fig. 2); and (2) the chemical composition of local gypsophile endemics (narrow gypsophiles) was more similar to the chemical composition of gypsovags than to the composition of regionally dominant gypsophiles. This model was also highly significant (F = 64·102, P = 0·002, TVE 50·2 %). Regionally dominant gypsophiles showed higher concentrations of ash, calcium and sulfur, while gypsovags and local gypsophile endemics displayed higher concentrations of carbon and greater C : N ratios, as shown in the corresponding biplots. Regionally dominant gypsophiles also tended to show higher N, Mg, P and Na concentrations than gypsovags (Fig. 2).
Fig. 2.
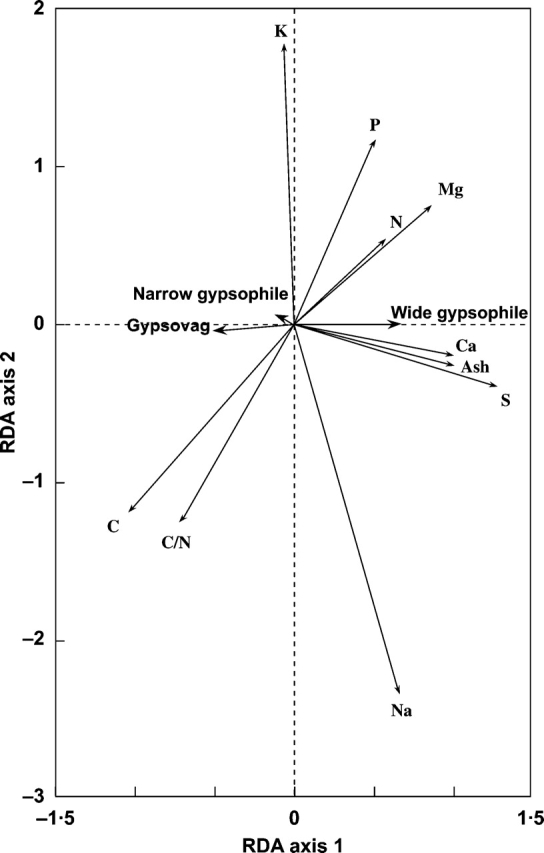
Biplot for the RDA model analysing the effect of the ‘gypsum plant type’ separately from the rest of classificatory variables. P = 0·002, F = 64·102, TVE = 50·2%. See text for further details of calculations.
Soil type also explained a significant fraction of the TVE when analysed separately from the rest of the dummy variables (TVE = 10·8 %, F = 7·719, P = 0·002; Fig. 3). Nevertheless, such effects disappeared when a partial RDA analysis was carried out and the variability explained by the gypsum plant type was removed (F = 0·800, P = 0·578). Finally, the type of origin and the type of distribution had no significant effect on the chemical composition of the study species when analysed separately from the rest of the dummy variables (F = 2·954, P = 0·055 and F = 2·456, P = 0·068, for the type of origin and the type of distribution, respectively).
Fig. 3.
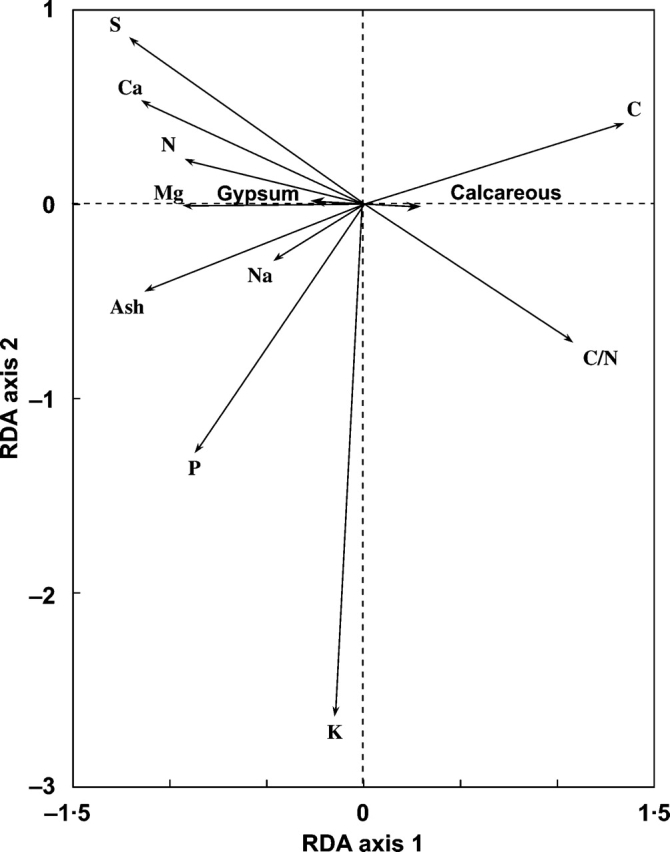
Biplot for the RDA model analysing the effect of the ‘type of soil’ separately from the rest of classificatory variables. P = 0·002, F = 7·718, TVE = 10·8%. See text for further details of calculations.
The four studied species of gypsovags showed different behaviour when growing on gypsum and calcareous soils (Tables 3 and 4). Thus, while no significant differences were found in the nutrient concentrations of plants of Teucrium polium subsp. capitatum growing on both soil types, the remaining species displayed different nutrient concentrations depending on the type of substrate they were growing on (Table 4). For example, concentrations of Mg where significantly higher in plants of Salvia lavandulifolia, Linum suffruticosum and Rosmarinus officinalis growing on gypsum soils. However, even within those species of gypsovags that changed their nutrient concentrations depending on the type of soil, metabolic strategies were diverse. Hence, whereas S concentrations were higher in plants of S. lavandulifolia and L. suffruticosum growing on gypsum soils, plants of R. officinalis had similar S concentrations irrespective of the type of soil on which they were growing (Tables 3 and 4).
Table 3.
Gaussian models for the two gypsovags occurring in gypsum and calcareous soils at both sites. The P-value of the corresponding analysis of deviance and the mean value of each variable in each class are indicated
| Variable | Salvia lavandulifolia |
Rosmarinus officinalis |
||||
|---|---|---|---|---|---|---|
| Gy/Caa | Siteb | Interaction termc | Gy/Caa | Siteb | Interaction termc | |
| Ash | 0·037 | ns | ns | 0·007 | ns | ns |
| Ca = 6·36; Gy = 7·23 | Ca = 5·43; Gy = 6·04 | |||||
| Ca | 0·001 | ns | ns | 0·019 | ns | 0·020 |
| Ca = 1·50; Gy = 1·94 | Ca = 1·20; Gy = 1·43 | Z-Ca = 1·17; Z-Gy = 1·64; M-Ca = 1·22; M-Gy = 1·22 | ||||
| C | 0·001 | 0·001 | ns | 0·032 | ns | 0·011 |
| Ca = 56·37; Gy = 54·94 | Z = 54·7; M = 56·6 | Ca = 56·35; Gy = 55·96 | Z-Ca = 56·21; Z-Gy = 56·30; M-Ca = 56·49; M-Gy = 55·62 | |||
| C/N | 0·0001 | ns | ns | ns | ns | ns |
| Ca = 37·53; Gy = 31·31 | ||||||
| Mg | 0·003 | 0·003 | 0·003 | 0·001 | ns | ns |
| Ca = 3·03; Gy = 3·28 | Z = 2·34; M = 3·97 | Z-Ca = 3·04; Z-Gy = 1·64; M-Ca = 3·01; M-Gy = 4·92 | Ca = 1·69; Gy = 2·77 | |||
| N | 0·0001 | ns | ns | ns | ns | ns |
| Ca = 15·08; Gy = 17·64 | ||||||
| Na | ns | 0·0001 | ns | ns | ns | ns |
| Z = 0·52; M = 0·35 | ||||||
| P | 0·006 | 0·003 | 0·003 | 0·004 | ns | ns |
| Ca = 0·85; Gy = 1·03 | Z = 1·03; M = 0·85 | Z-Ca = 0·85; Z-Gy = 1·21; M-Ca = 0·85; M-Gy = 0·84 | Ca = 0·85; Gy = 0·68 | |||
| K | ns | 0·005 | ns | 0·017 | ns | ns |
| Z = 6·80; M = 4·22 | Ca = 10·04; Gy = 8·05 | |||||
| S | 0·001 | ns | ns | ns | ns | ns |
| Ca = 0·05; Gy = 0·11 | ||||||
aCa = calcareous soil, Gy = gypsum soil.
bM = Madrid (central Spain, Middle Tajo Basin); Z = Zaragoza (north-east Spain, Middle Ebro Basin).
cZ-Ca = calcareous soils in Zaragoza; Z-Gy = gypsum soils in Zaragoza; Ca-M = calcareous soils in Madrid; Gy-M = gypsum soils in Madrid.
ns indicates that predictors do not explain a significant fraction of deviance. See text for details on statistics. n = 5.
Table 4.
Gaussian models for the gypsovags collected from both gypsum and calcareous soils exclusively in Madrid. The P-value of the corresponding analysis of deviance and the mean value of each variable in each class are indicated
| Variable | Teucrium polium subsp. capitatum | Linum suffruticosuma |
|---|---|---|
| Ash | ns | ns |
| Ca | ns | ns |
| C | ns | ns |
| C/N | ns | 0·001 |
| Ca = 17·84; Gy = 16·39 | ||
| Mg | ns | 0·001 |
| Ca = 2·71; Gy = 2·63 | ||
| N | ns | 0·0004 |
| Ca = 24·43; Gy = 27·9 | ||
| Na | ns | ns |
| P | ns | 0·004 |
| Ca = 1·44; Gy = 1·66 | ||
| K | ns | ns |
| S | ns | 0·0032 |
| Ca = 0·06; Gy = 0·07 |
aCa = calcareous soils; Gy = Gypsum soils.
ns indicates that predictors do not explain a significant fraction of deviance. See text for details on statistics. n = 5.
DISCUSSION
It is widely known that harsh and unusual soils constitute important havens of richness of native species and endemics (Anderson et al., 1999). Despite a great amount of work that has been devoted to serpentine endemics (Kruckeberg, 1992; Harrison et al., 2006a, b), knowledge about the factors controlling the existence of other edaphic specialists remains significantly lower. In a recent work, we showed that the physical properties of gypsum soils – i.e. the need for seedlings to be able to surpass the hard gypsum surface crust after germination – may explain, at least partially, the different distribution of gypsophiles and gypsovags (Romao and Escudero, 2005). However, widely distributed gypsophiles, such as Helianthemum squamatum, showed a better performance after emergence on gypsum than on normal soils, which also suggests that the chemical characteristics of gypsum soils may play an important role in the distribution of these species (Romao and Escudero, 2005). The results reported here help complete our understanding of gypsophily, especially on the chemical adaptation and flexibility of gypsum plants.
Different chemical compositions of regionally dominant gypsophiles and gypsovags
Our results agree with the observations of Duvigneaud and Denaeyer-De Smet (Duvigneaud and Denaeyer-De Smet, 1966, 1973; Duvigneaud, 1968), suggesting that gypsophiles and gypsovags can be statistically separated in terms of the chemical composition of their leaves. According to our results, regionally dominant gypsophiles show greater S, Ca, Mg, N, P and ash concentrations whereas gypsovags display greater C concentrations and C : N ratios. These differences were constant across the different provenances of plant samples. These results suggest that regionally dominant gypsophiles and gypsovags show distinct physiological adaptations to counteract the atypical chemical composition of gypsum soils. Accordingly, regionally dominant gypsophiles seem to selectively accumulate nutrients such as N and P, which are scarce on gypsum soils (Guerrero Campo et al., 1999b), whereas gypsovags show normal N and P concentrations. Indeed, some regionally dominant gypsophiles such as Lepidium subulatum showed a N concentration (50–52 mg g−1) up to 3-fold that of other Mediterranean woody species such as Olea europaea (15–25 mg g−1), while the leaf N concentrations of gypsovags were within the ranges reported for fruit cultivars and forest trees (Jones et al., 1991). In addition, regionally dominant gypsophiles seem to show a greater tolerance for the accumulation of Mg and S – elements found in excess in gypsum soils that can reduce the growth of non-gypsum species (Verheye and Boyadgiev, 1997). For example, the concentrations of S (49 mg g−1) and Mg (24–13 mg g−1) in the regionally dominant gypsophile Ononis tridentata were up to 10-fold those of common fruit trees and shrubs such as Malus sp. (2–4 mg g−1) or Vaccinium corymbosum (2–4 mg g−1; Jones et al., 1991). Similarly, previous studies have reported high S (Ruiz et al., 2003) and N (Alvarado et al., 2000) concentrations in regionally dominant gypsophiles from the Iberian Peninsula such as L. subulatum, Gypsophila struthium subsp. hispanica and H. squamatum, although, in accordance with our results, H. squamatum showed relatively lower concentrations of N. Nevertheless, in an analysis of the chemical composition of gypsum plants in Tunisia, Boukhris and Lossaint (1970, 1975) found that although most gypsophiles showed higher concentrations of sulfur than gypsovags, some gypsophiles had sulfur concentrations similar to those of non-gypsum plants. One possible explanation for these results might be the existence of contrasting metabolic strategies within gypsophiles, as suggested by the authors, or the inclusion of narrow endemics in the set of gypsophile species analysed (see below).
The physiological differences between gypsophiles and gypsovags might entail different ecological strategies in both groups of species. In particular, regionally dominant gypsophiles seem to fit the ‘specialist’ model, being species specifically adapted to gypsum substrates that accumulate in their leaves those elements found in excess in gypsum soils, while gypsovags seem to be stress-tolerant species fitting the ‘refuge’ model. Indeed, the greater C : N ratio of gypsovags seems to be indicative of their stress-tolerant nature as compared to the more competitive regionally dominant gypsophiles (Grime, 2001).
Our results demonstrate that gypsovags exhibit different strategies to cope with the atypical chemical composition of gypsum soils. Among the gypsovags studied, T. capitatum followed an exclusion strategy, avoiding the accumulation of toxic elements in its leaves and hence displaying similar leaf chemical compositions in gypsum and non-gypsum soils (Duvigneaud and Denaeyer-De Smet, 1973). On contrast, S. lavandulifolia and L. suffruticosum showed a certain tolerance to the accumulation of toxic elements such as Mg or S in their leaves, whereas R. officinalis followed a mixed strategy, accumulating some toxic elements (Mg) and excluding others (S). These results are in agreement with previous observations by Duvigneau and Denaeyer-de Smet on similar gypsum outcrops from north-east and central Spain (Duvigneaud and Denaeyer-De Smet, 1966, 1973; Duvigneaud, 1968).
Chemical composition of dominant gypsophiles, narrow-gypsophile endemics and gypsovags
According to the results, the narrow-gypsophile endemics studied here seem to follow a rather similar physiological strategy to gypsovags, showing a poor ability to counteract the low availability of N and P and the high S, Ca and Mg concentrations of gypsum soils. Therefore, it is suggested that these species also fit the ‘refuge’ model. Their restriction to gypsum soils could then be related to their low competitive ability linked to their nature as stress-tolerant plants, and not to their special adaptation to gypsum soils. According to this hypothesis, harsh and unusual soils might serve as havens of rare, low-competitive and highly stress-tolerant plants, sheltering them from competition with trees, shrubs and grass species, which perform better in more productive soils (Gankin and Major, 1964). Further studies assessing the competitive ability and stress tolerance of this type of gypsum species are needed to verify such a hypothesis.
Iberian forests reached a maximum expansion in the climatic optimum of the Atlantic period, dated approx. 8000–5000 BP (years before present; Carrión and Dupré, 1996). During this period, gypsum outcrops could have served as important refuges for stress-tolerant species of open areas, providing places away from the competition of trees and tall shrubs. The adverse physical and chemical features of gypsum outcrops could have impeded the development of forest communities in these areas (Guerrero Campo et al., 1999a, b). The distribution range and regional abundance of narrow-gypsophile endemics could have been further affected by a human presence since the Neolitic period, which had an extreme impact on the vegetation of the Iberian Peninsula (Burjachs et al., 1997). Stress-tolerant species have low growth and organ turnover rates, which makes them highly vulnerable to severe disturbances (Grime, 2001). Human impact has been particularly intense in the Ebro Depression since the establishment of Ibero-Roman populations (2500 BP; Peña et al., 2001), which might have accounted for the much lower occurrence of narrow-gypsophile endemics in this area as compared with central Spain.
The results of this study seem to indicate the different ecological strategies of gypsum species, and hence might have important implications for their conservation and management. If narrow-gypsophile endemics are stress-tolerant refugee species on gypsum soils, their conservation could be favoured by the reduction of disturbances such as ploughing, off-road traffic, overgrazing and trampling (Nelson and Harper, 1991; Guerra et al., 1995), and by avoiding the re-afforestation and the expansion of forest and dense shrub communities into these areas. In contrast, regionally dominant gypsophiles are competitive on gypsum soils and some species, such as L. subulatum and H. squamatum, even show a great ability to survive disturbances (Braun-Blanquet and Bolòs, 1957; Mota et al., 2003).
The different chemical composition of gypsovags and regionally dominant gypsophiles suggests that leaf chemistry is a useful tool to distinguish species specifically adapted to gypsum soils from those that take refuge in gypsum soils. Leaf chemical composition seems to be informative of the specialization of plants to gypsum substrates. However, further information on the physiological mechanisms that cause the distinct chemical composition of regionally dominant gypsophiles may be crucial for the understanding of their adaptation to gypsum soils. In addition, more information on the stress tolerance and competitive ability of the different types of gypsum plants would be required to test the hypotheses proposed in this study. In this regard, basic experiments with different types of soils and/or competition levels, and the quantification of some key functional traits of gypsum plants such as maximum relative growth rate, specific leaf area or leaf N concentration (Reich et al., 1992; Wilson et al., 1999), would be particularly useful.
CONCLUSIONS
Gypsum plants show differences in their leaf chemical composition that are suggestive of their different ecological strategies. Regionally dominant gypsophiles seem to be specifically adapted to gypsum soils whereas gypsovags seem to be stress-tolerant species that follow diverse strategies to counteract the chemical constraints of gypsum soils. The restriction of narrow-gypsophile endemics to gypsum soils could be due to their nature as stress-tolerant plants and not to their special adaptation to gypsum soils. The procedure used here seems to be a useful tool to identify plants' specialization to gypsum soils, provided comparable organs in similar phenological and developmental stages are considered.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Patricia Fustero and Adela Lamana for their assistance with plant sampling and chemical analyses, and José M. García Ruíz for his comments on earlier versions of the manuscript. R.M. and S.P. were funded by a Post-doctoral and a FPU fellowship, respectively (SEUI – MEC, Spain). This study was supported by the MEC (Spanish Government; research project RTA 2005–00100), the MCT (Spanish Government; research projects CGL2006–11619/HID, CGL2004–04919-C02-01/HID, REN2003–08678/HID) and by REMEDINAL, a research network financed by the Autonomous Government of Madrid.
APPENDIX
Chemical composition of study species. Species in bold are gypsovags. Mean values (and s.d.) are given
| Species | Soila | Siteb | Ca (%) | C (%) | C/N | Mg (mg g−1) | N (mg g−1) | Na (mg g−1) | P (mg g−1) | K (mg g−1) | S (%) | Ash (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Centaurea hyssopifolia | G (n = 4) | M | 2·9 (0·7) | 44·7 (0·9) | 11·1 (0·7) | 4·9 (1·3) | 40·2 (2·3) | 0·6 (0·03) | 2·7 (0·3) | 23·3 (1·5) | 0·8 (0·2) | 14·47 (1·29) |
| Gypsophila struthium subsp. hispanica | G (n = 5) | Z | 7·4 (0·5) | 37·0 (0·7) | 14·9 (0·79) | 12·1 (2·3) | 24·9 (1·3) | 0·6 (0·04) | 1·9 (0·2) | 11·8 (1·6) | 3·0 (0·5) | 26·67 (1·50) |
| Helianthemum marifolium subsp. conquense | G (n = 7) | M | 1·9 (0·1) | 49·1 (0·3) | 29·4 (2·4) | 2·6 (0·7) | 16·8 (1·5) | 0·3 (0·2) | 1·1 (0·2) | 3·9 (0·5) | 0·1 (0·03) | 6·49 (0·24) |
| Helianthemum squamatum | G (n = 7) | Z | 3·4 (0·3) | 43·7 (0·4) | 26·7 (4·7) | 6·1 (1·8) | 16·8 (2·7) | 0·8 (0·2) | 1·2 (0·3) | 6·4 (1·6) | 3·0 (0·2) | 14·1 (0·9) |
| G (n = 5) | M | 3·5 (0·5) | 43·4 (0·9) | 27·8 (2·9) | 7·6 (2·1) | 15·7 (1·7) | 0·6 (0·1) | 1·1 (0·3) | 5·6 (0·7) | 2·6 (0·2) | 15·0 (0·9) | |
| Herniaria fruticosa | G (n = 2) | Z | 2·9 (0·1) | 47·4 (0·3) | 18·8 (1·4) | 7·7 (0·6) | 25·3 (1·7) | 0·5 (0·04) | 1·1 (0·1) | 9·2 (1·2) | 1·1 (0·1) | 12·2 (0·04) |
| Lepidium subulatum | G (n = 5) | Z | 2·7 (0·2) | 44·9 (0·1) | 9·0 (0·8) | 2·0 (1·3) | 50·0 (4·6) | 0·6 (0·1) | 2·5 (0·1) | 13·2 (2·2) | 2·7 (1·1) | 12·8 (0·5) |
| G (n = 4) | M | 2·7 (0·3) | 45·4 (0·6) | 8·7 (0·4) | 5·5 (1·1) | 52·3 (3·0) | 0·5 (0·03) | 2·5 (0·2) | 6·3 (0·5) | 1·9 (0·1) | 12·2 (0·9) | |
| Linum suffruticosum | G (n = 5) | M | 2·4 (0·3) | 44·2 (0·5) | 13·5 (0·5) | 2·2 (0·5) | 32·9 (1·5) | 0·6 (0·1) | 1·9 (0·1) | 11·7 (2·7) | 0·02 (0·01) | 12·3 (0·9) |
| G (n = 5) | Z | 2·9 (0·4) | 44·2 (0·9) | 19·3 (1·8) | 2·7 (0·8) | 23·1 (2·2) | 0·6 (0·1) | 1·5 (0·3) | 6·6 (2·5) | 0·1 (0·04) | 14·4 (1·4) | |
| C (n = 5) | M | 2·7 (0·3) | 43·1 (1·2) | 17·8 (2·0) | 4·0 (0·7) | 24·4 (2·9) | 0·6 (0·1) | 1·4 (0·2) | 8·5 (2·7) | 0·1 (0·01) | 14·8 (2·3) | |
| Ononis tridentata | G (n = 5) | Z | 5·0 (0·8) | 35·6 (0·9) | 14·7 (0·9) | 23·8 (3·4) | 24·3 (1·9) | 0·7 (0·1) | 1·0 (0·1) | 3·1 (0·9) | 4·9 (0·4) | 23·5 (0·7) |
| G (n = 4) | M | 6·5 (0·6) | 35·8 (1·9) | 15·0 (1·4) | 12·9 (3·5) | 24·1 (3·4) | 1·0 (0·3) | 1·3 (0·2) | 2·5 (0·7) | 4·1 (0·5) | 24·7 (2·0) | |
| Rosmarinus officinalis | C (n = 5) | Z | 1·2 (0·2) | 56·2 (0·1) | 43·3 (7·1) | 1·3 (0·4) | 13·3 (2·3) | 0·6 (0·04) | 0·9 (0·1) | 10·3 (2·2) | 0·1 (0·02) | 5·4 (0·4) |
| C (n = 5) | M | 1·2 (0·1) | 56·5 (0·4) | 56·5 (5·7) | 2·1 (0·4) | 10·1 (1·1) | 0·5 (0·04) | 0·8 (0·2) | 9·8 (1·3) | 0·1 (0·01) | 5·4 (0·2) | |
| G (n = 5) | Z | 1·6 (0·2) | 56·3 (0·5) | 45·6 (2·0) | 2·2 (0·5) | 12·4 (0·5) | 0·6 (0·1) | 0·7 (0·1) | 8·5 (1·0) | 0·1 (0·04) | 6·5 (0·5) | |
| G (n = 5) | M | 1·2 (0·2) | 55·6 (0·5) | 60·1 (5·8) | 3·3 (0·5) | 9·3 (0·1) | 0·5 (0·1) | 0·7 (0·1) | 7·6 (2·0) | 0·1 (0·1) | 5·6 (0·6) | |
| Salvia lavandulifolia | C (n = 5) | Z | 1·3 (0·3) | 55·6 (0·6) | 37·1 (3·1) | 3·0 (1·4) | 15·1 (1·2) | 0·5 (0·03) | 0·9 (0·1) | 6·7 (2·5) | 0·1 (0·01) | 6·3 (1·0) |
| C (n = 5) | M | 1·7 (0·2) | 57·2 (1·4) | 38·0 (2·1) | 3·0 (0·4) | 15·1 (0·8) | 0·4 (0·1) | 0·9 (0·2) | 5·0 (0·8) | 0·03 (0·01) | 6·4 (0·9) | |
| G + C (n = 5) | Z | 1·5 (0·1) | 55·7 (0·4) | 37·0 (3·8) | 1·9 (0·6) | 15·3 (1·5) | 0·5 (0·1) | 0·8 (0·1) | 5·0 (1·4) | 0·05 (0·01) | 5·7 (0·4) | |
| G (n = 5) | Z | 2·0 (0·3) | 53·8 (0·6) | 29·8 (2·2) | 1·6 (1·2) | 18·2 (1·3) | 0·5 (0·02) | 1·2 (0·1) | 6·9 (2·0) | 0·1 (0·02) | 7·3 (0·6) | |
| G (n = 5) | M | 1·9 (0·3) | 56·0 (0·6) | 32·9 (2·2) | 4·9 (1·1) | 17·1 (1·4) | 0·4 (0·1) | 0·8 (0·1) | 3·4 (1·2) | 0·1 (0·06) | 7·1 (1·0) | |
| Teucrium polium subsp. capitatum | C (n = 4) | M | 1·8 (0·2) | 51·3 (1·0) | 22·4 (1·4) | 2·6 (1·2) | 23·0 (1·3) | 0·5 (0·1) | 1·5 (0·1) | 6·3 (1·6) | 0·05 (0·01) | 7·3 (1·3) |
| G (n = 4) | M | 1·9 (0·4) | 52·1 (0·8) | 19·9 (2·3) | 2·4 (0·9) | 26·5 (3·2) | 0·45 (0·1) | 1·3 (0·1) | 7·2 (1·9) | 0·06 (0·02) | 7·2 (0·8) | |
| Teucrium pumilum | G (n = 6) | M | 2·0 (0·1) | 52·3 (0·4) | 31·8 (4·4) | 6·1 (1·7) | 16·7 (2·3) | 0·5 (0·03) | 0·7 (0·3) | 7·6 (1·4) | 0·6 (0·2) | 8·1 (0·4) |
| Thymus lacaitae | G (n = 5) | M | 1·6 (0·2) | 51·7 (0·9) | 37·4 (6·7) | 4·0 (0·6) | 14·2 (2·8) | 0·5 (0·1) | 1·1 (0·1) | 5·6 (1·0) | 0·04 (0·02) | 8·6 (1·0) |
aG = gypsum soils; C = calcareous soils; G+C = mixed limestone and gypsum soils.
bM = Madrid (Central Spain, Middle Tajo Basin); Z = Zaragoza (north-east Spain, Middle Ebro Basin).
LITERATURE CITED
- Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research. 2000.;30:1–67. [Google Scholar]
- Allen SE. Chemical analysis of ecological materials. Oxford, UK: Blackwell Scientific Publications; 1989. [Google Scholar]
- Alonso C, Herrera CM. Patterns made of patterns: variation and covariation of leaf nutrient concentrations within and between populations of Prunus mahaleb. New Phytologist. 2001;150:629–640. [Google Scholar]
- Alvarado JJ, Ruiz JM, López-Cantarero I, Molero J, Romero L. Nitrogen metabolism in five plant species characteristic of Gypsiferous soils. Journal of Plant Physiology. 2000;156:612–616. [Google Scholar]
- Anderson R, Fralish JS, Baskin JM. Savannas, barrens, and rock outcrop plant communities of North America. New York: Cambridge University Press; 1999. [Google Scholar]
- Becker M. Análisis y valoración de piensos y forrajes. Zaragoza, Spain: Editorial Acribia; 1961. [Google Scholar]
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. [Google Scholar]
- Boukhris M, Lossaint P. Sur la teneur en soufre de quelques plantes gypsophiles de Tunisie. (Note préliminaire) Acta Oecologica. 1970;5:345–354. [Google Scholar]
- Boukhris M, Lossaint P. Aspects écologiques de la nutrition minérale des plantes gypsicoles de Tunisie. Revue d'Ecologie et de Biologie du Sol. 1975;12:329–348. [Google Scholar]
- ter Braak CJF. Canonical correspondence analysis, a new eigenvector technique for multivariate direct gradient analysis. Ecology. 1986;67:1167–1179. [Google Scholar]
- ter Braak CJF. Update notes: CANOCO version 3·1. Ithaca, NY: Microcomputer Power; 1990. [Google Scholar]
- ter Braak CJF, Prentice I. A theory of gradient analysis. Advances in Ecological Research. 1988;18:271–317. [Google Scholar]
- ter Braak CJF, Smilauer P. Canoco for Windows version 4·0. Wageningen, The Netherlands: Centre for Biometry; 1997. [Google Scholar]
- Braun-Blanquet J, Bolòs O. Les groupements végétaux du Bassin Moyen de l'Ebre et leur dynamisme. Anales de la Estación Experimental de Aula Dei. 1957;5:1–266. [Google Scholar]
- Bridges EM, Burnham CP. Soils of the state of Bahrain. Journal of Soil Science. 1980;31:689–707. [Google Scholar]
- Burjachs F, Giralt S, Roca JR, Seret G, Julià R. Palinología holocénica y desertización en el Mediterráneo occidental. In: Machado C, editor. El paisaje mediterráneo a través del espacio y del tiempo. Implicaciones en la desertificación. Logroño, Spain: Geoforma Ediciones; 1997. [Google Scholar]
- Carrión JS, Dupré M. Late Quaternary vegetational history at Navarrés, Eastern Spain, A two core approach. The New Phytologist. 1996;134:177–191. [Google Scholar]
- Cerrillo MI, Dana ED, Castro H, Rodriguez-Tamayo ML, Mota JF. Selection of priority areas for the conservation of gypsophilous flora in southeast Iberian Peninsula. Revista Chilena de Historia Natural. 2002;75:395–408. [Google Scholar]
- Duvigneaud P. Essai de classification chimique (éléments minéraux) des plantes gypsicoles du bassin de l'Ebre. Bulletin de la Société Royale de Botanique de Belgique. 1968;101:279–291. [Google Scholar]
- Duvigneaud P, Denaeyer-De Smet S. Accumulation du soufre dans quelques espèces gypsophiles d'Espagne. Bulletin de la Société Royale de Botanique de Belgique. 1966;99:263–269. [Google Scholar]
- Duvigneaud P, Denaeyer-De Smet S. Considérations sur l'écologie de la nutrition minérale des tapis végétaux naturels. Oecologia Plantarum. 1973;8 [Google Scholar]
- Escudero A, Somolinos RC, Olano JM, Rubio A. Factors controlling the establishment of Helianthemum squamatum, an endemic gypsophile of semi-arid Spain. Journal of Ecology. 1999;87:290–302. [Google Scholar]
- Escudero A, Albert MJ, Pita MJ, Pérez-García F. Inhibitory effects of Artemisia herba-alba on the germination of the gypsophyte Helianthemum squamatum. Plant Ecology. 2000a;148:71–80. [Google Scholar]
- Escudero A, Iriondo JM, Olano JM, Rubio A, Somolinos RC. Factors affecting establishment of a Gypsophyte: The case of Lepidium subulatum (Brassicaceae) American Journal of Botany. 2000b;87:861–871. [PubMed] [Google Scholar]
- European Community. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Brussels, Belgium: European Community; 1992. [Google Scholar]
- Gankin R, Major J. Arctostaphylos myrtifolia, its biology and relationship to the problem of endemism. Ecology. 1964;45:792–808. [Google Scholar]
- Gauch HG. Multivariate analysis in community ecology. London: Cambridge University Press; 1982. [Google Scholar]
- Gómez-Campo C. Libro rojo de especies vegetales amenazadas de España peninsular e Islas Baleares. Madrid, ICONA: Ministerio de Agricultura, Pesca y Alimentación; 1987. [Google Scholar]
- Grime JP. Plant strategies, vegetation processes, and ecosystem properties. Chichester, UK: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- Guerra J, Ros RM, Cano MJ, Casares M. Gypsipherous outcrops in SE Spain, refuges of rare, vulnerable and endangered Bryophytes and Lichens. Cryptogamie, Bryologie and Lichénologie. 1995;16:125–135. [Google Scholar]
- Guerrero Campo J, Alberto F, Hodgson J, García Ruiz JM, Montserrat Martí G. Plant community patterns in a gypsum area of NE Spain. I. Interactions with topographic factors and soil erosion. Journal of Arid Environments. 1999a;41:401–410. [Google Scholar]
- Guerrero Campo J, Alberto F, Maestro Martínez M, Hodgson J, Montserrat Martí G. Plant community patterns in a gypsum area of NE Spain. II. Effects of ion washing on topographic distribution of vegetation. Journal of Arid Environments. 1999b;41:411–419. [Google Scholar]
- Guisan A, Edwards TCJ, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecological Modelling. 2002;157:89–100. [Google Scholar]
- Harrison S, Safford HD, Grace JB, Viers JH, Davies KF. Regional and local species richness in an insular environment: Serpentine plants in California. Ecological Monographs. 2006a;76:41–56. [Google Scholar]
- Harrison S, Grace JB, Davies KF, Safford HD, Viers JH. Invasion in a diversity hotspot: exotic cover and native richness in the Californian serpentine flora. Ecology. 2006a;87:695–703. doi: 10.1890/05-0778. [DOI] [PubMed] [Google Scholar]
- Hill MO, Gauch HG. Detrended Correspondence Analysis: an improved ordination technique. Vegetatio. 1980;42:47–58. [Google Scholar]
- Hodgson J, Montserrat G, Alberto F, García Ruiz JM, Guerrero J, Colasanti R. A comparison of the functional characteristics of plants from sedimenting and eroded areas with particular reference to the gypsum hills of the Ebro Depression. In: Arnáez J, García Ruiz JM, Gómez Villar A, editors. Geomorfología en España. Logroño, Spain: Sociedad Española de Geomorfología; 1994. pp. 239–251. [Google Scholar]
- Johnston IM. Gypsophily among Mexican desert plants. Journal of the Arnold Arboretum. 1941;22:145–170. [Google Scholar]
- Jones JB, Wolf B, Mills HA. Plant analysis handbook: a practical sampling, preparation, analysis, and interpretation guide. Athens, GA: Micro-Macro; 1991. [Google Scholar]
- Kruckeberg AR. Plant life of western North America. In: Proctor J, editor. The ecology of areas with serpentinized rocks. A world view. Dordrecht: Kluwer Academic Publishers; 1992. pp. 31–73. [Google Scholar]
- Legendre P, Anderson MJ. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs. 1999;69:1–24. [Google Scholar]
- Legendre P, Legendre L. Numerical ecology. 2nd ed. Amsterdam: Elsevier Science BV; 1998. [Google Scholar]
- Margaris NS. Adaptive strategies in plants dominating Mediterranean-type ecosystems. In: Di Castri F, Goodall DW, Specht RL, editors. Mediterranean-type shrublands. Amsterdam: Elsevier; 1981. pp. 309–315. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. New York: Chapman and Hall; 1989. [Google Scholar]
- McCune B. Influence of noisy environmental data on canonical correspondence analysis. Ecology. 1997;78:2617–2624. [Google Scholar]
- Meyer SE. The ecology of gypsophile endemism in the Eastern Mojave desert. Ecology. 1986;67:1303–1313. [Google Scholar]
- Meyer SE, García-Moya E. Plant community patterns and soil moisture regime in gypsum grasslands of north central Mexico. Journal of Arid Environments. 1989;16:147–155. [Google Scholar]
- Meyer SE, García-Moya E, Lagunes-Espinoza LC. Topographic and soil surface effects on gypsophile plant community patterns in central Mexico. Journal of Vegetation Science. 1992;3:429–438. [Google Scholar]
- Mota JF, Rodriguez-Tamayo ML, Peñas J, Pérez F, Dana ED, Merlo ME. Exámen de la vegetación de los alquezales ibéricos con especial atención a la provincia de Almería. Investigación y Gestión. 1998;3:147–158. [Google Scholar]
- Mota JF, Sola AJ, Dana ED. Plant succession in abandoned gypsum quarries in SE Spain. Phytocoenologia. 2003;33:13–28. [Google Scholar]
- Nelson DR, Harper KT. Site characteristics and habitat requirements of the endangered dwarf bear-claw poppy (Arctomecon humilis Coville, Papaveraceae) Great Basin Naturalist. 1991;2:167–175. [Google Scholar]
- O'Dell RE, James JJ, Richards JH. Congeneric serpentine and nonserpentine shrubs differ more in leaf Ca:Mg than in tolerance of low N, low P, or heavy metals. Plant and Soil. 2006;280:49–64. [Google Scholar]
- Orshan G. Morphological and physiological plasticity in relation to drought. In: McKell CM, Blaisdell JP, Goodin JR, editors. Wildland shrubs – their biology and utilization. Ogden, Utah: USDA Forest Service General Technical Report INT-1; 1972. pp. 245–254. [Google Scholar]
- Orshan G. Plant pheno-morphological studies in Mediterranean type ecosystems. Dordrecht: Kluwer Academic Publishers; 1989. [Google Scholar]
- Palacio S, Montserrat-Martí G. Bud morphology and shoot growth dynamics in two species of Mediterranean sub-shrubs co-existing in gypsum outcrops. Annals of Botany. 2005;95:949–958. doi: 10.1093/aob/mci110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio S, Millard P, Maestro M, Montserrat-Martí G. Plant Biology. 2006. Non-structural carbohydrates and nitrogen dynamics in Mediterranean sub-shrubs: an analysis of the functional role of overwintering leaves. DOI: 10·1055/s-2006–924224. [DOI] [PubMed] [Google Scholar]
- Parsons RF. Gypsophily in plants. A review. American Midland Naturalist. 1976;96:1–20. [Google Scholar]
- Peña JL, Echeverría MT, Chueca J, Julián A. Processus d'accumulation et d'incision pendant l'Antiquité Classique dans la vallée de la Huerva (Basin de l'Ebre, Espagne) In: Vermeulen F, editor. Geoarchaeology of the landscapes of Classical Antiquity. Leuven: Peeters Publishers; 2001. pp. 151–159. [Google Scholar]
- Powell AM, Turner BL. Aspects of the plant biology of gypsum outcrops of the Chihuahuan desert. In: Riskinn DH, editor. Transactions of a Symposium on Biological Resources of the Chihuahuan desert. 1977. pp. 315–325. US Department of the Interior, National Park Service, Transactions Proceedings Series 3. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs. 1992;62:365–392. [Google Scholar]
- Rivas-Martínez S, Costa M. Comunidades gipsícolas del centro de España. Anales del Instituto Botánico Cavanilles. 1970;27:193–224. [Google Scholar]
- Romao RL, Escudero A. Gypsum physical soil crusts and the existence of gypsophytes in semi-arid central Spain. Plant Ecology. 2005;181:127–137. [Google Scholar]
- Ruiz JM, López-Cantarero I, Rivero RM, Romero L. Sulphur phytoaccumulation in plant species characteristic of gypsipherous soils. International Journal of Phytoremediation. 2003;5:203–210. doi: 10.1080/713779220. [DOI] [PubMed] [Google Scholar]
- Verdonschot PFM, ter Braak CJF. An experimental manipulation of oligochaete communities in mesocosms treated with chlorpyrifos or nutrient additions: multivariate analyses with Monte Carlo permutation tests. Hydrobiologia. 1994;278:251–266. [Google Scholar]
- Verheye WH, Boyadgiev TG. Evaluating the land use potencial of gypsiferous soils from field pedogenic characteristics. Soil Use and Management. 1997;13:97–103. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


