Abstract
Background and Aims
Plants from the family Lemnaceae are widely used in ecological engineering projects to purify wastewater and eutrophic water bodies. However, the biology of nutrient uptake mechanisms in plants of this family is still poorly understood. There is controversy over whether Lemnaceae roots are involved in nutrient uptake. No information is available on nitrogen (N) preferences and capacity of Landoltia punctata (dotted duckweed), one of the best prospective species in Lemnaceae for phytomelioration and biomass production. The aim of this study was to assess L. punctata plants for their ability to take up NH4+ and NO3− by both roots and fronds.
Methods
NO3− and NH4+ fluxes were estimated by a non-invasive ion-selective microelectrode technique. This technique allows direct measurements of ion fluxes across the root or frond surface of an intact plant.
Key Results
Landoltia punctata plants took up NH4+ and NO3− by both fronds and roots. Spatial distribution of NH4+ and NO3− fluxes demonstrated that, although ion fluxes at the most distal parts of the root were uneven, the mature part of the root was involved in N uptake. Despite the absolute flux values for NH4+ and NO3− being lower in roots than at the frond surface, the overall capacity of roots to take up ions was similar to that of fronds because the surface area of roots was larger. L. punctata plants preferred to take up NH4+ over NO3− when both N sources were available.
Conclusions
Landoltia punctata plants take up nitrogen by both roots and fronds. When both sources of N are available, plants prefer to take up NH4+, but will take up NO3− when it is the only N source.
Key words: Ammonium, eutrophication, ion fluxes, Landoltia punctata, nitrate, nitrogen uptake
INTRODUCTION
Cultivation of wetland plant species in wastewater serves two purposes. First, these plants purify water in a simple, cheap and energy-efficient method. Second, the biomass produced by the plants can be used as fodder for cattle.
Among wetland species, plants from the family Lemnaceae are of primary importance for water cleansing because they have high biomass production and can grow in sewage with high concentrations of nitrogen (N) (Cheng et al., 2002). During recent studies on secondary treatment of swine wastewater, several Lemnaceae genotypes were selected for high biomass production (Bergmann et al., 2000). The most productive genotype belonged to Landoltia punctata (former Spirodela punctata), a ‘dotted duckweed’; this is a small floating plant with two fronds (leaves) attached together and two descending roots. However, to date, no N uptake studies have been performed on L. punctata, which has genetic traits and metabolism distinct from its close relatives (Frick, 1994; Les and Crawford, 1999). Moreover, the only N uptake studies on Lemnaceae species, such as Lemna minor, were those with plants exposed to N concentrations close to their growing optimum at 50–400 µm NH4NO3 (Oscarson et al., 1988; Cedergreen and Madsen, 2002), which is lower than N concentrations found in wastewater.
Both roots and fronds of Lemnaceae plants are exposed to the surrounding media and, in theory, are able to take up nutrients (including NH4+ and NO3−). However, there is long-standing controversy about root involvement in nutrient uptake in Lemnaceae species (cf. Cedergreen and Madsen, 2002). Only recent studies on Lemna minor have demonstrated that roots are involved in N uptake and that plants can regulate NO3− uptake via fronds or roots depending on light intensity (Cedergreen and Madsen, 2002, 2003). However, the technique used in the studies mentioned above required mechanical separation of the organs: to separate root contribution from the overall uptake, roots were removed. This approach, although apparently successful, could modify root–frond ion transport and alter plant requirements for N supply.
The ion-selective microelectrode technique (MIFE) allows non-invasive, simultaneous measurement of fluxes of three specific ions at the surface of an intact plant. It has high temporal and spatial resolutions (Newman, 2001). Ion-selective microelectrodes have been used to study NH4+ and NO3− uptake by crops and trees. The spatial dynamics of fluxes of these ions, the relationship with the proton flux, comparison of root capacity to absorb NO3− and NH4+, and preferences for N sources have been established for a range of terrestrial plant species (Henriksen et al., 1992; Colmer and Bloom, 1998; Taylor and Bloom, 1998; Garnett et al., 2001). Recent MIFE measurements on canola roots demonstrated an NO3− effect on NH4+ transport dynamics (Babourina et al., 2006).
In the present study, the MIFE technique was used to determine: (1) the N uptake capacity of fronds and roots of intact L. punctata plants at three different concentrations of NH4NO3 comparable with N concentrations in wastewater; (2) the spatial distribution of NH4+ and NO3− fluxes along the root; and (3) plant preference for NH4+ or NO3− when supplied with different N sources.
MATERIALS AND METHODS
Plant materials
The free-floating macrophyte plant Landoltia punctata (G. Meyer) Les & D.J. Crawford was acquired from Lotus Blossom Garden, Perth, Western Australia (WA), and cultivated in a glasshouse at the University of WA.
The plants were cultured in 50-L opaque plastic containers on a modified Hoagland nutrient solution before treatment. The nutrient solution contained (in mm): NH4+, 0·143; NO3−, 0·143; Ca2+, 0·05; Mg2+, 0·025; Na+, 0·56; K+, 0·025; H2PO4−, 0·032; Cl−, 0·125; SO42−, 0·05; and CO3−, 0·2.
For ion flux measurements, plants in Petri dishes were placed on an inverted optical microscope. The position of the root cap and the meristem, and elongation and mature zones of the root were determined for each plant measured. Because cells in the meristem zone were the smallest, this zone was darker in colour. Cells in the elongation zone gradually increased in size and in the mature zone they reached their maximal size. Root radius (R) was also measured under an optical microscope. To estimate the average size of fronds and root length (L), plants were scanned, and the surface area was measured by using SigmaScan Pro software (SPSS Inc., Chicago, IL, USA), n=106. Root surface area was calculated as a cylinder surface (2πR×L) where R is radius and L root length.
Ion flux measurements
Ion fluxes were measured non-invasively using the MIFE® system (University of Tasmania, Hobart, Australia) generally as described by Newman (2001). Electrodes were pulled from borosilicate glass capillaries (GC150–10, Harvard Apparatus, Kent, UK), dried at 230 °C for about 5 h, and silanized with tributylchlorosilane (#90765, Fluka Chemicals, Buchs, Switzerland). The tips of dried and cooled electrode blanks were broken to a diameter of 1–10 µm, depending on the ionophore, and then back-filled with appropriate solutions. Back-filling solutions were 15 mm NaCl and 40 mm KH2PO4 for the hydrogen electrode, 500 mm NH4Cl for the NH4+ electrode, and 500 mm KNO3 and 100 mm KCl for the NO3− electrode. Immediately after back-filling, the electrode tips were front-filled with commercially available ionophore cocktails for measuring H+ (#95297, Fluka) and NH4+ (#09882, Fluka). The nitrate sensor contained 0·5 % (w/v) methyltridodecyl nitrate (MTDDA NO3−), 0·084 % (w/v) methyltriphenylphosphonium bromide (MTPPB) and 99·4 % (v/v) n-phenyloctyl ether (NPOE) (Zhen et al., 1992; Plassard et al., 2002). A reference electrode was fabricated in a similar way from a borosilicate glass capillary and filled with 0·1 m KCl in 1 % (w/v) agar. Electrodes were calibrated against a range of standards (pH from 5·3 to 7·2, NH4+ and NO3− from 1 to 10 mm). Electrodes with responses of less than 50 mV per decade were discarded.
Experimental procedures
Thirty minutes before ion flux measurements, plants were placed in the bathing solution containing (in mm): NH4NO3, 1·0; CaCl2, 0·05; MgSO4, 0·025; NaCl, 0·56; K2SO4, 0·025; NaH2PO4, 0·032; NaHCO3, 0·2. Ion fluxes were measured 60–120 µm from the root surface and 200 µm from the frond surface. In mapping experiments, 4–5 plants were measured and averaged. Experiments were performed at 20–22 °C under standard laboratory lighting. Fluxes of H+, NH4+ and NO3− were measured simultaneously.
For estimation of preferences of roots and fronds for NH4+ or NO3− supply, measurements were made at the border of the elongation and mature zones of roots and in the middle of the frond after 2 h exposure to the solution containing 0·5, 1·0 or 2·5 mm NH4 or NO3− in addition to other nutrients (in mm): CaCl2, 0·05; MgSO4, 0·025; NaCl, 0·56; K2SO4, 0·025; NaH2PO4, 0·032; NaHCO3, 0·2. Prior to measurements, the Petri dish was flushed with fresh solution containing the appropriate N source at the same concentration as in the pre-treatment solution. Flux measurements were performed 30 min after the transfer to a fresh solution to allow ion flux adjustment.
Statistical analysis
All treatments were performed in random order. To analyse the difference in ion fluxes in different zones of the root and at different NH4NO3 concentrations, the ion fluxes were evaluated by the Student's t-test.
RESULTS
The average surface area (one side,±s.e.) of a single frond in our experiments was 0·028±0·002 cm2, whereas the surface area of a single root was 0·054±0·003 cm2. Therefore, despite their small diameter of 0·018 ±0·001 cm, roots generally had a larger surface area than fronds to take up nutrients from the media.
Root morphology was similar to other wetland plant species (Landolt, 1986) with a large root cap covering the root apex, the meristem and the elongation zones. As with other members of the family Lemnaceae, Landoltia punctata does not have root hairs.
Mapping of ion fluxes along roots
As L. punctata is a floating plant and can potentially take up N by both roots and fronds (Cedergreen and Madsen, 2002), ion fluxes for both N forms at roots and fronds were compared. However, because the spatial distribution of ion fluxes along the plant root is uneven (cf. Newman, 2001), and there is no available information regarding NH4+ and NO3− flux distribution in L. punctata or its close relatives, it was necessary to map ion fluxes along the root.
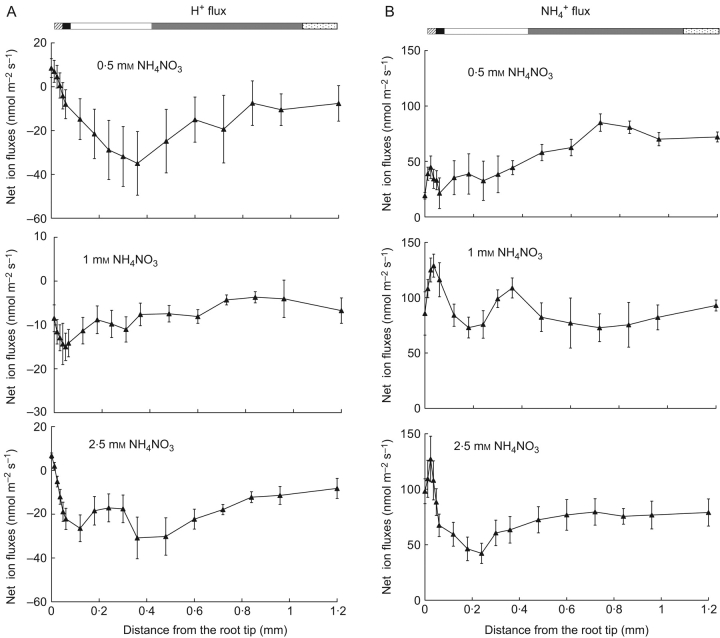
Exposure to different concentrations of NH4NO3 had some impact on H+ flux profile along L. punctata roots (Fig. 1A). The H+ influx zone, which is usually linked to the meristem zone, was more pronounced at higher NH4NO3 concentrations. The root tip had higher influx than the adjoining meristem zone. The mature zone had higher influx than the elongation zone. The fluctuations in H+ profiles were higher at higher concentrations of NH4NO3 (the largest fluctuations were found at 2·5 mm NH4NO3) than at the mid-range concentration of 1 mm NH4NO3.
Fig. 1.
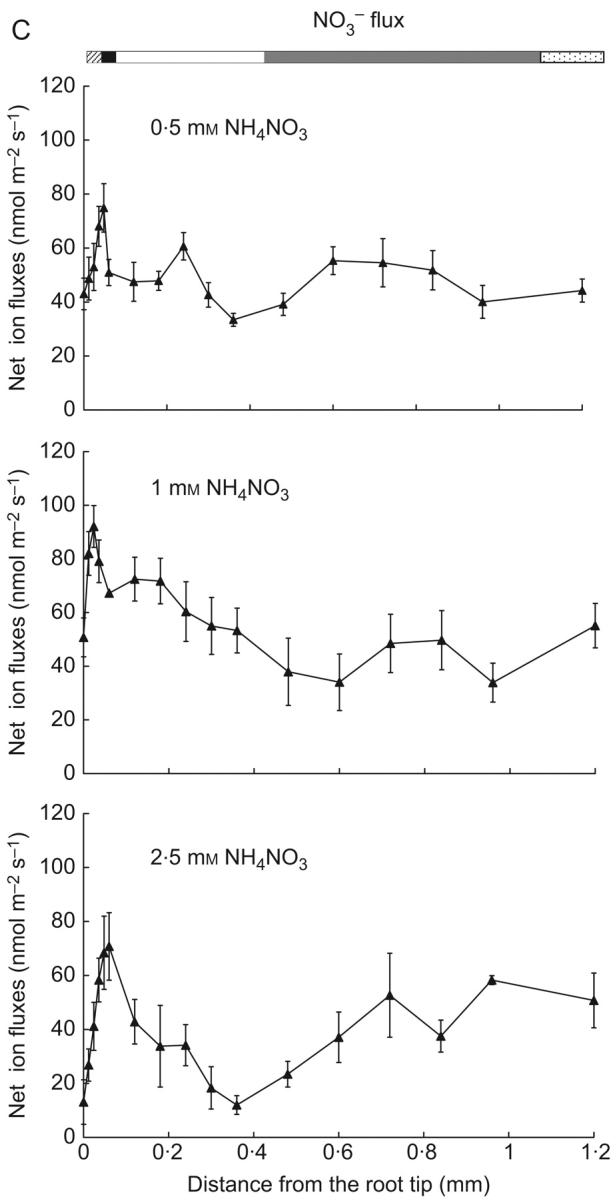
Net ion fluxes along the root length of Landoltia punctata as noted in the bars at the top: root cap (hatched), meristem zone (black), distal elongation zone (white), proximal elongation zone (grey) and mature zone (speckled). (A) H+, (B) NH4+, (C) NO3−. Numbers indicate the initial flux values. Flux measurements were taken while roots were exposed to the basal solution containing (in mm): NH4NO3, 0·5, 1·0 or 2·5; CaCl2, 0·05; MgSO4, 0·025; NaCl, 0·56; K2SO4, 0·025; NaH2PO4, 0·032; and NaHCO3, 0·2.
NH4+ flux mapping along roots indicated that the root tip had the highest influx (P<0·05), which decreased at the distal elongation zone (Fig. 1B). The net NH4+ influx at the root tip was lowest at the lowest concentration studied (0·5 mm NH4NO3); however, at the proximal elongation and mature zones, NH4+ fluxes were similar at all concentrations studied.
As with NH4+ fluxes, the root tip had higher NO3− influx than the neighbouring zones (P<0·05). The difference in NO3− flux values between different root zones was highest at 2·5 mm NH4NO3, with the smallest influx in the middle of the elongation zone (Fig. 1C).
Comparison of NH4+ and NO3− uptake by fronds and roots
Generally, NH4+ and NO3− fluxes were more stable at the end of the proximal elongation zone and at the mature zone of the root. The border between the elongation and the mature zones was chosen for further ion flux measurements to reduce variability between plants at different stages of their development. In fronds, ion fluxes were uniform when the microelectrodes were located at a relatively long distance (200 µm) from the frond surface.
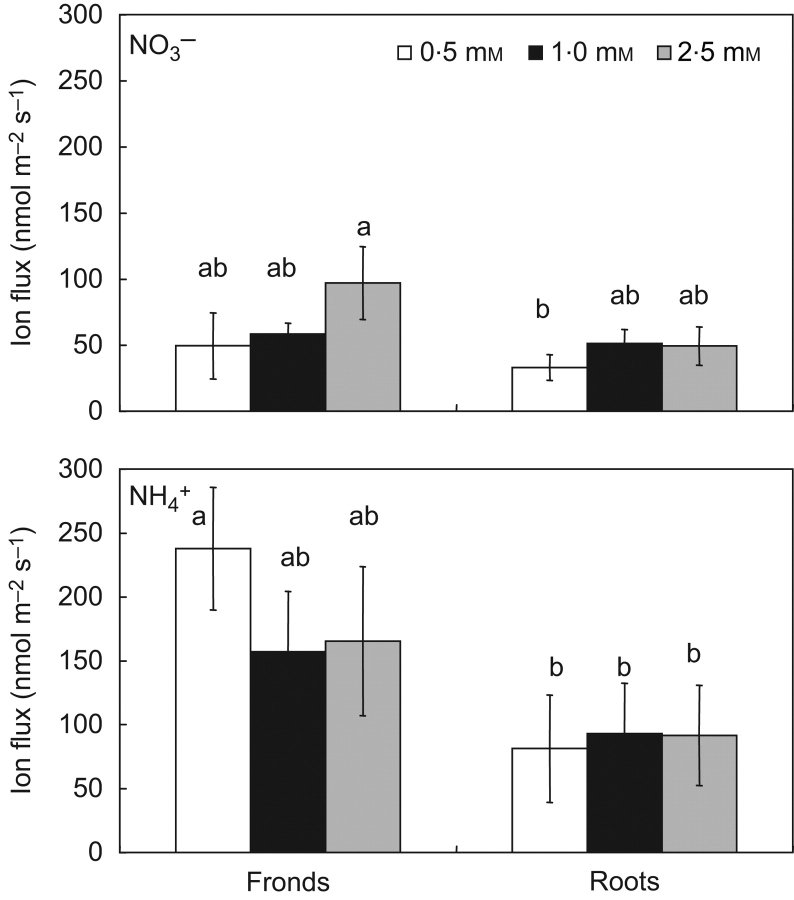
At all NH4NO3 concentrations studied, both roots and fronds took up NH4+ at a higher rate than NO3−. The concentration increase did not affect root uptake of NH4+ and NO3− whereas fronds took up NO3− at a higher rate at 2·5 mm NH4NO3, although this was statistically insignificant (Fig. 2).
Fig. 2.
Net fluxes (nmol m−2 s−1) of NH4+ and NO3− at roots (the mature zone, 1·2 mm from the root tip) and fronds of Landoltia punctata exposed to different media (0·5, 1·0 or 2·5 mm NH4NO3). Values are means±s.e. (n=5). Different lower-case letters indicate significant differences (P≤0·05).
In comparisons of N uptake by roots and fronds, uptake rate of NH4+ and NO3− by roots was generally lower, although not statistically so. The highest flux values were observed for NH4+ in fronds at 0·5 mm NH4NO3.
Overall, ion fluxes at the root surface were lower than at the frond surface. However, because the surface area of the single root was almost twice that of the frond, as calculated above, the total capacity of roots to take up nutrients was almost equal to that of the fronds. On average, the ratio of fronds/roots for N uptake was 1·09±0·20 (± s.e.) for NH4+ and 0·79±0·12 for NO3−.
NH4+ and NO3− flux in different N media
Net NO3− and NH4+ fluxes were measured at three positions along L. punctata roots, including the root tip (0 mm), the distal elongation zone (0·6 mm) and the mature zone (1·2 mm), in three different N media (1·0 mm NH4Cl, KNO3 or NH4NO3) (Table 1). NO3− flux in 1·0 mm KNO3 was larger than NH4+ flux in 1·0 mm NH4Cl. However, NH4+ uptake tended to be higher than NO3 uptake when NH4+ and NO3− were supplied together, especially at the root tip.
Table 1.
Net fluxes (nmol m−2 s−1) of NH4+ and NO3− at three specific points on the roots of Landoltia punctata exposed to different media (1·0 mm NH4Cl, KNO3 or NH4NO3)
| Distance from tip (mm) | 1 mm NH4Cl NH4+ flux | 1 mm KNO3 NO3− flux | 1 mm NH4NO3 NH4+ flux | NO3− flux |
|---|---|---|---|---|
| 0 | 90±10bc | 130±10b | 140±10b | 50±10d |
| 0·6 | 80±30bc | 260±40a | 80±10c | 30±40d |
| 1·2 | 30±10d | 270±50a | 90±30bc | 65±20cd |
The same plants were measured in all three bathing solutions in random order. Values are means±s.e.m. (n=4–5).
Different letters indicate significant differences estimated by Student's t-test (P≤0·05).
In 1·0 mm NH4Cl media, the root tip and the distal elongation zone showed three-fold higher NH4+ influx than in the mature zone. In 1·0 mm KNO3 media, the distal elongation zone and the mature zone showed two-fold higher NO3− uptake than the root tip.
When NO3− was the only source of N, the highest N influx was observed at the distal elongation zone and at the mature zone.
DISCUSSION
Comparison of NH4+ and NO3− uptake by fronds and roots
Hillman (1961) suggested that roots of floating macrophytes function mostly as anchors, whereas fronds and leaves are the main organs involved in nutrient uptake. Subsequent studies, covering fronds and roots of Spirodela polyrrhiza and Lemna minor (Lemnaceae) with paraffin in one set of experiments, and removing roots in another set of experiments, led to conclusions that the roots of duckweeds played only a small role in nutrient uptake (Muhonen et al., 1983; Ice and Couch, 1987). However, the researchers based their conclusions on the observation that plants with excised roots multiplied more rapidly than the rooted plants, and obviously roots were present in new generations of the plants (Muhonen et al., 1983). In earlier experiments, Gorham (1941) demonstrated that covering the undersides of fronds with lanolin decreased growth of duckweed plants, although he noticed that root length was increased. Recently, it has been found that increased ratio of the root surface to the frond surface led to increased NH4+ uptake rate in Lemna minor (Cedergreen and Madsen, 2002). These studies indicate that duckweed plants can regulate their life cycle, such as increased multiplication rate, and the surface area for nutrient absorption at the level of frond–root interactions.
The conclusion that roots are of low importance for N uptake in Lemnaceae was opposed by other studies, where it was shown that roots might have an important role in nutrient supply (Oscarson et al., 1988; Cedergreen and Madsen, 2002, 2003). Moreover, in Lemna minor, roots had a higher rate of uptake of both NH4+ and NO3− at low external concentration (5 µm NH4NO3) than fronds, whereas higher NH4NO3 supply (250 µm) reduced root uptake rates for both ions. This decreased uptake rate in roots at high NH4NO3 supply was compensated for by higher uptake rates in fronds (Cedergreen and Madsen, 2002). However, in all studies mentioned above, the researchers used mechanical approaches to separate the organs: covering fronds or roots with paraffin or lanolin, and removing roots. In the present study, intact plants were used to assess the relative contribution of fronds or roots to nutrient uptake by Lemnaceae plants, so that interactions between roots and fronds were not affected.
From the current study, roots of L. punctata contributed to N uptake at the same level as fronds. Even though the magnitude of ion fluxes in roots was lower than in fronds, the root surface area was two-fold greater than the frond surface area, and the ratio of fronds/roots in N uptake was close to 1:1·09 for NH4+ and 0·79 for NO3−. Therefore, plants have equal capacity to use fronds and roots for NO3− and NH4+ uptake.
Landoltia punctata preferences for different N sources
Different species have different preference for N forms. Picea glauca preferred NO3− (Kronzucker et al., 1995), whereas NH4+ uptake by maize roots was twice that of NO3− uptake when both N forms were present (Taylor and Bloom, 1998). Plant preference for different forms of N is affected by temperature, pH and element composition of the solution as well as plant growth stage (Ikeda, 1991).
Although Lemnaceae (Lemna minor) have been shown to prefer NH4+ as a nitrogen source (Porath and Pollock, 1982; Cedergreen and Madsen, 2002), high concentrations of NH4+ can inhibit their growth (Oron et al., 1985; Korner et al., 2001). A rise in external concentration from 50 to 250 µm NH4NO3 significantly decreased uptake of both N forms by roots and increased uptake of NO3− by fronds (Cedergreen and Madsen, 2002). In the present study, the capacity to take up NH4+ was two-fold higher than NO3− in both fronds and roots when both ions were supplied (Fig. 2) at external concentrations of NH4NO3 much higher than in the study of Cedergreen and Madsen (2002). These findings indicate that: (1) differences in NH4+ uptake might depend on Lemnaceae species requirements and (2) differences in NH4+ uptake may be found between an intact plant and a plant with the root removed.
Spatial distribution of ion fluxes in the root of Landoltia punctata
High H+ excretion at the root apex, which includes the apical initials, meristem and distal elongation zones, has been shown for many terrestrial plants (Taylor and Bloom, 1998; Newman, 2001). Lemnaceae plants have a large root cap covering the whole root apex, the full elongation zone and part of the mature zone, which makes them different from many other plants, including some wetland species (Landolt, 1986). In the current study, it was demonstrated that the root cap does not interfere with ion fluxes at the root surface: the H+ flux profile of the root of L. punctata is similar to other plants studied (cf. Newman, 2001). Higher NH4+ uptake at the root apex was established for rice adventitious roots and primary maize seminal roots (Colmer and Bloom, 1998; Taylor and Bloom, 1998). Using a higher spatial resolution (0·2 mm), the highest NH4+ influx at the root apex was found to be associated with the meristem zone rather than with the whole apex (Fig. 1B). Likewise, increased NO3− influx was noted at the root meristem zone, similar to observations made by Colmer and Bloom (1998) on maize primary seminal roots.
CONCLUSIONS
Landoltia punctata plants prefer to take up NH4+ when both N sources are available. Although the absolute flux values for NH4+ and NO3− in roots are lower than those at the frond surface, the overall capacity of roots to accumulate ions equalled that of fronds because of the greater surface area of roots compared with fronds.
ACKNOWLEDGEMENTS
We are grateful to Dr Ian Newman, University of Tasmania, who kindly lent us the MIFE system for selective ion measurements. The project was supported by the China Fund (administered by the Australian Department of Education, Science and Training) and the Australian Research Council. The Zhejiang University (China) and the University of Western Australia (Australia) contributed equally for the work presented in this paper.
LITERATURE CITED
- Babourina O, Voltchanskii K, McGann B, Newman I, Rengel Z. Journal of Experimental Botany. 2007. Nitrate supply affects ammonium transport in canola roots. in press. [DOI] [PubMed] [Google Scholar]
- Bergmann BA, Cheng J, Classen J, Stomp AM. In vitro selection of duckweed geographical isolates for potential use in swine lagoon effluent renovation. Bioresource Technology. 2000;73:13–20. [Google Scholar]
- Cedergreen N, Madsen TV. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytologist. 2002;155:285–292. doi: 10.1046/j.1469-8137.2003.00936.x. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Madsen TV. Light regulation of root and leaf NO3− uptake and reduction in the floating macrophyte Lemna minor. New Phytologist. 2003;161:449–457. doi: 10.1046/j.1469-8137.2003.00936.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Bergmann BA, Classen JJ, Stomp AM, Howard JW. Nutrient recovery from swine lagoon water by Spirodela punctata. Bioresource Technology. 2002;81:81–85. doi: 10.1016/s0960-8524(01)00098-0. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Bloom AJ. A comparison of NO3− and NH4+ net fluxes along roots of rice and maize. Plant, Cell and Environment. 1998;21:240–246. [Google Scholar]
- Frick H. Heterotrophy in the Lemnaceae. Journal of Plant Physiology. 1994;144:189–193. [Google Scholar]
- Garnett TP, Shabala SN, Smethurst PJ, Newman IA. Simultaneous measurement of ammonium, nitrate and proton fluxes along the length of eucalypt roots. Plant and Soil. 2001;236:55–62. [Google Scholar]
- Gorham PR. Measurement of the response of Lemna to growth-promoting substances. American Journal of Botany. 1941;28:98–101. [Google Scholar]
- Henriksen GH, Raman DR, Walker LP, Spanswick RM. Measurement of net fluxes of ammonium and nitrate at the surface of barley roots using ion-selective microelectrodes: patterns of uptake along the root axis and evaluation of the microelectrode flux estimation technique. Plant Physiology. 1992;99:734–747. doi: 10.1104/pp.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman WS. The Lemnaceae, or duckweeds. A review of the descriptive and experimental literature. Botanical Review. 1961;27:221–287. [Google Scholar]
- Ice J, Couch R. Nutrient absorption by duckweed. Journal of Aquatic Plant Management. 1987;25:30–31. [Google Scholar]
- Ikeda H. Utilization of nitrogen by vegetable crops. Japan Agricultural Research Quarterly. 1991;25:117–124. [Google Scholar]
- Korner S, Das SK, Veenstra S, Vermaat JE. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquatic Botany. 2001;71:71–78. [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY. Nitrate induction in spruce. An approach using compartmental analysis. Planta. 1995;196:683–690. [Google Scholar]
- Landolt E. The family of Lemnaceae – a monographic study. Zürich, Switzerland: Stiftung Rübel; 1986. [Google Scholar]
- Les DH, Crawford DJ. Landoltia (Lemnaceaee), a new genus of Lemnaceaes. Novon. 1999;9:530–533. [Google Scholar]
- Muhonen M, Showman J, Couch R. Nutrient absorption by Spirodela polyrrhiza. Journal of Aquatic Plant Management. 1983;21:101–109. [Google Scholar]
- Newman IA. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment. 2001;24:1–14. doi: 10.1046/j.1365-3040.2001.00661.x. [DOI] [PubMed] [Google Scholar]
- Oron G, Wildschut LR, Porath D. Waste water recycling by duckweed for protein production and effluent renovation. Water Science and Technology. 1985;17:803–817. [Google Scholar]
- Oscarson P, Ingemarsson B, Ugglas M, Larsson CM. Characteristics of NO3− uptake in Lemna and Pisum. Plant and Soil. 1988;111:203–205. [Google Scholar]
- Plassard C, Guérin-Laguette A, Véry AA, Casarin V, Thibaud JB. Local measurements of nitrate and potassium fluxes along roots of maritime pine. Effect of ectomycorrhizal symbiosis. Plant, Cell and Environment. 2002;25:75–84. [Google Scholar]
- Porath D, Pollock J. Ammonia stripping by duckweed and its feasibility in circulating aquaculture. Aquatic Botany. 1982;13:125–131. [Google Scholar]
- Taylor AR, Bloom AJ. Ammonium, nitrate, and proton fluxes along the maize root. Plant, Cell and Environment. 1998;21:1255–1263. [Google Scholar]
- Zhen RG, Smith SJ, Miller AJ. A comparison of nitrate-selective microelectrodes made with different nitrate sensors and the measurement of intracellular nitrate activities in cells of excised barley roots. Journal of Experimental Botany. 1992;43:131–138. [Google Scholar]