Abstract
Background and Aims
Herbivory on floral structures has been postulated to influence the evolution of floral traits in some plant species, and may also be an important factor influencing the occurrence and outcome of subsequent biotic interactions related to floral display. In particular, corolla herbivory may affect structures differentially involved in flower selection by pollinators and fruit predators (specifically, those ovopositing in ovaries prior to fruit development); hence floral herbivores may influence the relationships between these mutualistic and antagonistic agents.
Methods
The effects of corolla herbivory in Linaria lilacina (Scrophulariaceae), a plant species with complex flowers, were considered in relation to plant interactions with pollinators and fruit predators. Tests were made as to whether experimentally created differences in flower structure (resembling those occurring naturally) may translate into differences in reproductive output in terms of fruit or seed production.
Key Results
Flowers with modified corollas, particularly those with lower lips removed, were less likely to be selected by pollinators than control flowers, and were less likely to be successfully visited and pollinated. As a consequence, fruit production was also less likely in these modified flowers. However, none of the experimental treatments affected the likelihood of visitation by fruit predators.
Conclusions
Since floral herbivory may affect pollinator visitation rates and reduce seed production, differences among plants in the proportion of flowers affected by herbivory and in the intensity of the damage inflicted on affected flowers may result in different opportunities for reproduction for plants in different seasons.
Key words: Complex flowers, corolla herbivory, Linaria lilacina, pollination success, fruit predation
INTRODUCTION
Floral morphology of complex flowers may have evolved to accomplish two main, complementary, functions: first, to attract and permit access to efficient pollinators while restricting access to illegitimate or ineffective ones (which could negatively affect plant fitness via a reduction in male and/or female reproductive success); and secondly, to protect organs related to sexual functions, and the costly rewards produced for pollinator attraction, against unwanted visitors (including robbers, herbivores and predators). Under these assumptions, evolutionary biologists have been trying to assess the adaptive value of floral traits since the early 20th century using a wide variety of approaches, and numerous authors have presented results suggesting that specialized and complex floral morphologies may have evolved as a response to selective pressures imposed by particular biotic agents, with special reference to pollinators (e.g. Campbell et al., 1991; Mitchell et al., 1998; Goldblatt and Manning, 2000; Wilson et al., 2004, among others; but see Herrera, 1996; Waser et al., 1996).
However, in the last decade, a number of studies have suggested that form and integration of different traits in complex flowers are not always the result of the preferences of particular pollinators for certain floral phenotypes (e.g. Herrera, 1996, 2001; Aigner, 2001), and that floral adaptations may not be exclusively induced by the most effective pollinators (sensu Stebbins, 1970; see also Mayfield et al., 2001). In other words, selection may be achieved by a variable number of agents that differ in their ability as pollinators, which runs counter to the idea of flower specialization to a narrow range of pollinator species (see Waser et al., 1996; Fenster et al., 2004).
Furthermore, even assuming selection by particular pollinators on certain floral traits, it has been shown that post-pollination events (such us fruit or seed predation, or the action of pathogens) may strongly influence the results of the pollination services (e.g. Mutikainen and Delph, 1996; Krupnick et al., 1999; Lethilä and Strauss, 1999; Herrera, 2000a; Mothershead and Marquis, 2000; Gómez, 2003; Cariveau et al., 2004). Thus, an increasing number of papers are currently aimed at studying the likelihood of floral evolution not exclusively based upon the interaction between plants and pollinators, but using a multilevel interaction approach including several biotic agents (e.g. Strauss et al., 1996; Herrera et al., 2002; Strauss and Irwin, 2004; Newman and Thomson, 2005b).
Particularly interesting is the presumed influence of herbivores (see Strauss, 1997). Since the effect of herbivory on plant reproduction (either as a pre- or as a post-dispersive event) may be highly variable, depending on the species inflicting or suffering the damage, and the environmental and biological conditions of the interacting agents, the results of the interaction may eventually be detrimental (e.g. Herrera, 2000a), neutral (e.g. Sánchez-Lafuente, 2002) or even beneficial (e.g. Newman and Thomson 2005a) for plant fitness.
Herbivory on floral structures has been postulated to influence the evolution of floral traits in some species (e.g. Galen and Cuba, 2001; Herrera et al., 2002). While fruit predators may have a direct negative effect on plant fitness by reducing the number of seeds available for dispersal, corolla herbivory may also be an important factor influencing the occurrence and outcome of subsequent biotic interactions related to floral display (e.g. Strauss et al., 1996; Krupnik et al., 1999; Gómez, 2003). Thus, if corolla herbivory affects structures involved in flower selection by pollinators and fruit predators (specifically, those ovopositing in ovaries prior to fruit development), it may deter both these mutualists and antagonist agents, thus changing our predictions about the outcome of both interactors.
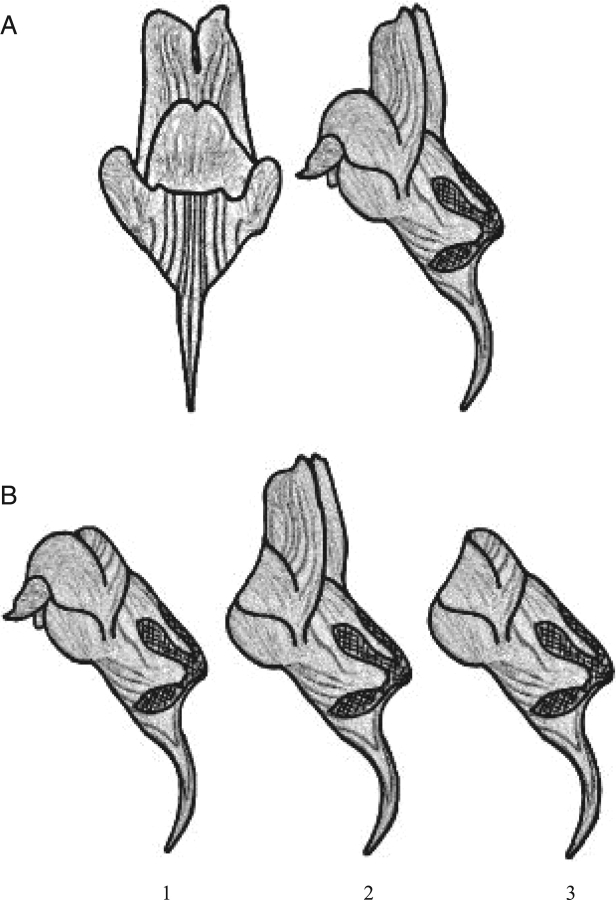
In this study, the functionality of the complex flower of Linaria lilacina Lange (Scrophulariaceae) is considered in relation to its interaction with pollinators, corolla herbivores and fruit predators, and an examination is made of whether experimental removal of flower parts to simulate herbivory may affect pollinator and herbivore visitation and plant female reproductive success. During the pre-dispersive stage, flowers of this plant species undergo herbivore damage affecting corolla structure, and fruit predation affecting seed production. Floral herbivores may damage the zygomorphic corolla (see Fig. 1A) in several ways, generally affecting the lower lips, but also the upper lips or both lips at the same time. While both upper and lower lips may have a role in pollinator attraction (standard in upper lips, nectar guides in lower lips), lower lips may also have a mechanical function to facilitate access to the corolla throat by both pollinators and fruit predators ovopositing in developing ovaries. Consequently, damage to the upper and/or lower lips may have different consequences for flower selection and likelihood of visitation.
Fig. 1.
(A) Front and side view of a Linaria lilacina flower. (B) Schematic representation of the simulations of corolla herbivory. 1, upper lips removed (UP); 2, lower lips removed (LO); 3, both lips removed (BL).
Specifically, the following questions are addressed. (a) Are modified flowers less attractive to flower visitors than intact ones? (b) Do modified flowers impose restrictions to pollinators or fruit predators when visiting such flowers? (c) Do such eventual restrictions translate into limitation to fruit and seed production at flower and plant levels? (d) Is there a conflicting selection between mutualists and/or antagonists originating from corolla herbivory?
MATERIALS AND METHODS
Study species and site
Linaria lilacina Lange is a perennial herb endemic to the Subbetic mountains in the South of the Iberian Peninsula (Valdés, 1970; Sáez and Crespo, 2005). Plants emerge every season from mid to late winter and produce from one to many simple or branched stems (mean length±s.d.: 22·07±7·65, n=68), with verticillate leaves, some of which may produce a variable number of flowers at their top. Morphologically, flowers are zygomorphic (Fig. 1A), consisting of a closed, tubular corolla with upper and lower lips (variable in colour, including white, blue or violet spots and yellow nectar guides) that obstruct the opening to the throat of the corolla. There is also a spur at the base of the lower lip which collects nectar produced by a nectary located under the ovary inside the corolla. Functionally, flowers are hermaphroditic and self-incompatible (A. M. Sánchez-Lafuente, unpubl. res.). In most cases, flowers are not dichogamous, but some slightly protogynous or protandrous flowers may be eventually found (pers. obs.). The calyx is small and the pedicel is short. The fruit is a two-locule capsule, usually dehiscing by valves.
This study was carried out in the Jaén mountains (Southern Spain), where plants usually grow on rocky cliffs, generally oriented northwards. Two populations were selected: Sillón del Rey (SR) and Otiñar (OT), with approx. 80 and 48 plants, respectively, separated by approx. 3 km. In these populations, flowers are approx. 23·01±1·62 mm long (mean±s.d.). Nectar length is 7·01±1·01 mm (n=80). In the study area, flowering generally begins in January–February and, depending on the population and weather conditions, it may extend for 2–4 months. After successful pollination, the capsule develops containing numerous small brown, flattened, winged seeds. Fruit maturation takes between 18 and 24 d.
Experimental manipulations and natural incidence of corolla herbivory
In January 2005, 31 experimental plants were randomly selected in SR and 27 in OT. Manipulations were carried out during the flowering peak when pollinators and seed predators were most abundant (early-April to mid-May), and were designed to imitate the damage caused to corollas by floral herbivores (currently, one unidentified Geometridae larva and a grasshopper are known to consume floral tissues). These herbivores may damage the corolla in several ways, affecting the upper and lower lips alone or both at the same time. Nectar robbery has been reported in several studies on L. vulgaris as a common type of damage to flowers (e.g. Stout et al., 2000; Newman and Thomson, 2005a, b). Nevertheless, no evidence of nectar robbery was found at the study site, and this does not seem to be an isolated example, since it is also absent in other distant (>150 km) L. lilacina populations (pers. obs.). For this reason, nectar robbery was not simulated as it does not occur naturally. Consequently, the type of floral herbivory simulated in this study is the only type of damage to reproductive tissues that was actually observed at the site.
Damage to corollas was simulated by assigning flowers to one of the following treatments (see Fig. 1B): UP, the upper lips were gently removed with a pair of scissors (Fig. 1B-1); LO, the lower lips were removed in the same way (Fig. 1B-2); BL, both upper and lower lips were removed (Fig. 1B-3); CTRL, flowers not suffering from either natural or experimental damage (i.e. resembling the absence of corolla herbivores, taken as controls and protected with bags when not under observation). All treatments were randomly replicated in at least four randomly selected flowers within each plant (range 4–8 flowers per treatment and plant, depending on flower availability and plant size). Treatments were applied approx. 1 d before the flowers opened (i.e. upper and lower lips expanded), and were fully available to pollinators. Overall, 928 flowers were tagged. Furthermore, 18 more plants in SR and 17 in OT were used to assess natural corolla herbivory during the study season. These plants were not manipulated, and were used to record the type and abundance of the different corolla herbivores, total flower production, total number of fully functional flowers damaged and type of damage inflicted (categorized as damage to lower lips, to upper lips or to both lips). Counts were made at weekly intervals throughout the experimental study period.
The unexpected and particularly adverse winter conditions experienced during the study season in the Mediterranean area in Southern Spain (unusual cold and dry weather during most of the late winter and spring) affected the phenology and physical conditions of many plants in the study site. Thus, some of them did not flower at all, while others were lost a few days after flowering began, or produced very few flowers. For these reasons, the initial number of plants was reduced to 27 in SR and 22 in OT; thus the number of tagged flowers was reduced by n=144. Further, in the plants left and due to such adverse weather, n=307 flowers were lost before fruit maturation. Thus, the final number of flowers considered to assess fruit set and seed production was 477 (n=262 in SR, range 52–76 flowers per treatment; and n=215 in OT, range 42–62 flowers per treatment).
Pollinator censuses and variables estimated
Use of the manipulated flowers by visitors was assessed by 5 min censuses conducted on all tagged plants in the two selected populations. Each day a plant was randomly chosen for the first count, then the rest were sequentially monitored several times a day. During each census, the species or genus of all visitors was noted, together with the floral treatment assigned to the visited flowers. Floral display (estimated as the number of functional flowers in each plant) was recorded every day before censuses started. The total number of censuses was 681 (298 at SR and 383 at OT), with 136 censuses yielding at least one visit (76 in OT and 60 in SR).
To assess whether experimental manipulations had an affect on insect visitation, a check was first made as to whether potential pollinators attempted to visit experimental flowers (i.e. whether corolla manipulation may be responsible for differences in flower selection by visitors). A visitation attempt was considered as being when an insect touched the corolla or landed on it. Secondly, an estimate was made of whether a visitation attempt resulted in a successful visitation (i.e. whether treatments imposed any limitations on insects actually to enter selected flowers). To this end, the flower handling time (HT) was recorded as the time elapsed between when an insect touched or landed on the flower and when it entered the corolla. If the visitor was finally unable to enter the corolla, this parameter estimates the time invested before the visitor rejected the flower (i.e. the visitation attempt failed). Finally, the time invested inside the flower in successful visitations was also recorded. Time was recorded using a digital stopwatch to the nearest 0·1 s. Overall, 528 visitation attempts were analysed.
Fruit set, fruit predation and seed production
Pollination success was assessed by estimating fruit and seed production. All flowers were surveyed every third day after the experimental treatment was applied to check for ovary enlargement (indicating a successful pollination). Thus, fruit production was considered as a binomial variable (fruit developed vs. not developed). Flowers with developing fruits were collected before fruit dehiscence, and the number of seeds produced was counted. Plant size, in terms of total flower production, was recorded at the end of the season.
Developing fruits of L. lilacina are used by Gymnaetron sp. (Curculionidae, Coleoptera) weevils for ovoposition before corolla abscission. For this purpose, weevils enter inside the flower through the throat in order to access the ovary. In order to test the effect of these herbivores on seed production, half of the experimental plants were sprayed with Syngenta Karate King (a lambda-cyhalothrin-based insecticide) when flowers were no longer functional. Spray was applied every third day. Previous observations have demonstrated that this product may be well tolerated by bees when diluted and applied according to the manufacturer's instructions, while it is effective against weevil attacks (although this effect may be highly variable). Fruit predation was detected by the presence of a weevil larva inside the mature fruit.
Data analyses
For the purposes of this study, experimental treatment, pollinator group (see Results), population and their interactions were considered as main effects in generalized linear mixed models. Since treatments were replicated within each plant, plants were used as a random grouping factor (i.e. as blocks, e.g. Herrera, 2000b), to account for non-independent data (see Pinheiro and Bates, 2000). Floral display (estimated as the daily number of functional flowers per plant) was used as a covariate in analyses involving pollinator visitation parameters, while plant size (estimated as total flower production) was used in analyses involving fruit and seed production. To account for multiple comparisons, P-values were calculated by model bootstrapping with 1000 repetitions. Statistical analyses were done using R 2·3·1. (R Development Core Team, 2005).
Plant visitation was analysed by testing the likelihood that a plant was visited or not during a given census (binomial response: visited vs. not visited). Use and handling of flowers within plants were analysed by linear models with logit link function, in order to test if experimental treatments imposed any limitation to insect visitors. Since the experimental groups were eventually unbalanced due to the flower losses mentioned above, the number of flowers left in each experimental group and in each plant was used as a factor weight. Fruit set and fruit predation events were also analysed by testing the proportion of experimental flowers developing fruit, the number of seeds produced per fruit and the likelihood that developing fruits were attacked by predators.
RESULTS
Incidence of natural corolla herbivory in the study populations
In SR, damaged flowers were detected in ten of the 18 plants used to asses natural incidence of corolla herbivory (55·55 %). In OT, 11 of the 17 plants used had damaged flowers (64·70 %). While the incidence of corolla herbivory did nor vary between populations (F1,292=0·59, P=0·44), the occurrence of the three types of damage differed (F2,292=9·37, P<0·001). Such differences were consistent between populations (type×population interaction: F2,292=0·46, P=0·63). Thus, removal of the lower lips, and the lower lips plus part of the upper lips, was the most frequent damage found (mean proportion±s.d.: 0·43±0·25 and 0·37±0·32, respectively; both populations pooled). Their occurrence was significantly higher than removal of the upper lips alone (0·07±0·12; both populations pooled; differences tested by post hoc Tukey tests).
Plant size (as measured by total flower production) had a significant effect on the absolute number of flowers affected by corolla herbivory (F1,292=28·66, P<0·001; larger plants had comparatively more flowers affected; coefficient: 0·045±0·008), but not on the proportion of flowers damaged (F1,292=3·25, P=0·08).
Flower visitors and overall plant visitation likelihood
Experimental plants were exclusively visited by Apis mellifera (n=324 records), Anthophora dispar (n=112), Anthophora acervorum (n=29) and Bombus terrestris (n=63). No other pollinators have been recorded visiting L. lilacina plants during three consecutive study seasons (A. M. Sánchez-Lafuente, unpubl. res). Both Anthophora species were pooled into a single group, given their similar size, and similar behaviour when visiting L. lilacina flowers.
The relative abundance of the three pollinator groups differed overall (F2,581=4·80, P<0·02) and between populations (F1,581=3·03, P<0·03), most probably due to differences in the relative abundance of A. mellifera. Nevertheless, there were no differences between populations in the probability that individual plants were visited (i.e. pollinators visiting any flower on a given plant; F1,581=0·07, P=0·79), although a larger floral display contributed to explain a higher visitation likelihood (F1,581=36·27, P<0·001; coefficient=0·015 ±0·004,±s.e.).
However, the number of flowers in a row visited in a plant (i.e. ‘geitonogamous’ visitations) was the highest for A. mellifera (8·29±5·24) followed by B. terrestris (4·20±3·41) and Anthophora (2·33±1·20). Differences between pollinator groups were significant (F2,581=4·38, P<0·04).
Visitation attempts and visitation success related to treatment and pollinator group
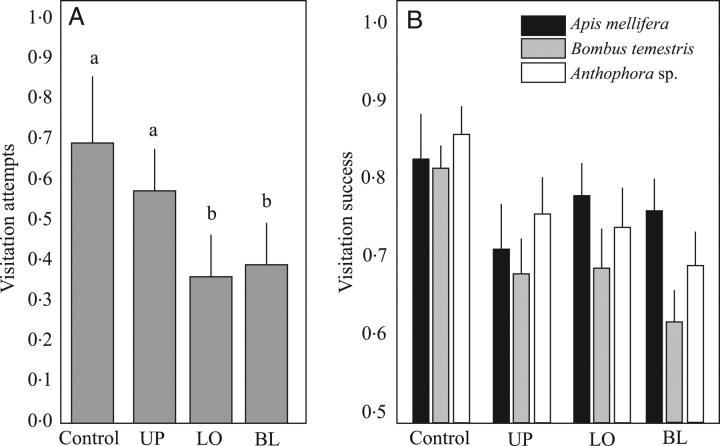
Flowers in the CTRL and UP groups received more visitation attempts than those in the BL and LO groups (post hoc Tukey tests; Table 1 and Fig. 2A). The effect of the treatment seemed to be consistent between populations and for all pollinator groups. An overall effect of the pollinator group was found, again most probably due to differences in the relative abundance of A. mellifera and its influence on visitation likelihood. Similarly, the interaction between pollinator group and population was presumably caused by differences in the relative abundance of A. mellifera between populations. Between-plant, variance (18·60) was considerably larger than that found within plants (8·54), and there were significant differences in the magnitude of the treatment effect among plants (Table 1). Larger floral display was again related to more visitation attempts (coefficient=0·018±0·006,±s.e.).
Table 1.
Results of the models testing for differences in visitation attempts (flower selection) and visitation success (flower visitation) related to experimental treatment, pollinator group and study population
| Fixed effects | Visitation attempts |
Visitation success |
||
|---|---|---|---|---|
| F3,438 | P | F3,408 | P | |
| Treatment (Tre) | 3·25 | 0·03 | 14·69 | 0·001 |
| Pollinator class (Pol) | 193·35 | 0·02 | 2·12 | 0·11 |
| Population (Pop) | 1·72 | 0·26 | 5·29 | 0·02 |
| Tre×Pol | 0·91 | 0·44 | 0·95 | 0·53 |
| Tre×Pop | 2·28 | 0·11 | 2·36 | 0·09 |
| Pol×Pop | 4·61 | 0·04 | 5·64 | 0·03 |
| Tre×Pol×Pop | 2·56 | 0·10 | 1·32 | 0·32 |
| Floral display | 6·09 | 0·01 | 0·29 | 0·59 |
| Block effect | L Ratio | P | L Ratio | P |
| Plant | 64·47 | 0·001 | 32·62 | 0·001 |
Individual plants were considered as blocks. P-values were calculated by bootstrapping (1000 repetitions). Significant effects are presented in bold.
Fig. 2.
(A) Mean number of flowers selected for a visitation attempt depending on the treatment (see Fig. 1 for abbreviations). All pollinators and populations pooled. Bars with different letters indicate significant differences. (B) Differences among pollinator groups and treatments in the likelihood that a visitation attempt resulted in a successful visitation. Both populations pooled. Data are means ±s.e.
A visitation attempt could be eventually successful (the flower is visited) or unsuccessful (the flower is rejected). Visitation attempts to control flowers were more likely to be successful compared with flowers with modified corollas (post hoc Tukey tests; Table 1 and Fig. 2B). The likelihood that a visitation attempt resulted in a successful visitation did not vary among pollinator groups (A. mellifera=0·78±0·41; Anthophora=0·81±0·42; B. terrestris=0·73±0·44). Successful visitations were more likely in the SR population (0·86±0·35) than in OT (0·67±0·47), while Anthophora achieved a higher visitation success in OT, and A. mellifera and B. terrestris in SR. Between-plant variance (1·09) was again larger than that found within plants (0·77). Differences in floral display did not contribute to explain the likelihood of a successful visitation.
Considering the likelihood of flower selection for a visitation attempt, and the likelihood that a visitation attempt was successful, flowers in the CTRL and UP groups were almost twice as likely to be successfully visited (54 and 40 %, respectively) than those in the LO and BL groups (25 and 26 %).
Differences in flower handling time between successful and unsuccessful visitations
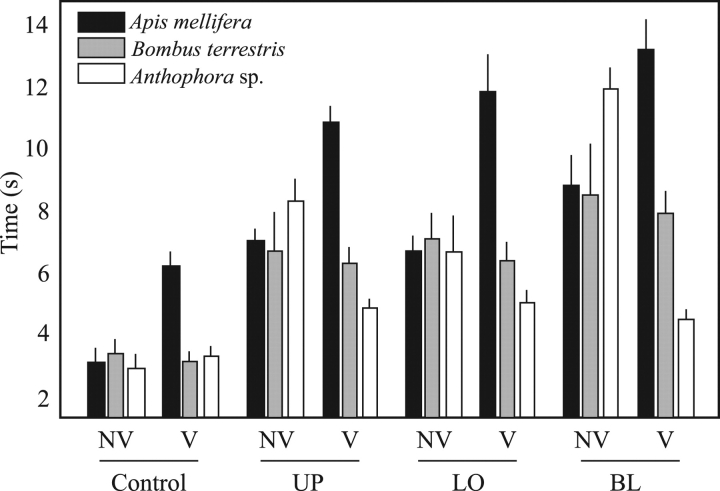
The difference in the time invested by visitors to achieve a successful visitation was strongly dependent on the pollinator group and the treatment (Table 2A). The overall trend suggests that Anthophora invested less time in achieving a successful visitation than in rejecting a flower, while A. mellifera did the opposite, and no difference was observed for B. terrestris (Fig. 3). The disproportionate HT of A. mellifera (Fig. 3) seemed responsible for the significant effects of pollinator group in the different interactions. However, the mean time that made the difference between a flower being visited or rejected was very short (about 1 s; mean±s.d.: successful=7·03±4·04; unsuccessful=6·06±4·69).
Table 2.
(A) Linear model testing the differences in HT as a function of the fate of the visitation attempts (i.e. whether a visitation attempt resulted in a flower visited or rejected), treatment, pollinator group and population; see also Fig. 3.
| Effects | Handling time (HT) |
|
|---|---|---|
| F3,408 | P | |
| Successful/unsuccessful (Suc) | 16·78 | 0·001 |
| Treatment (Tre) | 172·87 | 0·001 |
| Pollinator class (Pol) | 129·89 | 0·001 |
| Population (Pop) | 25·73 | 0·001 |
| Suc×Tre | 0·23 | 0·87 |
| Suc×Pol | 54·14 | 0·001 |
| Tre×Pol | 3·47 | 0·003 |
| Suc×Pop | 0·69 | 0·40 |
| Suc×Tre×Pol | 3·63 | 0·002 |
| Floral display | 54·28 | 0·001 |
| Block effect | L Ratio | P |
| Plant | 122·04 | 0·001 |
Fig. 3.
Differences in handling time between flowers visited (V, successful visitation attempt) and not visited (NV, failed visitation attempt) for each pollinator group and treatment (see Fig. 1 for other abbreviations). Both populations pooled. Data are means±s.e.
Significant interactions may reflect that pollinator groups are affected by the type of floral structure damaged in different ways, but only when flowers were rejected. However, considering successful visitations, the three manipulations imposed similar limitations to pollinators, with A. mellifera investing the most time, followed by B. terrestris and Anthophora (Fig. 3). Finally, the effect of floral display was significant, with flowers in plants with a larger floral display being handled for longer (coefficient=0·014±0·004, ±s.e.). Between-plant variance (28·04) was much larger than within-plant variance (6·33).
The time spent inside a flower also varied among pollinator groups (mean±s.d.: A. mellifera=6·34±4·35, Anthophora=2·03±1·22, B. terrestris=3·650±1·39; Table 2B) and between populations (mean±s.d.: SR=4·21±3·45, OT=3·05±3·17). The effect of floral display was again highly significant, with the duration of a visit to flowers in plants with a larger floral display being longer than those to plants with a smaller floral display (coefficient±s.e.=0·022±0·006). In contrast to previous findings, within-plant variance (37·48) was much higher than between-plant variance (6·89).
Table 2.
(B) Analyses of the time spent inside the flower experimental treatment, pollinator group and study population.
| Fixed effects | Time inside |
|
|---|---|---|
| F3,381 | P | |
| Treatment (Tre) | 1·18 | 0·31 |
| Pollinator class (Pol) | 22·78 | 0·001 |
| Population (Pop) | 4·81 | 0·03 |
| Tre×Pol | 0·61 | 0·72 |
| Tre×Pop | 0·96 | 0·41 |
| Pol×Pop | 0·60 | 0·55 |
| Tre×Pol×Pop | 1·17 | 0·33 |
| Floral display | 19·97 | 0·001 |
| Block effect | L Ratio | P |
| Plant | 12·42 | 0·001 |
Effects of treatment on flower and plant visitation rates
From the data obtained on the number of within-plant and between-plant visits to flowers with different treatments`, and the mean time invested to manipulate and visit flowers with different treatments, an estimate was made of the likely ‘visitation rate’ of each pollinator group on each flower depending on the treatment. Such visitation rates were defined according to two variables: number of flowers visited per minute (FVM) and number of plants visited per minute (PVM).
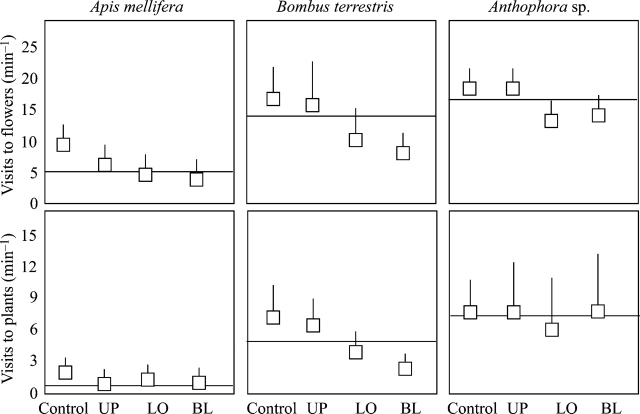
FVM varied depending on the treatment (F3,493=141·98, P<0·001), the pollinator group (F2,493=301·03, P<0·001) and the population (F1,493=43·01, P<0·001), although absolute differences between populations were subtle. Thus, pollinators visiting flowers in the CTRL group could achieve the highest flower visitation rate, while those visiting BL groups would achieve the lowest (Fig. 4). As for pollinator groups, A. mellifera could visit significantly fewer flowers per min (8·68±4·76) than Anthophora (17·58±5·83) and B. terrestris (16·66±8·88). The interaction between treatment and pollinator was significant (F3,493=3·43, P<0·001), suggesting that the treatments affected visitation rates in different ways (Fig. 4). No other interaction was significant, but the effect of floral display was significant (F1,493=8·89, P<0·004). Overall, flowers on plants with larger floral display may receive fewer visits per min than those on plants with a smaller floral display (coefficient±s.e.=–3·51×10−4±2·47×10−4).
Fig. 4.
Flower and plant visitation rates by each pollinator group depending on the treatment. See text for further considerations on these variables. Both populations pooled. Data are means±s.e. Horizontal lines indicate mean values across pollinator groups. See Fig. 1 for abbreviations.
Similar results were obtained from PVM. Pollinators visiting flowers in the CTRL group would achieve the highest plant visitation rates compared with those visiting other flowers (F3,493=44·19, P<0·001; Fig. 4). Again, pollinators differed in their ability in visiting plants (F2,493=415·09, P<0·001). While A. mellifera could visit on average 1·80±1·40 plants min−1, Anthophora could visit 7·90±4·78 and B. terrestris 4·52±3·29. The interaction between treatment and pollinator was again significant (F3,493=3·75, P<0·001); however, no differences were found between populations in plant visitation rates (F1,493=0·29, P=0·58). Floral display was significant in the same way as found above (F1,493=6·45, P<0·02, i.e. plant visitation rate was inversely related to floral display; coefficient±s.e.=–8·18×10−4±6·58×10−4).
Effects of treatments on fruit set, fruit predation and seed production
Flowers in the CTRL and UP groups were comparatively more likely to set fruit than those in the other treatment groups (Table 3; CTRL=0·65±0·47; UP=0·61±0·49; LO=0·48±0·50; BL=0·38±0·49). The treatment effect was consistent between populations (no significant treatment×population interaction). Further, there were no differences between populations (Table 3), with flowers in OT having similar probabilities to set fruit (0·60±0·50) to those in SR (0·55±0·48). The effect of plant size was not significant, and the variance explained was larger between (0·92) than within plants (0·58). Fruit set was not affected by spraying, indicating no detrimental effects of the insecticide used on pollination services.
Table 3.
Differences in fruit set, fruit predation likelihood and seed production related to experimental treatment and study population
| Fixed effects | Fruit set |
Fruit predation |
Seed production |
|||
|---|---|---|---|---|---|---|
| F3,408 | P | F3,408 | P | F3,392 | P | |
| Treatment (Tre) | 5·58 | 0·003 | 1·83 | 0·24 | 0·11 | 0·98 |
| Population (Pop) | 0·06 | 0·84 | 0·63 | 0·84 | 35·18 | 0·003 |
| Fruit set (FrS) | – | – | 9·75 | 0·002 | – | – |
| Sprayed | 0·33 | 0·56 | 38·19 | 0·001 | 0·16 | 0·70 |
| Fruit predation (FrP) | – | – | – | – | 15·54 | 0·001 |
| Tre×Pop | 2·34 | 0·24 | 1·52 | 0·38 | 0·58 | 0·67 |
| Tre×FrS | – | – | 0·38 | 0·82 | – | – |
| Tre×FrP | – | – | – | – | 1·09 | 0·36 |
| Plant size | 0·20 | 0·65 | 2·89 | 0·08 | 0·41 | 0·52 |
| Block effect | L Ratio | P | L Ratio | P | L Ratio | P |
| Plant | 14·45 | 0·001 | 6·79 | 0·01 | 1·23 | 0·27 |
Individual plants were considered as blocks. P-values were calculated by bootstrapping (1000 repetitions). Significant effects are presented in bold.
The likelihood of fruit predation did not depend on the treatment, but was related to fruit set (i.e. developing fruits were subject to much more predation than those not developing). In fact, it is possible that manyof the fruits which had been sujected to predation that were eventually considered as not developing were actually successfully pollinated flowers damaged or aborted at very early stages of fruit development. Fruits on sprayed plants were significantly less likely to suffer predation by weevils (Table 3; 0·26±0·14) than those on plants not sprayed (0·40±0·29). Again, no differences were found between populations regarding the significant effects. Between-plant variance was larger (0·87) than within-plant variance (0·33).
Finally, no effect of the treatment was detected on seed production per flower (24·78±10·39 seeds per fruit; Table 3), although significant differences were found between populations. Thus, mean seed production per flower was higher in SR (28·20±8·64) compared with OT (20·53±10·85). Fruits that had been subject to predation produced significantly fewer seeds (mean=11·61±9·78,±s.d.) than those that had not (27·26±8·77). Further, seeds from fruits which hade undergone predation have a very limited germination ability (pers. obs.). The effect of plant size was not significant, while the variance explained was much larger between (86·37) than within plants (4·11).
DISCUSSION
One of the major aims of this study was to examine whether artificial modifications of a complex flower, that mimicked natural damage, may alter visitation ability by pollinators and fruit predators, and whether such eventual limitations may affect plant fitness in terms of seed production. Among the most usual types of damage found in L. lilacina populations are those that were replicated experimentally in this study, affecting upper and/or lower corolla lips in different ways. This study shows that removal of the lower lips (either alone or in combination with the upper lips) may have detrimental effects on flower selection and visitation by pollinators, and consequently on fruit set (see below), compared with intact flowers or with flowers with only the upper lips removed. However, none of the experimental manipulations affected the likelihood of fruit predation, thus suggesting that access to flowers of specialized weevils ovopositing in developing fruits is not limited by the corolla modifications accounted for in this study. Thus, this study belongs with those addressing the additive and negative effects of floral herbivory and fruit predation on plant fitness via a reduction in seed production (e.g. Mahoro, 2003).
Effects of corolla herbivory on flower selection
Flowers with lower lips removed were overall less likely to be selected for a visitation attempt than those showing no damage or damage to the upper lips, although the magnitude of the effect varied among plants. Further, the likelihood that a visitation attempt was eventually successful was lower for flowers with lower lips removed, and this effect was consistent among plants. Consequently, L. lilacina flowers may be selected by pollinators according to cues related to the perception and use of certain corolla traits. However, although phenotypic selection of floral traits has been commonly reported in the literature (e.g. Andersson and Widen, 1993; Herrera, 1993), recent studies have suggested that visitors may not discriminate among flowers based exclusively upon morphological cues (e.g. Herrera, 1996, 2001). There are two known limitations of the present study. First, it was not specifically designed to detect pollinator preferences according to phenotypic differences among flowers. Hence, it is difficult to assess whether floral selection may be based exclusively upon pollinator perception of corolla traits or upon mechanical limitations imposed by removal of the lower lip. If the function of the lower corolla lips differs from that of the upper lips (see also Herrera, 2001), then pollinators' response may originate simply by the presence/absence of suitable structures on which to land. Alternatively, preferences may be caused by the presence/absence of nectar guides, located in the lower lips, and thus removed by experimental manipulations. Coloured nectar guides (bluish-to-yellow; variation is common among populations) are usually the first structures consumed by corolla herbivores. These results suggest a future direction in the study of the different functions of floral parts in this species. Secondly, the possibility that flower damage may be a direct cause of fruit losses was not explored. However, while green persistent sepals may contribute to fruit and seed development (Herrera, 2005), this role as a photosynthetic tissue is unlikely in this particular case, since natural corolla abscission occurs 2–4 d after successful pollination, while fruit maturation takes 14–24 d.
Effects of corolla herbivory on flower handling
The effects of corolla herbivory may also be estimated by analysing flower handling, which may be considered as an indirect estimator of the cost/benefit ratio to obtain a floral reward. Although floral herbivory may affect floral longevity (thus reducing the time the reward is available to pollinators), no influence of the treatments was assumed on the amount or quality of the reward (samples taken from naturally damaged and undamaged flowers did not differ; A. M. Sánchez-Lafuente, unpubl. res.). HT to access flowers was strongly dependent on pollinator group and treatment. This was particularly the case for A. mellifera, which disproportionately increased HT on modified flowers (Fig. 4). This behaviour may be explained by the absence of alternative flowering plants in the study site during most of the L. lilacina flowering season (except for Prunus dulcis, Rosaceae, heavily used by honey bees while flowering), which forced bees to visit the only flowering species available. Although HT was shorter overall in rejected than in visited flowers for all pollinators (except for A. mellifera), there is a cost associated with rejection, because visitors also invested some time on rejected flowers. However, despite the increased cost associated with handling of experimental flowers, all pollinator groups visited a similar proportion of flowers in each treatment (no significant treatment×pollinator interaction; Table 1). In other words, despite the significant differences among visitors in the cost associated with visiting flowers with modified corollas, all pollinators had the same ability to visit experimental flowers.
Effects of corolla herbivory on flower and plant visitation rates
Anthophora and B. terrestris were capable of much higher flower and plant visitation rates (for visitation rates of different Bombus species to L. vulgaris see Stout et al., 2000; Newman and Thomson, 2005a) than A. mellifera, particularly on damaged flowers. However, the likelihood of a successful visitation did not vary among pollinator groups, although available data suggest that there may be differences among them in their ability to set seeds (A. M. Sánchez-Lafuente, unpubl. res). Thus, these three pollinator groups vary in the number of flowers visited in a row within a given plant. Considering that L. lilacina is self-incompatible, the higher number of ‘geitonogamous’ visitations may render ineffective a number of visitations. As the likelihood of making ‘geitonogamous’ visitations increases, so does the likelihood of acting as an illegitimate pollinator, consuming rewards without successfully pollinating. Further, if plants in the study populations have a patchy distribution of genetic diversity (as found for a closely related species, e.g. Torres et al., 2003; see also Cresswell et al., 2002), pollinators visiting plants located within a short range may be more ineffective than those visiting plants separated by longer distances, as the risk of consanguinity may be inversely related to the distance between plants.
Effects of corolla herbivory on fruit predation and seed production
This study has shown that differences in experimental manipulations of corolla design, imitating natural floral herbivory, may cause differences in flower selection by potential pollinators, and differences in fruit set among plants. Since pollinator efficiency on a per fruit basis (as measured by the number of seeds per fruit) was similar among flowers, regardless of the treatment, the overall consequence is that individual differences in floral herbivory may promote a variable reduction in plants' overall seed production. Thus, differences in the proportion of flowers affected by herbivory, and in the intensity of the damaged inflicted on affected flowers, may result in different opportunities for reproduction for plants in different seasons.
Current data for the study species suggest that, while fruit set is positively correlated with plant size (in terms of total flower production), fruit predation rates only increase marginally as fruit set does (A. M. Sánchez-Lafuente, unpubl. res.). Some studies have suggested that seed predation may have stronger direct effects on seed set than pollination, and that plant size and the effects of seed predators are generally positively correlated (see Cariveau et al., 2004). Fruit predation thus appears as a major reason for seed losses in this species, as suggested by other related plant species in which pre-dispersal seed predation is frequent (Cariveau et al., 2004), although larger plants may have an advantage over smaller ones, since more fruits may escape herbivory as plant size increases.
At least two factors may attenuate the antagonistic effect of fruit predators on seed production: first, the flowering phenology. While this study was carried out during the peak of abundance of pollinators and herbivores for practical reasons, observations suggest that early flowers may escape from fruit predation at the cost of producing fewer seeds per fruit, since both pollinators and fruit predators are scarce and limited by temperature (pers. obs.). In contrast, later flowers may benefit from a more abundant pollinator assemblage and produce more seeds per fruit, but at the cost of a higher predation likelihood. Secondly, within-plant variation in floral traits during the flowering season may influence temporal variation in seed production (e.g. Breadmore and Kirk, 1998) associated with differences in preferences and abundance of pollinators and predators. The combined effect of flowering phenology and temporal variation in floral traits, on pollinator selection, fruit predation and maternal success is yet to be analysed.
The interaction between mutualisms and antagonisms
The interaction between pollinators and floral herbivores has been suggested to affect plant populations in both demographic and evolutionary terms (e.g. Galen and Cuba, 2001; Kelly and Dyer, 2002), with examples of conflicting selection between both interacting agents. Further, some hypotheses presume that correlated evolution of traits favouring mutualistic relationships while avoiding antagonistic ones may play an important role in evolutionary change (see Herrera et al., 2002). In the present study, a conflicting selection would be likely if traits contributing to herbivore avoidance also contributed to pollinator rejection. Alternatively, if herbivores and pollinators are attracted by different traits, then individual plants exhibiting the ‘right’ trait combination to avoid herbivores while attracting pollinators would be favoured. Although non-additive effects regarding different estimators of female fecundity have been found between mutualistic and antagonistic agents in plant populations (namely pollinators and herbivores; e.g. Herrera, 2000a; Gómez, 2005), studies addressing non-additive effects between antagonistic agents (i.e. herbivores) are rare (but see Ingvarsson and Ericson, 2000; Frey, 2004). However, it was found that both herbivory events analysed may have additive effects, and there seems to be no conflict between the pre-dispersive herbivory events accounted for in this study. However, although the most frequent corolla damage found in natural L. lilacina populations does not seem to have any direct effect on the likelihood of fruit predation, corolla herbivory has detrimental effects on fruit set via a reduction in visitation likelihood by pollinators; hence there may be an indirect effect on fruit predation caused by a reduced pollination success in flowers with modified corollas.
Supplementary Material
ACKNOWLEDGEMENTS
I am most grateful to J. Herrera, J. Alcántara, P. Rey and two anonymous reviewers for valuable comments on an earlier version of this manuscript, and to A. Simarro for her help during field work. This project is funded by the Spanish Ministerio de Educación y Ciencia (grant BOS2003-00292).
LITERATURE CITED
- Aigner PA. Optimality modelling and fitness trade-offs: when should plants become pollinator specialists? Oikos. 2001;95:177–184. [Google Scholar]
- Andersson S, Widen B. Pollinator-mediated selection on floral traits in a synthetic population of Senecio integrifolius (Asteraceae) Oikos. 1993;66:72–79. [Google Scholar]
- Breadmore KN, Kirk WDJ. Factors affecting floral herbivory in a limestone grassland. Acta Oecologica. 1998;19:501–506. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. Components of phenotypic selection: pollen export and flower corolla width in. Ipomopsis aggregata. Evolution. 1991;45:1458–1467. doi: 10.1111/j.1558-5646.1991.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Cariveau D, Irwin RE, Brody AK, Sevillano García-Mayeya L, Ohe A. Direct and indirect effects of pollinators and seed predators to selection on plant and floral traits. Oikos. 2004;104:15–26. [Google Scholar]
- Cresswell JE, Osborne JL, Bell SA. A model of pollinator-mediated gene flow between plant populations with numerical solutions for bumblebees pollinating oilseed rape. Oikos. 2002;98:375–384. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Frey FM. Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae) Evolution. 2004;58:2426–2437. doi: 10.1111/j.0014-3820.2004.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Galen C, Cuba J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot. Polemonium viscosum. Evolution. 2001;55:1963–1971. doi: 10.1111/j.0014-3820.2001.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Goldblatt P, Manning JC. The long-proboscid fly pollination system in southern Africa. Annals of the Missouri Botanical Garden. 2000;87:146–170. [Google Scholar]
- Gómez JM. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysimum mediohispanicum: consequences for plant specialization. American Naturalist. 2003;162:242–256. doi: 10.1086/376574. [DOI] [PubMed] [Google Scholar]
- Gómez JM. Non-additive effects of herbivores and pollinators on Erysimum mediohispanicum (Cruciferae) fitness. Oecologia. 2005;143:412–418. doi: 10.1007/s00442-004-1809-7. [DOI] [PubMed] [Google Scholar]
- Herrera CM. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs. 1993;63:251–275. [Google Scholar]
- Herrera CM. Floral biology. Studies on floral evolution in animal-pollinated plants. New York: Chapman & Hall; 1996. Floral traits and plant adaptations to insect pollinators: a devil's advocate approach. In: Lloyd DG, Barret SCH, eds; pp. 65–87. [Google Scholar]
- Herrera CM. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology. 2000a;81:2170–2176. [Google Scholar]
- Herrera CM. Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology. 2000b;81:15–29. [Google Scholar]
- Herrera CM. Deconstruction of a floral phenotype: do pollinators select for corolla integration in Lavandula latifolia? Journal of Evolutionary Biology. 2001;14:574–584. [Google Scholar]
- Herrera CM. Post-floral perianth functionality: contribution of persistent sepals to seed development in Helleborus foetidus (Ranunculaceae) American Journal of Botany. 2005;92:1486–1491. doi: 10.3732/ajb.92.9.1486. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Medrano M, Rey PJ, Sánchez-Lafuente AM, García MB, Guitián J, et al. Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits; Proceedings of tne National Academy of Sciences of the USA; 2002. pp. 16823–16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson PK, Ericson L. Exploitative competition between two seed parasites on the Common sedge. Carex nigra. Oikos. 2000;91:362–370. [Google Scholar]
- Kelly CA, Dyer RJ. Demographic consequences of inflorescence-feeding insects for Liatris cylindracea, an iteroparous perennial. Oecologia. 2002;132:350–360. doi: 10.1007/s00442-002-0948-y. [DOI] [PubMed] [Google Scholar]
- Krupnick GA, Weis AE, Campbell DR. The consequences of floral herbivory for pollinator service to. Isomeris arborea. Ecology. 1999;80:125–134. [Google Scholar]
- Lethilä K, Strauss SY. Effects of foliar herbivory on male and female reproductive traits of wild radish. Raphanus raphanistrum. Ecology. 1999;80:116–124. [Google Scholar]
- Mahoro S. Effects of flower and seed predators and pollinators on fruit production in two sequentially flowering congeners. Plant Ecology. 2003;166:37–48. [Google Scholar]
- Mayfield MM, Waser NM, Price MV. Exploring the ‘most effective pollinator principle’ with complex flowers: bumblebees and. Ipomopsis aggregata. Annals of Botany. 2001;88:591–596. [Google Scholar]
- Mitchell RJ, Shaw RG, Waser NM. Pollinator selection, quantitative genetics, and predicted evolutionary responses of floral traits in Penstemon centranthifolius (Scrophulariaceae) International Journal of Plant Sciences. 1998;159:331–337. [Google Scholar]
- Mothershead K, Marquis RJ. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in. Oenothera macrocarpa. Ecology. 2000;81:30–40. [Google Scholar]
- Mutikainen P, Delph LF. Effects of herbivory on male reproductive success in plants. Oikos. 1996;75:353–358. [Google Scholar]
- Newman DA, Thomson JD. Effects of nectar robbing on nectar dynamics and bumblebee foraging strategies in Linaria vulgaris (Scrophulariaceae) Oikos. 2005a;110:309–320. [Google Scholar]
- Newman DA, Thomson JD. Interactions among nectar robbing, floral herbivory and ant protection in. Linaria vulgaris. Oikos. 2005b;110:497–506. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-plus. New York: Springer Verlag; 2000. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2005 R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org . [Google Scholar]
- Sáez L, Crespo MB. A taxonomic revision of the Linaria verticillata group (Antirrhineae, Scrophulariaceae) Botanical Journal of the Linnean Society. 2005;148:229–244. [Google Scholar]
- Sánchez-Lafuente AM. Floral variation in the generalist perennial herb Paeonia broteroi (Paeoniaceae): differences between regions with different pollinators and herbivores. American Journal of Botany. 2002;89:1260–1269. doi: 10.3732/ajb.89.8.1260. [DOI] [PubMed] [Google Scholar]
- Stebbinns GL. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Stout JC, Allen JA, Goulson D. Nectar robbing, forager efficiency and seed set: bumblebee foraging on self incompatible plant Linaria vulgaris (Scrophulariaceae) Acta Oecologica. 2000;21:277–283. [Google Scholar]
- Strauss SY. Floral character link herbivores, pollinators, and plant fitness. Ecology. 1997;78:1640–1645. [Google Scholar]
- Strauss SY, Irwin RE. Ecological and evolutionary consequences of multispecies plant–animal interactions. Annual Review of Ecology, Evolution and Systematics. 2004;35:435–466. [Google Scholar]
- Strauss SY, Conner JK, Rush SL. Floral herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. American Naturalist. 1996;147:1098–1107. [Google Scholar]
- Torres E, Iriondo JM, Escudero A, Pérez C. Analysis of within-population spatial genetic structure in Antirrhinum microphyllum (Scrophulariaceae) American Journal of Botany. 2003;90:1688–1695. doi: 10.3732/ajb.90.12.1688. [DOI] [PubMed] [Google Scholar]
- Valdés B. Revisión de las especies europeas de Linaria con semillas aladas. 1970 Anales de la Universidad Hispalense no. 7, Universidad de Sevilla. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1070. [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.