Abstract
Background
Myrothamnus flabellifolia is unique as the only woody resurrection plant. It is an important plant in southern Africa because of its widespread occurrence and usage in African medicine and traditional culture. Many reports have investigated facets of its biology and the mechanisms associated with its desiccation tolerance.
Scope
The general biology of the woody resurrection plant Myrothamnus flabellifolia is reviewed. The review focuses on the geography and ecology, systematic placement, evolution, morphology and reproductive ecology of M. flabellifolia as well as the wood anatomy and re-filling mechanism. In addition, the desiccation tolerance, ethnobotanical importance and medicinal properties of the plant are reviewed. Also, future research avenues are suggested, in particular the necessity to research the biogeography and systematics of the species and the role of the polyphenols present, as well as the molecular basis of the plant's desiccation tolerance.
Key words: Myrothamnus flabellifolia, distribution, ecology, morphology, reproductive biology, wood anatomy, medicinal properties, desiccation tolerance, resurrection
INTRODUCTION
Myrothamnus flabellifolia (Welw.) is a relatively large resurrection plant, a woody shrub between 0·5 m and 1·5 m tall (Sherwin et al., 1998) that grows on rock inselbergs (Porembski and Barthlott, 2000) throughout southern Africa (Weimarck, 1936; Van Wyk et al., 1997; Glen et al., 1999). The plant was first recorded in 1859 by Friedrich Welwitsch, who named the plant Myrothamnus (myron meaning aromatic and thamnos meaning bush) flabellifolia (meaning fan-like leaves) (Puff, 1978a; Glen et al., 1999), the leaves having a balsamic-like odour (Puff, 1978a; Glen et al., 1999). Weiss (1906) was the first to note the ‘miraculous manner’ with which the desiccated plant revived when supplied with water (Fig. 1A, B). Myrothamnus flabellifolia occupies an important position in traditional African folklore and medicine (Watt and Breyer-Brandwijk, 1962; Hutchings, 1996; Van Wyk et al., 1997). The Zulu name for the plant is ‘uvukwabafile’ (wakes from the dead). The reviving ability is believed to be passed on to the ill person during treatment (Hutchings, 1996; Van Wyk et al., 1997). The plant is a geophyte possessing an extensive root system which extends into the crevices of the rocky slopes upon which it grows (Child, 1960; Glen et al., 1999). Myrothamnus flabellifolia can dehydrate its vegetative tissue, in particular its leaves, to an air-dry state. In this state, the leaves and stem segments curl and change colour from green to dull-brown (Farrant et al., 1999; Glen et al., 1999). When water is provided to the roots the plant re-hydrates its desiccated tissue and returns to its original colour and shape (Glen et al., 1999; Farrant et al., 2003). Since the last review on M. flabellifolia was written many years ago (Puff, 1978a) and since considerable work has been published in the last decade, this review focuses on recent advances in the understanding of the physiology, biochemistry and chemistry of M. flabellifolia.
Fig. 1.
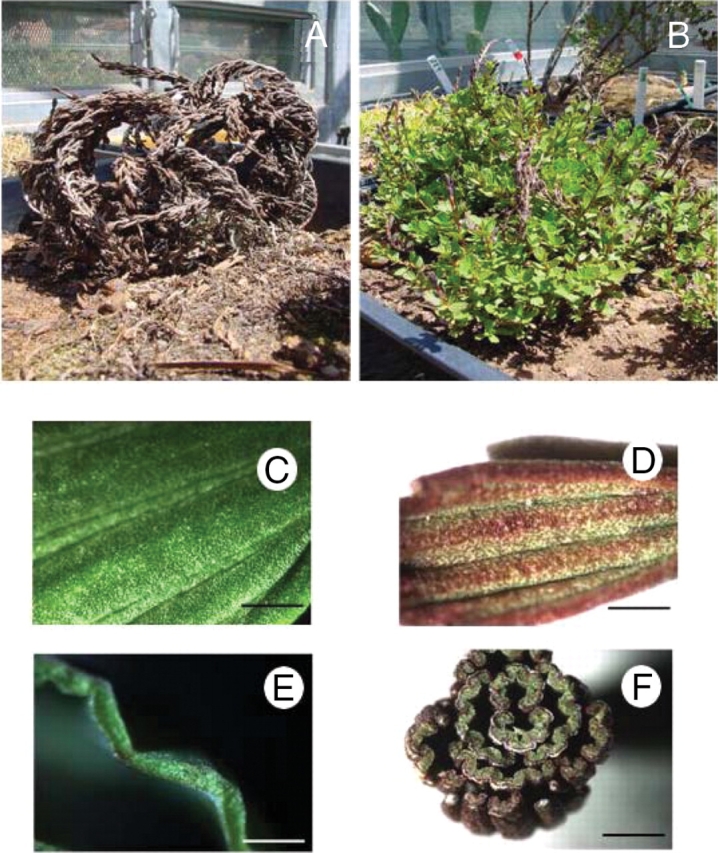
(A, B) The structure of the whole resurrection plant M. flabellifolia, in (A) the desiccated and (B) the hydrated state. (C–F) Light micrographs of surface and transverse sections of hydrated M. flabellifolia leaves. (C) Adaxial surface of hydrated leaf; (D) adaxial surface of desiccated leaf; (E) transverse section of a hydrated leaf; (F) transverse section of a desiccated leaf. Scale bars: C=1 mm; D=0·5 mm; E, F=3 mm.
GEOGRAPHICAL DISTRIBUTION AND ECOLOGY
Myrothamnus flabellifolia is usually found growing between 900 and 1200 m a.s.l. (Puff, 1978a) throughout the mountainous regions of central and southern Africa (Weimarck, 1936; Glen et al., 1999), although populations growing at altitudes greater than 2000 m have been reported (Schneider et al., 2000). The distribution of the plant is disjunct, with populations occurring along the west coasts of Namibia and Angola separated by the Kalahari Desert and low-lying central regions from the eastern populations. These occur in South Africa, Botswana, Zimbabwe, Mozambique, Malawi, Tanzania and Kenya (Weimarck, 1936; Puff, 1978a; Glen et al., 1999) with isolated populations reported to occur in Zambia and south-eastern Congo (Lisowski et al., 1970). Myrothamnus flabellifolia plants occur singly or in colonies with extensive root systems that extend into the crevices of the rocky outcrops with soil depths of around 15 cm (Child, 1960; Goldsworthy, 1992). Their roots are able to intercept water draining into these hollows after rainfall, thereby initiating re-hydration. Erosion debris and mammalian droppings have been commonly observed around the root network (Child, 1960) suggesting a means by which plants obtain vital nutrients such as nitrogen. The mammalian droppings and debris probably accumulate because of wind and rain as the plants are reportedly avoided by most herbivores and pests probably owing to the high phenolic content of the leaves. However, beetles have been observed chewing the leaves (Child, 1960) and beetle pupae have been found attached to plant stems (J. P. Moore, personal observation). Whereas the eastern populations are found in rock inselbergs, which have specific granitic and quartzitic geological sequences (Farrant and Kruger, 2001), Namibian plants are found growing in shale in mountainous regions such the Khomas Hochland and the Spitzkoppe (Puff, 1978a). Myrothamnus flabellifolia is considered to be an initial colonizer of the bare rock slopes and it has been suggested that, once established, it provides growth opportunities for other plant species such as grasses (Child, 1960; Porembski and Barthlott, 2000). The exposed rock outcrops where M. flabellifolia is found are exposed to considerable day/night temperature extremes, together with high light intensities and an erratic water supply (Child, 1960; Goldsworthy, 1992; Farrant and Kruger, 2001). The rainfall conditions of dry winter months with summer rains necessitate that plants must exist in a desiccated quiescent state for extended periods (Goldsworthy, 1992; Glen et al., 1999), the length of which varies between the geographic regions in which the plants occur (Puff, 1978a; Farrant and Kruger, 2001). Although most areas experience reliable annual rainfall, certain parts of Namibia are particularly arid, experiencing rain less frequently than biannually (Puff, 1978a).
SYSTEMATIC PLACEMENT AND EVOLUTION
The taxonomic position of the Myrothamnaceae has been uncertain ever since its discovery by Welwitsch (Welwitsch, 1859), who initially considered M. flabellifolia to belong in the family Saxifragaceae and to be closely related to the genus Myrica on account of its shrub-like shape and aromatic leaves. Sonder re-aligned Myrothamnus with the genus Cliffortia in the Saxifragaceae (Glen et al., 1999) as both plants possess superficial similarities, although the leaf shapes and arrangements are quite dissimilar. Reports of populations of M. flabellifolia occurring in the Cape Province (Bywater, 1984; Mendes, 1978) are incorrect and probably due to confusion with Cliffortia species. More recent anatomical studies of the stem and leaf structure suggested the placement of M. flabellifolia in the Hamamelidaceae (Glen et al., 1999). Although this had merit since many Hamamelidaceae species occur in southern Africa (Kubitzki, 1993), phylogenetic analysis led to the conclusion that M. flabellifolia was not a member of this family and was more closely related to the angiosperm genus Gunnera (Qui et al., 1998). Gunnera is one of the oldest genera of flowering plants, consisting of approx. 30–40 species primarily distributed in the southern hemisphere (Wanntorp et al., 2001; Wanntorp and Wanntorp, 2003). Gunnera perpensa, a large-leaved aquatic plant, has a similar geographical distribution to M. flabellifolia (Van Wyk et al., 1997). Fossil evidence combined with phylogenetic analysis has dated the Gunnera–Myrothamnus divergence at 120 million years ago (Wanntorp et al., 2001). However, recent morphometric analysis of the Gunnera has led Fuller and Hickey (2005) to disagree with this postulate.
The first systematic and biogeographical study of M. flabellifolia (Weimarck, 1936) proposed the presence of three subspecies, namely M. flabellifolia sensu stricto, M. flabellifolia elongata and M. flabellifolia robusta, although it was noted that M. flabellifolia is a phenotypically diverse species for which it was difficult to provide definitive classification. Since the former two subspecies were found on both sides of the Kalahari Desert divide (Weimarck, 1936), Puff reappraised this classification and proposed that M. flabellifolia elongata was in fact M. flabellifolia sensu stricto (Puff, 1978a). In addition, another member of the Myrothamnaceae, the rare M. moschata, is present in Madagascar (Glen et al., 1999). Little is known of this species other than that it resembles M. flabellifolia in morphology, as no extensive scientific investigation has been performed (Glen et al., 1999).
Recently, an analysis has been carried out on the polyphenols present and the DNA sequence of an intergenic region of the chloroplast genome of plants collected in Namibia and in South Africa (Moore et al., 2005a). It was found that all plants collected in Namibia had different polyphenols present and an altered DNA sequence compared with plants collected in South Africa, although the plants were very similar morphologically. Myrothamnus flabellifolia specimens from Mozambique and Malawi were morphologically similar, large plants with extremely large leaves and reproductive structures, in keeping with the description of M. flabellifolia robusta; in contrast, plants collected in Zimbabwe had extremely small leaves and may well be a new subspecies (J. P. Moore et al., unpubl. res.). At present, the polyphenol composition and the DNA sequences of all these populations are being analysed to ascertain whether they are biologically distinct.
GENERAL MORPHOLOGY AND REPRODUCTIVE BIOLOGY
The general morphology of M. flabellifolia has been reported previously (Mendes, 1978; Bywater, 1984; Goldsworthy, 1992; Glen et al., 1999). This reveals anatomical features suggestive of desiccation tolerance and reproductive ecology (Figs 2 and 3). The leaves are wedge-shaped with crenate–dentate leaf apices and are flabellate with five to seven folds. The unique arrangement of alternating ridges and furrows allows the leaves to fold parallel to the leaf face upon dehydration (Fig. 1). This probably facilitates a rapid return to the hydrated shape. The plants are dioecious (Glen et al., 1999) with diminutive catkin-like inflorescences usually occurring on short lateral branches. The male flowers, which consist of three to six stamens with reddish anthers that dehisce longitudinally, produce copious amounts of yellow tricolpate pollen grains at maturity. The female flowers are zygomorphic and consist of three basally attached carpels with papillose/spathulate stigmas that are reddish-purple and feather-like in appearance. Although the features of the male flowers would suggest wind pollination as the main mechanism of fertilization, zygomorphic female flowers would suggest insect pollination. It has been reported (Child, 1960) that bees visit the male flowers for pollen and that beetles and termites are found associated with the plant (J. P. Moore and G. G. Lindsey, personal observations). Thus the actual mode of pollination is not clear, although it has been suggested that M. flabellifolia is fairly promiscuous in its means of achieving fertilization (Puff, 1978a). Such a strategy might be required owing to the limited period within which the plant has to grow and reproduce. The fruits are three-lobed coriaceous, dehiscent capsules, which are slightly larger than the carpels at anthesis. The seeds, which are 0·3- to 0·5-mm-long ovoids possessing wrinkled coats, appear to be wind-dispersed and have been reported to germinate after 10–15 d at 25 ºC (Goldsworthy, 1992).
Fig. 2.
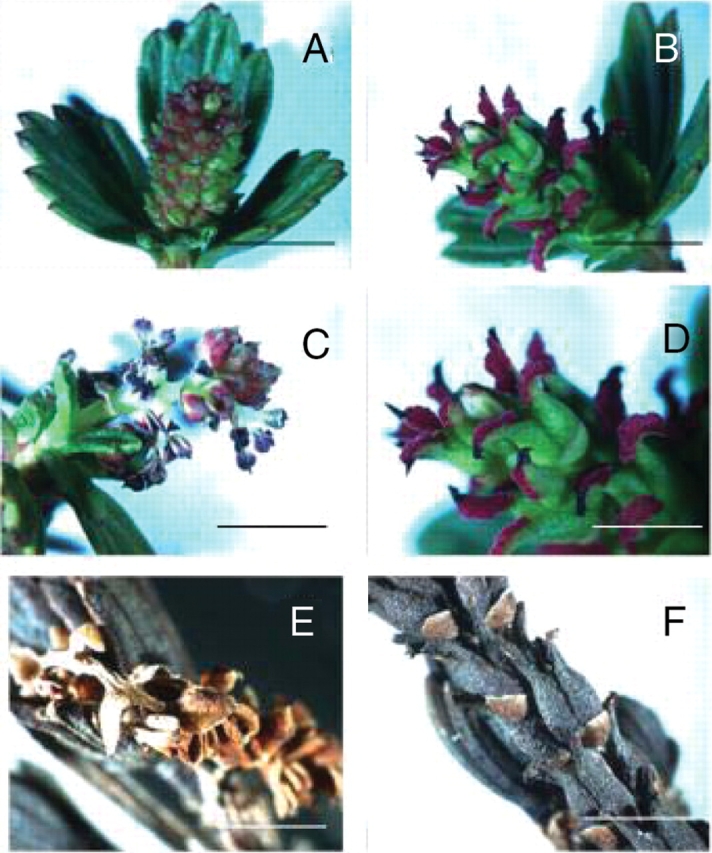
Hydrated male (A, C) and female (B, D) flowers and desiccated male (E) and female (F) flowers of Myrothamnus flabellifolia. (A) and (C) represent immature and mature (with pollen released) male flowers respectively; (B) and (D) represent mature female flowers with (D) at higher magnification showing the reddish-purple stigma. Scale bars: A–C=10 mm; D, F=5 mm; E=2·5 mm.
Fig. 3.
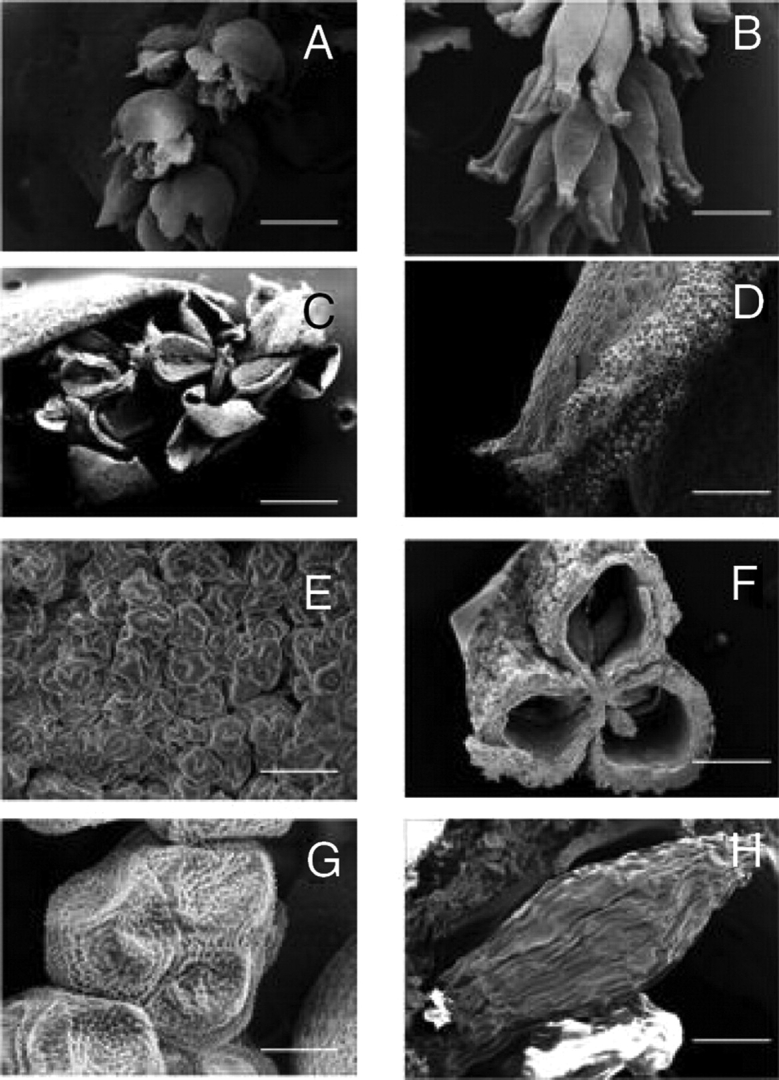
Scanning electron micrographs of flash-frozen male (A, C, E, G) and female (B, D, F, H) reproductive structures of Myrothamnus flabellifolia. Micrographs represent male flowers releasing pollen (A, C) and pollen grains (E, G) as well as female flowers at maturation (B, D), cross-section through a fruit showing the seed chambers (F) and a seed (H). Scale bars: A=1000 µm; B=1200 µm; C=600 µm; D=300 µm; E, H=50 µm; F=400 µm; G=10 µm.
WOOD ANATOMY AND REFILLING MECHANISM
Myrothamnus flabellifolia undergoes a rapid transformation after rainfall from an air-dry metabolically inactive state to a fully transpiring, re-hydrated state (Child, 1960; Glen et al., 1999). The absorption of water only occurs via the roots, desiccated leaves being unable to absorb water supplied to their external surfaces (Child, 1960). Since M. flabellifolia is the only resurrection plant possessing a woody stem, the ability to refill its xylem conduits and to transport water to the leaves is unique (Sherwin et al., 1998; Schneider et al., 2000). Water transport is probably aided by the presence of lipids in the xylem, these lipids preventing irreversible cavitation and facilitating re-hydration (Sherwin et al., 1998; Schneider et al., 2003). The very low hydraulic conductivity of the xylem vessels required for re-hydration of this species might also be due to the presence of these lipids (Sherwin et al., 1998).
NMR spectroscopy of hydrating branches revealed that lipids within the stem were distributed in two different zones: an inner zone of water non-dispersible lipids and an outer zone of water-dispersible lipids (Schneider et al., 2003). Lipids have been found to line the xylem element inner walls and lipid inclusions have been observed within the inter-vessel pits and tracheid edges (Wagner et al., 2000). The knob-like structures previously observed by SEM on the outer surface of the xylem vessels (Sherwin et al., 1998) are possibly these same lipophilic structures. Lipid-containing cells have been observed adjacent to leaf vascular tissue (Wilson and Drennan, 1992), and a unique wax-like filamentous network coating the exterior walls of spongy mesophyll cells of hydrated and desiccated leaves has been described (J. P. Moore et al., unpubl. res.). This network, which might link to the lipid structures present in the stem, may act to control the spatial pattern and temporal rate of re-hydration (J. P. Moore et al., unpubl. res.).
We consider that a combination of features contributes to the ability of M. flabellifolia to empty and refill its xylem conduits. These are the primitive vascular system, primarily consisting of tracheids, the narrow vessels and the presence of lipid-containing material lining these vessels (Carlquist, 1976; Puff, 1978b; Sherwin et al., 1998). We postulate that the reason that no other higher plants are both desiccation tolerant and woody is because the altered structure of the xylem vessels that permits efficient water transport is not conducive to surviving extreme dehydration.
ADAPTATIONS TO TOLERATE DESICCATION
Homoiochlorophyllous resurrection plants maintain their photosynthetic apparatus and chlorophyll intact during dehydration and re-hydration. The advantage of this strategy is a more rapid recovery of photosynthesis and growth upon re-hydration. Morphological adaptations associated with this strategy include leaf and stem folding to reduce the level of incident light reaching the photosystems (Farrant et al., 2003).
The most conspicuous change that occurs upon desiccation is that the leaves close in a fan-like manner with their adaxial surfaces appressed against the stem (Sherwin and Farrant, 1996; Farrant et al., 2003). The loss of protoplasmic water results in considerable anatomical and ultrastructural re-organization of the leaf tissue (Sherwin and Farrant, 1996; Moore et al., 2006). A recent study of the leaf cell wall of M. flabellifolia showed that the majority of leaf mesophyll cells folded their walls in response to desiccation whereas the sclerenchyma and vascular cells did not fold and thereby provided a rigid support. It is proposed that the high concentration of arabinose polymers, in the form of arabinans and arabinogalactan-proteins, associated with the pectin matrix was responsible for the high degree of flexibility of the mesophyll cell walls (Moore et al., 2006). An unusual feature of M. flabellifolia leaves is the stacking of the thylakoid membranes of the chloroplasts in desiccated leaf tissue, described as ‘staircase grana’ (Wellburn and Wellburn, 1976), which has been proposed to minimize photo-oxidative stress (Sherwin and Farrant, 1996; Koonjul et al., 2000; Farrant et al., 2003).
High tannin (polyphenol) contents have been reported to be present in the leaves of M. flabellifolia (Koonjul et al., 1999). These polyphenols are problematic in that they are co-extracted with nucleic acids and bind to proteins, thereby interfering with molecular biological studies (Koonjul et al., 1999). However, the use of added polyphenol-binding agents such as polyvinyl pyrrolidone together with anti-oxidants has overcome these obstacles (J. P. Moore et al., unpubl. res.). The report of Koonjul et al. (1999) led to the characterization of the main polyphenol present in plants sourced from Namibia. This compound, determined to be 3,4,5-tri-O-galloylquinic acid, was able to protect artificial membranes (liposomes) against desiccation and free-radical-induced oxidation (Moore et al., 2005b). A report that South African M. flabellifolia plants were able to survive in the desiccated state for only 9–12 months (Farrant and Kruger, 2001), whereas parts of Namibia where M. flabellifolia populations occur receive rainfall far less frequently (Puff, 1978a), led us to analyse the polyphenols present in South African plants. These were found not only to have a different polyphenol composition but were also genetically distinct (Moore et al., 2005a). The ability to survive in the desiccated state has been shown to depend on the levels of cellular antioxidants present in the leaves (Kranner et al., 2002; Moore et al., 2005a). The synthesis of the polyphenol pigment cyanidin 3-glucoside is induced upon desiccation and it is suggested that this functions in synergy with 3,4,5-tri-O-galloylquinic acid to protect the leaves against photo-oxidative stress (Moore et al., 2004). It is proposed that photo-oxidative stress is also reduced by the presence of waxes which may function to reflect light away from the leaf surface (J. P. Moore et al., unpubl. res.).
Early studies have reported contradictory data regarding the presence of soluble saccharides in desiccated M. flabellifolia leaves. Whereas arbutin and sucrose were reported to be the main saccharide constituents (Suau et al., 1991), other authors have reported the presence of sucrose and trehalose (Drennan et al., 1993) or sucrose and β-glucopyranosyl-(1→2)-glycerol, a known osmoprotectant (Bianchi et al., 1993). These conflicting data may result from the collection of plants from different locations in southern Africa, namely Zimbabwe, South Africa and Namibia, respectively. It has recently been confirmed that South African plants have high relative concentrations of sucrose and trehalose (J. P. Moore et al., unpubl. res.). A molecular study of the desiccation response of M. flabellifolia supports the hypothesis that saccharides play an important role in the desiccation tolerance of these plants as partial mRNA transcripts for putative carbohydrate biosynthesis and sugar transport proteins were found to be up-regulated upon desiccation (Koonjul, 1999). Concomitant with the increased soluble saccharide concentration reported upon desiccation, a decreased monovalent cation content has been observed in desiccated leaves. This supports the proposal that an increased soluble sugar:cation ratio is an important adaptation to desiccation (Schwab and Gaff, 1986); it is suggested that this is brought about by secretion of monovalent cations from the hydathode-like structures present at the leaf apices (J. P. Moore et al., unpubl. res.).
MEDICINAL PROPERTIES AND ETHNOBOTANICAL IMPORTANCE
Myrothamnus flabellifolia is a widely used medicinal plant, independently named by local tribal communities. These names often refer to its resurrection ability, regarded to be a symbol of hope in African tradition. Not only is it used as a psychological tool to treat severe depression but it is also used medicinally for a wide variety of ailments. These include the inhalation of smoke from burning leaves to treat chest complaints, the preparation together with butter of aromatic salves for wound sterilization, herbal teas and decoctions to treat coughs, influenza, mastitis, backache, kidney disorders, haemorrhoids and abdominal pains as well as the mastication of the leaves to treat scurvy, halitosis and Vincent's gingivitis (Watt and Breyer-Brandwijk, 1962; Hutchings, 1996; Van Wyk et al., 1997).
The first chemical analyses focused on the essential oils, compounds which are odorant molecules of ecological relevance and medicinal importance (Da Cunha and de Lurdes Rodriguez Roque, 1974; Gibbs, 1974; Harborne, 1998). Different studies have reported different compositions, probably as the plants were sourced locally from different regional populations. Major essential oils reported to be present include carvone and perillic acid (Da Cunha and de Lurdes Rodriguez Roque, 1974), 1,8-cineole and diosphenol (Gibbs, 1974), trans-pinocarvenol, pinocarvone, α-pinene and β-selinene (Chagonda et al., 1999), and pinocarvone and trans-pinocarvone (Viljoen et al., 2002). Viljoen et al. (2002) demonstrated that the hydro-distilled essential oil, which included 85 different compounds, had activity against a range of microbial pathogens.
Recently it has been found that 3,4,5-tri-O-galloylquinic acid is the predominant polyphenol in the leaves of plants sourced from Namibia, whereas a range of galloyl esters of quinic acid are present in the leaves of plants from South Africa (Moore et al., 2005a). Medicinally, gallotannins such as 3,4,5-tri-O-galloylquinic acid are known for their wound-healing properties (Onayade et al., 1996). In particular galloylquinic acids have been identified as compounds possessing high activity against bronchial hyper-reactivity and allergic reactions (Neszmelyi et al., 1993) as well as against HIV reverse transcriptase (Bokesch et al., 1996) and DNA polymerase (Koonjul et al., 1999). The properties of the essential oils and the galloylquinic acids correspond to the various medical applications in traditional medicine.
FUTURE RESEARCH
Our review has highlighted three main areas of potential future research. These are:
that different populations of M. flabellifolia are present throughout southern Africa. These may represent distinct genetically related species with different polyphenol compositions. This would have important implications regarding its conservation status since it is threatened by indiscriminate and excessive collection for commercial ventures and traditional medical applications.
the role of polyphenols in desiccation, anti-oxidative stress and the medicinal properties associated with these compounds. Further studies on M. flabellifolia variants might identify new useful polyphenol therapeutic agents since these compounds are present in high concentrations.
the molecular biology and proteomics of desiccation tolerance. The presence of high concentrations of polyphenols has prevented such work since these compounds bind to proteins and interfere with both protein extraction and added enzymes. However, recent advances in methodology using phenolic-binding compounds have allowed application of these techniques.
Such research might yield data of relevance to subsistence farming, agricultural biotechnology, medicinal chemistry and ethnobotany. A focus on such research might well initiate scientific endeavour in countries lacking sufficient research infrastructure.
ACKNOWLEDGEMENTS
J.P.M. would like to gratefully acknowledge the financial support provided by the University of Cape Town, Deutscher Akademischer Austauschdienst (Germany) and the National Research Foundation. J.M.F. and W.F.B. would like to acknowledge the financial support of the National Research Foundation. The authors would also like to thank Miranda Waldron and Mohammed Jaffer (UCT electron microscope unit) for their expert technical assistance.
LITERATURE CITED
- Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster R, Salamini F, et al. The unusual sugar composition in the leaves of the resurrection plant Myrothamnus flabellifolia. Physiologia Plantarum. 1993;87:223–226. [Google Scholar]
- Bokesch HR, McKee TC, Currens MJ, Gulakowski RJ, McMahon JB, Cardellina JH, II, et al. HIV-inhibitory gallotannins from Lepidoptrys staudtii. Natural Product Letters. 1996;8:133–136. [Google Scholar]
- Bywater M. In: Flora of Tropical East Africa: Myrothamnaceae. Polhill RM, editor. Rotterdam: Balkema; 1984. [Google Scholar]
- Carlquist S. Wood anatomy of Myrothamnus flabellifolia (Myrothamnaceae) and the problem of multiperforate perforation plates. Journal of Arnold Arboretum. 1976;57:119–126. [Google Scholar]
- Chagonda LS, Makanda C, Chalchat JC. Essential oils of four wild and semi-wild plants from Zimbabwe: Colophospermum mopane (Kirk ex Benth.) Kirk ex Leonard, Helichrysum splendidum (Thunb.) Less, Myrothamnus flabellifolia (Welw.) and Tagetes minute L. Journal of Essential Oil Research. 1999;11:573–578. [Google Scholar]
- Child GF. Brief notes on the ecology of the resurrection plant (Myrothamnus flabellifolia) with mention of its water-absorbing abilities. Journal of South African Botany. 1960;XXVI:1–8. [Google Scholar]
- Da Cunha AP, de Lurdes Rodriguez Roque O. Identificaçã e dosagem dos principais constituintes do óleo essencial do Myrothamnus flabellifolius Welw., de Angola. Boletim da Faculdade de Farmacia de ecimera Edicao cientifica. 1974;34:49–61. [Google Scholar]
- Drennan PM, Smith MT, Goldsworthy D, van Staden J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. Journal of Plant Physiology. 1993;142:493–496. [Google Scholar]
- Farrant JM, Kruger LA. Longevity of dry Myrothamnus flabellifolius in simulated field conditions. Plant Growth Regulation. 2001;35:109–120. [Google Scholar]
- Farrant JM, Cooper K, Kruger LA, Sherwin HW. The effect of drying rate on the survival of three desiccation-tolerant angiosperm species. Annals of Botany. 1999;84:371–379. [Google Scholar]
- Farrant JM, Vander Willigen C, Loffel DA, Bartsch S, Whittaker A. An investigation into the role of light during desiccation of three angiosperm resurrection plants. Plant, Cell and Environment. 2003;26:1275–1286. [Google Scholar]
- Fuller DQ, Hickey LJ. Systematics and leaf architecture of the Gunneraceae. Botanical Review. 2005;71:295–353. [Google Scholar]
- Gibbs RD. Chemotaxonomy of flowering plants. Vol. 3. Montreal: McGill-Queens University Press; 1974. [Google Scholar]
- Glen HF, Sherwin HW, Condy G. Myrothamnus flabellifolia. Flowering plants of Africa. Vol. 56. Pretoria: NBI Publications; 1999. pp. 62–68. [Google Scholar]
- Goldsworthy D-A. Desiccation tolerance in Myrothamnus flabellifolia Welw. Pietermaritzburg, South Africa: University of Natal; 1992. MSc thesis. [Google Scholar]
- Harborne JB. Phytochemical methods. 3rd edn. London: Chapman and Hall; 1998. [Google Scholar]
- Hutchings A. Zulu medicinal plants. Pietermaritzburg: Natal University Press; 1996. [Google Scholar]
- Koonjul PK. Investigating the mechanisms of desiccation tolerance in the resurrection plant Myrothamnus flabellifolius (Welw.) South Africa: University of Cape Town; 1999. PhD Thesis. [Google Scholar]
- Koonjul PK, Brandt WF, Farrant JM, Lindsey GG. Inclusion of polyvinylpyrrolidone in the polymerase chain reaction reverses the inhibitory effects of polyphenolic contamination of RNA. Nucleic Acids Research. 1999;27:915–916. doi: 10.1093/nar/27.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonjul PK, Brandt WF, Lindsey GG, Farrant JM. Isolation and characterisation of chloroplasts from the resurrection plant Myrothamnus flabellifolius Welw. Journal of Plant Physiology. 2000;156:584–594. [Google Scholar]
- Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW. Revival of a resurrection plant correlates with its antioxidant status. The Plant Journal. 2002;31:13–24. doi: 10.1046/j.1365-313x.2002.01329.x. [DOI] [PubMed] [Google Scholar]
- Kubitzki K. Myrothamnaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 2. Berlin: Springer-Verlag; 1993. pp. 468–469. [Google Scholar]
- Lisowski S, Malaisse F, Symoens JJ. Les Myrothamnaceae, nouvelle famille pour la flore phanérogamique du Congo-Kinshasa. Bulletin de la Jardin Botanique Nationale de Belgique. 1970;40:225–229. [Google Scholar]
- Mendes EJ. Myrothamnaceae. Flora Zambesica. 1978;4:68–71. [Google Scholar]
- Moore JP, Ravenscroft N, Lindsey GG, Farrant JM, Brandt WF. Galloylquinate ester:anthocyanin complexes in the leaves of the desiccated resurrection plant Myrothamnus flabellifolius. In: Hoikkalo A, Soidinsalo O, editors. Polyphenols Communications: XXII International Conference on Polyphenols; 25–28 August 2004; Helsinki, Finland. 2004. [Google Scholar]
- Moore JP, Farrant JM, Lindsey GG, Brandt WF. The South African and Namibian populations of resurrection plant Myrothamnus flabellifolius are genetically distinct and display variation in their galloylquinic acid composition. Journal of Chemical Ecology. 2005a;31:2823–2834. doi: 10.1007/s10886-005-8396-x. [DOI] [PubMed] [Google Scholar]
- Moore JP, Westall KL, Ravenscroft N, Farrant JM, Lindsey GG, Brandt WF. The predominant polyphenol in the leaves of the resurrection plant Myrothamnus flabellifolius, 3,4,5-tri-O-galloylquinic acid, protects membranes against desiccation and free radical-induced oxidation. Biochemical Journal. 2005b;385:301–308. doi: 10.1042/BJ20040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Nguema-Ona E, Chevalier L, Lindsey GG, Brandt WF, Lerouge P, et al. Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiology. 2006;141:651–662. doi: 10.1104/pp.106.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neszmelyi A, Kreher B, Muller A, Dorsch W, Wagner H. Tetragalloylquinic acid, the major anti-asthmatic principle of Galphimia glauca. Planta Medica. 1993;59:164–167. doi: 10.1055/s-2006-959635. [DOI] [PubMed] [Google Scholar]
- Onayade OA, Onayade AA, Sofowora A. Wound healing with plants: the African perspective. In: Hostettmann K, Chinyanganya F, Maillard M, Wolfender J-L, editors. Chemistry, biological and pharmacological properties of African medicinal plants. Vol. 1. Harare: University of Zimbabwe Publications; 1996. pp. 77–120. [Google Scholar]
- Porembski S, Barthlott W. Granitic and gneissic outcrops (inselbergs) as centres of diversity for desiccation-tolerant vascular plants. Plant Ecology. 2000;151:19–28. [Google Scholar]
- Puff C. Zur Biologie von Myrothamnus flabellifolius Welw. (Myrothamnaceae) Dinteria. 1978a;14:1–20. [Google Scholar]
- Puff C. The nodal anatomy of Myrothamnus flabellifolius (Myrothamnaceae): another example of a ‘split-lateral’ condition. Journal of the Arnold Arboretum. 1978b;59:192–196. [Google Scholar]
- Qiu Y-L, Chase MW, Hoot SB, Conti E, Crane PR, Sytsma KJ, et al. Phylogenetics of the Hamamelidae and their allies: parsimony analysis of nucleotide sequences of the plastid gene rbcL. International Journal of Plant Sciences. 1998;159:891–905. [Google Scholar]
- Schneider H, Wistuba N, Wagner H-J, Thürner F, Zimmermann U. Water rise kinetics in refilling xylem after desiccation in a resurrection plant. New Phytologist. 2000;148:221–238. doi: 10.1046/j.1469-8137.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- Schneider H, Manz B, Westhoff M, Mimietz S, Szimtenings M, Neuberger T, et al. The impact of lipid distribution, composition and mobility on xylem water refilling of the resurrection plant Myrothamnus flabellifolia. New Phytologist. 2003;159:487–505. doi: 10.1046/j.1469-8137.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- Schwab KB, Gaff DF. Sugar and ion content in leaf tissues of several drought tolerant plants under water stress. Journal of Plant Physiology. 1986;125:257–265. [Google Scholar]
- Sherwin HW, Farrant JM. Differences in re-hydration of three desiccation-tolerant angiosperm species. Annals of Botany. 1996;78:703–710. [Google Scholar]
- Sherwin HW, Lamenter NW, February E, Vander Willigen C, Farrant JM. Xylem hydraulic characteristics, water relations and wood anatomy of the resurrection plant Myrothamnus flabellifolius Welw. Annals of Botany. 1998;81:567–575. [Google Scholar]
- Suau R, Cuevas A, Values V, Reid MS. Arbutin and sucrose in the leaves of the resurrection plant Myrothamnus flabellifolia. Phytochemistry. 1991;30:2555–2556. [Google Scholar]
- Van Wyk BE, van Oudsthoorn B, Gericke N. Medicinal plants of Southern Africa. Pretoria: Briza Publications; 1997. [Google Scholar]
- Viljoen AM, Klepser ME, Ernst EJ, Keele D, Roling E, van Vuuren S, et al. The composition and antimicrobial activity of the essential oil of the resurrection plant Myrothamnus flabellifolius. South African Journal of Botany. 2002;68:100–105. [Google Scholar]
- Wagner H-J, Schneider H, Mimietz S, Wistuba N, Rokitta M, Krohne G, et al. Xylem conduits of a resurrection plant contain a unique lipid lining and refill after desiccation. New Phytologist. 2000;148:239–255. doi: 10.1046/j.1469-8137.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- Wanntorp L, Wanntorp H-E. The biogeography of Gunnera L.: vicariance and dispersal. Journal of Biogeography. 2003;30:979–987. [Google Scholar]
- Wanntorp L, Wanntorp H-E, Oxelman B, Källersjö M. Phylogeny of Gunnera. Plant Systematics and Evolution. 2001;226:85–107. [Google Scholar]
- Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and eastern Africa. 2nd edn. London: Livingstone; 1962. [Google Scholar]
- Weimarck Von H. Myrothamnus flabellifolia Welw., eine polymorphe Pflanzenart. Botaniska Notiser. 1936:451–462. [Google Scholar]
- Weiss FE. Sketches of vegetation at home and abroad. II. Some aspects of the vegetation of South Africa. New Phytologist. 1906;5 [Google Scholar]
- Wellburn FAM, Wellburn AR. Novel chloroplasts and cellular ultrastructure in the ‘resurrection’ plant Myrothamnus flabellifolia Welw. (Myrothamnaceae) Botanical Journal of the Linnean Society. 1976;72:51–54. [Google Scholar]
- Welwitsch FMJ. Myrothamnus flabellifolia Welw. Journal of the Linnean Society of London, Botany. 1859;3:155. [Google Scholar]
- Wilson L, Drennan PM. The distribution of lipids in Myrothamnus flabellifolius Welw. Proceedings of the Electron Microscopy Society of Southern Africa. 1992;22:99–100. [Google Scholar]


