Abstract
Sertoli cells undergo a maturation process during post-natal testicular development that leads to the adult-type Sertoli cell, which is required for spermatogenesis. Understanding Sertoli cell maturation is therefore necessary to gain insight into the underlying causes of impaired spermatogenesis and male infertility. The present study characterized the cellular and molecular differentiation of Sertoli cells in a xenograft model of mammalian testicular development. Immature rat Sertoli cells were cultured in a three-dimensional culture system to allow the formation of cord-like structures. The in vitro Sertoli cell cultures were then grafted into nude mice. Sertoli cell proliferation, morphological differentiation and mRNA expression of Sertoli cell maturation markers were evaluated in xenografts. Sertoli cell proliferation significantly decreased between 1 and 4 weeks (6.7 ± 0.9 versus 1.2± 0.1%, P < 0.001), and was maintained at low levels thereafter. Sertoli cell cord-like structures significantly decreased between 1 and 4 weeks (59.6 versus 21%, P < 0.05), whereas Sertoli cell tubules were more frequently observed after 4 weeks (13.3 versus 73.1%, P < 0.05). Furthermore, expression of androgen binding protein, transferrin and follicle stimulating hormone receptor, markers for mature Sertoli cells, was detected after 1 week of grafting and increased significantly thereafter. We conclude from these results that rat Sertoli cells continue maturation after xenografting to the physiological environment of a host. This model of in vitro tubule formation will be helpful in future investigations addressing testicular maturation in the mammalian testis.
Keywords: three-dimensional cell culture, Sertoli cell, testicular development, xenografting
Introduction
Post-natal testicular development depends on the proliferation of immature Sertoli cells to establish the final Sertoli cell number in the adult testis, thereby determining testicular size, and ultimately spermatogenic capacity (Orth et al., 1988). Sertoli cell numbers in rodents, non-human primates and humans are exclusively established in the pre- and peri-pubertal period when the cells are proliferating and differentiating. In the adult testis Sertoli cells are mitotically inactive in rodents as well as in rhesus monkeys and in humans (Clermont and Perey, 1957; Steinberger and Steinberger, 1971; Orth, 1982; Plant and Marshall, 2001). Concurrent with the cessation of proliferation, Sertoli cells undergo functional maturation during puberty, leading to the adult phenotype that is required for spermatogenesis. This maturation process is characterized by changes in cell morphology and polarity, the secretion of factors supporting spermatogenesis and by the establishment of the blood-testis barrier that divides the seminiferous epithelium into a basal and an adluminal compartment (Pelliniemi et al., 1984; Lui et al., 2003). Barrier formation is temporarily correlated with the development of a lumen within seminiferous tubules through fluid-secretion (Russell et al., 1989). Therefore, Sertoli cell maturation is marked by the transition from testicular cords to seminiferous tubules.
At the molecular level, functional maturation of Sertoli cells can be further characterized by the complement of genes that are up- and down-regulated during each stage of testicular development. Genes that are specifically expressed during the proliferative phase of Sertoli cell development include anti-Mullerian hormone (Amh), Gata4 and cytokeratin 18 (Krt18). AMH suppresses female development in XY embryos upon primary sex determination, thereby allowing testicular differentiation and the development of the male phenotype. AMH is expressed in premature Sertoli cells, starting around embryonic Day 15, and continues to be expressed until the onset of puberty. AMH expression declines as meiotic germ cells appear and as Sertoli cells gain increased androgen sensitivity (Rey et al., 2003). Postnatally, Gata4 expression in Sertoli cells peaks between 1 and 14 days and declines during testis maturation (Viger et al., 1998; Ketola et al., 1999). The intermediate filament marker Krt18 is detectable in pre-Sertoli cells as early as embryonic Day 15, and expression continuously decreases throughout post-natal development, disappearing by 14 days after birth (Paranko et al., 1986). Likewise, Krt18 is a robust marker for immature Sertoli cells in the pathological human testis (Steger et al., 1996, 1999). Cytochrome p450 aromatase (Cyp19a1) catalyzes the final step of androgen aromatization into estrogens (Simpson et al., 1994). In the rat, cytochrome p450 aromatase is first expressed in Sertoli cells during fetal and neonatal development (Sharpe et al., 2003) and expression is down-regulated after post-natal Day 10 during maturation of Sertoli cells (Ando et al., 2001; Carpino et al., 2001; Pezzi et al., 2001). In the adult testis, aromatase expression is mainly located to interstitial Leydig cells.
Adult-type Sertoli cells express transferrin (Trf), androgen binding protein (Shbg) and follicle stimulating hormone receptor (Fshr). Trf, a common marker for Sertoli cell differentiation, is present 10 days after birth and its levels continue to rise into adulthood (Skinner and Griswold, 1980; Perez-Infante et al., 1986). Shbg is present 14 days after birth and the greatest increase in Shbg levels occurs between the second and third weeks after birth (Hagenas et al., 1975; Tindall et al., 1975; Rich et al., 1983). FSHR levels begin to increase in number during the start of spermatogenesis, between 7 and 14 days after birth (Thanki and Steinberger, 1978).
We have recently developed a three-dimensional culture system for the de novo generation of testicular cord-like structures from single cell suspensions of rat Sertoli cells (Gassei et al., 2006). Subsequently, in vitro derived cord-like structures morphologically differentiated into seminiferous tubule-like structures after xenografting into immunodeficient host mice. In the present study, we add to these reports a detailed analysis of the Sertoli cell maturation process during the formation of tubule-like structures in xenografts. Previously, it remained unclear whether Sertoli cells in xenografts would continue proliferation, or if Sertoli cells would cease mitotic activity and mature after grafting to the physiological environment of a host. Therefore, in this study, we analysed cell proliferation, cellular composition of xenografts and the expression of Sertoli cell maturation markers in xenografts to determine the developmental status of Sertoli cells during a time course of up to 12 weeks in host mice.
Materials and Methods
Sertoli cell culture
Seven-day-old CD rats were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). All procedures were in compliance with the University of Pittsburgh Guidelines for the Care and Use of Laboratory Animals.
Testes from 10 rat pups per experiment were dissected, pooled and transferred to chilled Dulbecco's Minimum Essential Medium (DMEM, 4.5 g glucose/ml; Mediatech Inc., Herndon, VA, USA; No. 10-013-CV). Initial digestion was performed with 1 mg/ml collagenase I (Sigma, Saint Louis, MO, USA; No. C-2674) and 5 µg/ml DNAse (15 U/ml; Roche Applied Science, Indianapolis, IN, USA; No. 104132) in DMEM mixed 1:1 with Ham's F12 (Mediatech Inc.; No. 10-080-CV) and supplemented with 1% MEM non-essential amino acids (BioWhittaker Walkersville, MD, USA; No. 13-114E), 100 IU/ml Penicillin and 100 µg/ml Streptomycin (Mediatech Inc.; No. 30-002-CI) at 37°C for 15 min. Tubule fragments were separated from interstitial and peritubular cells by sedimentation at unit gravity. A second digestion with 1 mg/ml collagenase I, 5 µg/ml DNAse and 1 mg/ml hyaluronidase (Sigma-Aldrich, St. Louis, MO, No. H-3506) were carried out at 37°C for 20 min. Cells were resuspended in culture medium [DMEM 1 g glucose/ml supplemented with 1% non-essential amino-acids (NEAA) and 1% penicillin/streptomycin], and plated on a 24-well culture plate coated with 250 µl extracellular matrix proteins (Matrigel, BD Biosciences, Bedford, MA; No. 354234; 1:1 diluted with culture medium; 106 cells per well), and cultured at 35°C in 5% CO2.
This standard protocol yields a single cell suspension enriched for Sertoli cells, containing residual germ cells (25%), peritubular myoid cells (15%) and Leydig cells (0.3%), as described previously (Gassei et al., 2008).
Xenografting
Adult male nude mice (strain: nu/nu) were obtained from Charles River Laboratories and used as hosts for cell culture grafts. Castration was performed at the time of grafting. Rat Sertoli cells were injected under the dorsal skin of nude mice after 9 days of Matrigel culture. Each host received six injections of 250 µl extracellular matrix gel containing Sertoli cells under the dorsal skin. In the first study, xenografts were allowed to develop for up to 4 weeks (referred to as short-term xenografting). As the data derived from this study indicated a possibility for further changes in graft physiology with time, a second study was performed in which grafts were allowed to develop for up to 12 weeks. This second study is referred to as long-term xenografting. As the two studies each consist of a separate set of experiments, data where evaluated separately, although some of the time points overlap. Animal numbers for all groups are provided in Table I. At the time of graft removal, host mice received an intra-peritoneal injection of BrdU (100 mg/kg) and were killed 2 h later by exsanguinations under deep anaesthesia with Ketamine/Xylazine (Sigma-Aldrich, St. Louis, MO, No. K113). Xenografts were removed from the inner surface of the back skin and fixed in Bouin's fixative overnight for histology and immunohistochemistry, and then transferred to 70% (v/v) ethanol. For preparation of mRNA, xenografts were snap-frozen in liquid nitrogen. Body weights, seminal vesicle weights, as well as graft numbers and weights were recorded for each host animal.
Table I.
Animal numbers and recovered grafts
| Weeks | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| (a) Short-term xenografting | ||||||
| n (hosts) | 10 | 16 | 10 | 11 | ||
| n (grafts) | 71 | 157 | 132 | 121 | ||
| Weeks | 2 | 4 | 6 | 8 | 10 | 12 |
| (b) Long-term xenografting | ||||||
| n (hosts) | 8 | 10 | 9 | 7 | 8 | 9 |
| n (grafts) | 46 | 122 | 60 | 59 | 78 | 76 |
Histology
Standard histological analysis was performed on periodic acid/Schiff's reagent-stained (PAS) 4 µm sections prepared from resin embedded specimen (Technovit 7100; Heraeus Kulzer, Hanau, Germany). Gross anatomy and leukocyte infiltration of grafts was recorded.
Morphometric histological analysis was performed on coded slides in a randomized manner. A minimum of 50 tubules were scored per graft. In grafts with fewer than 50 tubules, all tubules were scored. Sertoli cells in xenografts were scored for the presence of the following morphologies: (a) cord-like structures, (b) tubule-like structures, (c) single layer epithelium and (d) lumen.
Immunostaining
For immunofluorescence staining, Sertoli cells were cultured in Matrigel for 9 days, incubated with BrdU for 2 h, and then isolated from Matrigel. Cell aggregates were plated on poly-l-lysin coated coverslips and fixed with 4% formalin. Paraffin sections (7 µm) of Bouin-fixed xenografts were deparaffinized and washed with Tris buffered saline (TBS) before proceeding with immunohistochemistry. Coverslips and sections blocked with TBS containing 0.5% bovine serum albumin (BSA) and 10% goat serum were incubated overnight at 4°C with antibodies against bromodeoxyuridine (BrdU, Biomeda, Foster City, CA, USA; diluted 1:50 in TBS containing 0.1% BSA), α-smooth muscle actin (αSMA; peritubular myoid cell marker; Sigma no. A2547; 1:2000 dilution), and cytochrome P450 side-chain cleavage enzyme (p450scc, Leydig cell marker; Chemicon no. AB1244; 1:500). Samples stained with anti-BrdU antibody were incubated with 1 M HCl for 15 min prior to antibody incubation. Negative controls were performed in all experiments by omitting primary antibodies to ensure antibody specificity. Sections from a 7-day-old rat, adult rat or adult mouse testes served as positive controls. Slides were washed and incubated with secondary goat anti-mouse IgG (for αSMA and BrdU detection) or secondary goat anti-rabbit IgG (for p450scc), either conjugated to horseradish peroxidase or AlexaFluor-488 fluorophore. Colorimetric detection with diaminobenzidine (DAB; SigmaFast™, Sigma No. D4168) was performed according to the manufacturer's specifications. Samples were counterstained with Mayer's hematoxylin or DAPI, depending on the experiment.
Proliferation index
BrdU labelling indices were determined by scoring BrdU-negative and BrdU-positive cells in paraffin embedded xenograft sections. At least six grafts from three or more recipients were scored per time point, and 500 cells were scored in each xenograft. In sections with fewer than 500 total cells, all cells were scored.
RNA isolation and cDNA preparation
RNA was obtained from freshly isolated Sertoli cells, Sertoli cell aggregates after 9 days of culture in Matrigel, or liquid nitrogen-frozen xenografts (50–150 mg tissue per sample, n = 3 for each time point) by ultracentrifugation (40 000g for 16 h at 16°C) in a guanidine isothiocyanate caesium chloride (GITC/CsCl) gradient. The RNA pellet was resuspended in 0.3 M NaAcO, pH 6.0/0.1% SDS for ethanol precipitation. The RNA was treated with RNase-free DNase (Promega Madison, WI, USA; No. M6101) and subjected to reverse transcription. One microgram total RNA was used for reverse transcription in a reaction mix containing 5 mM MgCl2, 40 U RNasin ribonuclease inhibitor (Promega No. N2111), 0.4 mM deoxynucleotide triphosphates (Promega, No. U1330), 45 µM random hexamers (Integrated DNA Technologies, Coralville, IA, USA) and 10 U AMV reverse transcriptase (Promega, No. M5101). To control for contaminant DNA, parallel reactions were carried out without reverse transcriptase. All samples were incubated at 25°C for 10 min, at 42°C for 45 min and at 95°C for 10 min.
RT–PCR
Sertoli cell immaturity and differentiation markers were amplified using 2 µl cDNA in a PCR mix containing 1.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphates, 1.25 U GoTaq DNA polymerase (Promega No. M3005) and 10 mM forward and reverse primer. Parallel reactions were performed without template cDNA to control for contaminant DNA. PCR conditions were as follows: 5 min at 95°C (30 s at 95°C, 30 s at the annealing temp and 90 s at 72°C) × 35–40, and 5 min at 72°C. Oligonucleotide sequences, annealing temperatures and expected product sizes are listed in Table II. PCR products were analysed on a 2% (w/v) agarose gel for large fragments, or a 10% (w/v) poly-acrylamide gel for small fragments (∼100 bp). Intensity of RT–PCR bands was quantified with ImageJ software (Abramoff et al., 2004) and data from three independent experiments were combined to a mean ± standard error of the mean (SEM).
Table II.
Oligonucleotide primer sequences for the amplification of Sertoli cell maturation markers
| Gene | Sequence | TA (°C) | Region amplified | Product size (bp) |
|---|---|---|---|---|
| Ppia | Forward 5′-ATGGTCAACCCCACCGTGT-3′ | 60 | 43–143 | 100 |
| Reverse 5′-TCTGCTGTCTTTGGAACTTTGTCT-3′ | ||||
| Trf | Forward 5′-ATCTGGGAGATCCTCAAAGTGGCTC-3′ | 59 | 874–1277 | 403 |
| Reverse 5′-GGCACTAGTCCACACTGGCCTGCTA-3′ | ||||
| Shbg | Forward 5′-GACGGACCCTGAGACACATT-3′ | 57.3 | 107–674 | 590 |
| Reverse 5′-GAACAGTCCAGGTTGCAGGT-3′ | ||||
| Wt1 | Forward 5′-GCCACCCCACTCCTTCATCAAAC-3′ | 61.8 | 210–788 | 601 |
| Reverse 5′-GTTGCTCTGCCCTTCTGTCCATT-3′ | ||||
| Fshr | Forward 5′-TCTGTCCTGTAAATCTGGGCTTGC-3′ | 55 | 1709–2037 | 328 |
| Reverse 5′-TGCTATACCCACATCTACCTC-3′ | ||||
| Gata4 | Forward 5′-CTGTCATCTCACTATGGGCAC-3′ | 56.1 | 1669–1905 | 257 |
| Reverse 5′-CCAAGTCCGAGCAGGAATTTG-3′ | ||||
| Amh | Forward 5′-CCTTTCTGTTTGGCTCTGATTCC-3′ | 61.2 | 683–1024 | 341 |
| Reverse 5′-GCGGGTGACAGCAGCAGTAATAG-3′ | ||||
| Krt18 | Forward 5′-TCCACCACCTTCTCCACCAACTA-3′ | 60.8 | 59–308 | 272 |
| Reverse 5′-TTCTCGGTTTCCAGGTTCTTCAC-3′ | ||||
| Cyp19a1 | Forward 5′-TGCACAGGCTCGAGTATTTCC-3′ | 56 | 1411–1714 | 304 |
| Reverse 5′-ATTTCCACAATGGGGCTGTCC-3′ |
Statistical analysis
All data are presented as mean ± SEM. Descriptive statistical analysis and detection of significant differences were performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA, USA). Data sets were first tested for normality and equality of variances and further analyzed using One Way ANOVA. The expected level of statistical significance was P < 0.05.
Results
Sertoli cell aggregation in vitro and expression of maturation markers
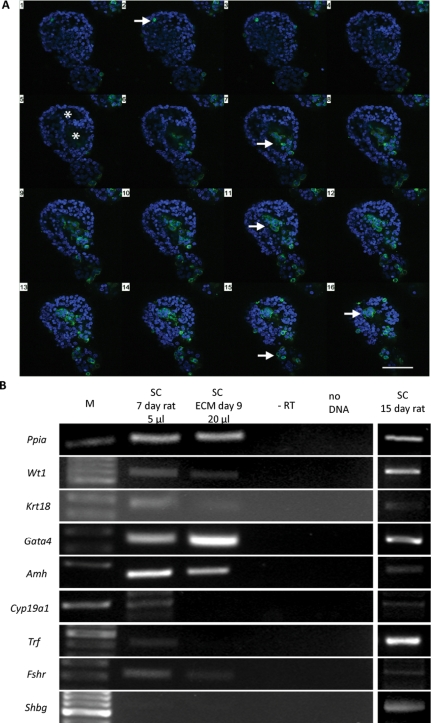
Immature Sertoli cells aggregate in three-dimensional culture systems to form cord-like structures. Confocal fluorescence microscopy revealed that Sertoli cells and peritubular myoid cells that are present in the primary cell suspension co-operate during the morphogenetic cascade (Fig. 1A). Further, it was observed that Sertoli cells are organized in single-layer epithelia, and that most aggregates were hollow, showing a central cavity lined by smooth-muscle actin positive myoid cells. In contrast, p450-positive cells were never observed to participate in cord formation. Interestingly, neither Sertoli cells nor myoid cells appeared to be mitotically active in Matrigel cultures, as indicated by the absence of BrdU-positive S-phase cells.
Figure 1.
Three-dimensional Sertoli cell culture in Matrigel.
(A) Sertoli cell aggregates isolated from Matrigel culture. Sequential z-axis confocal scan through a spherical aggregate stained for α-smooth muscle actin (SMA, myoid cell marker, green) and DAPI nuclear stain (blue). Sixteen planes in 2 µm intervals were taken from the top (1) to the bottom (16) of the aggregate. Scale bar = 100 µm. Peritubular cells are located at the outer and inner periphery of the aggregate (arrows). The sequential scan also reveals the hollow structure of the aggregate and epithelial-like arrangement of the SMA negative Sertoli cells (asterisks). (B) Expression of Sertoli cell maturation markers in Sertoli cells isolated from 7-day-old rats and from Sertoli cell aggregates cultured in Matrigel for 9 days prior to xenografting (ECM Day 9). Sertoli cells isolated from 15-day-old rats and cultured for 3 days were used as positive controls for PCR efficiency.
Analysis of Sertoli cell maturation marker expression showed that the markers of undifferentiated Sertoli cells Krt18, Gata4 and Amh were expressed in immature Sertoli cells isolated from 7-day-old rats, and expression was maintained during Matrigel culture, albeit at low levels (Fig. 1B). Trf, Fshr and Cyp19a1, markers of mature Sertoli cells, were also found to be expressed in isolated Sertoli cells, but expression was weak or absent after 9 days of 3D culture. Finally, Shbg was not detected in isolated Sertoli cells or in Sertoli cell aggregates after Matrigel culture. In control studies using Sertoli cell mRNA from 15-day-old rats after 3 days in a conventional culture system, the expression pattern of Sertoli cell markers was as expected for this post-natal age.
Differentiation of Sertoli cell tubules after xenografting
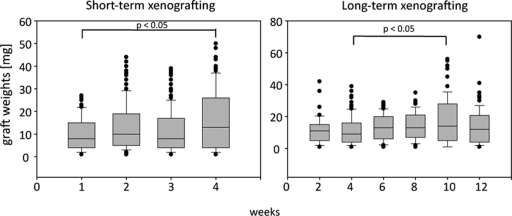
Xenografts were recovered from 7 to 16 recipients per time point. Xenografts could be distinguished as flat to round patches of soft tissue attached to the inner side of the dorsal skin, most prominently along dorsal blood vessels. Due to the low viscosity of the Matrigel that served as vehicle for the Sertoli cells during xenografting, we found that in some recipients a higher number of grafts could be recovered than initially injected, due to the spreading of Matrigel away from the injection side, and that graft weights varied widely (Table I and Fig. 2). Overall, graft weights significantly increased between 1 and 4 weeks during short-term xenografting, and between 4 and 10 weeks during long-term xenografting. Leukocyte infiltration into xenografts was observed in one out of five hosts after 3 weeks, two out of five hosts after 4 weeks, one out of three hosts after 8 weeks and one out of three hosts after 12 weeks.
Figure 2.
The distribution of graft weights in xenografts after short- and long-term grafting.
The median of the graft weights (black line) and lower and upper quartiles (gray box) are presented. Whiskers extend to the 5th and 95th percentile, respectively. Left: graft weights significantly increased between 1 and 4 weeks (ANOVA on ranks, n = 71–157). Right: graft weights significantly increased between 4 and 10 weeks (ANOVA on ranks, n = 46–122).
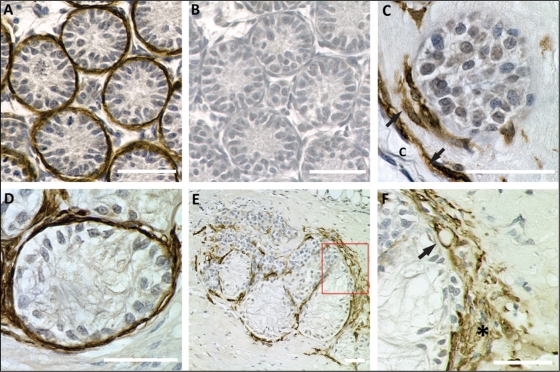
Histological analysis revealed the occurrence of four Sertoli cell phenotypes, representing progressive maturation judged by the morphology and appearance of Sertoli cell aggregates. First, Sertoli cells with an immature phenotype were organized in small, round or oval groups without a pronounced basement membrane (cord-like structure, Fig. 3A). Second, Sertoli cell tubule-like structures were characterized by a larger diameter than the cord-like structures, and the presence of a basement membrane. Peritubular myoid cells were observed aligned around tubules (Fig. 3B). Third, Sertoli cells were observed to be aligned as a single layer epithelium. Finally, the formation of a fluid-filled lumen was observed and persisted throughout all later time points tested (Fig. 3C–F). Semi-quantitative morphometric evaluation revealed a morphological cascade of progressive Sertoli cell tubule development. After 1 week of xenografting, cord-like structures represented the most commonly observed developmental stage (59.6 ± 5.3%), but they were significantly decreased after 2 weeks (38.6 ± 3.3%), 3 weeks (28.3 ± 2.8%) and 4 weeks (21 ± 3.7%) (n = 16–31 grafts). In contrast, tubule-like structures were only rarely observed at 1 week (13.3 ± 4.2%) and became more frequent after 2, 3 and 4 weeks (44.9 ± 4.5, 61.7 ± 3.6, 73.1 ± 4.2%).
Figure 3.
Development of seminiferous tubules in xenografts.
Resin embedded sections were stained with periodic acid/Schiff's reagent for histological analysis. Scale bars = 50 µm. (A) After 1 week, small cord-like structures are present, and secretion of carbohydrate-rich basement membrane components is evident (arrow). (B) Xenograft after 2 weeks. Tubule-like structured with single-layered Sertoli cell epithelium are predominantly observed. Peritubular cells are aligned along tubules (arrows). (C) Maturation of Sertoli cells and formation of tight junctional complexes is indicated by the formation of fluid-filled lumen (asterisk, xenograft after 3 weeks). (D) Sertoli cell tubules after 4 weeks. Tubules are organized in clusters and show differentiated basement membranes and recruitment of peritubular myoid cells. Endothelial (asterisk) and fibroblast-like cell (arrow) migration into xenografts. (E) Xenograft after 8 weeks are associated with extratubular cells (arrows), resembling the interstitial compartment in vivo. (F) After 12 weeks, xenografts become fibrotic (asterisk). Sertoli cell tubules begin to degenerate and the epithelial organization deteriorates.
These data suggest a progressive maturation of Sertoli cells, leading to the development of tubule-like structures from cord-like structures. Tubules with single layered epithelia were observed in 38.4 ± 5.4% of Sertoli cell cords and tubules after 1 week, and significantly increased between 2, 3 and 4 weeks (61.4 ± 3.3, 71.8 ± 1.8, 78.9 ± 3.7%). In 2.9 ± 1.16% of all structures, lumen formation was observed after 1 week. Tubules with a fluid-filled lumen were only rarely observed after 1 week (2.9 ± 1.2%) and 2 weeks (7.9 ± 1.5%) but were more common after 3 weeks (14.1 ± 2%) and 4 weeks (19.9 ± 3.4%). Finally, after 4 weeks of xenografting, Sertoli cell tubules became increasingly complex and were organized in clusters of several tubules (Fig. 3D). Most tubules showed differentiated basement membranes and recruitment of peritubular myoid cells. Endothelial and fibroblast-like cells migrated into and resided in Matrigel residues between Sertoli cell tubules.
Increased migration of cells from the host into the Matrigel was observed after 8 and 12 weeks (Fig. 3E and F). At all late time points investigated, Sertoli cell tubules were associated with extra-tubular cells. After 12 weeks of development in the host, some Sertoli cell tubules began to degenerate and the epithelial organization deteriorated (Fig. 3F).
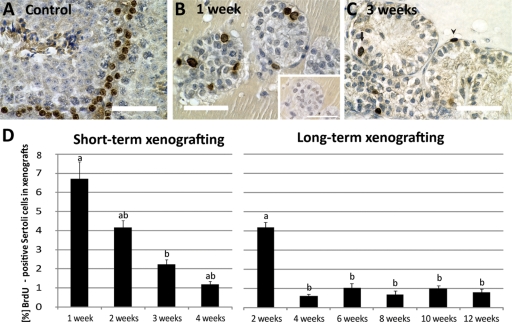
Proliferation of Sertoli cells in xenografts
After 1 week of grafting, Sertoli cells in cord-like structures were actively proliferating (Fig. 4B). The Sertoli cell labelling index (LI) for BrdU was 6.7 ± 0.9%. In xenografts after 3 weeks we observed only few cells per tubule that were labelled with BrdU (Fig. 4C), and the BrdU LI was significantly lower after 3 and 4 weeks (LI 2.2 ± 0.3 and 1.2 ± 0.1%, respectively) (n = 10–24 grafts; Fig. 4D). In xenografts after 2 and 3 weeks we also observed peritubular myoid cells occasionally labelled with BrdU. These cells were excluded from the BrdU-LI. Consistent with this result we also observed a decrease of the BrdU-labelling during long-term xenografting. The Sertoli cell LI was significantly higher at 2 weeks (4.2 ± 0.3%) than after 4, 6, 8, 10 and 12 weeks (0.62 ± 0.1, 1 ± 0.2, 0.7 ± 0.2, 1 ± 0.2, 0.8 ± 0.2%, respectively; n = 5–13 grafts) (Fig. 4D).
Figure 4.
Proliferation of Sertoli cells in xenografts.
Sertoli cells in S-phase were labelled by BrdU-incorporation and detected using an anti-BrdU primary antibody and visualized with DAB (brown precipitate in cell nucleus). Sections were counterstained with hematoxylin. Scale bars = 50 µm. (A) Positive control. In adult mouse testis, type A and B spermatogonia are the only proliferating cell types. (B) Sertoli cell cords actively proliferate in xenografts after 1 week. Inset: negative control. The same cord in an adjacent cross section that was incubated without primary antibody. No specific staining was visible in Sertoli cells in this cord. (C) Xenograft after 3 weeks. Sertoli cell proliferation decreased (arrow). Proliferating peritubular cells surrounding the Sertoli cell tubule were frequently observed (arrowhead). (D) BrdU-LI after short-term and long-term xenografting. Left panel: in the short-term study, Sertoli cell proliferation significantly declined from Week 1 to Week 3 (P < 0.05, ANOVA on ranks, n = 10–24 xenografts). Right panel: similarly, Sertoli cell proliferation was significantly higher at 2 weeks than in later time points (P < 0.05, ANOVA, n = 5–13 grafts). A low proliferation rate (∼1%) was observed at all time points up to 12 weeks.
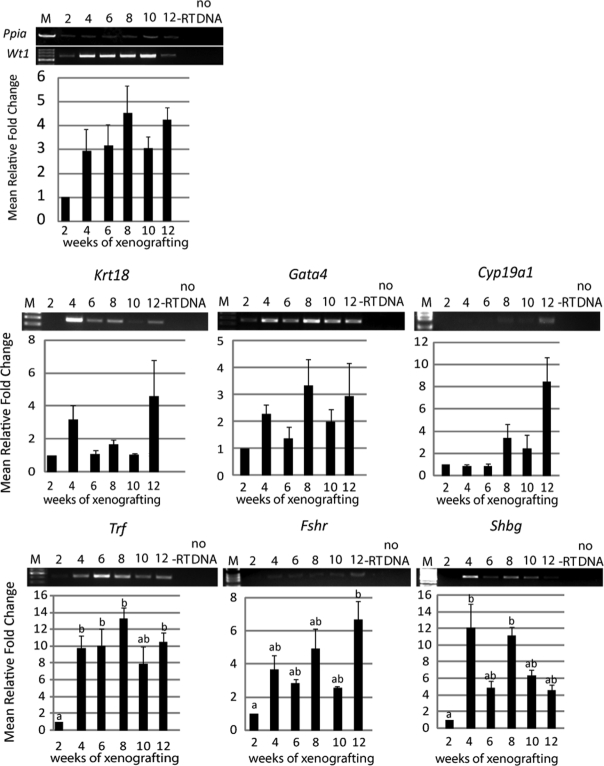
Expression of Sertoli cell immaturity and differentiation markers in xenografts
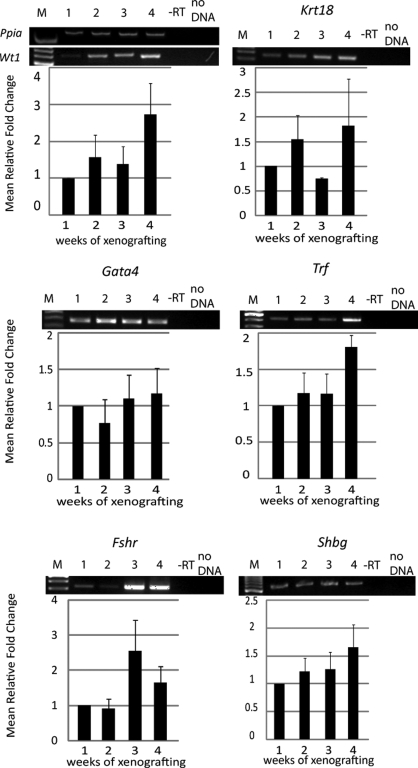
The presence of Sertoli cell differentiation markers were assessed to determine whether populations of proliferating or differentiating Sertoli cells are present within the developing xenografts. Wt1 mRNA expression was assayed as a positive control for Sertoli cell presence and was detected at all time points tested (Figs 5 and 6). During short-term grafting, markers of less mature, proliferating Sertoli cells Gata4 and Krt18 were expressed at relative constant levels throughout the development of the xenografts (Fig. 5). In contrast, Amh expression was low and could only be observed sporadically (in two out of five experiments) during the first 3 weeks of grafting, and could not be detected thereafter (data not shown). Cyp19a1 expression was not observed after 1 week of xenografting, and weak expression was occasionally observed after 2 weeks (in one out of five experiments) and 4 weeks (in three out of five experiments, data not shown). The expression of differentiation markers Trf, Fshr and Shbg were observed at all time points (Fig. 5). Overall, for the short-term xenografts mRNA levels remained low and semi-quantitative RT–PCR evaluation did not reveal statistically significant changes over the course of the study for any of the markers.
Figure 5.
Expression of Sertoli cell differentiation markers during short-term grafting.
mRNA isolated from xenografts after 1–4 weeks was analyzed by RT–PCR (n = 3–5). In control experiments, PCR reactions were performed on RT reactions without reverse transcriptase (−RT) and in the absence of cDNA (no DNA). Average expression levels were normalized to Ppia, and the mean (±SEM) fold change expressed relative to 1 week (scaled to 1). M = 100 bp DNA ladder. Expression of Wt1, known to be expressed in Sertoli cells at all developmental stages was detected at all time points. The expression patterns of Krt18, Gata4, Trf, Fshr and Shbg were examined.
Figure 6.
Expression of Sertoli cell differentiation markers during long-term grafting.
mRNA isolated from xenografts after 2–12 weeks was analyzed by RT–PCR (n = 3). In control experiments, PCR reactions were performed on RT reactions without reverse transcriptase (−RT) and in the absence of cDNA (no DNA). Average expression levels were normalized to Ppia, and the mean (±SEM) fold change expressed relative to 2 weeks (scaled to 1). M = 100 bp DNA ladder. Expression of Wt1 was detected throughout all time points. Expression of Krt18, Gata4, Cyp19a1 Trf, Fshr and Shbg were examined. Different lower case letters indicate statistically significant changes of expression (P < 0.05).
During long-term xenografting, Krt18 and Gata4 levels remained constant at low levels during the course of the study (Fig. 6). Interestingly, Cyp19a1 was initially absent but expression increased after 8 weeks. Similar to the results obtained during short-term grafting, Amh expression could not be detected in long-term xenografts (data not shown). In contrast, up-regulation of Trf, Fshr and Shbg expression was observed during xenografting. Trf mRNA became up-regulated after 4 weeks and significantly increased between 2, 8 and 12 weeks. Fshr expression significantly increased between 2 and 12 weeks, and Shbg expression was up-regulated between 2, 8 and 12 weeks. Overall, expression of these markers was stronger at later time points than observed during the short-term experiment.
Recruitment of peritubular myoid cells to xenografts
Peritubular myoid cells were identified by cytoplasmic αSMA-staining (Fig. 7). After 1 week, only few cord-like structures were associated with αSMA-positive cells. The connective tissue capsule surrounding xenografts was also lined by myoid cells (Fig. 7C). After 4 weeks most Sertoli cell tubules were surrounded by a thick layer of peritubular myoid cells (Fig. 7D and E).
Figure 7.
Development of peritubular myoid cells in xenografts.
Xenografts were stained for α-smooth muscle actin (SMA, brown precipitate), a myoid and smooth muscle cell marker. Scale bars = 50 µm. (A) Positive control. A representative cross-section from a 7-day-old rat testis. Immature testicular cords are surrounded by peritubular myoid cell. (B) The same tubules as shown in A in an adjacent section that was incubated with secondary antibody only. No staining is visible in the control section. (C) Sertoli cell cord in a xenograft after 1 week. The cord is partly surrounded by peritubular myoid cells (arrow). The connective tissue capsule (c) next to the cord is aligned by myoid cells (arrow). (D) After 4 weeks, Sertoli cell tubules are surrounded by peritubular myoid cells, as indicated by positive staining for SMA. (E) Cluster of Sertoli cell tubules and inter-tubular compartment in a xenograft after 12 weeks. Peritubular myoid cells are located around Sertoli cell tubules. Single myoid cells or small groups are located within the inter-tubular compartment. (F) Higher magnification of the boxed area in E. The connective tissue capsule is covered with multi-layered myoid cells (asterisk). A SMA-positive vascular wall cell, indicating vascularization, is shown (arrow).
In addition, αSMA could be seen in cells that appeared to be vascular smooth muscle cells or pericytes as judged by their morphology, and therefore could be used as an indicator of vasculature distribution throughout the grafts. Further, the connective tissue capsule surrounding xenografts was covered with multi-layered myoid cells in many grafts (Fig. 7F).
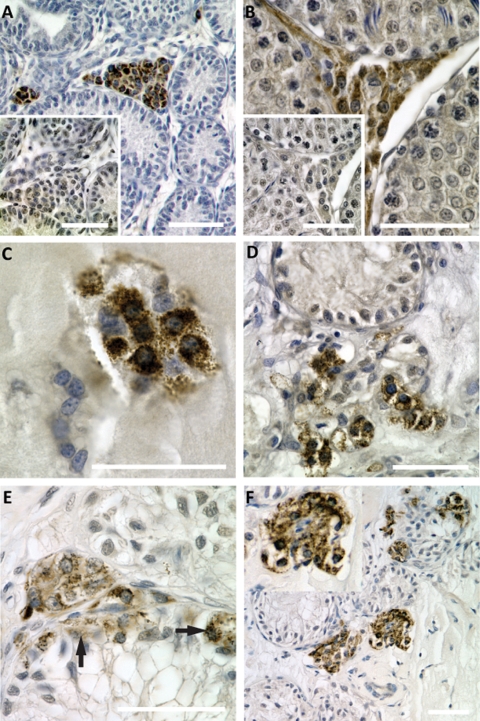
Differentiation of Leydig cells in xenografts
Small groups of Leydig cells were occasionally observed in xenografts after 1 week (Fig. 8), but the overall level of p450scc-positive cells remained low within the first 3 weeks of xenografting. After 4 weeks, Leydig cells became more abundant and were mostly located in proximity to Sertoli cell tubules (Fig. 8D). Leydig cells were also observed in xenografts after 8 weeks (Fig. 8E) and 12 weeks (Fig. 8F). Although most p450scc-positive cells were located in the interstitial compartment, we also observed intratubular p450scc-positive cells after prolonged xenografting.
Figure 8.
Development of Leydig cells in xenografts.
Paraffin embedded sections of xenografts were stained for cytochrome p450 side-chain cleavage enzyme (p450scc, Leydig cell marker, brown precipitate), and counterstained with hematoxylin. Scale bars = 50 µm. (A, B) Positive controls. Testicular cross-sections from post-natal (A) and adult (B) rats show specific staining of Leydig cells in the interstitial compartment. Insets: negative controls. The same areas as captured in A and B in adjacent sections were incubated with secondary antibody only and do not show staining for Leydig cells. (C) Small groups of p450scc positive cells were observed in xenografts after 1 week. (D) Leydig cells become more abundant in xenografts after 4 weeks, and are located close to Sertoli cell tubules. (E, F) Progressive development of Leydig cells in long-term xenografts after 8 weeks (E) and 12 weeks (F). Most p450scc-positive cells are located in the interstitial compartment. After prolonged grafting periods, p450scc-poitive cells are also observed within Sertoli cell tubules (arrows in E).
Anatomical data
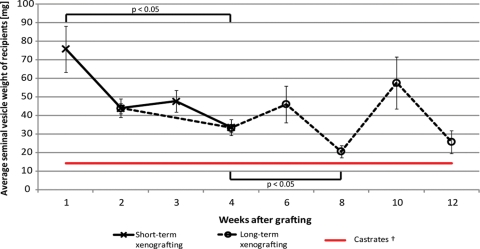
Seminal vesicle weights in graft recipients decreased between 1 and 4 weeks after castration in the short-term xenografting study (75.8 ± 12.5 versus 33.5 ± 4.2 mg; n = 10–15 mice). During long-term graft development, seminal vesicles further decreased between 2 and 8 weeks after castration (43.8 ± 2.7 versus 20.5 ± 3.2 mg; n = 6–10 mice), but remained slightly higher than the seminal vesicles weights in castrated control animals (Fig. 9).
Figure 9.
Seminal vesicle weights of castrated graft recipients.
Seminal vesicle sizes significantly decreased between 1 and 4 weeks after castration in the short-term xenografting study (ANOVA on ranks, n = 10–15; black line). During long-term graft development, seminal vesicles decreased between 2 and 8 weeks after castration (ANOVA on ranks, n = 6–10; dashed line). †Castrate level seminal vesicles derived from previous studies. Average SVW = 14.4 mg (±2.4, n = 16; red line).
Discussion
In this study we analysed Sertoli cell maturation in xenografts as a model of mammalian testicular development. We hypothesized that limited maturation of Sertoli cells in vitro is due to the lack of environmental input and can be overcome in a xenografting model. Immune rejection by the hosts in this model was rare, leading to successful growth of xenografts at all time points. The wide range of graft sizes observed could be due to the physical properties of the Matrigel vehicle. In future studies, encapsulation of Sertoli cells in alternative matrices such as alginate could improve the xenografting technique for greater reproducibility. Nevertheless, we show that Sertoli cells initiate proliferation immediately after xenografting, and that proliferation is reduced to low levels as Sertoli cell maturation is initiated. This process is complemented by the recruitment of peritubular myoid cells and Leydig cells to newly-formed seminiferous tubules.
The Sertoli cell proliferation studies indicate that Sertoli cell mitosis is transiently re-established after xenografting. BrdU-labelled cells could not be detected during three-dimensional culture, although Sertoli cells isolated from rats of this age group are highly proliferative in vivo and in conventional cell culture (Orth, 1982, Schlatt et al., 1996). In contrast, Sertoli cells initiated mitotic activity after xenografting and proliferation was maintained at high levels within the first 3 weeks after grafting. Proliferating Sertoli cells were observed at a low level for up to 12 weeks. This is in contrast to the in vivo situation, where Sertoli cell proliferation ceases around Day 15 in the rat (Clermont and Perey, 1957; Steinberger and Steinberger, 1971, Orth, 1982). These data are also correlated with the observation of immature Sertoli cell cords in xenografts throughout the study period until they start to deteriorate. Therefore, our results suggest that Sertoli cell tubules progressively differentiated in xenografts up to 12 weeks. Further, the data suggest that a heterogeneous Sertoli cell population consisting of proliferating and differentiated Sertoli cells is present in xenografts, and that Sertoli cell maturation occurs in a non-synchronized manner. This could be caused by local differences in the graft environment due to uneven vascular development. A similar effect was reported before by Rathi et al. (2008) for the maturation of immature rhesus monkey testicular grafts.
This observation is further supported by data obtained from mRNA expression studies of xenografts. Specifically, the expression of immature Sertoli cell markers Krt18 and Gata4 persisted at low levels for at least 12 weeks after xenografting. In the case of Gata4 it has been proposed that Gata4 expression remains constant in adult Sertoli cells (Imai et al., 2004), and it was speculated that the previously reported decline of Gata4 after post-natal Day 14 might be due to a dilution effect caused by increasing numbers of germ cells. Accordingly, the absence of germ cells in this model might be accountable for the persistence of constant Gata4 mRNA in xenografts. Cyp19a1 mRNA expression increased with time, counter to the expected down-regulation during Sertoli cell differentiation. However, Cyp19a1 is also expressed in Leydig cells and germ cells in the adult testis, but is absent from peritubular myoid cells (Levallet et al., 1998; Lambard et al., 2005). Taking into account the increasing number of Leydig cells at the later time points, it seems plausible that these cells might account for the increase in total aromatase mRNA expression in xenografts.
The gene expression studies further indicated that Sertoli cells matured after xenografting, in contrast to Sertoli cells during in vitro culture that showed low levels or absent expression of Trf, Fshr and Shbg. In contrast, expression of Trf, Shbg and Fshr mRNA indicated the presence of differentiated Sertoli cells. These genes have been shown to be expressed at low levels during early post-natal life and to become up-regulated as Sertoli cells mature and cease proliferation (around Day 15 post-partum in the rat). Up-regulation of these markers during long-term xenografting therefore indicates that Sertoli cells in the testis and in xenografts develop with similar kinetics.
The importance of peritubular myoid cells for tubulogenesis becomes apparent in this study. Immunohistochemistry showed an increase in peritubular myoid cells aligned along Sertoli cell tubules with time, coinciding with the emergence of polarized Sertoli cell epithelial organization and tubule growth. The formation of a basement membrane in cooperation by Sertoli cells and peritubular myoid cells appears to be of crucial importance for continuing Sertoli cell maturation. In in vivo studies it has been shown that impaired peritubular myoid cell proliferation and differentiation during post-natal testicular development causes decreased testicular size and disturbed longitudinal growth of seminiferous tubules in the adult testis (Nurmio et al., 2007). This is in line with observations from knock-out models that show testicular pathology due to abnormal peritubular tissue differentiation. In Dhh (desert hedgehog)-null mice, a focally absent basal lamina leads to ectopical gonocytes and apolar Sertoli cells in the adult testis (Clark et al., 2000). Targeted deletion of Dax1 (dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X chromosome, gene 1) in mice results in decreased numbers of peritubular myoid cells at embryonic Day 13.5 testis (Park et al., 2005) and the resulting phenotype shows a disrupted basal lamina and incompletely formed testicular cords.
It has long been speculated that precursor cells for peritubular myoid cells are among the migrating mesonephric cell population that enters the gonad during embryonic cord formation, a crucial event for testicular morphogenesis (Tilmann and Capel, 1999). More recently it was reported that peritubular myoid cells do not enter the gonad from the mesonephros (Cool et al., 2008), but rather are established through cell proliferation of a resident precursor cell population within the gonad. Both mechanisms were observed in our model. Firstly, peritubular myoid cells were detected in the initial cell suspension used for three-dimensional cell culture and were found to be important for in vitro morphogenesis of cord-like structures (Gassei et al., 2006). Additionally, immunofluorescence staining for αSMA on Sertoli cell aggregates isolated from Matrigel revealed the integration of myoid cells in the cord-like structures at the time of grafting. These observations lead to the conclusion that Sertoli cells and myoid cells likewise contribute to cord-formation in vitro and in xenografts. In addition, proliferating peritubular cells were occasionally observed for up to 3 weeks in xenografts. On the other hand, myoid cells were present in grafts after 1 week, and these cells were found to be aligned along the mesenchymal capsule of xenografts, independent of Sertoli cell cords. At later time points, these cells appeared to migrate into xenografts together with fibroblast-like cells and endothelial cells. Thus, it is possible that the peritubular cells present in aggregates at the time of grafting initially differentiate and contribute to tubule differentiation. However, the increasing number of invading mesenchymal precursor cells could also be responsible for the formation of intact layers of myoid cells surrounding the Sertoli cell tubules in xenografts.
In vivo, steroidogenic Leydig cells reside within the interstitium. We used cytochrome p450 side-chain cleavage enzyme (p450scc), the initial and rate-limiting enzyme during androgen biosynthesis, as a marker for Leydig cells (Payne and Youngblood, 1995) to examine if they would be recruited to the interstitial compartment in xenografts. Histological analysis showed the development of interstitial compartments around 4 weeks after grafting. Within this compartment, the presence of p450scc-positive putative Leydig cells was low during the first weeks of xenografting and high at 8–12 weeks. The initial single cell suspension used for three-dimensional culture contained only a low number of Leydig cells, as previously determined (Gassei et al., 2008). Furthermore, immunohistochemistry for p450scc on isolated Sertoli cell aggregates did not reveal the integration of Leydig cells in Sertoli cell aggregates at the time of grafting (data not shown). However, as the presence of the p450scc enzyme alone is not sufficient for androgen production, we also analysed the size of seminal vesicles from castrated host mice. As expected, seminal vesicles rapidly decreased in size after castration, but after prolonged xenografting their weights were slightly above those reported in castrated nude mice in previous studies (Schlatt et al., 2003l; Ehmcke et al., 2008), indicating low levels of bioactive testosterone in the castrated host mice. We therefore suggest that a small population of steroidogenic Leydig cells differentiated from mesenchymal precursor cells that migrated into xenografts.
We conclude that immature Sertoli cells in xenografts continue to mature beyond the developmental state at post-natal Day 7, the day of cell isolation, and we further show that this maturation could not be achieved by in vitro culture alone. Sertoli cells in xenografts cease to proliferate and morphologically mature, and this is concurrent with the up-regulation of Fshr, Shbg and Trf. We further conclude that progressive migration of fibroblast-like cells into xenografts is crucial for the differentiation of peritubular myoid cells and Leydig cells in xenografts and the overall differentiation of a somatic testicular environment. However, seminiferous tubules in this study showed a ‘Sertoli cell only’ (SCO)-phenotype, and it remains unclear if these tubules would be potentially capable of maintaining spermatogonia. Nevertheless, the presence of all somatic testicular components that are seen in vivo indicate that this model will be helpful for future questions addressing Sertoli cell maturation and testicular differentiation in mammals.
Funding
This work was supported by start up funds from the University Münster, the National Institute of Health (U54 HD 008610 Center grant to S.S. and W.H.W.), a doctoral scholarship from the Ernst Schering Research Foundation (to K.G.), and a Young Investigator Grant from the Lance Armstrong Foundation (to J.E.).
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Ando S, Sirianni R, Forastieri P, Casaburi I, Lanzino M, Rago V, Giordano F, Giordano C, Carpino A, Pezzi V. Aromatase expression in prepuberal Sertoli cells: effect of thyroid hormone. Mol Cell Endocrinol. 2001;178:11–21. doi: 10.1016/s0303-7207(01)00443-9. [DOI] [PubMed] [Google Scholar]
- Carpino A, Pezzi V, Rago V, Bilinska B, Ando S. Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell. 2001;33:349–353. doi: 10.1054/tice.2001.0186. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat. 1957;100:241–267. doi: 10.1002/aja.1001000205. [DOI] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2:128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmcke J, Gassei K, Schlatt S. Ectopic testicular xenografts from newborn hamsters (Phodopus sungorus) show better spermatogenic activity in aged compared with young recipients. J Exp Zool. 2008;309:278–287. doi: 10.1002/jez.459. [DOI] [PubMed] [Google Scholar]
- Gassei K, Schlatt S, Ehmcke J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl. 2006;27:611–618. doi: 10.2164/jandrol.05207. [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Schlatt S. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction. 2008;136:459–469. doi: 10.1530/REP-08-0241. [DOI] [PubMed] [Google Scholar]
- Hagenas L, Ritzen EM, Plooen L, Hansson V, French FS, Nayfeh SN. Sertoli cell origin of testicular androgen-binding protein (ABP) Mol Cell Endocrinol. 1975;2:339–350. doi: 10.1016/0303-7207(75)90021-0. [DOI] [PubMed] [Google Scholar]
- Imai T, Kawai Y, Tadokoro Y, Yamamoto M, Nishimune Y, Yomogida K. In vivo and in vitro constant expression of GATA-4 in mouse postnatal Sertoli cells. Mol Cell Endocrinol. 2004;214:107–115. doi: 10.1016/j.mce.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999;140:1470–1480. doi: 10.1210/endo.140.3.6587. [DOI] [PubMed] [Google Scholar]
- Lambard S, Silandre D, Delalande C, Denis-Galeraud I, Bourguiba S, Carreau S. Aromatase in testis: Expression and role in male reproduction. J Steroid Biochem Mol Biol. 2005;95:63–69. doi: 10.1016/j.jsbmb.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol Reprod. 1998;58:919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- Lui WY, Mruk D, Lee WM, Cheng CY. Sertoli cell tight junction dynamics: their regulation during spermatogenesis. Biol Reprod. 2003;68:1087–1097. doi: 10.1095/biolreprod.102.010371. [DOI] [PubMed] [Google Scholar]
- Nurmio M, Toppari J, Zaman F, Andersson AM, Paranko J, Soder O, Jahnukainen K. Inhibition of tyrosine kinases PDGFR and C-Kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int J Androl. 2007;30:366–376. doi: 10.1111/j.1365-2605.2007.00755.x. [DOI] [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- Paranko J, Kallajoki M, Pelliniemi LJ, Lehto VP, Virtanen I. Transient coexpression of cytokeratin and vimentin in differentiating rat Sertoli cells. Dev Biol. 1986;117:35–44. doi: 10.1016/0012-1606(86)90345-3. [DOI] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132:2415–2423. doi: 10.1242/dev.01826. [DOI] [PubMed] [Google Scholar]
- Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- Pelliniemi LJ, Paranko J, Grund SK, Frojdman K, Foidart J-M, Lakkala-Paranko T. Morphological differentiation of Sertoli cells. In: Saez JJM, Forest MG, DaZord A, Bertrand J, editors. Les colloques de l'INSERM: Recent Progress in Cellular Endocrinology of the Testis. Vol. 123. Paris, France: INSERM; 1984. pp. 121–140. [Google Scholar]
- Perez-Infante V, Bardin CW, Gunsalus GL, Musto NA, Rich KA, Mather JP. Differential regulation of testicular transferrin and androgen-binding protein secretion in primary cultures of rat Sertoli cells. Endocrinology. 1986;118:383–392. doi: 10.1210/endo-118-1-383. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Panno ML, Sirianni R, Forastieri P, Casaburi I, Lanzino M, Rago V, Giordano F, Giordano C, Carpino A, et al. Effects of tri-iodothyronine on alternative splicing events in the coding region of cytochrome P450 aromatase in immature rat Sertoli cells. J Endocrinol. 2001;170:381–393. doi: 10.1677/joe.0.1700381. [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- Rathi R, Zeng W, Megee S, Conley A, Meyers S, Dobrinski I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149:5288–5296. doi: 10.1210/en.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol. 2003;211:21–31. doi: 10.1016/j.mce.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Rich KA, Bardin CW, Gunsalus GL, Mather JP. Age-dependent pattern of androgen-binding protein secretion from rat Sertoli cells in primary culture. Endocrinology. 1983;113:2284–2293. doi: 10.1210/endo-113-6-2284. [DOI] [PubMed] [Google Scholar]
- Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat. 1989;184:179–189. doi: 10.1002/aja.1001840302. [DOI] [PubMed] [Google Scholar]
- Schlatt S, de Kretser DM, Loveland KL. Discriminative analysis of rat Sertoli and peritubular cells and their proliferation in vitro: evidence for follicle-stimulating hormone-mediated contact inhibition of Sertoli cell mitosis. Biol Reprod. 1996;55:227–235. doi: 10.1095/biolreprod55.2.227. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorder of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Griswold MD. Sertoli cells synthesize and secrete transferrin-like protein. J Biol Chem. 1980;255:9523–9525. [PubMed] [Google Scholar]
- Steger K, Rey R, Kliesch S, Louis F, Schleicher G, Bergmann M. Immunohistochemical detection of immature Sertoli cell markers in testicular tissue of infertile adult men: a preliminary study. Int J Androl. 1996;19:122–128. doi: 10.1111/j.1365-2605.1996.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Steger K, Rey R, Louis F, Kliesch S, Behre HM, Nieschlag E, Hoepffner W, Bailey D, Marks A, Bergmann M. Reversion of the differentiated phenotype and maturation block in Sertoli cells in pathological human testis. Hum Reprod. 1999;14:136–143. doi: 10.1093/humrep/14.1.136. [DOI] [PubMed] [Google Scholar]
- Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod. 1971;4:84–87. doi: 10.1093/biolreprod/4.1.84. [DOI] [PubMed] [Google Scholar]
- Thanki KH, Steinberger A. Effect of age and hypophysectomy on FSH binding by rat testes. Andrologia. 1978;10:195–202. doi: 10.1111/j.1439-0272.1978.tb03016.x. [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Tindall DJ, Vitale R, Means AR. Androgen binding protein as a biochemical marker of formation of the blood-testis barrier. Endocrinology. 1975;97:636–648. doi: 10.1210/endo-97-3-636. [DOI] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125:2665–2675. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]