Abstract
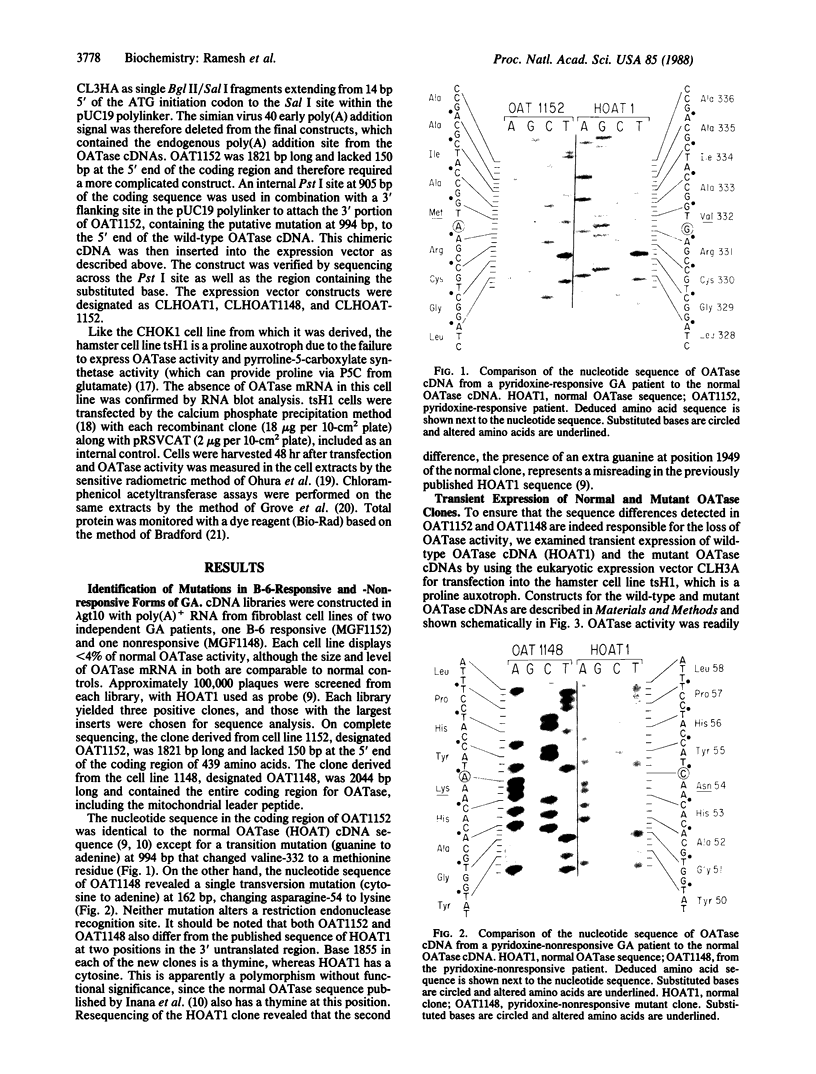
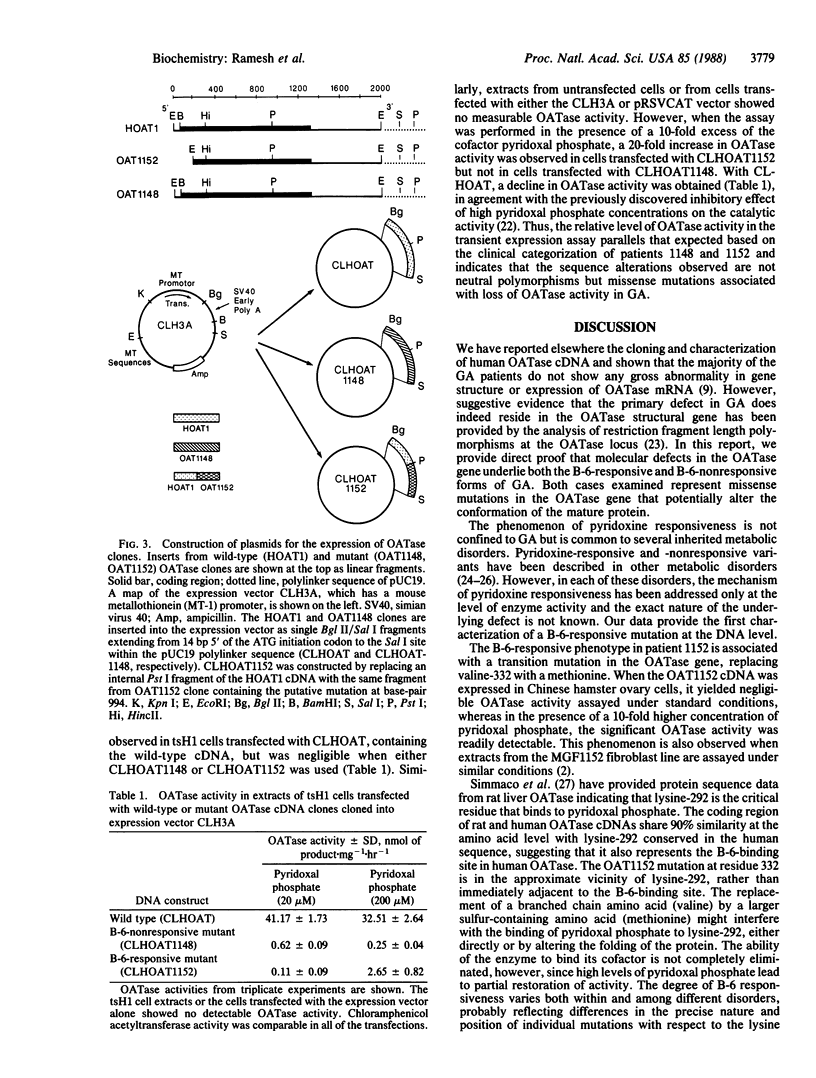
Gyrate atrophy (GA), a recessive eye disease involving progressive loss of vision due to chorioretinal degeneration, is associated with a deficiency of the mitochondrial enzyme ornithine aminotransferase (OATase; ornithine-oxo-acid aminotransferase; L-ornithine:2-oxo-acid aminotransferase, EC 2.6.1.13) with consequent hyperornithinemia. Genetic heterogeneity of GA has been suggested by the demonstration that administration of pyridoxine to increase the level of pyridoxal phosphate, a cofactor of OATase, reduces hyperornithinemia in a subset of patients. We have cloned and sequenced cDNAs for OATase from two GA patients, one responsive and one nonresponsive to pyridoxine treatment. The respective cDNAs contained different single missense mutations, which were sufficient to eliminate OATase activity when each cDNA was tested in a eukaryotic expression system. However, like the enzyme in fibroblasts from the pyridoxine-responsive patient, OATase encoded by the corresponding cDNA from this individual showed a significant increase in activity when assayed in the presence of an increased pyridoxal phosphate concentration. These data firmly establish that both pyridoxine responsive and nonresponsive forms of GA result from mutations in the OATase structural gene. Moreover, they provide a molecular characterization of the primary lesion in a pyridoxine-responsive genetic disorder.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Schmidt S. Y., Shih V. E. Ocular and biochemical abnormalities in gyrate atrophy of the choroid and retina. Ophthalmology. 1978 Oct;85(10):1018–1027. doi: 10.1016/s0161-6420(78)35588-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Fowler B. Recent advances in the mechanism of pyridoxine-responsive disorders. J Inherit Metab Dis. 1985;8 (Suppl 1):76–83. doi: 10.1007/BF01800664. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Price D. J., Goodman H. M., Avruch J. Recombinant fragment of protein kinase inhibitor blocks cyclic AMP-dependent gene transcription. Science. 1987 Oct 23;238(4826):530–533. doi: 10.1126/science.2821622. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Inana G., Totsuka S., Redmond M., Dougherty T., Nagle J., Shiono T., Ohura T., Kominami E., Katunuma N. Molecular cloning of human ornithine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1203–1207. doi: 10.1073/pnas.83.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway N. G., Weleber R. G., Buist N. R. Gyrate atrophy of the choroid and retina with hyperornithinemia: biochemical and histologic studies and response to vitamin B6. Am J Hum Genet. 1980 Jul;32(4):529–541. [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J., Rosenberg L. E. On the mechanism of pyridoxine responsive homocystinuria. II. Properties of normal and mutant cystathionine beta-synthase from cultured fibroblasts. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4821–4825. doi: 10.1073/pnas.71.12.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Looney J., Steel G., Suchanek M., Dowling C., Der Kaloustian V., Kaiser-Kupfer M., Valle D. An initiator codon mutation in ornithine-delta-aminotransferase causing gyrate atrophy of the choroid and retina. J Clin Invest. 1988 Feb;81(2):630–633. doi: 10.1172/JCI113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H. Pyridoxine-responsive genetic disease. Fed Proc. 1971 May-Jun;30(3):970–976. [PubMed] [Google Scholar]
- O'Donnell J. J., Sandman R. P., Martin S. R. Deficient L-ornithine: 2-oxoacid aminotransferase activity in cultured fibroblasts from a patient with gyrate atrophy of the retina. Biochem Biophys Res Commun. 1977 Nov 21;79(2):396–399. doi: 10.1016/0006-291x(77)90170-x. [DOI] [PubMed] [Google Scholar]
- Ohura T., Kominami E., Katunuma N. A new sensitive and convenient assay of ornithine aminotransferase. J Nutr Sci Vitaminol (Tokyo) 1983 Apr;29(2):123–128. doi: 10.3177/jnsv.29.123. [DOI] [PubMed] [Google Scholar]
- Pascal T. A., Gaull G. E., Beratis N. G., Gillam B. M., Tallan H. H., Hirschhorn K. Vitamin B6-responsive and -unresponsive cystathioninuria: two variant molecular forms. Science. 1975 Dec 19;190(4220):1209–1211. doi: 10.1126/science.1198108. [DOI] [PubMed] [Google Scholar]
- Peraino C. Functional properties of ornithine-ketoacid aminotransferase from rat liver. Biochim Biophys Acta. 1972 Nov 10;289(1):117–127. doi: 10.1016/0005-2744(72)90114-3. [DOI] [PubMed] [Google Scholar]
- Ramesh V., Benoit L. A., Crawford P., Harvey P. T., Shows T. B., Shih V. E., Gusella J. F. The ornithine aminotransferase (OAT) locus: analysis of RFLPs in gyrate atrophy. Am J Hum Genet. 1988 Feb;42(2):365–372. [PMC free article] [PubMed] [Google Scholar]
- Ramesh V., Eddy R., Bruns G. A., Shih V. E., Shows T. B., Gusella J. F. Localization of the ornithine aminotransferase gene and related sequences on two human chromosomes. Hum Genet. 1987 Jun;76(2):121–126. doi: 10.1007/BF00284906. [DOI] [PubMed] [Google Scholar]
- Ramesh V., Shaffer M. M., Allaire J. M., Shih V. E., Gusella J. F. Investigation of gyrate atrophy using a cDNA clone for human ornithine aminotransferase. DNA. 1986 Dec;5(6):493–501. doi: 10.1089/dna.1.1986.5.493. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Garramone A. J., Sasak H., Wei C. M., Watkins P., Galli J., Hsiung N. Expression of human uterine tissue-type plasminogen activator in mouse cells using BPV vectors. DNA. 1987 Oct;6(5):461–472. doi: 10.1089/dna.1987.6.461. [DOI] [PubMed] [Google Scholar]
- Shih V. E., Berson E. L., Mandell R., Schmidt S. Y. Ornithine ketoacid transaminase deficiency in gyrate atrophy of the choroid and retina. Am J Hum Genet. 1978 Mar;30(2):174–179. [PMC free article] [PubMed] [Google Scholar]
- Simmaco M., John R. A., Barra D., Bossa F. The primary structure of ornithine aminotransferase. Identification of active-site sequence and site of post-translational proteolysis. FEBS Lett. 1986 Apr 7;199(1):39–42. doi: 10.1016/0014-5793(86)81219-4. [DOI] [PubMed] [Google Scholar]
- Sipilä I., Simell O., O'Donnell J. J. Gyrate atrophy of the choroid and retina with hyperornithinemia: characterization of mutant liver L-ornithine:2-oxoacid aminotransferase kinetics. J Clin Invest. 1981 Jun;67(6):1805–1807. doi: 10.1172/JCI110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Phang J. M. The importance of ornithine as a precursor for proline in mammalian cells. J Cell Physiol. 1979 Mar;98(3):475–481. doi: 10.1002/jcp.1040980306. [DOI] [PubMed] [Google Scholar]
- Tada K., Yokoyama Y., Nakagawa H., Arakawa T. Vitamin B6 dependent xanthurenic aciduria (the second report). Tohoku J Exp Med. 1968 Jun;95(2):107–114. doi: 10.1620/tjem.95.107. [DOI] [PubMed] [Google Scholar]
- Valle D., Kaiser-Kupfer M. I., Del Valle L. A. Gyrate atrophy of the choroid and retina: deficiency of ornithine aminotransferase in transformed lymphocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5159–5161. doi: 10.1073/pnas.74.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]




